Abstract
Rationale: Ivacaftor (VX-770), a cystic fibrosis transmembrane conductance regulator (CFTR) potentiator, has been shown to improve lung function, pulmonary exacerbation rate, respiratory symptoms, and weight gain compared with placebo in patients with cystic fibrosis aged 12 years or older with a G551D-CFTR mutation.
Objectives: This randomized, double-blind, placebo-controlled trial evaluated ivacaftor in patients with cystic fibrosis aged 6–11 years with a G551D-CFTR mutation on at least one allele.
Methods: Patients were randomly assigned to receive ivacaftor administered orally at 150 mg (n = 26) or placebo (n = 26) every 12 hours for 48 weeks in addition to existing prescribed cystic fibrosis therapies.
Measurements and Main Results: Despite near-normal mean baseline values in FEV1, patients receiving ivacaftor had a significant increase in percent predicted FEV1 from baseline through Week 24 versus placebo group (treatment effect, 12.5 percentage points; P < 0.001). Effects on pulmonary function were evident by 2 weeks, and a significant treatment effect was maintained through Week 48. Patients treated with ivacaftor gained, on average, 2.8 kg more than those receiving placebo at Week 48 (P < 0.001). The change from baseline through Week 48 in the concentration of sweat chloride, a measure of CFTR activity, with ivacaftor was −53.5 mmol/L (P < 0.001) versus placebo. The incidence of adverse events was similar in the two groups.
Conclusions: In patients who are younger and healthier than those in previously studied populations, ivacaftor demonstrated a significant improvement in pulmonary function, weight, and CFTR activity compared with placebo. Clinical trial registered with www.clinicaltrials.gov (NCT00909727).
Keywords: cystic fibrosis; cystic fibrosis transmembrane conductance regulator protein; cystic fibrosis, pulmonary; G551D-CFTR mutation; sweat test
At a Glance Commentary
Scientific Knowledge on the Subject
Ivacaftor is a small-molecule cystic fibrosis transmembrane conductance regulator (CFTR) potentiator that is approved in the United States, European Union, and Canada for the treatment of cystic fibrosis in patients aged 6 years and older with a G551D-CFTR mutation. The ivacaftor clinical program evaluated efficacy and safety in adolescents and adults (≥12 years of age) and in children 6–11 years of age. The adolescent and adult study, previously published, reported significant improvements in lung function, patient-reported quality of life, and weight gain, all associated with markedly reduced sweat chloride concentrations compared with placebo.
What This Study Adds to the Field
Here we report findings from the second of these Phase 3 placebo-controlled trials of ivacaftor. Despite a milder degree of lung disease at baseline, children (6–11 years of age) demonstrated significant improvements in lung function, of a magnitude similar to that in the older cohort; there were also significant increases in weight after 48 weeks of treatment with ivacaftor. This report shows efficacy with this small-molecule CFTR potentiator in this age group and underscores the clinical improvements that can be achieved even in a population with low disease burden at baseline.
Cystic fibrosis (CF), a genetic disorder affecting approximately 70,000 children and adults worldwide (1–3), is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene (4, 5). This gene encodes an epithelial chloride ion channel protein (CFTR), which contributes to the regulation of salt and water absorption and secretion in various tissues. The G551D-CFTR mutation, a class III mutation, results in functional impairment of CFTR channels that reach the cell surface (6). Approximately 4–5% of patients with CF have this mutation on at least one allele (1, 6). Although CF affects multiple organs, progressive loss of lung function is the leading cause of mortality.
Ivacaftor (VX-770; Vertex Pharmaceuticals Inc., Cambridge, MA) is an orally bioavailable CFTR potentiator. Ivacaftor increased the probability of CFTR channel opening in recombinant cells bearing the G551D gating mutation; it also enhanced chloride secretion while reducing excessive sodium and water reabsorption in cultured human CF bronchial epithelial cells carrying one G551D allele (7). By addressing the underlying protein defect, ivacaftor may help to maintain adequate airway hydration, and may be able to modify disease progression (8, 9).
This randomized, placebo-controlled, double-blind, multicenter trial evaluated the efficacy and safety of ivacaftor in a young (6–11 yr) and more mildly affected group of patients with CF and at least one G551D-CFTR mutation. Some of the results of these studies have been previously reported in the form of abstracts (10–12).
Methods
Study Oversight
The protocol was reviewed and approved by the institutional review board or ethics committee at each participating center, and each patient or caregiver provided written informed assent or consent.
