Abstract
Myosin Va is associated with discrete vesicle populations in a number of cell types, but little is known of the function of myosin Vb. Yeast two-hybrid screening of a rabbit parietal cell cDNA library with dominant active Rab11a (Rab11aS20V) identified myosin Vb as an interacting protein for Rab11a, a marker for plasma membrane recycling systems. The isolated clone, corresponding to the carboxyl terminal 60 kDa of the myosin Vb tail, interacted with all members of the Rab11 family (Rab11a, Rab11b, and Rab25). GFP-myosin Vb and endogenous myosin Vb immunoreactivity codistributed with Rab11a in HeLa and Madin-Darby canine kidney (MDCK) cells. As with Rab11a in MDCK cells, the myosin Vb immunoreactivity was dispersed with nocodazole treatment and relocated to the apical corners of cells with taxol treatment. A green fluorescent protein (GFP)-myosin Vb tail chimera overexpressed in HeLa cells retarded transferrin recycling and caused accumulation of transferrin and the transferrin receptor in pericentrosomal vesicles. Expression of the myosin Vb tail chimera in polarized MDCK cells stably expressing the polymeric IgA receptor caused accumulation of basolaterally endocytosed polymeric IgA and the polymeric IgA receptor in the pericentrosomal region. The myosin Vb tail had no effects on transferrin trafficking in polarized MDCK cells. The GFP-myosin Va tail did not colocalize with Rab11a and had no effects on recycling system vesicle distribution in either HeLa or MDCK cells. The results indicate myosin Vb is associated with the plasma membrane recycling system in nonpolarized cells and the apical recycling system in polarized cells. The dominant negative effects of the myosin Vb tail chimera indicate that this unconventional myosin is required for transit out of plasma membrane recycling systems.
INTRODUCTION
The class V myosins are one of the best characterized groups of unconventional myosins (Reck-Peterson et al., 2000), and myosin Va has been implicated in the regulation of vesicle trafficking in neurons and melanocytes (Evans et al., 1998; Tabb et al., 1998; Wu et al., 1998). The dilute mutation in myosin Va leads to mental retardation and impaired melanocyte function (Griscelli syndrome in humans) (Mercer et al., 1991; Provance et al., 1996). Myosin Va regulates melanosome distribution along microfilaments (Provance et al., 1996; Nascimento et al., 1997; Rogers and Gelfand, 1998) and is also found in association with centrosomes (Bonafe and Sellers, 1998). In addition, myosin Va has been implicated in the regulation of endoplasmic reticulum-to-Golgi trafficking in neurons (Tabb et al., 1998). A number of studies suggest that class V myosins associate with membrane vesicles through their carboxyl-terminal tails (Catlett and Weisman, 1998; Schott et al., 1999; Tsakraklides et al., 2000). Miller and Sheetz (2000) have reported recently a stoichiometry of 100 myosin Va molecules per brain vesicle.
Although the association of myosin Va with membrane-bounded organelles is well established, the precise function of myosin Va in regulating vesicle trafficking has been more obscure. Recently, Bretscher and colleagues (Schott et al., 1999) have suggested, based on genetic evidence, that the yeast Rab protein Sec4 interacts with the carboxyl terminus of Myo2p, a yeast class V myosin. These results suggested that the association of myosin V subclass family members with discrete vesicle populations may be regulated by Rab small GTP-binding protein family members. Although it is well-established that Rab proteins are localized to specific subcellular vesicle populations, the identities of their downstream effectors remain generally unknown.
Recycling of internalized plasma membrane receptors, pumps, and channels is fundamental to the maintenance of normal membrane composition in both nonpolarized and polarized cells (Mostov et al., 1992). The Rab11 family of small GTP-binding proteins in mammalian cells consists of three proteins: Rab11a (Kikuchi et al., 1988; Chavrier et al., 1990; Goldenring et al., 1994), Rab11b (Lai et al., 1994), and Rab25 (Goldenring et al., 1993). A number of investigations in both polarized and nonpolarized cells have demonstrated that Rab11a is associated with plasma membrane recycling systems. In nonpolarized cells, transferrin trafficks into Rab11a-containing vesicles (Ullrich et al., 1996; Green et al., 1997) and a dominant-negative mutant (Rab11aS25N) inhibits plasma membrane recycling (Ullrich et al., 1996; Ren et al., 1998). In polarized Madin-Darby canine kidney (MDCK) cells, Rab11a and Rab25 are both associated with the apical recycling system and overexpression of Rab25 inhibits transcytosis and apical recycling (Casanova et al., 1999). Gastric parietal cells appear to possess the highest endogenous levels of Rab11a of any eukaryotic cell (Goldenring et al., 1994). Rab11a is contained on tubulovesicles that mediate the regulated apical recycling of the H/K-ATPase responsible for luminal acid secretion from gastric parietal cells (Calhoun and Goldenring, 1997). Thus, the parietal cell represents an example of an amplified apical recycling system (Forte and Yao, 1996). Transfection of rabbit parietal cells with a dominant negative Rab11a inhibits regulated apical trafficking of the H/K-ATPase (Duman et al., 1999).
With the use of yeast two-hybrid screening of a parietal cell cDNA library, we have now identified myosin Vb as a putative regulator of Rab11a-containing plasma membrane recycling systems. Myosin Vb associates with plasma membrane recycling systems in both polarized and nonpolarized cells. In addition, we have found that expression of the carboxyl terminal 60 kDa of myosin Vb, lacking the motor domain, retards trafficking through plasma membrane recycling systems. These studies indicate that myosin Vb is involved in mediating transit out of the plasma membrane recycling system.
MATERIALS AND METHODS
Yeast Two-Hybrid Screening
A rabbit gastric parietal cell cDNA library was constructed in pADGAL (Stratagene, La Jolla, CA) from polyA+ mRNA isolated from 98% purity parietal cells. The library was constructed from cDNAs primed with oligo-dT and the average clone length was 2.0 kb. The Rab11aS20V, Rab25S21V, Rab11aS25N, Rab11aT43A, Rab11aI44N mutations were constructed by single base pair (bp) replacements in wild-type Rab11a or Rab25 cloned into pBDGAL(CaM) with the use of a two complementary oligonucleotide method with Pfu polymerase (Stratagene). Rab11aS20VΔC, Rab25ΔC, rabbit Rab2, rat Rab3a, and murine Rab11b were amplified with Pfu polymerase with the use of sense and antisense primers containing restriction endonuclease sites and the cloned products were ligated into pBDGAL(CaM). Ezrin was cloned into pBDGAL(CaM) as previously described (Bhartur and Goldenring, 1998). The veracity of all constructs was confirmed by automated sequencing in the Medical College of Georgia Molecular Biology Core Facility. The Y190 yeast strain was transfected with pBDGAL(CaM)-Rab11aS20VΔC and a stable bait line was isolated on tryptophan-deficient media. The bait strain was then transfected with 10 μg of library plasmid and plated on media deficient in leucine, tryptophan, and histidine with 2.5 mM 3-aminotriazole. After 4 d of growth, colonies were lifted onto filter paper and incubated with Xgal. Interactions were considered positive if blue β-galactosidase reaction product was evident after 4 h of incubation at room temperature. Positive clones were regrown on triple deficient media and beta-galactosidase activity was reconfirmed. Plasmids were then rescued from yeast and transformed into XL-1 Blue bacteria with selection on ampicillin. Isolated plasmids were sequenced by automated sequencing with the use of flanking vector sequences and primer walking.
For binary screening, the pADGAL-myosin Vb tail plasmid was dual transfected into Y109 yeast with Rab bait plasmids. Yeast were plated on leucine and tryptophan dual deficient media and allowed to grow for 4 d. Colonies were lifted onto filter paper and screened as described above. Expression of bait proteins was confirmed in lysates of yeast proteins on Western blots with the use of antibodies against the binding domain of GAL4 (CLONTECH, Palo Alto, CA). The paralogous region of chicken myosin Va (a gift of Dr. Richard Cheney, University of North Carolina, Chapel Hill, NC) was amplified with Pfu polymerase and cloned into pADGAL. The pADGAL-myosin Va tail plasmid was dual transfected into yeast with selected pBDGAL(CaM)-Rab11 family member plasmids and interactions were tested on filter paper lifts. For truncation of amino acids 1–100 of the myosin Vb tail, pADGAL-myosin Vb was cut with PvuI and SmaI and religated. Similarly, amino acids 1–188 of the myosin Vb were generated with SmaI and SfoI digestion of pADGAL-myosin Vb and religation. All other myosin Vb truncations were constructed by amplification of specific sequences with Pfu polymerase and cloning into pADGAL. The veracity of all amplified sequences was confirmed by automated sequencing.
