Abstract
Rationale: Characterization of bacterial populations in infectious respiratory diseases will provide improved understanding of the relationship between the lung microbiota, disease pathogenesis, and treatment outcomes.
Objectives: To comprehensively define lung microbiota composition during stable disease and exacerbation in patients with bronchiectasis.
Methods: Sputum was collected from patients when clinically stable and before and after completion of antibiotic treatment of exacerbations. Bacterial abundance and community composition were analyzed using anaerobic culture and 16S rDNA pyrosequencing.
Measurements and Main Results: In clinically stable patients, aerobic and anaerobic bacteria were detected in 40 of 40 (100%) and 33 of 40 (83%) sputum samples, respectively. The dominant organisms cultured were Pseudomonas aeruginosa (n = 10 patients), Haemophilus influenzae (n = 12), Prevotella (n = 18), and Veillonella (n = 13). Pyrosequencing generated more than 150,000 sequences, representing 113 distinct microbial taxa; the majority of observed community richness resulted from taxa present in low abundance with similar patterns of phyla distribution in clinically stable patients and patients at the onset of exacerbation. After treatment of exacerbation, there was no change in total (P = 0.925), aerobic (P = 0.917), or anaerobic (P = 0.683) load and only a limited shift in community composition. Agreement for detection of bacteria by culture and pyrosequencing was good for aerobic bacteria such as P. aeruginosa (κ = 0.84) but poorer for other genera including anaerobes. Lack of agreement was largely due to bacteria being detected by pyrosequencing but not by culture.
Conclusions: A complex microbiota is present in the lungs of patients with bronchiectasis and remains stable through treatment of exacerbations, suggesting that changes in microbiota composition do not account for exacerbations.
Keywords: bronchiectasis, lung microbiome, exacerbation, bacteria
At a Glance Commentary
Scientific Knowledge on the Subject
Molecular techniques have emerged that can provide a comprehensive description of an entire population of bacteria at a body site. Despite the importance of the microbial–host relationship in the pathogenesis and progression of infectious diseases, these approaches have not been applied to define the lung microbiota in patients with bronchiectasis.
What This Study Adds to the Field
Diverse polymicrobial communities are present in the lungs of patients with bronchiectasis when clinically stable and during an exacerbation. A surprising degree of stability was observed in both microbial load and community composition before and after antibiotic treatment of patients with acute pulmonary exacerbations. This finding suggests that changes in lung microbiota composition do not account for pulmonary exacerbations in patients with bronchiectasis.
Bronchiectasis is a chronic airway disease characterized by irreversible dilation of one or more bronchi (1). This results in poor mucus clearance and a vicious cycle of persistent bacterial colonization, airway obstruction, inflammation, and progressive tissue destruction (2). Patients with bronchiectasis frequently develop acute infective pulmonary exacerbations characterized by symptoms of fever, purulent sputum, and dyspnea (3), with attendant adverse effects on both their morbidity and health-related quality of life (4–6). Studies using classical aerobic-based microbial culture techniques to examine airway specimens from patients with stable bronchiectasis have reported Haemophilus influenzae (14–47% of cases), Pseudomonas aeruginosa (5–31%), and Streptococcus pneumoniae (2–14%) as the most frequently isolated pathogens (7–10). Significantly, in a large proportion of patients, no organisms were isolated from purulent sputum, even though repeated cultures were performed in patients not receiving antibiotics.
Using aerobic and strict anaerobic bacteriological techniques, we have detected a spectrum of potentially pathogenic anaerobic species in sputum samples from patients with cystic fibrosis (CF) when stable (11) and during antibiotic treatment of acute infective exacerbations (12). Furthermore, using a range of culture-independent methods, our group and others have shown that a much more complex and diverse polymicrobial community exists in the lungs of patients with CF than can be detected by culture (12–15).
Despite the emergence of DNA sequence–based molecular techniques, the role of the lung microbiota in the pathogenesis and progression of bronchiectasis remains poorly defined. Moreover, the British Thoracic Society guidelines for non-CF bronchiectasis highlighted that only a few studies offered a comprehensive cross-sectional analysis of bacterial isolation, and that these studies were all performed by routine diagnostic microbiology techniques (16). Clearly, a better understanding of airway infection in patients with bronchiectasis could inform studies that assess the impact of antimicrobial therapy and ensure that rational treatment strategies can be devised to treat colonizing bacteria.
