Abstract
Rationale: Natural killer (NK) cells are innate lymphocytes that target virus-infected and tumor cells. Much less is known about their ability to limit adaptive immune responses.
Objectives: Thus, we investigated to what extent NK cells can influence mouse lung allograft rejection.
Methods: For this purpose, we employed an orthotopic lung transplantation model in mice.
Measurements and Main Results: We demonstrate here that NK cells infiltrate mouse lung allografts before T cells and thereby diminished allograft inflammation, and that NK-cell deficiency enhanced allograft rejection. In contrast, expansion of recipient NK cells through IL-15/IL-15Rα complex treatment resulted in decreased T-cell infiltration and alloreactive T-cell priming as well as improved function of the allogeneic lung transplant. Only perforin-competent, but not perforin-deficient, NK cells were able to transfer these beneficial effects into transplanted NK cell–deficient IL-15Rα−/− mice. These NK cells killed allogeneic dendritic cells (DCs) in vitro and significantly decreased the number of allogeneic DCs in transplanted lungs in vivo. Furthermore, DC-depleted lung allografts presented decreased signs of rejection.
Conclusions: These results suggest that NK cells favor allograft acceptance by depleting donor-derived DCs, which otherwise would prime alloreactive T-cell responses. Thus, conditioning regimens that augment NK-cell reactivity should be clinically explored to prepare lung allograft recipients.
Keywords: natural killer cells, dendritic cells, lung, mouse transplantation, acute rejection
At a Glance Commentary
Scientific Knowledge on the Subject
This study builds on our initial findings that natural killer (NK) cells can eliminate dendritic cells (DCs) by cytotoxicity. Since then, this editing function toward the hematopoietic lineage has been described in ameliorating graft-versus-host disease after bone marrow transplantation. During solid organ transplantation, however, prevention of transplant rejection due to NK cell–mediated killing of donor DCs has been less explored.
What This Study Adds to the Field
Our study describes, in an orthotopic allogeneic transplantation model of a large blood-perfused organ, namely the lung, that recipient NK cells can eliminate donor antigen-presenting cells, mainly DCs, to ameliorate allograft rejection. These donor-derived DCs prime direct alloreactive immune responses and, accordingly, DC-depleted lung allografts are less rejected. We demonstrate that recipient NK cells, especially after IL-15/IL-15Rα complex expansion, can regulate donor DC content of the transplant via their cytotoxicity, and that this affects recipient leukocyte infiltrates, alloreactive T-cell responses, and transplant function as indicated by magnetic resonance imaging as well as blood oxygenation. These findings suggest a role for recipient NK cells in the regulation of lung allograft rejection, and suggest novel avenues for conditioning of transplant recipients for better graft acceptance.
Nearly 50 years after the first lung transplantation (1), this medical procedure has been performed more than 32,000 times worldwide (2). The main indications are nonmalignant lung diseases such as cystic fibrosis, chronic obstructive pulmonary diseases, and idiopathic pulmonary fibrosis. Although donor organ preparation, surgical procedures, and immune suppression management have continuously improved, long-term survival of transplant recipients does not exceed 60% beyond 5 years after transplantation. In addition, numbers of suitable major histocompatibility complex (MHC)–matched donor organs remain low, but this limitation could be overcome by limiting immunological transplant rejection of MHC-mismatched allografts, which could serve as an additional donor organ source.
Although immune-suppressive treatments are essential for lung graft acceptance and maintenance, and are a standard for clinical care after transplantation, the immunomodulatory ability of the immune system itself has so far not been explored to improve lung transplant survival. Meanwhile, natural killer (NK) cells, which are innate lymphocytes that restrict viral infections and are able to target tumor cells (3), have been shown to regulate immune responses by editing populations of antigen-presenting cells (APCs) that induce these responses, and T cells that mediate them (4–7). Along these lines, NK cells can eliminate dendritic cell (DCs) and T-cell populations by perforin-mediated lysis, limiting in this way autoimmunity and antiviral immunity (8–14). In contrast to virally infected or transformed target cells, these leukocytes are thought to be recognized by NK cells not on loss of MHC class I ligands for inhibitory NK-cell receptors (missing self-recognition), but via high expression of ligands for activating NK-cell receptors (altered self-recognition), such as NKG2D and natural cytotoxicity receptors (8, 15, 16). Thus, NK cells can directly kill APCs and T cells to limit immune responses.
Alternatively, however, they can also augment adaptive immune responses via cytokines. In particular, NK cell–secreted tumor necrosis factor (TNF)-α can mature DCs, and IFN-γ of NK cells promotes helper T-cell type 1 (Th1) polarization during antigen presentation (17). This NK-cell assistance for adaptive immunity improves DC-dependent T-cell priming and Th1-mediated restriction of Leishmania infection in mice (18–20). In addition, NK cell–derived IFN-γ augments Th1 polarization of DC-induced alloreactive T-cell responses (21). Therefore, cytokine production by NK cells can shape adaptive immune responses and enhance Th1-polarized T-cell immunity.
In the present study we investigated the net outcome of these opposing functions of NK cells in regulating the adaptive T-cell response in a mouse model of allogeneic lung transplantation. We found that NK-cell activity prolonged graft function and limited alloreactive immune responses and, therefore, rejection by targeting donor-derived DCs, which are known to be inducers of graft-reactive T-cell responses. Our results suggest that NK-cell alloreactivity against DCs can be used to purge allogeneic solid organ transplants of these APCs, which otherwise would initiate direct allograft rejection.
Methods
Mouse Models of Orthotopic Single-Lung Transplantation and Interventions
Orthotopic single-lung transplantation was performed as previously described (22) and the respective manipulations in this model, including IL-15/IL-15Rα (IL-15 receptor, α chain) administration (23), are described in the online supplement.
Histology and Immunohistochemistry
Histology and immunohistochemistry were performed as described in the online supplement.