Study Patients
Patients were eligible for inclusion if they were 6–11 years of age at screening; had a confirmed diagnosis of CF (13); had the G551D-CFTR mutation on at least one allele; had an FEV1 of 40–105% of the predicted value for persons of their age, sex, and height (14); and had a body weight greater than or equal to 15 kg. Patients were randomly assigned in a 1:1 ratio to receive ivacaftor at a dose of 150 mg every 12 hours or matched placebo for 48 weeks. The dose of ivacaftor was selected based on Phase 2a data and an initial, single-dose pharmacokinetic analysis conducted in nine patients, as described in the online supplement. Throughout the study, all patients continued to take their prestudy medications, with the exception of inhaled hypertonic saline, which was not an approved therapy in the United States and was not permitted. Additional information about patient eligibility, study visits, and assessments are provided in the online supplement.
Endpoints
The primary efficacy endpoint was the absolute change from baseline through Week 24 in percent of predicted FEV1. Secondary endpoints included the absolute changes from baseline in (1) percent of predicted FEV1 through Week 48; (2) weight at Weeks 24 and 48; (3) concentration of sweat chloride (a measure of CFTR function) through Weeks 24 and 48; and (4) patient-reported respiratory symptoms through Weeks 24 and 48 as assessed by the respiratory domain of the child version of the Cystic Fibrosis Questionnaire-revised (CFQ-R) (15) (scored on a 100-point scale, with higher numbers indicating less of an effect of symptoms on the patient’s quality of life and four points considered to be a minimal clinically important difference in clinically stable patients) (16). Safety was also evaluated (see online supplement). Subgroups based on pulmonary function (<70%, 70–90%, >90%, and ≤90% of the predicted FEV1), geographic region, and sex were used in specific analyses. Occurrence of pulmonary exacerbation (as defined in the online supplement) was monitored; however, in expectation of limited events, no formal analyses were performed.
Statistical Analyses
A minimum sample size of 30 patients was based on the anticipated available population rather than formal power calculations. The primary analysis was based on a mixed-effects model for repeated measures and adjusted for the baseline value of percent predicted FEV1. The primary endpoint and key secondary endpoints (changes from baseline in weight, sweat chloride, and CFQ-R respiratory domain score for all time points up to and including Week 24) were analyzed with the use of a multistage gatekeeping procedure (see online supplement).
Results
Study Population
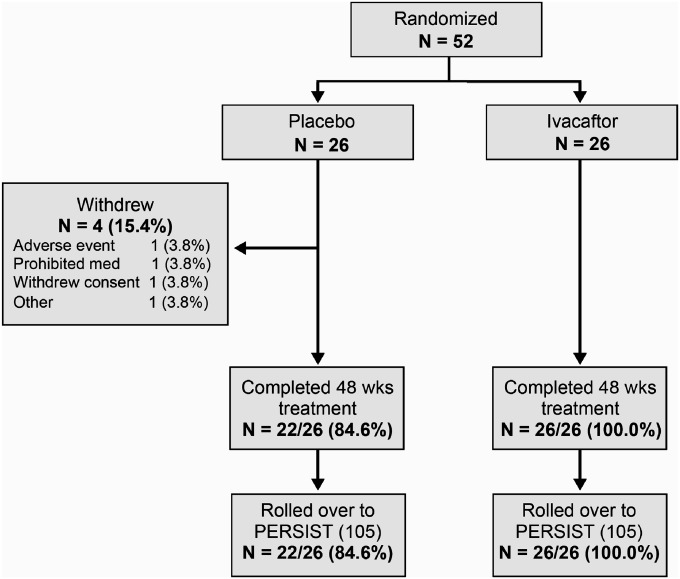
The study was conducted from March 2010 through April 2011. The screening, randomization, and follow-up of patients are shown in Figure 1. The study population consisted of 52 patients who underwent randomization and received at least one dose of ivacaftor (26 patients) or placebo (26 patients). Baseline characteristics are shown in Table 1. The mean age was 8.9 years, and the mean percent of predicted FEV1 was 84.2. Overall, 52% of patients were female; sex distribution was uneven within the groups. The mean values for percent of predicted FEV1, sweat chloride concentration, and body weight were similar in the two groups at baseline.
Figure 1.
Patient disposition.
TABLE 1.