In this study, we collected sputum samples from patients with clinically stable bronchiectasis and from patients receiving antibiotic treatment for acute infective exacerbations. Strict anaerobic bacteriological culture techniques and pyrosequencing were used to provide an in-depth analysis of the microbiome in sputum samples from these patients. Furthermore, we determined whether microbial load and community composition changed during and after antibiotic treatment of an exacerbation. We also assessed the relationship between pulmonary function and both microbial density and community diversity. Some of the results of these studies have been previously reported in the form of an abstract (17).
Methods
Subjects and Sample Collection
Patients with confirmed disease were recruited during a 23-month period (July 2008 to May 2010). Additional details with respect to patient recruitment are provided in the online supplement. Patients recruited during stable disease (cross-sectional group) were sampled at a single time point with clinical stability defined as no exacerbation or antibiotic treatment of a respiratory infection in the previous 4 weeks. Patients presenting with an exacerbation (longitudinal group) were also recruited, with exacerbations defined according to the criteria of Fuchs and colleagues (18). For this longitudinal group, samples were collected at initiation (24 h before to a maximum of 48 h after first dose of antibiotics) and completion of antibiotic treatment (24 h before to a maximum of 48 h after last dose of antibiotics) and 4–8 weeks posttreatment when clinically stable.
A single expectorated or induced sputum sample was collected from each patient at each time point and transported to the laboratory for processing in an anaerobic cabinet. If sufficient sample weight was collected the sample was divided, with one aliquot immediately processed for culture and the second aliquot frozen at −80°C for subsequent molecular analysis.
Clinical Data
Demographic data (age, sex), clinical measures (lung function [FEV1] and serum inflammatory markers [C-reactive protein, CRP; white cell count, WCC]), and details of chronic maintenance therapy were recorded from patient notes.
Bacterial Isolation and Identification
Culture and subsequent detection of isolates in sputum samples were performed as previously described (11, 12), with all bacteria detected quantified (colony-forming units per gram sputum; cfu/g) by total viable count (TVC) and identified by PCR and Sanger sequencing of 16S ribosomal genes.
Pyrosequencing
DNA was extracted from sputum samples as previously described (12). Bar-coded pyrosequencing of the 16S ribosomal RNA (rRNA) V1–V3 hypervariable region was performed at the University of North Carolina (Chapel Hill, NC) High-Throughput Sequencing Facility. A full description of the protocols used for pyrosequencing is provided by Fodor and colleagues (19; Methods) with minor modifications described in the online supplement.
Data Analysis
A detailed description of the statistical methods is provided in the online supplement. Briefly, in the cross-sectional group, aerobe and anaerobe viable counts were compared by Wilcoxon signed-rank test. In the longitudinal group, participant characteristics before and after antibiotic treatment were compared by paired-samples t test or Wilcoxon signed-rank tests as appropriate. Viable counts were compared at each time point and before and after antibiotic treatment, using Wilcoxon signed-rank tests. Comparison between three time points simultaneously was done by Friedman test.
Sample diversity metrics were assessed on the basis of the nonparametic Shannon-Wiener (SW) diversity index. Bray-Curtis (BC) metrics were used to generate pairwise comparison of community structure and plotted in two-dimensional space, using nonmetric multidimensional scaling (nMDS). Bacterial diversity (SW index) was compared in samples from clinically stable patients and those with an exacerbation, using the Mann-Whitney test. A Spearman rank correlation coefficient was used to measure the strength of the linear association between lung function and microbial load (aerobic, anaerobic, and total viable counts) and diversity (SW index). Diversity in samples grouped according to the predominant genera was compared by Kruskal-Wallis test. κ statistics were calculated to measure agreement between the presence or absence of bacteria detected by culture and pyrosequencing.
Results
Subjects and Sputum Samples
Forty patients were enrolled in the cross-sectional study, with a single sample collected. According to the Global Initiative for Obstructive Lung Disease (GOLD) guidelines (20), patients were categorized as having either mild (GOLD 1, n = 17), moderate (GOLD 2, n = 17), or severe (GOLD 3, n = 6) disease. Patient characteristics for this cohort are presented in Table 1.
TABLE 1.
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS OF PARTICIPANTS IN CROSS-SECTIONAL STUDY
| Mean (SD) | |
|---|---|
| Number |
40 |
| Age, yr |
65.5 (7.3) |
| Sex |
13M/27F |
| FEV1, L |
1.76 (0.63) |
| CRP, mg/L* |
4.0 (2.0–6.0) |
| WCC, × 109/L* | 6.6 (5.43–8.60) |
Definition of abbreviations: CRP = C-reactive protein; F = female; M = male; WCC = white cell count.
As CRP and WCC are skewed, the median and interquartile range are presented.