Magnetic Resonance Imaging
All magnetic resonance imaging (MRI) measurements were performed at the indicated time points as described previously (24) and as specified in the online supplement.
Transplant Oxygenation Analysis
Graft oxygenation was evaluated by sampling blood (∼250 μl) directly from the pulmonary vein of the transplanted (3 min after clamping the hilum of the right lung) or naive lung at the indicated time points through a heparinized needle, which was inserted proximal to the anastomotic cuff.
Cell Isolation and Mixed Leukocyte Reactions
The respective experiments were conducted as outlined in the online supplement.
CD107a Degranulation Analysis and NK-Cell Adoptive Transfer
The online supplement contains the experimental information for these analyses.
Statistical Analysis
Statistical analysis was performed with GraphPad Prism software (GraphPad Software, San Diego, CA). A nonparametric unpaired two-tailed Student t test, Mann-Whitney test, and one- or two-way analysis of variance with Bonferroni post-test were used if not otherwise indicated. P values less than 0.05 were considered statistically significant.
Results
NK Cells Infiltrate and Become Activated in Rejected Allogeneic Lung Transplants
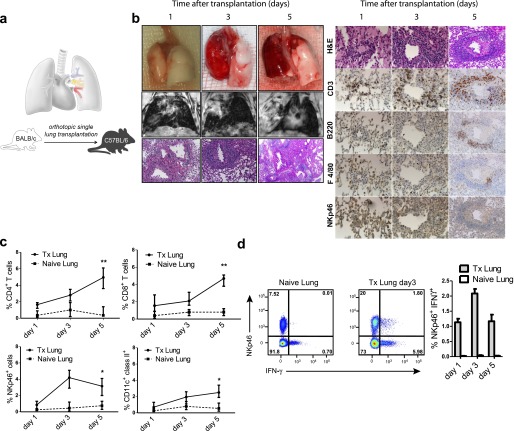
We have established a mouse model of orthotopic single-lung transplantation (Tx) (Figure 1a) (22), a technique that physiologically mimics the human lung Tx settings (see video in the online supplement). To induce a vigorous allogeneic rejection, we employed a fully MHC class I and class II–mismatched strain combination, using BALB/c as donors and C57BL/6 as recipients of orthotopically transplanted lungs. In this strain combination, recipients developed typical acute cellular rejection patterns reminiscent of those found in human acute pulmonary allograft rejection (25). Allografts analyzed 1 day after Tx displayed macroscopically a slightly swollen and reddish surface. To properly analyze changes in lung parenchyma and to be able to monitor the development of graft rejection, we performed magnetic resonance imaging (MRI). MRI allows the depiction of increased fluid and/or cell infiltration into the lung parenchyma. Applying regular echo times in MRI (5,000 ms), normal lung appears black without yielding a signal. In contrast, fluid or cell infiltration is reflected by a decrease in transparency. By shortening the echo time sequences from 5,000 to 50 milliseconds, the transplanted lung can be evaluated in an objective manner by measuring the proton density. Allograft rejection is characterized by enhanced density of the transplanted organ. On Day 1, the transplanted lung appeared transparent in MRI when compared with naive lung (Figure 1b, middle row), indicating a viable and functional graft. The histology showed that macrophages and neutrophils dominated in the initial graft infiltrates, followed, however, over time by increased numbers of T and B lymphocytes, which characteristically accumulated around arteries and venules because the endothelium is the primary target of alloreactive responses. On Day 5 post-Tx, the morphology of the allograft displayed features of a nonfunctional organ, showing an inflamed and severely swollen surface. These findings were confirmed by MRI, which revealed an almost complete loss of transparency (Figure 1b). These data were further supported by kinetic analysis of immunohistological scoring (see Figure E1 in the online supplement). Moreover, by flow cytometry we could observe that on Day 5 after Tx, CD4+ and CD8+ T cells were the main cell infiltrates of the Tx lung whereas NK cells had already reached their maximum on Day 3 post-Tx followed by a moderate decrease (Figure 1c). The amount of CD11c+ dendritic cells, presumably of recipient origin, increased mildly in a time-dependent fashion. We then performed intracellular staining of IFN-γ to study NK-cell activation and effector functions. We could already observe a massive increase in IFN-γ secretion on Day 3 post-Tx when compared with the naive lung, and this difference reached a peak on Day 3 post-Tx (Figure 1d). Three subsets of NK cells differing in expression of CD11b and CD27 have been described (26), with CD11b+CD27dull NK cells being the most mature. On Tx, we found that NK cells acquired the CD11b+CD27dull phenotype in contrast to those NK cells found within naive lungs (data not shown). Collectively, these data show the characteristic pattern of CD4+ and CD8+ T-cell infiltration during lung allograft rejection and describe NK cells as activated and differentiated effector cells that home to the transplanted organ early after lung Tx.
Figure 1.
Mouse lung allografts are rapidly rejected in the MHC class I and II–mismatched mouse model of orthotopically transplanted lung allografts. (a) Left lungs from donors were removed from the donor; and the artery, bronchus, and vein were cuffed and subsequently telescopically introduced into the respective recipient structures. (b) Representative images: Left: Macroscopic appearance (top row), magnetic resonance imaging (middle row), and histology, hematoxylin and eosin (H&E) staining (bottom row; magnification, ×100) on Days 1, 3, and 5 after transplantation (Tx) (data are representative of six experiments). Right: In line with the corresponding H&E stainings (top row), lung sections were immunohistochemically further characterized on respective days for T cells (CD3), B cells (B220), macrophages (F4/80), and natural killer cells (NKp46) (magnification, ×200; data are representative of six experiments). (c) Kinetics of CD45+ infiltrating lung cells by flow cytometric analysis for CD4+, CD8+, NKp46+, and CD11c+ dendritic cells in transplanted versus naive lungs (six independent experiments, means ± SEM, *P < 0.05, **P < 0.01). (d) Flow cytometric analysis by intracellular staining for IFN-γ, produced by CD45+NKp46+ NK cells on Day 3, and kinetics from Day 1 to 5 after allogeneic lung Tx compared with naive lungs (data are representative of six independent experiments).