BASELINE CHARACTERISTICS OF THE STUDY POPULATION*
| Characteristic | Placebo (n = 26) | Ivacaftor (n = 26) | Total (n = 52) |
|---|---|---|---|
| Sex, n (%) | |||
| Male |
16 (62) |
9 (35) |
25 (48) |
| Female |
10 (38) |
17 (65) |
27 (52) |
| Non-Hispanic, n (%)† |
24 (92) |
23 (88) |
47 (90) |
| Geographic distribution, n (%) | |||
| North America |
15 (58) |
12 (46) |
27 (52) |
| Europe |
5 (19) |
6 (23) |
11 (21) |
| Australia |
6 (23) |
8 (31) |
14 (27) |
| Age, yr | |||
| Mean |
8.9 |
8.9 |
8.9 |
| Range |
6 to 12‡ |
6 to 12‡ |
6 to 12‡ |
| Age distribution, n (%) | |||
| 6–8 yr |
13 (50) |
12 (46) |
25 (48) |
| 9–11 yr |
12 (46) |
11 (42) |
23 (44) |
| 12 yr‡ |
1 (4) |
3 (12) |
4 (8) |
| Height, cm | |||
| Mean |
132.6 |
134.9 |
133.8 |
| Range |
110.5 to 155.8 |
115.0 to 168.6 |
110.5 to 168.6 |
| Weight, kg | |||
| Mean |
30.0 |
31.8 |
30.9 |
| Range |
17.8 to 46.3 |
18.8 to 62.6 |
17.8 to 62.6 |
| Body mass index,§ kg/m2 | |||
| Mean |
16.8 |
17.1 |
17.0 |
| Range |
13.8 to 22.1 |
14.2 to 26.0 |
13.8 to 26.0 |
| Body mass index-for-age z score | |||
| Mean |
0.08 |
0.09 |
0.08 |
| Range |
−1.8 to 1.4 |
−1.5 to 2.1 |
−1.8 to 2.1 |
| Percent of predicted FEV1 | |||
| Mean |
83.7 |
84.7 |
84.2 |
| Range |
44.0 to 116.3 |
52.4 to 133.8 |
44.0 to 133.8 |
| Percent of predicted FEV1 distribution, n (%) | |||
| <70% |
8 (31) |
4 (15) |
12 (23) |
| ≥70% to ≤90% |
6 (23) |
12 (46) |
18 (35) |
| >90% |
12 (46) |
10 (38) |
22 (42) |
| Sweat chloride, mmol/L | |||
| Mean |
104.8 |
104.3 |
104.6 |
| Range |
92.0 to 121.0 |
54.0 to 128.0 |
54.0 to 128.0 |
| CFQ-R, child version, score for respiratory domain | |||
| Mean |
80 |
78 |
79 |
| Range |
25.0 to 100.0 |
33.3 to 100.0 |
25.0 to 100.0 |
| CFQ-R, parent/caregiver version, score for respiratory domain | |||
| Mean |
81 |
81 |
81 |
| Range | 38.9 to 100.0 | 33.3 to 100.0 | 33.3 to 100.0 |
None of the characteristics differed significantly between the groups (P > 0.05 for all comparisons).
Race or ethnic group was self-reported; information not provided for four patients in accordance with local regulations. One patient self-identified as “Other.”
All participants met inclusion criteria at screening (four children turned 12 yr of age between screening and baseline visits).
The body mass index is the weight in kilograms divided by the square of the height in meters.
Twenty-six patients in the ivacaftor group (100%) and 22 in the placebo group (85%) completed 48 weeks of treatment. The mean rate of on-study compliance (see online supplement) with study drug was 94.4% in the ivacaftor group and 95.7% in the placebo group. All patients, placebo or ivacaftor group, who completed 48 weeks of treatment enrolled in an open-label extension study (Study VX08–770–105; PERSIST).