Targeting the Green Fluorescent Protein (GFP)-Myosin Vb Tail Construct and GFP-Myosin Vb
The rabbit myosin Vb carboxyl-terminal coding sequence isolated from the two-hybrid screen was amplified from the pADGAL plasmid with the use of Pfu polymerase and was ligated into EGFP-C1 plasmid (CLONTECH). The paralogous region of chicken myosin Va (a gift of Dr. R. Cheney) was similarly amplified and cloned into EGFP-C2. The full-length rat myosin Vb cDNA was cloned into SacII and SmaI sites of EGFP-C2. The GFP-myosin Vb and GFP-myosin Va tail plasmids were transfected transiently with the use of Effectene (Qiagen, Chatsworth, CA) into 50% confluent HeLa cells grown on glass coverslips. For HeLa cells, Effectene/DNA complexes (0.5 μg of plasmid DNA per 25-mm coverslips in 6-well plates) were incubated with cells for 4 h and the cells were then allowed to recover for 24 h in normal serum-containing medium at which time GFP fluorescence was present in >80% of HeLa cells and >20% of MDCK cells. Cells were fixed in 4% paraformaldehyde for 30 min at 4°C. Cells were permeabilized and blocked with 5% donkey serum, 0.3% Triton X-100 in phosphate-buffered saline for 30 min and then incubated for 2 h at room temperature with primary antibodies. HeLa cells were stained with rabbit polyclonal anti-Rab11a (1:100; Zymed, South San Francisco, CA), monoclonal anti-human transferrin receptor (1:25; Chemicon, Temecula, CA), monoclonal antip58 (1:100; Sigma, St. Louis, MO), polyclonal anti-Rab4 (1:100; QCB, Hopkinton, MA) or polyclonal anti-Rab5 (1:100; QCB) for 2 h at room temperature followed by incubation with multiply absorbed secondary antibodies conjugated with either Cy3 or Cy5 (Jackson Immunoresearch, West Grove, PA). Coverslips were mounted with Prolong antifade (Molecular Probes, Eugene, OR). Cells were visualized with a Zeiss Axiophot microscope outfitted with a Spot camera or a Molecular Dynamics (Sunnyvale, CA) scanning confocal microscope.
For MDCK cell transfection, trypsinized cells in suspension were transfected with Effectene as described above and then seeded onto Transwell filters. At 24 h after transfection, the confluent monolayers were fixed in 4% paraformaldehyde. Cells were stained with polyclonal anti-Rab11a and rat anti-ZO-1 (1:200; Chemicon), to visualize the location of tight junctions, followed by secondary antibodies conjugated with Cy5 and Cy3, respectively. Cells were visualized with scanning confocal fluorescence microscopy (Molecular Dynamics). For these and all other triple labeling experiments, images were collected as previously described (Casanova et al., 1999) with filters that abolish interchannel contamination. Thus, Cy2 and Cy5 were determined simultaneously by excitation with dual 488- and 647-nm laser wavelengths with fluorescence emmission detected through a 650-nm beamsplitter by a 530-nm DF30 filter for Cy2 fluorescence and a 660-nm EFLP filter for Cy5. The cells were then reimaged with excitation at 568 nm with fluorescence detection through a 565-nm beamsplitter followed by a D605 nm/55 M filter for Cy3. Thirty 0.29-μm optical sections were collected for all three labels and section series were rendered with look-through projections with the use of Imagespace software on a Silicon Graphics Indy workstation (Medical College of Georgia Imaging Core Facility, Augusta, GA).
Transferrin Trafficking in HeLa Cells
To assess trafficking in HeLa cells transiently transfected with the GFP-myosin Vb tail or GFP-myosin Va tail, cells grown on coverslips were transfected as described above. Twenty-four hours after transfection, cells were serum starved in serum-free media containing 0.6% bovine serum albumin for 30 min. Coverslips were then incubated for 1 h with 10 μg/ml Cy3-human transferrin (a gift of Dr. James Casanova, Massachusetts General Hospital, Boston, MA) in serum-free media. Cells were then washed and fixed in 4% paraformaldehyde or allowed to recover for 4 h in media containing 10% fetal bovine serum before fixation. Fixed cells were stained with either polyclonal anti-Rab11a or monoclonal anti-transferrin receptor and visualized by fluorescence microscopy as described above.
To assess quantitatively trafficking in HeLa cells, HeLa cells grown in 24-well plates were transiently transfected with either GFP-myosin Va tail or GFP-myosin Vb tail (40–50% transfection). Twenty-four hours after transfection, cells were serum starved for 2 h in serum-free medium containing 1.0% bovine serum albumin (trafficking media) at 37°C. Cells were then loaded for 40 min with 5 μg/ml biotinylated-transferrin (Sigma) at 37°C. Cells were washed in four changes of trafficking media. Then, trafficking media with 0.1% bovine serum albumin and 5-μg/ml unlabeled human holotransferrin (Sigma) was added and cells were allowed to complete internalization of transferrin for either 5 min at 37°C (baseline transferrin uptake) or 20 min at 37°C (chase) in a water bath. At the two time points, media were removed, cells were rapidly washed in ice cold phosphate-buffered saline, and then lysed in 1.5% SDS containing buffer. 2-Mercaptoethanol was then added to the lysates at a final concentration of 5% and samples were heated to 70°C for 15 min. All determinations were performed in triplicate wells. To quantitate the amount of transferrin within cells, 1/10 of each lysate was resolved on 10% SDS-PAGE gels and transferred to Immobilon-P (Millipore, Bedford, MA). Blots were blocked in 5% nonfat dry milk in Tris-buffered saline with 0.05% Tween 20 and the amount of biotinylated transferrin was then determined by incubation of blots with horseradish peroxidase-conjugated streptavidin (Jackson Immunoresearch) for 60 min at room temperature. Binding was visualized by enhanced chemiluminescence (Supersignal; Pierce, Rockford, IL). Multiple film exposures were done to ensure linearity of signal. Quantification of films was performed by image digitization with analysis by IPLab Gel (Scanalytics, Fairfax, VA). Results were expressed as a percentage of transferrin retained in cells after chase compared with the initial uptake at 5 min. The values for transferrin uptake were compared with the use of a Mann-Whitney test.
Trafficking in MDCK Cells
To assess trafficking of polymeric IgA in polarized MDCK cells, MDCK cells stably transfected with the polymeric IgA receptor (pWE) (Brown et al. 2000) were transfected with GFP-myosin Vb tail and seeded onto permeable filters as described above except regular Transwell filters were used instead of Transwell-clear filters. Cells were grown at confluence for 4 d. Transwells were incubated with Texas Red-polymeric IgA in the basal media (20 μg/ml) for 60 min. After loading with IgA, the cells were either fixed or allowed to recover for 60 min in fresh media without IgA and then fixed. Cells were stained with polyclonal anti-Rab11a and examined by confocal fluorescence microscopy. In some cases, polarized cells transfected with either GFP-myosin Va tail or GFP-myosin Vb tail were fixed and dual stained with anti-Rab11a and sheep anti-polymeric IgA receptor (a gift of Dr. Curtis Okamoto, University of Southern California, Los Angeles, CA).
To assess trafficking of transferrin in polarized MDCK cells, MDCK cells were transiently transfected and seeded onto Transwell filters as described above. Cells were grown at confluence for 4 d. Cells were serum starved as described above for HeLa cells and then loaded for 60 min with 50 μg/ml Cy3-human transferrin in basolateral serum-free media. After loading, cells were fixed and then stained with polyclonal Rab11a antibodies.
MDCK Cells Stably Transfected with GFP-Rab11a
Rabbit Rab11a was amplified with Pfu polymerase (Stratagene) and cloned into the EGFP-C2 vector. MDCK cells were transfected with Effectene as described above and plated on 110-mm dishes. Two days after transfection, cells were trypsinized into solution, replated with serial dilutions in 110-mm dishes, and grown in media supplemented with G418. Cell colonies growing under selection after 5–7 d were observed under fluorescence microscopy to identify colonies expressing GFP-Rab11a constructs. Individual clones were then transferred to 24-well plates and clones were expanded. Three clones were isolated for expression of GFP-Rab11a. The distribution of GFP-chimeric protein was identical among individual cell lines.
Anti-myosin Vb Antibodies
Polyclonal antibodies against myosin Vb were raised in rabbit against the sequence SDSNYPSISTSEIGD with an additional terminal cysteine. This sequence from myosin Vb (myr 6) was not present in the sequence of myosin Va. The peptide was coupled with MBS to keyhole lympet hemocyanin. Rabbits were injected and boosted three times. The antiserum was affinity purified with the use of the peptide coupled to Sulfo-Link resin (Pierce). Bound antibody was eluted with Tris-buffered saline/glycine pH 2.8 into equal volumes of 1 M Tris/HCl, pH 8.0, and dialyzed against Tris-buffered saline/2 mM NaN3. This antibody recognized a single 200-kDa band on Western blot (Huber et al., 2000) and showed a different tissue distribution from myosin Va (Bähler, unpublished results).