IL-15 Mediates NK-Cell Expansion and Promotes Lung Allograft Acceptance
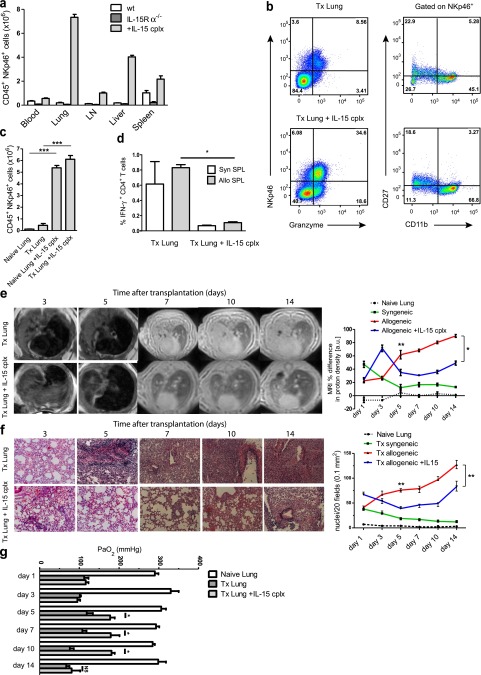
IL-15 is a regulator of memory CD8+ T-cell maintenance and NK-cell development, expansion, and activation (27). To investigate the influence of NK-cell expansion in the context of lung transplantation, we injected wild-type C57BL/6 (WT) mice with IL-15/IL-15Rα complexes (23) and analyzed their effect on NK (CD45+NKp46+) cells by flow cytometry. On treatment, we found a considerable expansion of NK cells in various organs (Figure 2a). Whereas we observed a 2- to 5-fold increase in the number of NK cells in blood, lymph nodes, and spleen of IL-15 complex–treated mice, the number of NK cells within the lung increased more than 20-fold when compared with untreated naive animals. A considerable increase in NK cells was also observed in the liver. In contrast and as expected, NK cells were missing in all the organs of IL-15Rα−/− mice.
Figure 2.
Natural killer (NK)-cell expansion by IL-15 complexes favors transplantation outcome. (a) Amount of CD45+NKp46+ cell content in the indicated tissues from untreated, IL-15Rα(IL-15 receptor, α chain)−/−, and IL-15 complex–treated wild-type C57BL/6 mice (lung cells from three mice each are shown, means ± SEM; LN = cervical and mediastinal lymph nodes). (b) Flow cytometric analysis of CD45+NKp46+ lung cells from transplanted mice with and without IL-15 complex treatment stained for granzyme B, CD27, and CD11b expression by NKp46+ NK cells (representative data of six mice per group). (c) Lung cells gated on CD45+NKp46+ cells from naive and transplanted mice compared with IL-15 complex–treated mice (Tx Lung: Day 3 after transplantation, six mice per group, means ± SEM, ***P < 0.001). (d) Total numbers of IFN-γ-producing CD4+ T cells from transplanted lungs with (Tx IL-15 complex [cplx] lung) and without (Tx lung) IL-15 complex treatment of the recipient after 3 days of culture with syngeneic (syn SPL) or allogeneic (allo SPL) T cell-depleted splenocytes (composite data of three independent experiments, three mice per group, means ± SEM, *P < 0.05). (e) Representative magnetic resonance images from allotransplanted and IL-15 complex–treated allotransplanted mice on Days 3, 5, 7, 10, and 14 after transplantation. Infiltrations into lungs were assessed in four regions of interest for each lung and expressed by proton density in arbitrary units comparing transplanted with contralateral, normal lungs (percent differences). Naive lungs and syngeneically transplanted mice were also assessed (seven mice per group for Days 3 and 5, three mice per group for Days 7, 10, and 14 are shown, means ± SEM, *P < 0.05, **P < 0.01). (f) Representative lung sections stained with hematoxylin and eosin in untreated and IL-15 complex–stimulated allotransplanted mice on Days 3, 5, 7, 10, and 14 after transplantation. Quantification of the histologic analysis was performed by counting nuclei of infiltrating cells in 20 fields, each measuring 0.1 mm2 (six independent experiments per group for Days 3 and 5, three independent experiments per group for Days 7, 10, and 14 are shown, means ± SEM, Mann-Whitney, **P < 0.01). (g) Oxygenation measurements from blood samples taken from the left pulmonary vein at the indicated time points after Tx are shown for naive (naive lung), transplanted (Tx lung), and IL-15 complex–treated recipient of transplanted (Tx lung + IL-15 cplx) lungs (three independent experiments per group are shown, means ± SEM, Mann-Whitney, *P < 0.05, NS = no statistical significance).