Clinical Efficacy
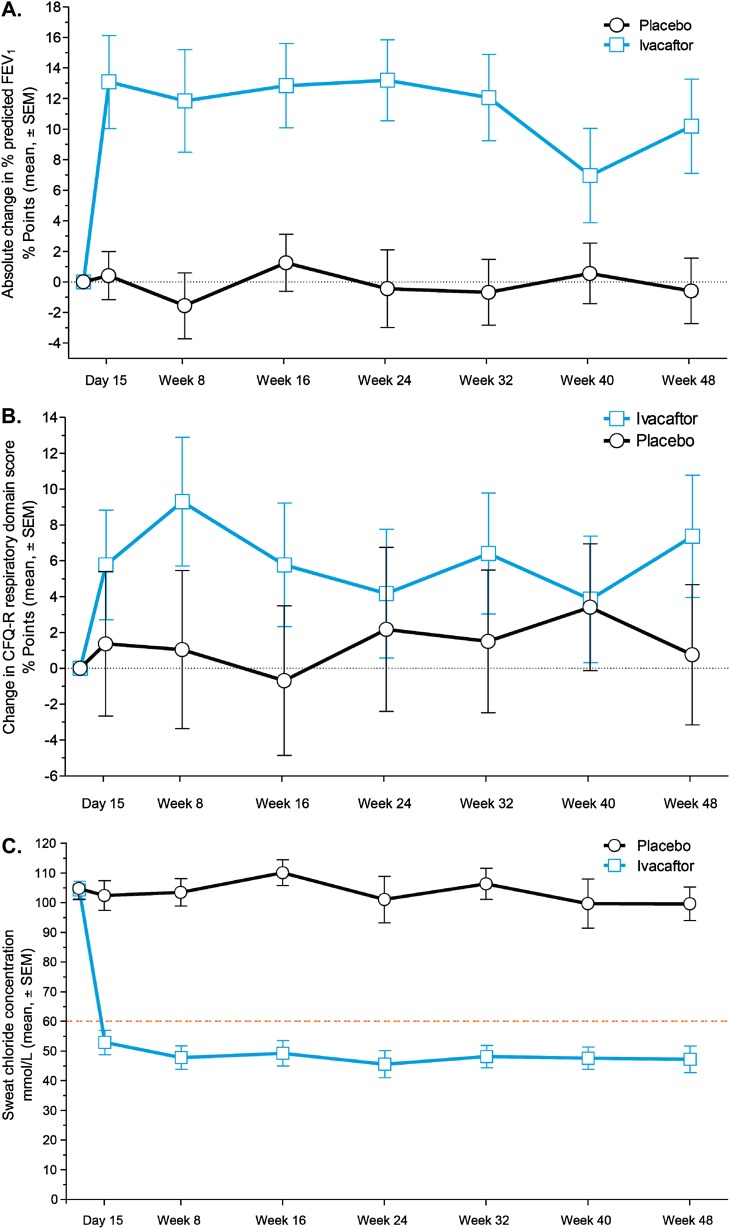
Mean percent of predicted FEV1 values at baseline were 84.7 in the ivacaftor group and 83.7 in the placebo group. Through Week 24, the model-adjusted, mean absolute increase from baseline was 12.6 percentage points in the percent of predicted FEV1 in the ivacaftor group, compared with an increase of 0.1 percentage points in the placebo group, a treatment effect of 12.5 percentage points (P < 0.001) (Figure 2A; see Table E1 in the online supplement). An effect of ivacaftor was noted by Day 15 of treatment, and a significant treatment effect was maintained throughout the study. The adjusted change in percent of predicted FEV1 from baseline through Week 48 was 10.0 percentage points greater with ivacaftor than with placebo (10.7 vs. 0.7; P < 0.001) (see Table E1). The absolute change from baseline in percent of predicted forced mid-expiratory flow rate was significantly greater for the ivacaftor group versus the placebo group through Week 24 (treatment effect, 22.3 percentage points; P < 0.001), which was consistent with the change in percent of predicted FEV1 (see Table E2).
Figure 2.
Absolute changes from baseline in percent of predicted FEV1 and Cystic Fibrosis Questionnaire-revised (CFQ-R) respiratory domain scores, and absolute sweat chloride concentrations by study group. (A) Absolute mean change from baseline (± SEM) in percent of predicted FEV1. (B) Absolute mean change from baseline (± SEM) in the CFQ-R respiratory domain score (child questionnaire version). (C) Sweat chloride concentrations (± SEM) from baseline to Week 48. The orange dashed line indicates 60 mmol/L, the recognized diagnostic cut-off. In A, B, and C, estimates are model-based.
The change in percent of predicted FEV1 was analyzed within predefined subgroups based on geography, sex, and baseline FEV1. For all subgroups, FEV1 outcomes through 24 weeks showed evidence of a treatment effect with ivacaftor (range, 4.2–24.6 percentage points). The differences in FEV1 remained statistically significant for ivacaftor versus placebo within the following subgroups: females, European centers, and patients with percent of predicted FEV1 less than or equal to 90% at baseline (see Figure E1). In a post hoc analysis of FEV1 outcomes by tertiles of baseline FEV1/forced vital capacity ratio (a lower ratio being associated with greater severity), ivacaftor treatment was associated with a greater treatment effect and absolute improvements among patients with more severe lung function impairment at baseline compared with other tertiles (see Figure E2).
