Tertiary lymphoid organs (TLO) or ectopic lymphoid follicles are lymphoid aggregates with specialized structures that form under chronic inflammatory processes (1). Their denomination comes from the resemblance these structures bear with secondary lymphoid organs (SLOs), and include the presence of defined B- and T-cell areas, follicular dendritic cells, the promotion of lymphatic development, and the acquisition of high endothelial venule properties by blood vessels (1). TLOs, however, differ from SLOs in that they are not developmentally preprogrammed and appear after exposure to antigen. TLOs have been described in a variety of inflammatory and infectious settings, including influenza (2) and Mycobacterium tuberculosis (3) infections as well as autoimmune conditions such as rheumatoid arthritis (4), multiple sclerosis (5), and systemic lupus erythematosus (6). There is accumulating evidence suggesting that TLOs may be involved in local immune responses that may occur under inflammatory conditions. As such, development of TLOs often correlates with disease aggravation and promotion of autoreactive T- and B-cell activation in autoimmune conditions (1). However, in some infectious diseases, as is the case in tuberculosis (7), their presence may be associated with development of protective immune responses and improved pathogen control.
Chronic obstructive pulmonary disease (COPD) is a progressive, not fully reversible inflammatory respiratory disorder characterized by limitation of airflow in the lung, including obstructive bronchitis and emphysema (8). Although the main etiological factor is smoking, coal dust exposure and α1-antitrypsin deficiency have also been associated with COPD development (9). In patients with COPD (10) and in mouse models (11), previous reports have described the presence of lymphoid follicles in the lung; however, the precise mechanisms mediating lymphoid follicle development in the COPD lung remain unknown. In this issue of the Journal, Litsiou and colleagues (pp. 1194–1202) seek to address the role of CXCL13 in TLOs during COPD in the human lung (12). Although other studies have demonstrated a role for CXCL13 and lymphotoxin (LT) in lymphoid neogenesis in a mouse model of COPD (11), the current study is one of the first studies to examine the dynamics of CXCL13 expression in samples from patients with COPD. Here, the authors report elevated levels of CXCL13 in the lung follicles of patients with COPD and show a positive correlation between the area occupied by lymphoid follicles and lung CXCL13 levels. Interestingly, no correlation was seen between lung CXCL13 levels and the levels of proinflammatory cytokines such as TNFα and IL-8, cytokines used as a measure of severity of lung inflammation. Importantly, and unlike other pathologies where follicular dendritic cells, macrophages, and monocytes are the main producers of CXCL13 (13), B cells were identified as the main source of CXCL13 in COPD lung samples. B cells produced LT, exhibited the highest expression levels of LTβR, and were chemotactic toward CXCL13 gradients. CXCL13, in turn, increased the expression of membrane-bound LT in B cells, further stimulating CXCL13 production and establishing a positive feedback loop that perpetuates lymphoid follicle formation (Figure 1).
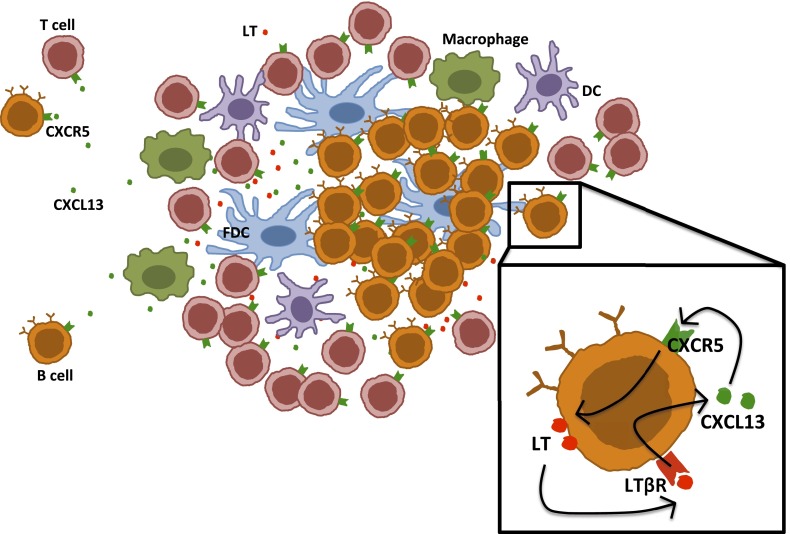
Figure 1.