For immunostaining, MDCK cells stably transfected with GFP-Rab11a were grown on permeable filters for 3 d. Cells were then fixed in 4% paraformaldehyde after no treatment, treatment with 33 μM nocodazole, or treatment with 5 μM taxol, as previously described (Casanova et al., 1999). The cells were then stained as described above with a combination of affinity-purified rabbit anti-myosin Vb antibodies (1:100) and rat anti ZO-1.
RESULTS
Identification of Myosin Vb as a Rab11a-interacting Protein
We have previously demonstrated that the Q70L mutation in Rab11a does not alter GTPase activity (Casanova et al., 1999). Such a mutation has been used for the study of dominant active mutations in a number of other small GTP-binding proteins (Hoffenberg et al., 1995). We have recently demonstrated that an S20V mutation in Rab11a inactivates the GTPase activity and functions as a dominant-active mutation (Wang et al., 2000). We therefore screened a parietal cell yeast two-hybrid cDNA library with Rab11aS20V truncated to remove the carboxyl-terminal cysteines (Rab11aS20VΔC). In screening 5 × 105 clones, we obtained two identical 1979 nucleotide clones for a cDNA sequence with homology to the carboxyl-terminal tail of myosin Vb (Figure 1). The rabbit sequence exhibited 89% amino acid sequence identity with the rat myosin Vb sequence (myr 6) (Zhao et al., 1996). The rabbit myosin Vb cDNA fragment is missing a 26 amino acid coding region that is also absent in a partial murine myosin Vb sequence originally isolated erroneously as a putative glutamic acid decarboxylase sequence (Huang et al. 1990). This difference is likely consistent with alternative splice variants. The rabbit clone comprises the carboxyl-terminal 60 kDa of the myosin Vb tail region and lacks both calmodulin-binding and motor domains.
Figure 1.
Partial amino acid sequence of rabbit myosin Vb compared with rat myosin Vb (myr 6), a murine myosin Vb fragment mistakenly identified as glutamate decarboxylase (GAD) and chicken myosin Va. Underlined regions denote amino acid sequences required for association with Rab11a (Figure 2).
Interaction of Myosin Vb Tail with Rab11 Family Members and Mutants
To characterize the association of Rab11 family members with myosin Vb, we examined their interaction with the rabbit myosin Vb tail clone with the use of the yeast two-hybrid assay (Table 1). Wild-type Rab11a, Rab11aS20V, and Rab11aS20VΔC all interacted with the myosin Vb tail sequence. Rab11a and Rab11b differ only in their carboxyl-terminal hypervariable regions (Lai et al. 1994), and Rab11b interacted with the myosin Vb tail in the two-hybrid assay. Similarly, Rab25 and Rab25ΔC both interacted with myosin Vb tail. In contrast, the myosin Vb tail failed to interact with wild-type Rab2 or Rab3A. The GTP-binding mutant Rab11aS25N also failed to interact with the myosin Vb tail. Similarly, mutations in the Rab11a effector domain (Rab11aT43A and Rab11aI44N) blocked interaction with myosin Vb tail. These results suggested that myosin Vb interacts with the GTP-bound form of Rab11a. Interestingly, a chicken myosin Va construct corresponding to the paralogous region from myosin Vb failed to interact with any of the Rab11 family members (Table 1). Thus, Rab11a appears to interact with only a single myosin V family member, myosin Vb.
Table 1.
Yeast two-hybrid interaction of Rab11 constructs with myosin Vb and myosin Va A positive interaction was indicated by development of blue color within 4 h of incubation with Xgal. Expression of noninteracting bait proteins was confirmed on Western blots of yeast lysates by using antibodies directed against the GAL4 binding domain.
| Target construct | Interaction with myosin Vb | Interaction with myosin Va |
|---|---|---|
| Rab11aWT | + | − |
| Rab11aS20V | + | − |
| Rab11aS20VΔC | + | − |
| Rab11aS25N | − | − |
| Rab11aT43A | − | − |
| Rab11aI44N | − | − |
| Rab11bWT | + | − |
| Rab25WT | + | − |
| Rab25ΔC | + | − |
| Rab2 | − | − |
| Rab3a | − | − |
| Ezrin | − |
Mapping of Requirements for Rab11a Interaction with Myosin Vb Tail
As Figure 1 shows, the tail regions of myosin Va and myosin Vb show regions of extensive identity and similarity in their tails. We have attempted to map the regions on myosin Vb that interact with Rab11a with the use of amino and carboxyl-terminal truncations (Figure 2). Deletion of the 35 carboxyl-terminal amino acids, containing sequence unique to the myosin Vb tail, did not alter the interaction with Rab11a. In contrast, truncation of the carboxyl-terminal region by a further 15 amino acids eliminated interaction with Rab11a (Figures 1 and 2). Truncation of the amino terminal 138 amino acids containing the major predicted coiled-coil dimerization region (amino acids 82–124 in the rabbit clone) did not alter the interaction with Rab11a. However, truncation of a further 22 amino acids eliminated binding to Rab11a. Constructs containing up to 345 amino acids of the amino terminal region all failed to interact with Rab11a. The results therefore suggest that the binding site for Rab11a is complex and requires elements from both the amino and carboxyl regions of the tail of myosin Vb.
Figure 2.
Rab11a interaction with truncated myosin Vb tail constructs. The structure of the myosin Vb tail is shown schematically including a region of coiled-coil structure and the terminal globular region. Amino acid numbers in parentheses correspond to the sequence of murine myosin Vb (myr 6), whereas the nonbracketed numbers refer to the amino acid sequence of the rabbit myosin Vb tail clone shown in Figure 1. Truncations of the myosin Vb tail sequence were assembled in pADGAL and cotransfected into yeast with either pBDGAL-Rab11a or pBDGAL-Rab11aS20V. Lifts of yeast colonies were then exposed to Xgal and development of blue color was interpreted as a positive interaction (+). Results with Rab11aS20V were identical to those with Rab11a wild type.
Myosin Vb Localizes with Rab11a
To investigate the association of myosin Vb with Rab11a in situ, we transiently transfected nonpolarized HeLa cells and MDCK cells polarized on permeable filters with a GFP chimera with full-length rat myosin Vb (GFP-myosin Vb). In HeLa cells (Figure 3, a–c), GFP-myosin Vb localized to the perinuclear region of cells, colocalizing with endogenous Rab11a and endogenous transferrin receptor. In cells overexpressing the GFP-myosin Vb, immunostaining for both Rab11a and transferrin receptor appeared enhanced in the perinuclear region, although their overall distribution throughout the cell was similar to that in nontransfected cells (Figure 3). In addition, we observed bright staining of GFP-myosin Vb along with Rab11a and transferrin receptor in projections from spreading cells (arrows, Figure 3, a–c). In polarized MDCK cells (Figure 3, d–f), we also observed prominent overlap of GFP-myosin Vb with endogenous Rab11a. As in HeLa cells, overexpression of GFP-myosinVb elicited enhanced staining of endogenous Rab11a without a change in the overall distribution of Rab11a within the cell.
Figure 3.
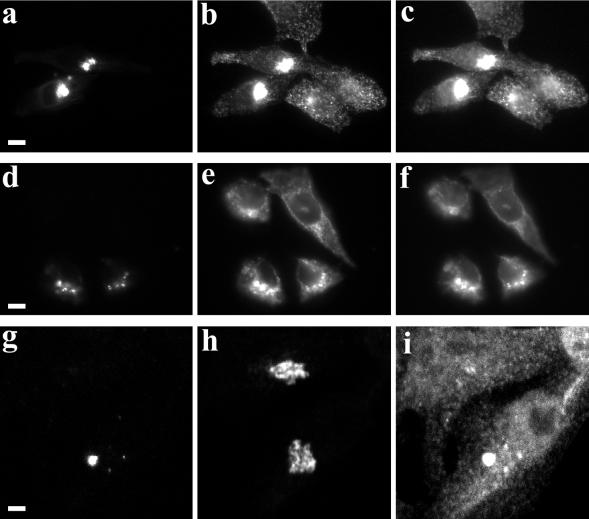
Localization of GFP-myosin Vb in transfected HeLa and MDCK cells. HeLa cells (a–c) were transiently transfected with a full-length GFP-myosin Vb chimera. Cells were triple imaged for GFP-myosin Vb (a), Rab11a (b), and transferrin receptor (c). Arrowheads indicate the positions of cell-labeled extensions in transfected cells. MDCK cells (d–f) were transfected with the full-length GFP-myosin Vb chimera and grown at confluence for 4 d on permeable filters. Cells were triple imaged for GFP-myosin Vb (d), Rab11a (e), and ZO-1 (f). Images in MDCK cells represent projections assembled from confocal optical section series. The results are representative of three separate experiments. Bar, 5 μm (a–c), 1 μm (d–f).