Degranulation of NK cells leads to the release of lytic granules containing perforin and granzymes (e.g., granzyme B). When comparing NK cells from lung transplants of IL-15 complex–stimulated or untreated recipients, 86% of the NK cells from IL-15 complex–treated recipients were granzyme B positive, whereas 70% of NK cells from control transplants carried this cytotoxic marker (Figure 2b). Moreover, further characterization of NK subsets within transplanted lungs in IL-15 complex–treated recipients showed that they had matured into CD11b+CD27dull cells (Figure 2b). Thus, IL-15 complex–stimulated NK cells were not only increased in number in the transplanted and naive lungs (Figure 2c) but they acquired a more differentiated effector phenotype within the transplant. To investigate the effect of the expanded NK cells on alloreactive T-cell responses, we first analyzed infiltrating total cell numbers by counting and flow cytometry and we observed that the large amounts of infiltrating NK cells on Day 1 were followed by a progressive decrease, and that initially accumulating T cells also decreased over time, together with a mild reduction of dendritic cells (Figure E2). Furthermore, ex vivo analysis of IFN-γ and TNF-α production by T cells showed a significant decrease in the T-cell effector function of those mice treated with IL-15 complex (Figures E2b–E2d). In addition, stimulation of these lung-infiltrating T cells with allogeneic T cell–depleted splenocytes revealed that these cells had reduced alloreactivity and secreted significantly less IFN-γ (Figure 2d; and Figures E2e and E2f) or TNF-α (Figure E2f) when compared with lung T cells of untreated transplanted mice. To correlate these observations to a functional outcome, we performed MRI of the lungs of transplanted mice with or without previous IL-15 complex treatment (Figure 2e). In both conditions on Day 3 post-Tx, the lung transplants from untreated and IL-15 complex–treated mice revealed increased proton density even though IL-15–treated transplanted recipients had fewer cellular infiltrates by histology and flow cytometry. This phenomenon is most likely related to the ischemia–reperfusion injury that inevitably occurs during the first 48 hours after transplantation. Indeed, the proton density decreased over time in syngeneically transplanted recipients, underlining the role of edema that usually diminishes by 3 days after Tx. However, from Day 5 up to 14 days post-Tx, lungs from IL-15 complex–stimulated mice showed significantly decreased proton density (Figure 2e). These results were supported by the amount of cell infiltrates in the Tx lung and a partial reconstitution of pulmonary architecture detected by histology (Figure 2f). As a functional test for the pulmonary capacity, we next measured the oxygenation levels. On Days 5, 7, and 10, we detected significant higher oxygen levels in IL-15 complex–treated allograft recipients than in those that were untreated (Figure 2g). However, on Day 14 this improved functionality was no longer observed. Taken together, these data indicate that IL-15 complex–mediated NK-cell expansion ameliorates lung allograft rejection up to 14 days.
IL-15Rα Deficiency Leads to Enhanced Lung Allograft Rejection
To demonstrate further that NK cells have a beneficial effect on the Tx outcome, we used a mouse strain deficient for the α chain of the IL-15 receptor, which displays a functional deficiency of IL-15 by its inability to trans-present to NK and memory CD8+ T cells (28, 29). Thus, similar to IL-15−/− mice (27), IL-15Rα−/−mice lack NK cells and memory CD8+ T cells (28, 29). This approach allowed us to study the course of allograft rejection in the absence of NK cells. As shown in Figure 2a, NK cells were missing in the various organs of IL-15Rα−/− mice. Three days after lung transplantation, NK cells of WT mice reached the highest numbers in the graft, followed by a decrease on Day 5 post-Tx (Figure 3a). As expected, transplanted lungs of IL-15Rα−/− mice contained no NK cells. Interestingly, over time, the transplanted lungs in IL-15Rα−/− mice showed higher numbers of CD4+ and CD8+ T cell infiltrates than lungs transplanted into WT recipients, as demonstrated by flow cytometry (Figure E3a). Also, DCs within the transplant increased over time to even higher amounts compared with WT transplanted mice, indicating that these DCs survive in the absence of NK cells (Figure E3a). Confirming these flow cytometric results, the allograft of IL-15Rα−/− recipients displayed more cell infiltrates over time by histology, which continuously increased up to Day 5 post-Tx (Figure 3b; and Figure E3b). In line with these observations, transplanted IL-15Rα−/− mice had a high proton density by MRI on Day 1 after Tx, whereas WT transplanted mice displayed a more modest loss of transparency (Figure 3c). Interestingly, during the course of allograft rejection, IL-15Rα−/− transplant recipients displayed a higher increase in proton density than did WT allograft recipient mice (Figure 3c). We also assessed the functionality of the grafted lung and noticed a gradual decrease in the oxygenation capacity of the engrafted lung in IL-15Rα−/− recipients compared with transplanted WT recipients (Figure E3c). These differences, although not statistically significant, demonstrate a trend to worsened functionality of the grafted lung in the absence of NK cells. From these data we suggest that decreased NK-cell numbers lead to enhanced lung allograft rejection.
Figure 3.
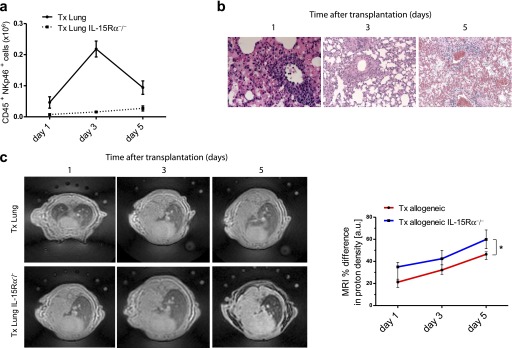
IL-15 receptor, α chain (IL-15Rα)−/− transplant recipients demonstrate enhanced lung allograft rejection in the absence of natural killer (NK) cells. (a) Kinetics of lung-infiltrating CD45+NKp46+ NK cells in allotransplanted wild-type versus IL-15Rα−/− mice from Day 1 to Day 5 after transplantation. (b) Representative lung sections stained with hematoxylin and eosin in IL-15Rα−/− allotransplanted mice on the indicated days after transplantation (representative images for five mice per group). (c) Representative magnetic resonance images from allotransplanted wild-type and IL-15Rα−/− mice on Days 1, 3, and 5 after transplantation. Infiltrations into lungs were assessed in four regions of interest for each lung and expressed by proton density in arbitrary units comparing transplanted with contralateral, normal lungs (percent differences, seven mice per group were analyzed, means ± SEM, *P < 0.05).