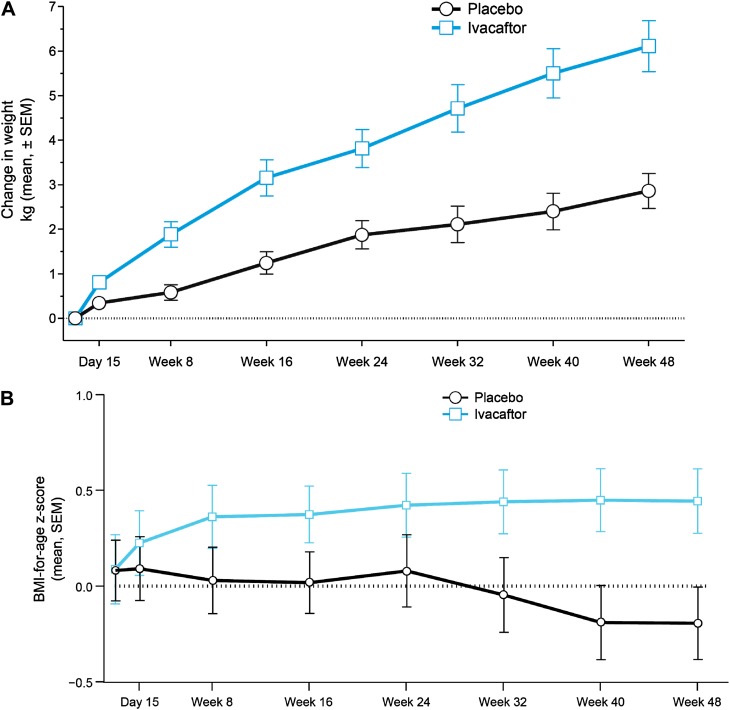
At Week 24, patients in the ivacaftor group had gained an average of 3.7 kg, compared with 1.8 kg in the placebo group (treatment effect, 1.9 kg; P < 0.001) (Figure 3A; see Table E1). The treatment effect of ivacaftor relative to placebo was greater by Week 48 (2.8 kg; P < 0.001) (see Table E1). Body mass index (BMI)-for-age z scores improved in the ivacaftor group through Week 8, and were sustained for the remainder of the 48-week treatment period, whereas BMI-for-age z scores declined in the placebo group (wk 24 treatment effect, 0.34, P < 0.001; wk 48 treatment effect, 0.45, P < 0.001) (Figure 3B).
Figure 3.
Changes from baseline in weight, and absolute body mass index (BMI)-for-age z scores by study group. (A) Absolute mean change from baseline (± SEM) in weight. (B) Absolute mean (± SEM) BMI-for-age z scores. Z scores are adjusted for normal growth by age and sex and are presented as the standard deviation from the population mean. In A and B, estimates are model-based.
Patients treated with ivacaftor had an increase in scores on the child version of the CFQ-R respiratory domain, indicating improvement in respiratory symptoms compared with those receiving placebo. Mean baseline scores were 78 points in the ivacaftor group and 80 points in the placebo group. From baseline to Week 24, the model-adjusted scores increased by 6.3 points in the ivacaftor group and 0.3 points in the placebo group (treatment effect, 6.1 points; P = 0.109) (Figure 2B; see Table E1). Parents or caregivers also completed a version of the CFQ-R (mean baseline value of 81 points in both groups), and the mean adjusted absolute change in the respiratory domain from baseline to Week 24 was higher in the ivacaftor group (treatment effect, 5.9 points; P = 0.033).
The protocol-defined pulmonary exacerbation rate was low and did not differ between ivacaftor-treated and placebo groups (four events and three events, respectively). Sweat chloride concentrations dropped rapidly (treatment effect observed at the first on-treatment visit, Day 15) in the ivacaftor group and was stable through Weeks 24 and 48 (Figure 2C), which is consistent with improved CFTR function. The mean change from baseline in sweat chloride was −55.5 mmol/L in the ivacaftor group and −1.2 mmol/L in the placebo group (treatment effect, −54.3 mmol/L; P < 0.001) and was maintained through Week 48 (see Table E1). Only one patient was homozygous for the G551D mutation; the absolute change from baseline in sweat chloride at Week 48 was −54.5 mmol/L for this patient.
Safety and Adverse Event Profile
The incidence of adverse events through Week 48 was similar in the two groups (Table 2). One patient in the ivacaftor group had an event leading to study drug interruption, as did three patients in the placebo group. One patient in the placebo group withdrew from the study because of psychological issues and anxiety. No subjects withdrew in the ivacaftor group. Adverse events occurring in greater than or equal to 10% of subjects in either arm are shown in Table E3 in the online supplement. Compared with the placebo group, cough, productive cough, vomiting, rales, and decreased pulmonary function test were less common (≥5% incidence differential between groups) in the ivacaftor group, whereas oropharyngeal pain, headache, nasopharyngitis, upper respiratory tract infection, otitis media, diarrhea, and increased blood eosinophil count were more common in the ivacaftor group.