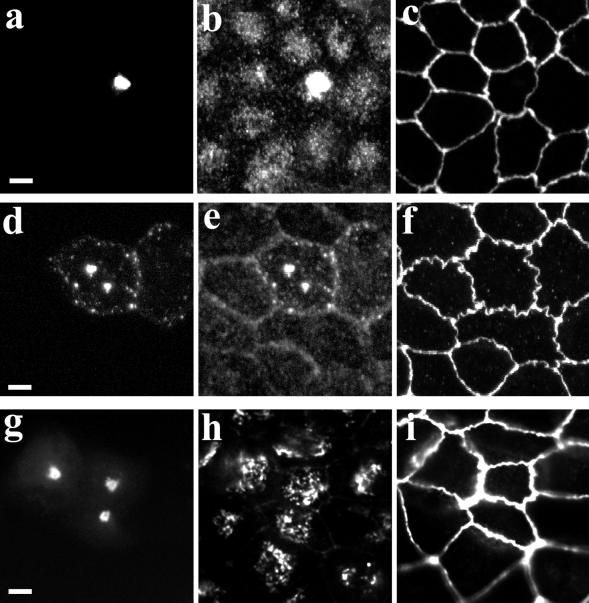
To confirm these findings, MDCK cells stably transfected with GFP-Rab11a were stained with an affinity-purified rabbit polyclonal antibody raised against a peptide sequence specific for myosin Vb and not contained in the sequence of myosin Va (Huber et al., 2000). As seen with the GFP-myosin Vb chimera, myosin Vb immunoreactivity overlapped considerably with GFP-Rab11a (Figure 4, a–c). In MDCK cells, we have previously demonstrated that the microtubule depolymerizing agent nocodazole disperses Rab11a-containing vesicles (Casanova et al., 1999). Figure 4, d–f, demonstrates that treatment with nocodazole dispersed both GFP-Rab11a and myosin Vb immunoreactivity. After treatment with nocodazole, there was a loss of colocalization between Rab11a and myosin Vb. Because microtubules are required for entry into the recycling system (Apodaca et al., 1994), these results suggest that the association of myosin Vb with the recycling system vesicles may require organized assembly of the tubulovesicular system.
Figure 4.
Localization of endogenous myosin Vb in MDCK cells stably expressing GFP-Rab11a. GFP-Rab11a/MDCK cells were grown at confluence for 4 d on permeable filters. Cells were fixed after no treatment (a–c), treatment with nocodazole (d–f), or treatment with taxol (g–i). Cells were triple imaged for GFP-Rab11a (a, d, and g), endogenous myosin Vb immunostaining (b, e, and h), and ZO-1 immunostaining (c, f, and i). Each panel represents X-Y confocal projection reconstructions. Arrowheads indicate the position used for X-Z reconstructions shown in the bottom portion of each panel. The results are representative of two separate experiments. Bar, 1 μm (a–c), 2 μm (d–i).
In a previous investigation, we noted that the microtubule-stabilizing drug taxol causes relocation of Rab11a-containing vesicles to the perijunctional apical corners of polarized MDCK cells (Casanova et al., 1999). This effect of taxol is an important assay for proteins associated with the apical recycling system, because taxol did not have a similar effect on the distribution of early and late endosomal markers (Casanova et al., 1999). Taxol treatment of polarized MDCK cells stably transfected with GFP-Rab11a caused relocation of both GFP-Rab11a and myosin Vb to the apical corners of cells (Figure 4, g–i). GFP-myosin Vb transiently transfected into MDCK cells also redistributed to the apical corners of polarized cells in response to taxol treatment (our unpublished results). These results suggest that myosin Vb is dynamically associated with Rab11a containing vesicles.
Targeting of GFP-Myosin Vb Tail in HeLa Cells
To examine further the association of myosin Vb and Rab11a in situ, we transiently expressed a chimera of GFP and the rabbit myosin Vb tail sequence (GFP-myosin Vb tail) in HeLa cells. Because this construct does not contain a motor domain, we reasoned that it may act as a targeted dominant-negative inhibitor of vesicle movement. Figure 5 demonstrates that the GFP-myosin Vb tail completely colocalized with endogenous Rab11a. Interestingly, transfection of the GFP-myosin Vb tail caused a redistribution of Rab11a, resulting in compaction of staining in the perinuclear area and a relative loss of peripheral Rab11a-immunoreactive vesicles (Figure 5b). Transfection of the GFP-myosin Vb tail also led to concentration of the endogenous transferrin receptor, colocalizing with both the GFP-myosin Vb tail and Rab11a in the perinuclear region (Figure 5c). Similar to the pattern observed for Rab11a, GFP-myosin Vb tail transfection led to loss of peripheral transferrin receptor containing vesicles.
Figure 5.
Morphological effects of overexpression of GFP-myosin Vb tail in HeLa cells. (a–f) HeLa cells were transiently transfected with GFP-myosin Vb tail and allowed to recover for 24 h. Cells then received either no treatment (a–c) or treatment with nocodazole (d–f). Cells were then fixed and in addition to imaging GFP (a and d) cells were stained for endogenous transferrin receptor (b and e) and endogenous Rab11a (c and f). (g and h) HeLa cells were transiently transfected with GFP-myosin Vb tail as described above. Fixed cells were triple imaged for GFP (g), p58 immunostaining, a Golgi marker (h), and Rab11a immunostaining (i). Images in g–i represent projections assembled from fifteen 0.29-μm optical sections obtained in confocal microscopy. The results are representative of three separate experiments. Bar, 5 μm (a–f), 2 μm (g–i).
Because nocodazole had altered the distribution of endogenous myosin Vb, we examined the effects of nocodazole on HeLa cells transfected with the GFP-myosin Vb tail chimera (Figure 5, d–f). Nocodazole caused a partial dispersal of the GFP-myosin Vb tail staining, although the resulting vesicles were considerably larger than the fine vesicle pattern for endogenous Rab11a in nontransfected cells.
Whereas the GFP-myosin Vb tail altered the structure of the recycling system vesicles, there were no apparent changes in the distribution or morphology of Golgi membranes (as assessed with either p58 immunostaining, Figure 5h; or γ-adaptin immunostaining, our unpublished results). In addition, we observed no overlap of GFP-myosin Vb tail with Golgi markers. Transfection with GFP-myosin Vb tail also had no effect on the distribution of endogenous Rab4, Rab5, Rab7, or Rab9 (our unpublished results).
GFP-Myosin Vb Tail Retards Exit of Transferrin from Recycling System in HeLa Cells
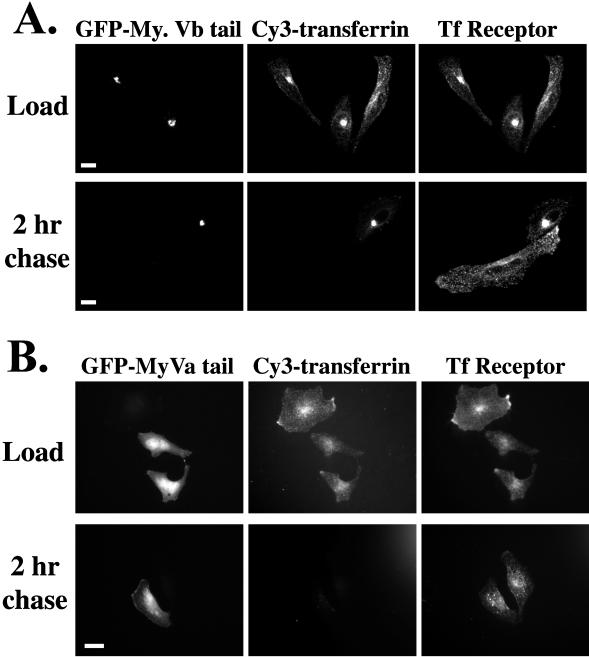
Accumulation of both Rab11a and transferrin receptor staining with the GFP-myosin Vb tail suggested that the carboxyl-terminal myosin Vb tail construct was inhibiting trafficking through the plasma membrane recycling system. We therefore sought to assess transferrin trafficking by evaluating the uptake of Cy3-transferrin into HeLa cells transiently transfected with the GFP-myosin Vb tail. Serum-starved cells were pulsed with Cy3-transferrin for 60 min and then chased into complete serum-containing medium. Cy3-transferrin was taken up by all cells and colocalized with both endogenous transferrin receptor (Figure 6A) and endogenous Rab11a (our unpublished results). However, in GFP-myosin Vb tail-transfected cells, the transferrin was more concentrated in the perinuclear region (Figure 6A). Importantly, although chasing of transferrin with serum-containing media led to the loss of Cy3-transferrin in nontransfected cells, in GFP-myosin Vb tail-transfected cells Cy3-transferrin was retained in the perinuclear region at 2 h after addition of serum-containing medium (Figure 6A). This pattern was maintained for at least 8 h after serum chase (our unpublished results). These studies indicate that, although transferrin and transferrin receptor were able to enter the recycling system in GFP-myosin Vb tail-transfected HeLa cells, expression of the myosin Vb tail chimera markedly retarded recycling of transferrin receptor back to the plasma membrane.