A role for CXCL13 in a feedback loop that promotes the maintenance of chronic obstructive pulmonary disease (COPD) lymphoid follicles. Lymphoid follicles with defined B- and T-cell areas, a network of follicular dendritic cells, and infiltrating dendritic cells and macrophages have been described in COPD. In these structures, CXCL13 production is instrumental for guiding T- and B-cell migration. CXCL13 signaling stimulates membrane-bound lymphotoxin expression by B cells, which in turn stimulates CXCL13 production by these cells. This positive feedback mechanism contributes to the recruitment of additional cells to lung follicles and likely promotes tertiary lymphoid organ permanence in patients with COPD. DC = dendritic cell; FDC = follicular dendritic cell; LT = lymphotoxin.
Thus, the findings described in this article add clinical relevance to findings from the COPD mouse model, and suggest that mechanisms leading to ectopic lymphoid follicle formation are possibly conserved across species. Namely, LT overexpression in different organs has been shown to up-regulate the expression of the homeostatic chemokines CXCL13 and CCL21, which promote the development of organized B- and T-cell zones, and CCL19, which favors lymphocyte and dendritic cell migration (13). In this article, B cells were identified as the main source of CXCL13 in lung samples from patients with stage 2 COPD who underwent surgery and exhibited moderate to severe persistent inflammation and established lymphoid follicles. The described feedback loop involving CXCL13 and LT may thus occur at a later phase of lymphoid neogenesis, and likely feeds into the innate mechanisms that initiate the early stages of lung inflammation. Mouse studies have identified that innate cells, including macrophages, neutrophils, and dendritic cells, are the first cells to be recruited to the lung after cigarette smoke exposure (14). Innate immune cells thus respond to proinflammatory molecules induced by cigarette smoke, including the up-regulation of IL-17 production through stimulation by the aryl hydrocarbon receptor (15). One such response is likely the IL-17–driven increase of CXCL13 production (16), through either LT-dependent or -independent pathways. Elucidating the kinetics of lymphoid follicle formation and the cytokines and chemokines responsible for their induction and maintenance will provide valuable information to understand the biology and clinical progression of COPD.
The role of TLOs in COPD and its impact on disease progression remains controversial. It is possible that lymphoid follicle formation is associated with the development of protective responses against infectious agents in COPD, evidenced by the suspension of the clinical trial involving the use of rituximab (a human/mouse monoclonal antibody directed against the CD20 antigen) for COPD treatment due to increased infectious complications (14). Other studies, however, associate lymphoid follicle formation in COPD to the development of pathogenic autoantibodies and the persistence of inflammation (14). Indeed, the number of lymphoid follicles present in small airways is increased in patients with severe COPD when compared with those with mild COPD or healthy control subjects. It would be interesting to determine whether smoking cessation induces the regression of these structures or if they contribute to the continued inflammation and lung damage seen in patients with COPD who have fully stopped smoking. B cell populations present in ectopic lymphoid follicles in COPD have been shown to be oligoclonal, suggesting that antigen-specific responses may be involved in lymphoid neogenesis (14). Elucidating the antigen specificity of these B cells will shed further light on the still-debated etiological factors leading to COPD.
In summary, defining the mechanisms leading to lymphoid follicle formation in different stages of COPD, as well as the characteristics of the cells recruited and the reversibility of the process, will contribute to the global understanding of the nature of the antigenic signals that perpetuate inflammation and development of TLOs in COPD. Importantly, gaining knowledge on these processes may suggest novel therapeutic avenues, including the use of CXCL13 antagonists, for the treatment of COPD. The continued use of relevant animal models and validation in human samples from patients is required to address these questions and progress the field forward.