TABLE 2.
PATIENTS WITH ADVERSE EVENTS AND DRUG DISCONTINUATIONS CAUSED BY ADVERSE EVENTS BY TREATMENT GROUP
| Adverse Event Category | Placebo (n = 26) n (%) | Ivacaftor (n = 26) n (%) |
|---|---|---|
| Any adverse event |
25 (96.2) |
26 (100) |
| Adverse event leading to study drug interruption |
3 (11.5) |
1 (3.8) |
| Adverse event leading to permanent discontinuation of study drug |
1 (3.8) |
0 |
| Any serious adverse event |
6 (23.1) |
5 (19.2) |
| Death | 0 | 0 |
Eleven patients reported serious adverse events (five patients in the ivacaftor group and six in the placebo group). Only pulmonary exacerbation (two patients in the ivacaftor group and three in the placebo group) and productive cough (one patient in each group) were reported more than once. No deaths occurred during the study. No clinically important trends attributable to ivacaftor were identified in results on clinical laboratory tests (serum chemistry, hematology, coagulation studies, liver function tests, and urinalysis); vital signs; digital electrocardiograms; ambulatory electrocardiograms; or physical examinations.
Discussion
Ivacaftor, an oral CFTR potentiator, significantly improved FEV1 and most secondary endpoints in children with CF 6–11 years of age who have a G551D-CFTR mutation on at least one allele. The results of this study confirm that the efficacy of ivacaftor shown previously in adolescents and adults extended to this younger, healthier patient population (17). During the preparation of this manuscript, ivacaftor was approved in the United States, Europe, and Canada for the treatment of CF in patients greater than or equal to 6 years of age who have a G551D mutation.
The improvements in percent of predicted FEV1 were similar in magnitude to those seen in the trial of patients with CF greater than or equal to 12 years of age despite the fact that the children in the current study entered with less impairment in lung function than the adolescents and adults enrolled in the earlier trial, who had a mean FEV1 of 63.6%. Many children in the current study started with lung function within the normal range and still displayed improvements in percent of predicted FEV1 and other key secondary endpoints. In these children with CF, the mean treatment effect with ivacaftor through 24 week was an absolute improvement of 12.5 percentage points of predicted FEV1. Dornase alfa, which is part of the standard of care in CF, was associated with a treatment effect of 3.2 percentage points in FEV1 at Week 96 in children 6–10 years of age in a clinical trial (18). Inhaled tobramycin, another standard-of-care CF therapy, resulted in a nonstatistically significant treatment effect relative to baseline of 8.6% in FEV1 versus placebo at Week 20 in a subgroup of children 6–12 years of age enrolled in a clinical study (19). Most children in the current study were receiving these agents (dornase alfa, 77%; tobramycin, 50%); thus, the substantial treatment effect of ivacaftor was achieved within the setting of these ongoing, standard-of-care medications.
A comparison of changes in pulmonary function observed in this trial and the earlier study of ivacaftor in adolescents and young adults (17) suggests a greater magnitude of improvement in this younger population. A post hoc analysis of patients with baseline FEV1 50–80% of predicted across the two trials showed that, at every time point, younger patients from the current trial had a greater mean improvement in FEV1 compared with older patients with similar impairments in pulmonary function (see Figure E3).
Impairment in FEV1 in patients with CF can be related to a number of factors, including mucus plugging, airway wall thickening caused by inflammation or remodeling, and established bronchiectasis. The rapid improvements in FEV1 followed by a plateauing of effect with ivacaftor in the current study population were similar to those observed in the earlier trial in patients with CF greater than or equal to 12 years of age (17). This suggests that the primary mechanism of ivacaftor may involve a CFTR-mediated effect on airway surface secretions, which may have resulted in decreased inflammation and/or less mucus plugging rather than an effect on airway remodeling processes. Additional follow-up is needed to determine whether the effects of CFTR modulation by ivacaftor over multiple years produce physiologic changes that further improve airway function in patients with CF. Although placebo-controlled trials long enough and with a large enough sample size to show such an impact are impractical to execute, data from the ongoing, open-label extension study may be a first step in elucidating answers to these questions.