Perforin-Competent, but Not Perforin-Deficient, NK Cells Reconstitute Restriction of Alloreactive T-Cell Responses during Allogeneic Lung Transplantation
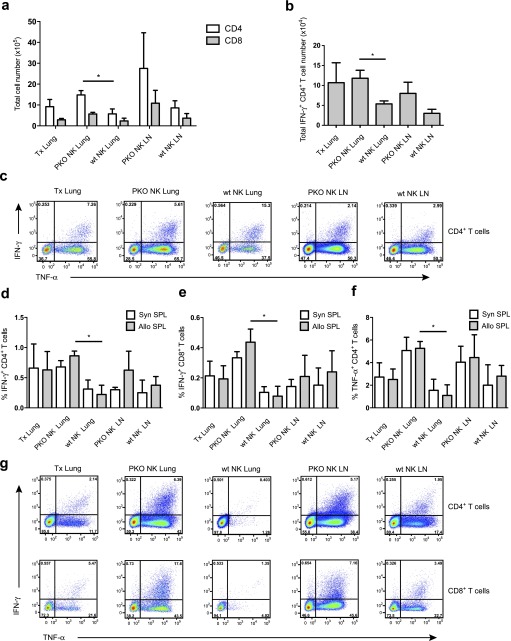
To confirm that NK cells were responsible for the observed beneficial effects of IL-15/IL-15Rα complex treatment in the graft outcome, we adoptively transferred NK cells isolated from IL-15/IL-15Rα complex–treated WT or perforin−/− mice into IL-15Rα−/− lung transplant recipients. Interestingly, perforin-positive, but not perforin-deficient, NK-cell transfer diminished the T-cell infiltration of the transplanted lungs (Figure 4a). In addition, lungs and draining lymph nodes of mice with WT NK-cell transfer contained decreased numbers of ex vivo IFN-γ– and TNF-α–secreting effector T cells (Figures 4b and 4c). Furthermore, mixed lymphocyte reaction cultures of these T cells with allogeneic splenocytes displayed reduced alloreactive responses as demonstrated by the reduced production of IFN-γ (Figures 4d, 4e, and 4g) and TNF-α (Figures 4f and 4g). The reactivity against syngeneic splenocytes in these assays probably reflects persisting activation after in vivo stimulation in this 3-day in vitro assay. These experiments establish that NK-cell transfer can diminish alloreactive T-cell responses during allogeneic lung transplantation and that NK cells require perforin to exert this beneficial effector function.
Figure 4.
Natural killer (NK) cells require perforin to limit the induction of alloreactive T-cell responses. (a) Total numbers of infiltrating CD4+ and CD8+ T cells were analyzed in transplanted lungs (lung) and draining lymph nodes (LN) of IL-15Rα(IL-15 receptor, α chain)–deficient recipients with and without NK-cell transfer from wild-type (wt NK) or perforin-deficient (PKO NK) animals. Compiled data from three experiments and three mice per group are shown (means ± SEM, *P < 0.05). (b) Ex vivo IFN-γ production was measured by intracellular cytokine staining in CD4+ T cells of transplanted lungs and draining lymph nodes in the same experimental groups as in (a) (means ± SEM, *P < 0.05). (c) Representative dot plots for intracellular cytokine staining of IFN-γ and tumor necrosis factor (TNF)-α in CD4+ T cells for the experimental groups as outlined in (a). (d and e) IFN-γ and (f) TNF-α production as assessed by intracellular cytokine staining in (d and f) CD4+ T cells and (e) CD8+ T cells from the same experimental groups as in (a) after in vitro expansion with syngeneic (syn SPL) or allogeneic (allo SPL) T cell–depleted splenocytes. Compiled data from three experiments and three mice per group are shown (means ± SEM, *P < 0.05). (g) Representative dot blots of intracellular IFN-γ and TNF-α staining in CD4+ (top row) and CD8+ (bottom row) T cells of transplanted lung and draining lymph nodes after expansion with allogeneic splenocytes.
Depletion of Donor Dendritic Cells by NK Cells Improves Allograft Acceptance
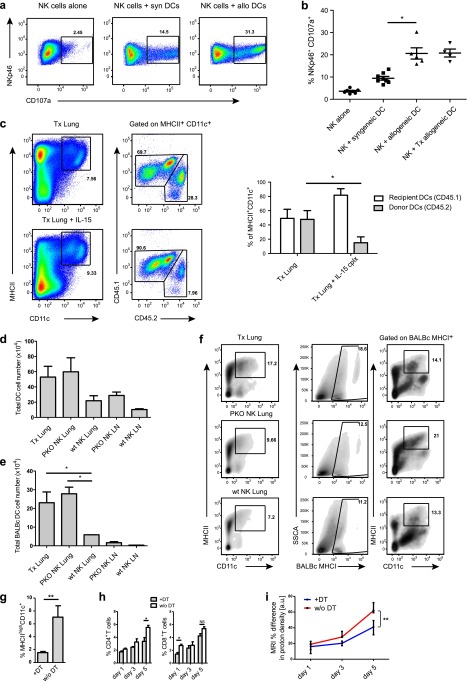
To understand the mechanism through which NK cells could improve transplant viability, we used a CD107a degranulation assay to evaluate the cytotoxic properties of NK cells toward dendritic cells of the donor allograft, because these antigen-presenting cells have been implicated to initiate direct alloreactivity during acute allograft rejection (30). CD107a or lysosome-associated membrane protein (LAMP)-1 has been described as a marker of lymphocyte cytotoxicity, present on surfaces after exocytosis of cytotoxic granules (31). Using this assay, the cytotoxic ability of NK cells was determined in the absence of target cells to detect spontaneous degranulation and in the presence of syngeneic and allogeneic donor dendritic cells to assess the specific cytotoxicity. We tested these conditions, analyzing NK cells after in vivo IL-15 complex administration and additional in vitro IL-2 stimulation. Whereas spontaneous degranulation was observed on less than 5% of lung NK cells, it increased to about 10% on incubation with immature syngeneic DCs. Furthermore, allogeneic DCs elicited degranulation of about 20% of NK cells (Figures 5a and 5b). To demonstrate that the level of NK-cell reactivity also affected donor DC content in transplanted lungs, we used congenic markers (CD45.1 and CD45.2) to trace and quantify the amount of donor and recipient DCs. On Day 3 after Tx, we could detect a more than twofold reduction of CD11c+ MHC class II+ donor (CD45.2+) DCs in the transplanted lungs of IL-15 complex–treated recipients by flow cytometry (Figure 5c). Furthermore, total donor-derived DC numbers were also reduced in allogeneic transplants of mice that had received perforin-positive NK cells (Figures 5d–5f). Strikingly, there was also a significant decrease in recipient-derived (CD45.1+) infiltrating CD4+ and CD8+ T cells into the allograft of IL-15 complex–treated mice (Figure E4). To evaluate whether the NK cell–dependent donor DC depletion plays a role in direct alloantigen presentation during the early course of lung allograft rejection, we assessed the rejection process after DC depletion in lung transplants from CD11c-DTR transgenic BALB/c mice. We successfully depleted lung DCs in CD11c-DTR donor mice more than fourfold by diphtheria toxin (DT) administration (Figure 5g). On Day 5 post-Tx we could observe a significant increase in CD4+, but not in CD8+, T-cell infiltrates in the transplanted lung of those mice without DC depletion compared with DT-treated mice before Tx (Figure 5h). Furthermore, lung infiltration on Day 5, as assessed by MRI, was significantly decreased in DC-depleted allografts (Figure 5i). However, these results were less pronounced than after IL-15/IL-15Rα treatment, possibly because of incomplete DC depletion before transplantation and/or cotransplanted DC precursors, which could develop into potent antigen-presenting cells during the course of the experiments. Nevertheless, these results collectively indicate that donor dendritic cells are the target for recipient NK cells and that their depletion reduces T-cell alloreactivity, resulting in an improved allograft outcome.