Footnotes
Supported by the Children’s Hospital of Pittsburgh, NIH grants AI083541 and HL105427 to S.A.K., and a Research Advisory Committee Grant from the Children’s Hospital of Pittsburgh of the UPMC Health System to L.M.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 2012;33:297–305. doi: 10.1016/j.it.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GeurtsvanKessel CH, Willart MA, Bergen IM, van Rijt LS, Muskens F, Elewaut D, Osterhaus AD, Hendriks R, Rimmelzwaan GF, Lambrecht BN. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J Exp Med. 2009;11:2339–2349. doi: 10.1084/jem.20090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ulrichs T, Kosmiadi GA, Trusov V, Jörg S, Pradl L, Titukhina M, Mishenko V, Gushina N, Kaufmann SH. Human tuberculosis granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. J Pathol. 2004;204:217–228. doi: 10.1002/path.1628. [DOI] [PubMed] [Google Scholar]
- 4.Takemura S, Braun A, Crowson C, Kurtin PJ, Cofield RH, O’Fallon WM, Goronzy JJ, Weyand CM. Lymphoid neogenesis in rheumatoid synovitis. J Immunol. 2001;167:1072–1080. doi: 10.4049/jimmunol.167.2.1072. [DOI] [PubMed] [Google Scholar]
- 5.Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14:164–174. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang A, Henderson SG, Brandt D, Liu N, Guttikonda R, Hsieh C, Kaverina N, Utset TO, Meehan SM, Quigg RJ, et al. In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol. 2011;186:1849–1860. doi: 10.4049/jimmunol.1001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slight SR, Rangel-Moreno J, Gopal R, Lin Y, Fallert Junecko BA, Mehra S, Selman M, Becerril-Villanueva E, Baquera-Heredia J, Pavon L, et al. CXCR5+ T helper cells mediate protective immunity against tuberculosis. J Clin Invest. 2013;123:712–726. doi: 10.1172/JCI65728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnes PJ. Chronic obstructive pulmonary disease: a growing but neglected global epidemic. PLoS Med. 2007;4:e112. doi: 10.1371/journal.pmed.0040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8:183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 10.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 11.Demoor T, Bracke KR, Maes T, Vandooren B, Elewaut D, Pilette C, Joos GF, Brusselle GG. Role of lymphotoxin-α in cigarette smoke-induced inflammation and lymphoid neogenesis. Eur Respir J. 2009;34:405–416. doi: 10.1183/09031936.00101408. [DOI] [PubMed] [Google Scholar]
- 12.Litsiou E, Semitekolou M, Galani IE, Morianos I, Tsoutsa A, Kara P, Rontogianni D, Bellenis I, Konstantinou M, Potaris K, et al. CXCL13 production in B cells via toll-like receptor/lymphotoxin receptor signaling is involved in lymphoid neogenesis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:1194–1202. doi: 10.1164/rccm.201208-1543OC. [DOI] [PubMed] [Google Scholar]
- 13.Carragher DM, Rangel-Moreno J, Randall TD. Ectopic lymphoid tissues and local immunity. Semin Immunol. 2008;20:26–42. doi: 10.1016/j.smim.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brusselle GG, Demoor T, Bracke KR, Brandsma C-A, Timens W. Lymphoid follicles in (very) severe COPD: beneficial or harmful? Eur Respir J. 2009;34:219–230. doi: 10.1183/09031936.00150208. [DOI] [PubMed] [Google Scholar]
- 15.Chen K, Pociask DA, McAleer JP, Chan YR, Alcorn JF, Kreindler JL, Keyser MR, Shapiro SD, McGarry Houghton A, Kolls JK, et al. IL-17RA is required for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smoke. PLoS ONE. 2011;6:e20333. doi: 10.1371/journal.pone.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, Hwang JY, Kusser K, Hartson L, Kolls JK, Khader SA, Randall TD. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol. 2011;12:639–646. doi: 10.1038/ni.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]