Figure 6.
Effects of overexpression of GFP-myosin Vb tail and GFP-myosin Va tail on transferrin trafficking in HeLa cells. To assess the effects on transferrin trafficking GFP-myosin Vb tail (A) or GFP-myosin Va tail (B) were transiently transfected into HeLa cells. Twenty-four hours after transfection, cells were serum starved for 30 min and then allowed to endocytose Cy3-transferrin for 60 min. After uptake, some cells were allowed to recover in 10% fetal bovine serum-containing medium for 2 h. Cells were imaged for GFP, Cy3-transferrin, and immunostaining for endogenous transferrin receptor. The results are representative of four separate experiments. Bar, 5 μm.
In contrast with the results with myosin Vb tail, transfection of GFP-myosin Va tail into HeLa cells produced a more diffuse distribution (Figure 6B). In addition, the GFP-myosin Va tail did not elicit the concentration of either transferrin receptor (Figure 6B) or Rab11a (our unpublished results). Additionally, cells transfected with the myosin Va tail displayed a normal pattern for transferrin distribution after loading with fluorescent ligand. Finally, as seen in nontransfected cells, labeled-transferrin was completely chased from cells overexpressing the myosin Va tail (Figure 6B). These results indicate that the effects of the myosin Vb tail on transferrin receptor trafficking are specific among the myosin V classes.
To evaluate more quantitatively the effects of myosin Vb tail expression on transferrin receptor recycling, we studied the recycling of biotinylated-transferrin in nontransfected cells compared with those transiently transfected with either the GFP-myosin Va tail or the GFP-myosin Vb tail. Figure 7 demonstrates that although transfection of the GFP-myosin Va tail had no significant effect on the recycling of transferrin, the GFP-myosin Vb tail caused a significant retention of biotinylated transferrin within cells, consistent with the inhibition of recycling observed with the use of fluorescent transferrin.
Figure 7.
Effects of GFP-myosin Vb on recycling of biotinylated transferrin in HeLa cells. Nontransfected HeLa cells (NT) or HeLa cells transfected with either GFP-myosin Va tail (Va) or GFP-myosin Vb tail (Vb) were loaded with biotinylated-human transferrin. After washing, cells were allowed to recover for either 5 or 20 min in media containing unlabeled transferrin. Electrophoretic transfers of cell lysates were incubated with horseradish peroxidase-streptavidin to visualize transferrin (A) Triplicate samples are shown. (B) Results were quantified and expressed as a percentage of transferrin retained within cells after 20 min (mean + SEM; n = 6). *p < 0.01 compared with both NT and myosin Va by Mann-Whitney test.
GFP-Myosin Vb Tail Alters Recycling System in Polarized MDCK Cells
Because Rab11a also regulates plasma membrane recycling in polarized cells, we studied the effects of GFP-myosin Vb tail transfection in polarized MDCK cells (Figure 8). Similar to the pattern seen in HeLa cells, the GFP-myosin Vb tail colocalized with endogenous Rab11a and elicited an accumulation of Rab11a in the pericentriolar region (Figure 8b).
Figure 8.
GFP-myosin Vb tail alters apical recycling system morphology in MDCK cells. MDCK were transiently transfected with GFP-myosin Vb tail and grown for 3 d at confluence on permeable filters. (a–f) Cells were then exposed to either no treatment (a–c) or treatment with nocodazole (d–f). Fixed cells were triple imaged for GFP (a and d), endogenous Rab11a (b and e), and ZO-1 (c and f). (g–i) GFP-myosin Vb tail transfected MDCK cells were fixed and triple imaged for GFP (g), the Golgi marker p58 (h), and ZO-1 (i). The results are representative of three separate experiments. Bar, 5 μm.
We also studied the effects of nocodazole on transfected GFP-myosin Vb tail in polarized MDCK cells. Figure 8d demonstrates that, as in HeLa cells, nocodazole only partially dispersed the GFP-myosin Vb tail-labeled vesicles, compared with the effects seen for the endogenous Rab11a in nontransfected cells. These results are consistent with a role for microtubules in trafficking into the apical recycling system. Taxol had no effect on the distribution of GFP-myosin Vb tail in transiently transfected MDCK cells (our unpublished results). Thus, the effects of taxol on the apical recycling system vesicles probably requires a myosin Vb-dependent process.
As in HeLa cells, GFP-myosin Vb tail transfection did not alter significantly the structure of the Golgi complex (Figure 8h). Also as in HeLa cells, GFP-myosin Vb tail had no effect on the distribution of endogenous Rab4, Rab5, Rab7, or Rab9 (our unpublished results). The GFP-myosin Vb tail had a similar effect of concentrating Rab11a immunoreactivity in polarized HCA-7 colonic adenocarcinoma cells (our unpublished results).
GFP-Myosin Vb Tail Alters Transcytosis in Polarized MDCK Cells
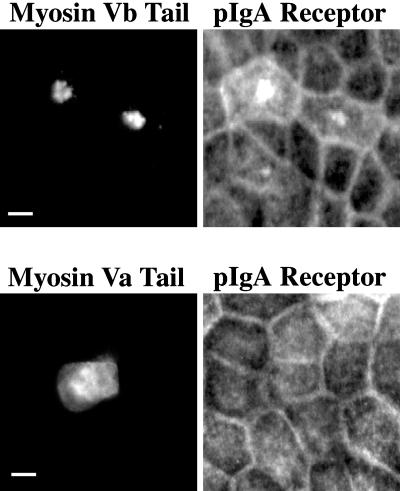
Because Rab11a is a critical regulator of transcytosis in polarized epithelial cells, we sought to evaluate the effects of the GFP-myosin Vb tail chimera on transcytosis in MDCK cells stably transfected with the polymeric IgA receptor (pIgR). We first studied the effects of GFP-myosin Vb tail on the distribution of the pIgR in these MDCK cells. Figure 9 demonstrates that expression of GFP-myosin Vb tail caused an accumulation of pIgR in a pericentrosomal compartment and this accumulation also colocalized with Rab11a (our unpublished results). In contrast, transfection of GFP-myosin Va tail elicited a diffuse distribution in the MDCK cells and had no effect on the distribution of either pIgR (Figure 9) or Rab11a (our unpublished results). These results suggested that the myosin Vb tail could retard trafficking through the apical recycling system of polarized MDCK cells.
Figure 9.
GFP-myosin Vb tails alters the distribution of polymeric IgA receptor. MDCK cells were transiently transfected with either GFP-myosin Vb tail or GFP-myosin Va tail and grown for 3 d at confluence on permeable filters. Cells were then fixed and dual imaged for GFP and pIgR immunostaining. The results are representative of three separate experiments. Bar, 5 μm.
To evaluate trafficking in the polarized MDCK cells expressing pIgR, cells were loaded with Texas Red-polymeric IgA from the basolateral medium. In cells overexpressing the GFP-myosin Vb tail, fluorescent IgA accumulated with the GFP-myosin Vb tail and endogenous Rab11a (Figure 10, a–c). In addition, when cells were returned into fresh medium for 1 h, fluorescent IgA was chased out of nontransfected cells, whereas fluorescent IgA was retained in transfected cells (Figure 10e). Transfection of GFP-myosin Va tail had no effect on fluorescent IgA trafficking (our unpublished results). In contrast with the results for IgA, when cells were loaded with Cy3-transferrin from the basolateral medium, no accumulation of fluorescent transferrin was observed in association with GFP-myosin Vb tail (Figure 10h). Finally, we were unable to observe trafficking of Cy3-transferrin into Rab11a immunoreactive vesicles. These results are consistent with previous findings (Low et al., 1999; Brown et al., 2000) and support the conclusion that transferrin does not enter the Rab11a-containing apical recycling system in polarized MDCK cells.
Figure 10.
Alteration of trafficking in MDCK cells by GFP-myosin Vb tail. (a–f) MDCK cells stably expressing the polymeric IgA receptor were transiently transfected with GFP-myosin Vb tail and allowed to grow at confluence on permeable filters for 4 d. The cells were then loaded with Texas Red-IgA from the basolateral media for 60 min and then either fixed (a–c) or allowed to recover in fresh medium as a chase for 60 min (d–f). Cells were triple imaged for GFP (a and d), Texas Red-IgA (b and e), and endogenous Rab11a immunostaining (c and f). (g–i) MDCK cells were transiently transfected with GFP-myosin Vb tail and allowed to grow at confluence on permeable filters for 4 d. Cells were loaded with Cy3-transferrin for 60 min and fixed. Cells were triple imaged for GFP (g), Cy3-transferrin (h), and endogenous Rab11a immunostaining (i). All panels represent X-Y reconstructions of confocal optical section series. Arrowhead marks the location used for assembly of X-Z projections seen below X-Y projections in g–i. Bar, 5 μm (a–f), 2 μm (g–i).