As was expected in this age group, children in both study arms gained weight during the trial period. However, children who received ivacaftor gained, on average, 2.8 kg more than those allocated to placebo at Week 48, which is consistent with the 2.7 kg greater weight gain with ivacaftor compared with placebo observed in the earlier study (17). It is striking that z scores for BMI improved rapidly in the ivacaftor group over the first 8 weeks of treatment (with sustained improvements through 48 wk), whereas BMI z scores declined in the placebo arm. Suboptimal weight gain is a common problem in CF and may be attributed to multiple factors, including pancreatic insufficiency leading to intestinal malabsorption, nutritional deficiencies, reduced appetite, diabetes, direct consequences of reduced CFTR function on gut absorption, and higher metabolic rate caused by chronic lung inflammation and the increased work of breathing (20–23). It seems unlikely that any agent given after 6 years of age could have a significant impact on pancreatic damage, which is thought to occur earlier in life. CF therapies that improve lung function, such as tobramycin and dornase alfa, did not lead to improvements in nutritional status in published studies (18, 19). Taken together, this suggests that additional mechanisms may be contributing to the nutritional findings in the current study of ivacaftor.
Although respiratory symptoms (measured with the CFQ-R) decreased from baseline with ivacaftor treatment, these reductions were not statistically significant compared with placebo, except for the Week 24 findings with the parent-caregiver questionnaire. In the study conducted in patients greater than or equal to 12 years of age, ivacaftor was associated with significant improvements in CFQ-R respiratory domain scores through Week 48, compared with placebo (17). This apparent difference between studies may reflect the differences in baseline respiratory severity in the respective study cohorts; the higher CFQ-R at baseline in the current study (mean 79 points, child version, vs. 70 points, pooled adolescent/adult and child versions in the adult study); or simply the small sample size in this study (n = 52 vs. n = 161 in the adult study).
Correlations between outcomes, such as sweat chloride and FEV1, were not observed in the much larger adolescent and adult trial of this agent and therefore were not explored in this study.
Ivacaftor had an acceptable safety profile during twice-daily administration for 48 weeks, a finding consistent with those from the earlier study. Individual adverse events with ivacaftor were reported at similar frequencies as in the earlier study, with cough, pulmonary exacerbation, oropharyngeal pain, and headache as the most common events. Serious adverse events were not increased by ivacaftor, compared with placebo, in either study. However, this novel agent has to date been administered to a relatively small population and for a limited period of time. Ongoing post-marketing surveillance is essential to confirm longer-term safety, particularly because the drug is intended for lifetime use. In addition to pharmacovigilance activities and to better understand the long-term safety of ivacaftor, a 5-year safety surveillance study is ongoing in the United States and Europe. Also, because cataracts were observed in newborn rats, and although cataracts have not been observed in human trials to date, an observational study on ocular safety seeks to provide information on potential ophthalmologic side effects.
Ivacaftor has been tested in clinical studies only in patients with the G551D mutation and in those homozygous for F508del (24); in the latter, as expected, there was no beneficial effect. Several ongoing studies are underway to explore response in patients with mutations beyond G551D and F508del. It may be instructive to observe whether similar outcomes occur in patients with G551D-like mutations and in those where a more limited response might be expected based on the current in vitro understanding of the genetic defects.
In this study, treatment with ivacaftor produced statistically significant and clinically meaningful improvements in pulmonary function, weight, and CFTR activity (as determined by sweat chloride) in 6- to 11-year-old patients carrying a G551D-CFTR mutation on at least one allele. Importantly, these children were younger and healthier than previously studied populations. Effects of ivacaftor treatment were seen in addition to standard CF therapies and the adverse event profile was consistent with that seen in other studies. The consistency of therapeutic efficacy with ivacaftor across the spectrum of CF disease supports continued testing of this agent in children younger than 6 years of age, because this is the population with the greatest potential for modification of disease progression.
Acknowledgments
Acknowledgment
The authors thank all the patients involved in this study and the study coordinators (see the online supplement for a complete list). The Royal Brompton Hospital acknowledges the support of the National Institutes for Health Research through the Medicines for Children Research Network. The authors thank the following employees and previous employees of Vertex: Elizabeth Dorn, Ph.D., Adrienne A. Aiello, Pharm.D. (previous employee) for writing support; Mark De Rosch, Ph.D. (previous employee), Lisa DeTora, Ph.D. (previous employee), Geoffrey Gilmartin, M.D., Charles Johnson, M.D., Po-Shun Lee, M.D. (previous employee), Paul Negulescu, M.D., and Christopher Wright, M.D., for scientific and editorial advice; Gabrielle Poirier, B.S., for clinical operations support; Jiuhong Zha, Ph.D. (previous employee), for pharmacokinetic analyses; and James O’Hearn, M.S., and Hongyu Liu, Ph.D., for statistical programming support. Writing support was provided by the fmP group of Fallon Medica and was funded by Vertex.