A further 14 patients were enrolled in a longitudinal study when they presented with an exacerbation. For these patients, samples were collected at both initiation and completion of antibiotic treatment and for 13 of 14 patients, 4–8 weeks posttreatment (mean, 45 d; range, 28–63 d) when clinically stable. For 11 of 14 patients, the initiation of treatment sample was collected before antibiotic treatment, starting with samples collected from the other 3 patients within 24 hours of commencing antibiotics. Patient characteristics for this cohort are presented in Table 2. Patients with an exacerbation were treated with either oral ciprofloxacin or cefaclor (n = 4 patients) or intravenous (n = 10 patients) antibiotic therapy (see Table E1 in the online supplement) for a mean of 14.5 days (range, 14–21) with all patients improving clinically. After antibiotic treatment, there was no change (P = 0.383) in lung function (FEV1). However, CRP (P = 0.018) and WCC (P = 0.035) both decreased significantly by medians of 12 mg/L and 1.40 × 109/L, respectively (Table 2).
TABLE 2.
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS OF PARTICIPANTS RECEIVING ANTIBIOTIC TREATMENT FOR AN INFECTIVE EXACERBATION IN LONGITUDINAL STUDY
| |
|
Completion of IV Antibiotics |
|
Stable |
|
||
|---|---|---|---|---|---|---|---|
| Start of IV Antibiotics Mean (SD) | Mean (SD) | Mean Diff. from Start* (95% CI) | P Value | Mean (SD) | Mean Diff. from Start* (95% CI) | P Value | |
| Number |
14 |
14 |
|
|
13 |
|
|
| Age, yr |
63.8 (6.2) |
63.8 (6.2) |
|
|
64.3 (6.1) |
|
|
| Sex |
6M/8F |
6M/8F |
|
|
5M/8F |
|
|
| FEV1, L |
1.52 (0.44) |
1.58 (0.46) |
+0.04 (−0.095 to 0.195) |
0.383 |
1.54 (0.38) |
+0.21 (−0.035 to 0.41) |
0.186 |
| CRP, mg/L* |
19.0 (5.75 to 37.25) |
7.0 (3.75 to 11.75) |
−12.0 (−22.0 to −1.0) |
0.035 |
4.0 (1.5 to 7.5) |
−17.0 (−36.0 to −4.0) |
0.003 |
| WCC, × 109/L* | 8.55 (6.08 to 10.53) | 6.95 (5.18 to 8.45) | −1.40 (−3.5 to −0.2) | 0.018 | 6.8 (5.6 to 9.6) | −0.15 (−1.8 to 0.8) | 0.722 |
Definition of abbreviations: CI = confidence interval; CRP = C-reactive protein; Diff. = difference; F = female; IV = intravenous; M = male; WCC = white cell count.
*As CRP and WCC are skewed, the median and interquartile range are presented, along with the median difference from start (95% CI) and the P value from the Wilcoxon signed-rank test.
Culture Analysis of Lung Microbiota Composition in Clinically Stable Patients
Bacteria were detected in high abundance (up to 1.3 × 109 cfu/g sputum) in sputum samples from clinically stable patients in the cross-sectional study. A mean (range) of 5.97 (2–12) different genera were cultured per sample with a median (range) total viable count of 3.93 × 107 (1.52 × 104 to 1.96 × 109) cfu/g sputum. Aerobic bacteria were isolated in high abundance (up to 1.3 × 109 cfu/g sputum) in all samples with P. aeruginosa (10 of 40 patients), H. influenzae (12 of 40 patients), and S. aureus (6 of 40 patients) the predominant species detected (see Table E2). Anaerobes from 5 different genera were also detected in high numbers (up to 1 × 108 cfu/g) from 33 of 40 samples (83%), with Prevotella (18 of 40 patients) and Veillonella (13 of 40 patients) most frequently isolated (see Table E2). Aerobic bacteria were present in significantly greater numbers than anaerobic bacteria (median difference in TVC, 1.36 × 107 cfu/g [95% confidence interval [CI], 5.53 × 106, 32.7 × 106]; Wilcoxon signed-rank test, P < 0.001). There was a positive correlation between CRP and both total viable count (ρ = 0.340, P = 0.032) and anaerobic viable count (ρ = 0.346, P = 0.020) but no significant correlation with aerobic viable count (ρ = 0.289, P = 0.070). Also, no correlations were apparent between viable counts and either FEV1 or WCC.