DISCUSSION
Plasma membrane recycling is a fundamental cellular process regulating the trafficking of membrane receptors, channels, and pumps. In nonpolarized cells, the transferrin receptor is internalized and recycled to the plasma membrane through a pericentrosomal vesicle system. In polarized cells, plasma membrane recycling is more complicated and a pericentrosomal vesicular recycling system is thought to mediate both basolateral and apical membrane recycling as well as coordinating the process of basolateral-to-apical transcytosis (Mostov et al., 1992; Apodaca et al., 1994). Studies over the past several years have established that Rab11a is a ubiquitous marker of plasma membrane recycling systems (Ullrich et al., 1996). The results presented here indicate that myosin Vb also is associated with the plasma membrane recycling system in both nonpolarized and polarized cells. In both HeLa and MDCK cells, we observed considerable overlap in the distribution of Rab11a and myosin Vb as assessed by immunostaining of endogenous myosin Vb, as well as in cells transfected with a full-length GFP-myosin Vb chimera. Most importantly, we have established in situ correlates of myosin Vb association with Rab11a-containing recycling system vesicles. First, both Rab11a and myosin Vb were dispersed by nocodazole. Second, both proteins relocated to a perijunctional apical corner of MDCK cells treated with taxol. Third, in both nonpolarized and polarized cells, the GFP-myosin Vb tail chimera lacking a motor domain caused accumulation with Rab11a of recycling cargoes (transferrin receptor and pIgR, respectively). Fourth, in HeLa cells, the GFP-myosin Vb tail construct retarded trafficking of transferrin through plasma membrane recycling system. Fifth, in MDCK cells, consistent with a diminution of trafficking through the apical recycling system, the GFP-myosin Vb tail chimera caused accumulation with Rab11a of IgA endocytosed from the basolateral membrane. To date, however, we have been unable to demonstrate, either by immunoprecipitation or other direct isolation techniques, a biochemical association of Rab11a with myosin Vb. Brown et al. (2000) have reported similar difficulty in demonstrating association of myo2p with a yeast kinesin. As in that case, we presently hypothesize that the interaction of Rab11a and myosin Vb is labile in the detergent lysis conditions required for extraction of Rab11a from the membrane. Alternatively, it is possible that the interaction of Rab11a with myosin Vb could require a third protein that is provided in the yeast two-hybrid assay. Nevertheless, all of the evidence noted above supports an association of myosin Vb with the plasma membrane recycling vesicles and its likely association with Rab11a.
Although strong evidence supports the association of myosin Va with vesicle movement, far less is known about myosin Vb, which was originally cloned from rat and is present in a number of epithelial tissues and the brain (Zhao et al., 1996). Myosin Vb demonstrates 59% amino acid sequence similarity with myosin Va (Zhao et al., 1996). Recently, Schott et al. (1999) have suggested through genetic evidence an interaction of the yeast Rab protein Sec4 with the carboxy terminus of the yeast class V myosin Myo2p. Our studies suggest that the tail region of myosin Vb, but not myosin Va, interacts with Rab11a. Previous studies reported that the transfected myosin Va tail distributed to a pericentrosomal region in melancoytes (Tsakralides et al., 1999). However, we have observed a more diffuse pattern of expression for the GFP-myosin Va tail. In addition, the GFP-myosin Va tail did not inhibit plasma membrane recycling in either HeLa or MDCK cells. At least two other proteins also appear to associate with the tail regions of myosin V members. Previous studies had suggested that a kinesin-related protein acts as a suppressor of mutations in Myo2p (Lillie and Brown, 1992). Jenkins and colleagues have demonstrated that myosin Va interacts with a ubiquitous kinesin molecule (Huang et al., 1999) through a region that is highly homologous within myosin V family members (the carboxyl-terminal AF-6 homology domain). Thus, this kinesin would be expected to interact with all myosin V family members. Similarly, as noted above, the yeast class V myosin myo2p interacts with a yeast kinesin (Beningo et al., 2000). Recently, a RING finger protein (BERP) has also been identified that interacts with both myosin Va and myosin Vb, likely through shared sequences in their carboxyl-terminal AF-6 homology domain (El-Husseini and Vincent, 1999). The role of BERP in regulating vesicle trafficking is presently unclear. Thus, a number of putative regulators of vesicle trafficking may be associating with class V myosin tails.
The association of Rab11a with myosin Vb required two separate regions in the myosin Vb tail. These two regions are divergent from the two regions of myo2p that were implicated in vesicle targeting in yeast with the use of genetic screens (Catlett et al., 2000). Interestingly, the deletion of the major putative coiled-coil domain, predicted to be responsible for dimerization of the tail construct, did not alter association of the myosin Vb tail with Rab11a in our two-hybrid assay. In addition, because the differences in the two binding regions between myosin Va and myosin Vb are small, we would predict that myosin Va may also bind with an as yet unidentified Rab protein. Previous studies have focused on the presence of mutations in myosin Va in mouse dilute mutants (Wu et al., 1998; Bridgman, 1999) and human Griscelli syndrome patients. However, a number of recent investigations have identified a mutation in Rab27a responsible for the phenotype of the ashen mouse (Wilson et al., 2000) as well as in a subset of Griscelli syndrome patients (Menasche et al., 2000). The similarity of these phenotypes in the mouse mutants and Griscelli syndrome patients suggest that Rab27a may be the specific binder for the myosin Va tail. Another unconventional myosin, myr4, has also recently been implicated in the regulation of early steps in trafficking of recycling cargoes (Huber et al., 2000). Nevertheless, it is clear from the data presented here that the myosin Va tail had no effect on recycling of either transferrin or IgA receptor in either nonpolarized or polarized cells. In contrast, the myosin Vb tail acted as a potent dominant-negative construct, causing pericentrosomal accumulation of transferrin receptor in nonpolarized HeLa cells and of pIgR in polarized MDCK cells.
As in the study of all Rab proteins, the identification of upstream and downstream regulators is critical for an understanding of the function of Rab11 family members. Two groups (Mammoto et al., 1999; Zeng et al., 1999) have identified a novel protein (Rab11-BP/Rabphillin-11), which interacts with Rab11a as assessed by protein overlay. Although the protein does not strictly colocalize with Rab11a, transfection and overexpression of an amino or carboxyl-truncated protein alters transferrin trafficking in nonpolarized cells (Mammoto et al., 1999; Zeng et al., 1999). Like myosin Vb, this protein appears to interact with Rab11a in its GTP-bound form. Also like myosin Vb, Rab11-BP did not associate with Rab11a effector domain mutants (Mammoto et al., 1999; Zeng et al. 1999). These studies and the present data suggest that Rab11a may be interacting with multiple protein effectors and modulators along the recycling pathway. Indeed, in further yeast two-hybrid screens with different Rab11a and Rab25 baits, we have now identified four further putative Rab11-interacting proteins (Goldenring, unpublished data).
Previous studies have also established the importance of the microtubule cytoskeleton in the regulation of the plasma membrane recycling system in both nonpolarized and polarized cells. Depolymerization of microtubules causes dispersal of the recycling system (Apodaca et al., 1994; Casanova et al., 1999). In MDCK cells, stabilization of microtubules with taxol causes relocation of the Rab11a-containing recycling vesicles to a position adjacent to the junctional complex (Casanova et al., 1999). Our results suggest that the distribution of myosin Vb also is affected by the state of microtubule polymerization. In MDCK cells, myosin Vb staining dispersed along with GFP-Rab11a after treatment with nocodazole, and both colocalized to the apical corners after taxol treatment. Because rearrangements with taxol are especially characteristic of Rab11a recycling system vesicles (Casanova et al., 1999), these results provide a functional confirmation of myosin Vb association with the recycling system. Previous investigations in melanocytes have shown that microtubule polymerization affects myosin Va distribution (Tsakraklides et al., 2000). In these studies, as in our own, an association of myosin V species with a kinesin to form a multifunctional motor complex (Huang et al., 1999; Beningo et al., 2000) would explain the dispersal of vesicles and myosin Vb staining by nocodozole treatment.