Figure 5.
IL-15–stimulated natural killer (NK) cells target allogeneic dendritic cells (DCs) in vitro and in vivo, and DC-depleted allografts are better accepted. (a) NK cells were stimulated with IL-15 complexes in vivo and with IL-2 ex vivo. Their degranulation was determined by CD107a surface expression in response to syngeneic or allogeneic DCs. NK cells were purified from lungs of naive or transplanted mice (representative flow cytometric plots from nine independent experiments are shown). (b) Compiled data of degranulation experiments (nine mice per allogeneic group, and four mice per allogeneic transplanted group, means, *P < 0.05, Mann-Whitney). (c) Representative flow cytometric analysis of congenic markers on DCs of lung transplants with CD45.1 indicating recipient origin (C57BL/6) and CD45.2 donor origin (BALB/c). Lung cells were gated on MHC class IIhighCD11c+ dendritic cells from allotransplanted untreated versus IL-15 complex–treated mice (three mice per group are shown, means ± SEM, *P < 0.05, two-way analysis of variance with Bonferroni post-test). (d) Total DC number in transplanted lungs (lung) and draining lymph nodes (LN) of IL-15Rα (IL-15 receptor, α chain)–deficient recipients with and without NK-cell transfer from wild-type (wt NK) or perforin-deficient (PKO NK) animals. Compiled data of three experiments and three animals per group are shown (means ± SEM, *P < 0.05). (e) Total cell numbers of donor-derived DCs (BALB/c MHCI+) in transplanted lungs and draining lymph nodes of the experimental groups as in (d) (means ± SEM, *P < 0.05). (f) Representative contour plots of total (left column) and donor-derived (right column) DCs in the same experimental groups as in (d). (g) Dendritic cells of CD11c-DTR mice were depleted with diphtheria toxin 24 hours before flow cytometric analysis (three mice per group, means ± SEM, **P < 0.01). (h) Recipient CD4+ and CD8+ T-cell infiltrates on Days 1, 3, and 5 in lung allotransplants from mice with and without prior diphtheria toxin treatment (four mice per group, means ± SEM, *P < 0.05). (i) Infiltrations into lungs were assessed by magnetic resonance imaging in four regions of interest in each lung and expressed by proton density in arbitrary units comparing transplanted to contralateral, normal lungs (percent differences, four mice per group are shown, means ± SEM, **P < 0.01).
Discussion
Our study documents that recipient NK cells are able to purge donor DCs from transplanted lungs and may thereby prolong graft acceptance and function. Graft-invading NK cells target allogeneic DCs and DC-depleted grafts display a survival benefit. These studies suggest that preconditioning of lung transplant recipients with regimens that augment NK-cell reactivity could be beneficial for transplantation outcome.
This observation is somewhat counterintuitive because NK cells have been implicated in enhancing Th1 polarization of cell-mediated immune responses via the production of cytokines (17) as also evidenced in direct allograft rejection of heart transplants (32–34). In contrast, during transplantation of allogeneic pancreatic islet, skin, and in this study of lung, NK cells seem to play a beneficial role in graft outcome (35, 36). During pancreatic islet transplantation, a cytolytic NK-cell function was identified as critical for the survival of allogeneic transplants (35). However, it remained unclear whether NK cells targeted donor-derived antigen-presenting cells (APCs) or effector T cells during pancreatic islet allograft rejection. Instead, in a model of allogeneic skin transplantation, it was shown that allograft-derived donor APC dissemination was restricted by NK cells (36). These APCs, among which also DCs were identified, primed directly alloreactive T-cell responses. In a similar model of allogeneic skin transplantation, these findings were extended to demonstrate that donor DCs were eliminated by NK cells in secondary lymphoid organs by perforin-mediated killing (30). Thus, especially early after allograft transplantation, NK cells seem to promote allograft acceptance by eliminating donor APCs, which otherwise would directly prime recipient-derived alloreactive T cells. However, this beneficial effect seems to be transient and might be compromised after mature recipient DC infiltration, which could then initiate indirect alloreactivity by presenting mismatched antigens on self-MHC molecules for graft rejection. In addition to an early NK-cell influence on DCs during transplantation and only in some transplantation settings, primarily documented for cardiac allografts, somatic cells of the transplant might later up-regulate activating ligands for NK cells and recruit these innate lymphocytes to the rejection reaction.