Footnotes
Supported by Vertex Pharmaceuticals Incorporated.
Author Contributions: The study sponsor (Vertex Pharmaceuticals, Incorporated) designed the protocol in collaboration with the academic authors. Site investigators collected the data, which were analyzed by the sponsor. All authors had full access to the study data. J.C.D. developed the initial draft of the manuscript, with writing assistance from the sponsor. All authors participated in subsequent revisions. After consultation with their coauthors, J.C.D and R.A. made the decision to submit the manuscript for publication.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201301-0153OC on April 3, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Cystic Fibrosis Foundation. Bethesda, MD: Cystic Fibrosis Foundation; 2011. Cystic fibrosis foundation patient registry: 2010 annual data report. [Google Scholar]
- 2.Farrell PM. The prevalence of cystic fibrosis in the European Union. J Cyst Fibros. 2008;7:450–453. doi: 10.1016/j.jcf.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 4.Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 5.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 6.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 7.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, Turnbull A, Singh A, Joubran J, Hazlewood A, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA. 2009;106:18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 9.Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest. 2007;132:1631–1636. doi: 10.1378/chest.07-0288. [DOI] [PubMed] [Google Scholar]
- 10.Ahrens R, Rodriguez S, Yen K, Davies JC. VX-770 in subjects 6 to 11 years with cystic fibrosis and the G551D-CFTR mutation. Pediatr Pulmonol. 2011;46:212–428. [Google Scholar]
- 11.Davies JC, Li H, Yen K, Ahrens R. Ws6.5 ivacaftor in subjects 6 to 11 years of age with cystic fibrosis and the G551D-CFTR mutation. J Cyst Fibros. 2012;11:S13. [Google Scholar]
- 12.Elborn S, Wainwright C, Sermet-Gaudelus I, Nasr S, Rodriguez S, Yen K, Ramsey B. Pulmonary effects of the investigational CFTR potentiator, ivacaftor, in two phase 3 trials in subjects with CF who have the G551D-CFTR mutation. Am J Respir Crit Care Med. 2012;185:A2464. [Google Scholar]
- 13.Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, Durie PR, Legrys VA, Massie J, Parad RB, et al. Cystic Fibrosis Foundation. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153:S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 15.Modi AC, Quittner AL. Validation of a disease-specific measure of health-related quality of life for children with cystic fibrosis. J Pediatr Psychol. 2003;28:535–545. doi: 10.1093/jpepsy/jsg044. [DOI] [PubMed] [Google Scholar]
- 16.Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire-Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest. 2009;135:1610–1618. doi: 10.1378/chest.08-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW, et al. VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quan JM, Tiddens HA, Sy JP, McKenzie SG, Montgomery MD, Robinson PJ, Wohl ME, Konstan MW Pulmozyme Early Intervention Trial Study Group. A two-year randomized, placebo-controlled trial of dornase alfa in young patients with cystic fibrosis with mild lung function abnormalities. J Pediatr. 2001;139:813–820. doi: 10.1067/mpd.2001.118570. [DOI] [PubMed] [Google Scholar]
- 19.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, Vasiljev-K M, Borowitz D, Bowman CM, Marshall BC, et al. Cystic Fibrosis Inhaled Tobramycin Study Group. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med. 1999;340:23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 20.Borowitz D, Baker RD, Stallings V. Consensus report on nutrition for pediatric patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2002;35:246–259. doi: 10.1097/00005176-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Pencharz PB, Durie PR. Pathogenesis of malnutrition in cystic fibrosis, and its treatment. Clin Nutr. 2000;19:387–394. doi: 10.1054/clnu.1999.0079. [DOI] [PubMed] [Google Scholar]
- 22.Zemel BS, Jawad AF, FitzSimmons S, Stallings VA. Longitudinal relationship among growth, nutritional status, and pulmonary function in children with cystic fibrosis: analysis of the Cystic Fibrosis Foundation National CF Patient Registry. J Pediatr. 2000;137:374–380. doi: 10.1067/mpd.2000.107891. [DOI] [PubMed] [Google Scholar]
- 23.Peterson ML, Jacobs DR, Jr, Milla CE. Longitudinal changes in growth parameters are correlated with changes in pulmonary function in children with cystic fibrosis. Pediatrics. 2003;112:588–592. doi: 10.1542/peds.112.3.588. [DOI] [PubMed] [Google Scholar]
- 24.Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, Sagel SD, Hornick DB, Konstan MW, Donaldson SH, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]