The present investigations support the hypothesis that myosin Vb, likely through an interaction with Rab11a, is involved in exiting the recycling system. Consistent with this hypothesis, the myosin Vb tail construct without a motor domain acts as a dominant-negative protein, associating with Rab11a-containing recycling system vesicles, but preventing their interaction with cytoskeletal elements required for transit out of the recycling system. Furthermore, nocodazole partially dispersed GFP-myosin Vb tail concentration of the recycling system in both HeLa and MDCK cells. Thus, it is likely that microtubules are required for steps proximal to the function of myosin Vb. This model would explain the accumulation of cargoes trafficking through the recycling system within GFP-myosin Vb tail containing vesicles. Recent studies have provided evidence that microfilaments and Rac1 may be associated with the recycling system in MDCK cells (Jou and Nelson, 1998; Jou et al., 1998). Dominant active Rac1 colocalizes with Rab11a and inhibits apical recycling (Jou and Nelson, 1998; Jou et al., 1998). Indeed, the presumption that myosin Vb also would interact with a kinesin (Huang et al., 1999) suggests that a myosin Vb/kinesin complex could mediate trafficking through the recycling system. Thus, although the microtubule system is involved in trafficking of vesicles into the plasma membrane recycling systems, microfilaments and myosin Vb may be responsible, at least in part, for trafficking of vesicles out of the recycling system. A similar mechanism may be involved in Golgi trafficking where Goud and colleagues have recently identified another novel kinesin as an effector for Rab6 (Echard et al., 1998).
In polarized cells, controversy continues to exist over the role of Rab11a in basolateral trafficking of transferrin receptor versus apical recycling and transcytosis. With the use of electron microscopic examination, Hopkins and colleagues (Hughson and Hopkins, 1990; Knight et al., 1995; Odorizzi et al., 1996) have noted colocalization of endocytosed transferrin and polymeric IgA in tubular endosomes. However, in polarized MDCK cells, Dunn and colleagues (Brown et al., 2000) have recently reported that fluorescently labeled transferrin and polymeric IgA were sorted away from each other before the entry of IgA into a Rab11a-containing apical recycling compartment. In addition, we have demonstrated in polarized MDCK cells that dominant-negative Rab11a mutants inhibit transcytosis of IgA, but have no effect on the basolateral recycling of transferrin (Wang et al., 2000). The studies of Dunn (Brown et al., 2000) have established an orderly progression of sorting whereby transferrin and IgA endocytosed from the basolateral membrane enter a common recycling endosome after segregation away from low-density lipoprotein receptors. This recycling endosome is likely the common recycling endosome originally described by Hopkins and colleagues in MDCK and HeLa cells (Hughson and Hopkins, 1990; Knight et al., 1995; Odorizzi et al., 1996). From the recycling endosome, transferrin and IgA are separated with transferrin receptor recycling to the basolateral membrane, whereas transcytosing IgA receptor moves into an apical recycling endosome containing Rab11a. We have verified these findings here and, additionally, we have found that transferrin was not trafficked into GFP-myosin Vb tail containing vesicles in MDCK cells, as it was in HeLa cells. These data support the hypothesis that, in polarized epithelial cells, transcytosing cargoes are sorted away from the majority of transferrin receptor before entry into the Rab11a-containing apical recycling system.
In summary, the results presented here demonstrate that myosin Vb associates with Rab11a-containing plasma membrane recycling systems in both nonpolarized and polarized cells. The data indicate that myosin Vb is required for trafficking out of plasma membrane recycling systems. Because myosin Va does not interact with any Rab11 family member, further studies will be required to discern whether other classes of Rab proteins interact with the myosin Va tail. The dominant-negative myosin Vb tail fragment should prove to be of great utility in the identification of proteins that traffic through the plasma membrane recycling systems in both nonpolarized and polarized cells.
ACKNOWLEDGMENTS
We thank Dr. Richard Cheney for the chicken myosin Va plasmid, Dr. James Casanova for Cy3-transferrin and pWE cells, Dr. Curtis Okamoto for antibodies against the polymeric IgA receptor, and Dr. Ken Dunn for Texas Red-polymeric IgA. We thank Christa Baradoy for excellent technical assistance. This work was supported by grants to J.R.G. from awards from National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (DK-48370 and DK-43405) and a Veterans Administration Merit Award. J.A.M. is supported by National Science Foundation Award 9874907.
REFERENCES
- Apodaca G, Katz LA, Mostov KE. Receptor-mediated transcytosis of IgA in MDCK cells is via apical recycling endosomes. J Cell Biol. 1994;125:67–86. doi: 10.1083/jcb.125.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beningo KA, Lillie SH, Brown SS. The yeast kinesin-related protein Smy1p exerts its effects on class V myosin Myo2p via a physical interaction. Mol Biol Cell. 2000;11:691–702. doi: 10.1091/mbc.11.2.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhartur SG, Goldenring JR. Mapping of ezrin dimerization using yeast two-hybrid screening. Biochem Biophys Res Commun. 1998;243:874–877. doi: 10.1006/bbrc.1998.8196. [DOI] [PubMed] [Google Scholar]
- Bonafe N, Sellers JR. Molecular characterization of myosin V from Drosophila melanogaster. J Muscle Res Cell Motil. 1998;19:129–141. doi: 10.1023/a:1005356511634. [DOI] [PubMed] [Google Scholar]
- Bridgman PC. Myosin Va movements in normal and dilute-lethal axons provide support for a dual filament motor complex. J Cell Biol. 1999;146:1045–1060. doi: 10.1083/jcb.146.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PS, Wang E, Aroeti B, Chapin SJ, Mostov KE, Dunn KW. Definition of distinct compartments in polarized Madin-Darby canine kidney (MDCK) cells for membrane-volume sorting, polarized sorting and apical recycling. Traffic. 2000;1:124–140. doi: 10.1034/j.1600-0854.2000.010205.x. [DOI] [PubMed] [Google Scholar]
- Calhoun BC, Goldenring JR. Two Rab proteins, VAMP-2, and SCAMPs are present on immunoisolated gastric tubulovesicles. Biochem J. 1997;325:559–564. doi: 10.1042/bj3250559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova JE, Wang X, Kumar R, Bhartur SG, Navarre J, Woodrum JE, Ray GS, Goldenring JR. Rab11a and Rab25 association with the apical recycling system of polarized MDCK cells. Mol Biol Cell. 1999;10:47–61. doi: 10.1091/mbc.10.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlett NL, Duex JE, Tang F, Weisman LS. Two distinct regions in a yeast myosin-V tail domain are required for the movement of different cargoes. J Cell Biol. 2000;150:513–525. doi: 10.1083/jcb.150.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlett NL, Weisman LS. The terminal tail region of a yeast myosin-V mediates its attachment to vacuole membranes and sites of polarized growth. Proc Natl Acad Sci USA. 1998;95:14799–14804. doi: 10.1073/pnas.95.25.14799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P, Vingron M, Sander C, Simons K, Zerial M. Molecular cloning of YPT1/SEC4-related cDNAs from an epithelial cell line. Mol Cell Biol. 1990;10:6578–6585. doi: 10.1128/mcb.10.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman JG, Tyagarajan K, Kolsi MS, Moore HH, Forte JG. Expression of rab11a N124I in gastric parietal cells inhibits stimulatory recruitment of the H+-K+-ATPase. Am J Physiol. 1999;277:C361–C372. doi: 10.1152/ajpcell.1999.277.3.C361. [DOI] [PubMed] [Google Scholar]
- Echard A, Jollivet F, Martinez O, Lacapere J-J, Rousselet A, Janoueix-Lerosey I, Goud B. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science. 1998;279:580–585. doi: 10.1126/science.279.5350.580. [DOI] [PubMed] [Google Scholar]
- El-Husseini AE, Vincent SR. Cloning and characterization of a novel RING finger protein that interacts with class V myosins. J Biol Chem. 1999;274:19771–19777. doi: 10.1074/jbc.274.28.19771. [DOI] [PubMed] [Google Scholar]
- Evans LL, Lee AJ, Bridgman PC, Mooseker MS. Vesicle-associated brain myosin-V can be activated to catalyze actin-based transport. J Cell Sci. 1998;111:2055–2066. doi: 10.1242/jcs.111.14.2055. [DOI] [PubMed] [Google Scholar]