In addition to their immunoregulatory role during solid organ transplantation, NK cells are currently being explored for their beneficial role during allogeneic bone marrow transplantation. In patients with acute myeloid leukemia, NK cells from those donors with a mismatch in MHC class I ligands for inhibitory NK-cell receptors were found not only to mediate potent graft-versus-leukemia effects against residual disease, but also to control graft-versus-host disease (37). The latter was attributed to donor NK cell–mediated killing of allogeneic recipient DCs, which otherwise would prime alloreactive donor T cells to attack the host. Indeed, this beneficial effect has now been observed in several clinical studies (38–40), and seems to be greatest, if not only the recipient is lacking some of the inhibitory MHC class I ligands for killer immunoglobulin-like receptors of donor NK cells, but also if the donor carries distinct NK-cell populations that rely on the mismatched inhibitory ligand for self-tolerance. Thus, these studies complement the findings in solid organ transplantation models and suggest that NK-cell alloreactivity should be harnessed for clinical benefit to target donor DCs in solid organ transplants and recipient DCs to prevent graft-versus-host disease during bone marrow transplantation.
Our study describes one such possible intervention by boosting NK-cell function with IL-15, an essential cytokine for NK-cell development and activation (27, 28, 41–43). Although this cytokine seems to be an attractive candidate for conditioning allogeneic recipients before transplantation, several caveats need to be considered. First, IL-15 also promotes survival and proliferation of memory CD8+ T cells in mice, which could also include alloreactive memory T cells in the transplant setting (27, 29). Second, in addition, this cytokine might also affect CD4+ T-cell lineages in humans (44). And third, in recipients that have been rendered lymphopenic by the conditioning regimen before transplantation NK cells were found to restrict alloreactive T-cell proliferation by constituting a sink for available IL-15 (45), which would be overcome by IL-15 supplementation. Thus, NK cells constitute an attractive target for immunomodulatory treatments during transplantation and cytokines that regulate their activity as well as expansion should be cautiously evaluated for clinical benefits in allograft acceptance.
Acknowledgments
Acknowledgment
The authors thank Maries van den Broek for providing CD11c-DTR transgenic BALB/c mice depleted of DCs by diphtheria toxin treatment, Manfred Welti for assistance in preparing single-cell suspensions, and the FACS core facility for technical assistance with flow cytometry experiments and cell sorting.
Footnotes
Supported by the Center for Clinical Research, University Hospital and University of Zurich (DFL_MF 1134) to W.J. and W.W., and by grants from the National Cancer Institute (R01CA108609), the Sassella Foundation (10/02), Cancer Research Switzerland (KFS-02652-08-2010), the Association for International Cancer Research (11-0516), KFSPMS and KFSPHLD of the University of Zurich, the Vontobel Foundation, the Baugarten Foundation, the EMDO Foundation, the Sobek Foundation, Fondation Acteria, Novartis, and the Swiss National Science Foundation (310030_126995 and CRSII3_136241) to C.M.
Author Contributions: Guarantors of integrity of entire study, W.J., C.M.; study concepts/study design and data acquisition, C.M., W.J., L.C.; analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; approval of final version of submitted manuscript, all authors.
This article has an online supplement, which is available from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201209-1749OC on April 12, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hardy JD, Webb WR, Dalton ML, Jr, Walker GR., Jr Lung homotransplantation in man. JAMA. 1963;186:1065–1074. doi: 10.1001/jama.1963.63710120001010. [DOI] [PubMed] [Google Scholar]
- 2.Christie JD, Edwards LB, Kucheryavaya AY, Aurora P, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult lung and heart–lung transplant report—2010. J Heart Lung Transplant. 2010;29:1104–1118. doi: 10.1016/j.healun.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol. 2002;2:957–964. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- 5.Shi FD, Van Kaer L. Reciprocal regulation between natural killer cells and autoreactive T cells. Nat Rev Immunol. 2006;6:751–760. doi: 10.1038/nri1935. [DOI] [PubMed] [Google Scholar]
- 6.Chijioke O, Münz C. Interactions of human myeloid cells with natural killer cell subsets in vitro and in vivo. J Biomed Biotechnol. 2011;2011:251679. doi: 10.1155/2011/251679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Degli-Esposti MA, Smyth MJ. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol. 2005;5:112–124. doi: 10.1038/nri1549. [DOI] [PubMed] [Google Scholar]
- 8.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Münz C. Human dendritic cells activate resting NK cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayakawa Y, Screpanti V, Yagita H, Grandien A, Ljunggren HG, Smyth MJ, Chambers BJ. NK cell trail eliminates immature dendritic cells in vivo and limits dendritic cell vaccination efficacy. J Immunol. 2004;172:123–129. doi: 10.4049/jimmunol.172.1.123. [DOI] [PubMed] [Google Scholar]
- 11.Bielekova B, Catalfamo M, Reichert-Scrivner S, Packer A, Cerna M, Waldmann TA, McFarland H, Henkart PA, Martin R. Regulatory CD56bright natural killer cells mediate immunomodulatory effects of IL-2Rα–targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci USA. 2006;103:5941–5946. doi: 10.1073/pnas.0601335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2012;481:394–398. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabinovich BA, Li J, Shannon J, Hurren R, Chalupny J, Cosman D, Miller RG. Activated, but not resting, T cells can be recognized and killed by syngeneic NK cells. J Immunol. 2003;170:3572–3576. doi: 10.4049/jimmunol.170.7.3572. [DOI] [PubMed] [Google Scholar]