- Forte JG, Yao X. The membrane-recruitment-and-recycling hypothesis of gastric HCl secretion. Trends Cell Biol. 1996;6:45–48. doi: 10.1016/0962-8924(96)81009-9. [DOI] [PubMed] [Google Scholar]
- Goldenring JR, Shen KR, Vaughan HD, Modlin IM. Identification of a small GTP-binding protein, Rab25, expressed in the gastrointestinal mucosa, kidney and lung. J Biol Chem. 1993;268:18419–18422. [PubMed] [Google Scholar]
- Goldenring JR, Soroka CJ, Shen KR, Tang LH, Rodriguez W, Vaughan HD, Stoch SA, Modlin IM. Enrichment of rab11, a small GTP-binding protein, in gastric parietal cells. Am J Physiol. 1994;267:G187–G194. doi: 10.1152/ajpgi.1994.267.2.G187. [DOI] [PubMed] [Google Scholar]
- Green EG, Ramm E, Riley NM, Spiro DJ, Goldenring JR, Wessling-Resnick M. Rab11 is associated with transferrin-containing recycling compartments in K562 cells. Biochem Biophys Res Commun. 1997;239:612–616. doi: 10.1006/bbrc.1997.7520. [DOI] [PubMed] [Google Scholar]
- Hoffenberg S, Sanford JC, Liu S, Daniel DS, Tuvin M, Knoll BJ, Wessling-Resnick M, Dickey BF. Biochemical and functional characterization of a recombinant GTPase, Rab5, and two of its mutants. J Biol Chem. 1995;270:5048–5056. doi: 10.1074/jbc.270.10.5048. [DOI] [PubMed] [Google Scholar]
- Huang J-D, Brady ST, Richards BW, Stenoien D, Resau JH, Copeland NG, Jenkins NA. Direct interaction of microtubule- and actin-based transport motors. Nature. 1999;397:267–270. doi: 10.1038/16722. [DOI] [PubMed] [Google Scholar]
- Huang WM, Reed-Fourquet L, Wu E, Wu JY. Molecular cloning and amino acid sequence of brain L-glutamate decarboxylase. Proc Natl Acad Sci USA. 1990;87:8491–8495. doi: 10.1073/pnas.87.21.8491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber LA, Fialka I, Paiha K, Hunziker W, Sacks DB, Bahler M, Way M, Gagescu R, Gruenberg J. Both calmodulin and the unconventional myosin myr4 regulate membrane trafficking along the recycling pathway of MDCK cells. Traffic. 2000;1:494–503. doi: 10.1034/j.1600-0854.2000.010607.x. [DOI] [PubMed] [Google Scholar]
- Hughson EJ, Hopkins C. Endocytic pathways in polarized caco-2 cells: identification of an endosomal compartment accessible from both apical and basolateral surfaces. J Cell Biol. 1990;110:337–348. doi: 10.1083/jcb.110.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou T-Z, Nelson JW. Effects of regulated expression of mutant RhoA and Rac1 small GTPases on the development of epithelial (MDCK) cell polarity. J Cell Biol. 1998;142:85–100. doi: 10.1083/jcb.142.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou TS, Schneeberger EE, Nelson WJ. Structural and functional regulation of tight junctions by rhoA and rac1 small GTPases. J Cell Biol. 1998;142:101–115. doi: 10.1083/jcb.142.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A, Yamashita T, Kawata M, Yamamoto K, Ikeda K, Tanimoto T, Takai Y. Purification and characterization of a novel GTP-binding protein with a molecular weight of 24,000 from bovine brain membranes. J Biol Chem. 1988;263:2897–2904. [PubMed] [Google Scholar]
- Knight A, Hughson E, Hopkins CR, Cutler DF. Membrane protein trafficking through the common apical endosome compartment of polarized Caco-2 cells. Mol Biol Cell. 1995;6:597–610. doi: 10.1091/mbc.6.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F, Stubbs L, Artzt K. Molecular analysis of mouse Rab11b: a new type of mammalian YPT/Rab protein. Genomics. 1994;22:610–616. doi: 10.1006/geno.1994.1434. [DOI] [PubMed] [Google Scholar]
- Lillie SH, Brown SS. Suppression of a myosin defect by a kinesin-related gene. Nature. 1992;356:358–361. doi: 10.1038/356358a0. [DOI] [PubMed] [Google Scholar]
- Low SH, Chapin SJ, Wimmer C, Whiteheart SW, Komuves LG, Mostov KE, Weimbs T. The SNARE machinery is involved in apical plasma membrane trafficking in MDCK cells. J Biol Chem. 1999;141:1503–1513. doi: 10.1083/jcb.141.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto A, Ohtsuka T, Hotta I, Sasaki T, Takai Y. Rab11BP/Rabphillin-11, a downstream target of Rab11 small G protein implicated in vesicle recycling. J Biol Chem. 1999;274:25517–25524. doi: 10.1074/jbc.274.36.25517. [DOI] [PubMed] [Google Scholar]
- Menasche G, Pastural E, Feldman J, Certain S, Ersoy F, Dupuis S, Wulffraat N, Bianchi D, Fischer A, Le Deist F, de Saint Basile G. Mutations in Rab27a cause Griscelli syndrome associated with hemophagocytic syndrome. Nature Genet. 2000;25:173–176. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- Mercer JA, Seperack PK, Strobel MC, Copeland NG, Jenkins NA. Novel myosin heavy chain encoded by murine dilute coat color locus. Nature. 1991;349:709–712. doi: 10.1038/349709a0. [DOI] [PubMed] [Google Scholar]
- Miller KE, Sheetz MP. Characterization of myosin V binding to brain vesicles. J Biol Chem. 2000;275:2598–2606. doi: 10.1074/jbc.275.4.2598. [DOI] [PubMed] [Google Scholar]
- Mostov K, Apodaca G, Aroeti B, Okamoto C. Plasma membrane protein sorting in polarized epithelial cells. J Cell Biol. 1992;116:577–583. doi: 10.1083/jcb.116.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento AAC, Amaral RG, Bizario JCS, Larson RE, Espreafico EM. Subcellular localization of myosin-V in the B16 melanoma cells, a wild-type cell line for the dilute gene. Mol Biol Cell. 1997;8:1971–1988. doi: 10.1091/mbc.8.10.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi G, Pearse A, Domingo D, Trowbridge IS, Hopkins CR. Apical and basolateral nedosomes of MDCK cells are interconnectedand contain a polarized sorting mechanism. J Cell Biol. 1996;135:139–152. doi: 10.1083/jcb.135.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provance DW, Jr, Wei M, Ipe V, Mercer JA. Cultured melanocytes from dilute mice exhibit dendritic morphology and altered melanosome distribution. Proc Natl Acad Sci USA. 1996;93:14554–14558. doi: 10.1073/pnas.93.25.14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck-Peterson SL, Provance DW, Mooseker MS, Mercer JA. Class V myosins. Biochem Biophys Acta. 2000;1496:36–51. doi: 10.1016/s0167-4889(00)00007-0. [DOI] [PubMed] [Google Scholar]
- Ren X, Xu G, Zeng J, De Lemos-Chiarandini C, Adesnik M, Sabatini DD. Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc Natl Acad Sci USA. 1998;95:6187–6192. doi: 10.1073/pnas.95.11.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SL, Gelfand VI. Myosin cooperates with microtubule motors during organelle transport in melanophores. Curr Biol. 1998;8:161–164. doi: 10.1016/s0960-9822(98)70063-6. [DOI] [PubMed] [Google Scholar]
- Schott D, Ho J, Pruyne D, Bretscher A. The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. J Cell Biol. 1999;147:791–807. doi: 10.1083/jcb.147.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabb JS, Molyneaux BJ, Cohen DL, Kuznetsov SA, Langford GM. Transport of ER vesicles on actin filaments in neurons by myosin V. J Cell Sci. 1998;111:3221–3234. doi: 10.1242/jcs.111.21.3221. [DOI] [PubMed] [Google Scholar]
- Tsakraklides V, Krogh K, Bizario JC, Larson RE, Espreafico EM, Wolenski JS. Subcellular localization of GFP-myosin V in live mouse melanocytes. J Cell Sci. 2000;112:2853–2865. doi: 10.1242/jcs.112.17.2853. [DOI] [PubMed] [Google Scholar]
- Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kumar R, Navarre J, Casanova JE, Goldenring JR. Regulation of vesicle trafficking in Madin-Darby Canine Kidney cells by Rab11a and Rab25. J Biol Chem. 2000;275:29138–29146. doi: 10.1074/jbc.M004410200. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Yip R, Swing DA, O'Sullivan N, Zhang Y, Novak EK, Swank RT, Russell LB, Copeland NG, Jenkins NA. A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc Natl Acad Sci USA. 2000;97:7933–7938. doi: 10.1073/pnas.140212797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Bowers B, Rao K, Wei Q, Hammer JA. Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function In vivo. J Cell Biol. 1998;143:1899–1918. doi: 10.1083/jcb.143.7.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Ren M, Gravotta D, De Lemos-Chiarandini C, Lui M, Erdjument-Bromage H, Tempst P, Xu G, Shen TH, Morimoto T, Adesnick M, Sabatini DD. Identification of a putative effector protein for rab11 that participates in transferrin recycling. Proc Natl Acad Sci USA. 1999;96:2840–2845. doi: 10.1073/pnas.96.6.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LP, Koslovsky JS, Reinhard J, Bahler M, Witt AE, Provance DW, Jr, Mercer JA. Cloning and characterization of myr6, an unconventional myosin of the dilute/myosin-V family. Proc Natl Acad Sci, USA. 1996;93:10826–10831. doi: 10.1073/pnas.93.20.10826. [DOI] [PMC free article] [PubMed] [Google Scholar]