- 14.Lu L, Ikizawa K, Hu D, Werneck MB, Wucherpfennig KW, Cantor H. Regulation of activated CD4+ T cells by NK cells via the Qa-1–NKG2A inhibitory pathway. Immunity. 2007;26:593–604. doi: 10.1016/j.immuni.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 16.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell–mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 17.Strowig T, Brilot F, Münz C. Noncytotoxic functions of NK cells: direct pathogen restriction and assistance to adaptive immunity. J Immunol. 2008;180:7785–7791. doi: 10.4049/jimmunol.180.12.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-γ for TH1 priming. Nat Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 19.Bajenoff M, Breart B, Huang AY, Qi H, Cazareth J, Braud VM, Germain RN, Glaichenhaus N. Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. J Exp Med. 2006;203:619–631. doi: 10.1084/jem.20051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-β controls T helper type 1 cell development through regulation of natural killer cell interferon-γ. Nat Immunol. 2005;6:600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 21.Morandi B, Bougras G, Muller WA, Ferlazzo G, Münz C. NK cells of human secondary lymphoid tissues enhance T cell polarization via IFN-γ secretion. Eur J Immunol. 2006;36:2394–2400. doi: 10.1002/eji.200636290. [DOI] [PubMed] [Google Scholar]
- 22.Jungraithmayr W, Weder W. The technique of orthotopic mouse lung transplantation as a movie-improved learning by visualization. Am J Transplant. 2012;12:1624–1626. doi: 10.1111/j.1600-6143.2011.03980.x. [DOI] [PubMed] [Google Scholar]
- 23.Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, Sprent J. Converting IL-15 to a superagonist by binding to soluble IL-15Rα. Proc Natl Acad Sci USA. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jungraithmayr W, Chuck N, Frauenfelder T, Weder W, Boss A. MR imaging by using very short echo time sequences after syngeneic lung transplantation in mice. Radiology. 2012;265:753–761. doi: 10.1148/radiol.12112679. [DOI] [PubMed] [Google Scholar]
- 25.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, Glanville A, Gould FK, Magro C, Marboe CC, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 26.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176:1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15–deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koka R, Burkett PR, Chien M, Chai S, Chan F, Lodolce JP, Boone DL, Ma A. Interleukin (IL)-15Rα-deficient natural killer cells survive in normal but not IL-15Rα–deficient mice. J Exp Med. 2003;197:977–984. doi: 10.1084/jem.20021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burkett PR, Koka R, Chien M, Chai S, Chan F, Ma A, Boone DL. IL-15Rα expression on CD8+ T cells is dispensable for T cell memory. Proc Natl Acad Sci USA. 2003;100:4724–4729. doi: 10.1073/pnas.0737048100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laffont S, Seillet C, Ortaldo J, Coudert JD, Guery JC. Natural killer cells recruited into lymph nodes inhibit alloreactive T-cell activation through perforin-mediated killing of donor allogeneic dendritic cells. Blood. 2008;112:661–671. doi: 10.1182/blood-2007-10-120089. [DOI] [PubMed] [Google Scholar]
- 31.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 32.Maier S, Tertilt C, Chambron N, Gerauer K, Huser N, Heidecke CD, Pfeffer K. Inhibition of natural killer cells results in acceptance of cardiac allografts in CD28−/− mice. Nat Med. 2001;7:557–562. doi: 10.1038/87880. [DOI] [PubMed] [Google Scholar]
- 33.McNerney ME, Lee KM, Zhou P, Molinero L, Mashayekhi M, Guzior D, Sattar H, Kuppireddi S, Wang CR, Kumar V, et al. Role of natural killer cell subsets in cardiac allograft rejection. Am J Transplant. 2006;6:505–513. doi: 10.1111/j.1600-6143.2005.01226.x. [DOI] [PubMed] [Google Scholar]
- 34.Uehara S, Chase CM, Kitchens WH, Rose HS, Colvin RB, Russell PS, Madsen JC. NK cells can trigger allograft vasculopathy: the role of hybrid resistance in solid organ allografts. J Immunol. 2005;175:3424–3430. doi: 10.4049/jimmunol.175.5.3424. [DOI] [PubMed] [Google Scholar]
- 35.Beilke JN, Kuhl NR, Van Kaer L, Gill RG. NK cells promote islet allograft tolerance via a perforin-dependent mechanism. Nat Med. 2005;11:1059–1065. doi: 10.1038/nm1296. [DOI] [PubMed] [Google Scholar]
- 36.Yu G, Xu X, Vu MD, Kilpatrick ED, Li XC. NK cells promote transplant tolerance by killing donor antigen-presenting cells. J Exp Med. 2006;203:1851–1858. doi: 10.1084/jem.20060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 38.Hsu KC, Keever-Taylor CA, Wilton A, Pinto C, Heller G, Arkun K, O'Reilly RJ, Horowitz MM, Dupont B. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105:4878–4884. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leung W, Iyengar R, Turner V, Lang P, Bader P, Conn P, Niethammer D, Handgretinger R. Determinants of antileukemia effects of allogeneic NK cells. J Immunol. 2004;172:644–650. doi: 10.4049/jimmunol.172.1.644. [DOI] [PubMed] [Google Scholar]
- 40.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 41.Ferlazzo G, Thomas D, Pack M, Paludan C, Schmid D, Strowig T, Muller WA, Moretta L, Münz C. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci USA. 2004;101:16606–16611. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koka R, Burkett P, Chien M, Chai S, Boone DL, Ma A. Cutting edge: murine dendritic cells require IL-15Rα to prime NK cells. J Immunol. 2004;173:3594–3598. doi: 10.4049/jimmunol.173.6.3594. [DOI] [PubMed] [Google Scholar]
- 43.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huntington ND, Alves NL, Legrand N, Lim A, Strick-Marchand H, Mention JJ, Plet A, Weijer K, Jacques Y, Becker PD, et al. IL-15 transpresentation promotes both human T-cell reconstitution and T-cell–dependent antibody responses in vivo. Proc Natl Acad Sci USA. 2011;108:6217–6222. doi: 10.1073/pnas.1019167108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zecher D, Li Q, Oberbarnscheidt MH, Demetris AJ, Shlomchik WD, Rothstein DM, Lakkis FG. NK cells delay allograft rejection in lymphopenic hosts by downregulating the homeostatic proliferation of CD8+ T cells. J Immunol. 2010;184:6649–6657. doi: 10.4049/jimmunol.0903729. [DOI] [PubMed] [Google Scholar]