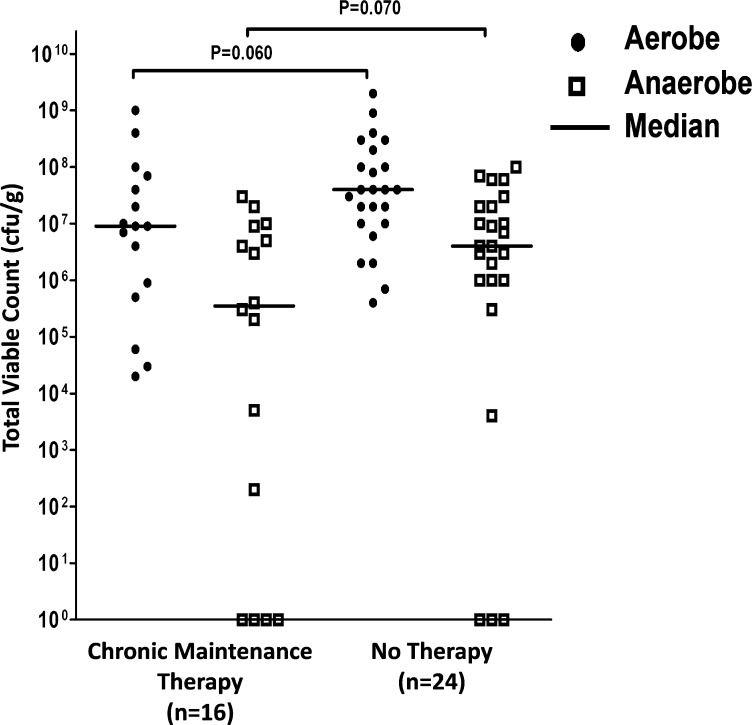
Of the 40 patients enrolled in the cross-sectional study, 16 were prescribed chronic maintenance therapy (azithromycin and colistin, n = 2 patients; azithromycin, n = 9 patients; colistin, n = 5 patients). Lung function (FEV1) was significantly less (P = 0.022, independent t test) in patients prescribed maintenance therapy (mean [SD], range: 1.94 [0.70], 0.79–2.27) compared with those not prescribed maintenance therapy (mean [SD], range: 1.49 [0.38], 0.80–3.54). However, although not statistically significant, total (P = 0.064, Mann-Whitney test), aerobic (P = 0.060), and anaerobic (P = 0.070) counts were less in patients receiving chronic maintenance therapy (Figure 1).
Figure 1.
Comparison of total viable counts per gram sputum of aerobic and anaerobic bacteria cultured from sputum samples collected from patients with clinically stable bronchiectasis receiving (n = 16) and not receiving (n = 24) chronic maintenance antibiotic therapy. Although not statistically significant, total (P = 0.064, Mann-Whitney test), aerobic (P = 0.060), and anaerobic (P = 0.070) counts were less in patients receiving chronic maintenance therapy.
There was also no significant difference in total (P = 0.913, Kruskal-Wallis test), aerobic (P = 0.995), and anaerobic (P = 0.580) viable counts across the GOLD categories described previously. However, CRP was significantly related to GOLD category (P = 0.002).
Culture Analysis of Lung Microbiota Composition in Patients with an Exacerbation
Aerobic bacteria were detected in high abundance (up to 4.6 × 108 cfu/g sputum) in all samples from patients with an exacerbation; those bacteria previously deemed to be pathogens were present in 9 of 14 patients at the start of treatment (P. aeruginosa, 5; H. influenzae, 4; Strep. pneumoniae, 1; methicillin-resistant Staphylococcus aureus [MRSA], 1), 7 of 14 patients at completion of treatment (P. aeruginosa, 1; H. influenzae, 4; Strep. pneumoniae, 1; MRSA, 1), and 5 of 13 patients when stable (P. aeruginosa, 3; Strep. pneumoniae, 2) (see Table E3). H. influenzae was detected in three patients at the start and end of treatment and in one patient at the start but not at the end of treatment. P. aeruginosa was only cultured in one patient at the start and end of treatment with an additional four patients positive by culture at initiation but not completion of treatment.
Anaerobic bacteria were also detected in high numbers (up to 7.5 × 107 cfu/g sputum) in 12 of 14 patients at the start of treatment (Prevotella, 6; Veillonella, 5), 9 of 14 patients at completion of treatment (Prevotella, 3; Veillonella, 3), and 7 of 13 patients when stable (Prevotella, 1; Veillonella, 3; Actinomyces, 3) (see Table E3).
Aerobic bacteria were present in significantly greater numbers than anaerobic bacteria at completion of antibiotic treatment (P = 0.001, Wilcoxon signed-rank test) and when the same patients were clinically stable (P = 0.001) but less so at the start of antibiotic treatment (P = 0.064) (Figure 1). However, there was no change in total (P = 0.925, Wilcoxon signed-rank test), aerobic (P = 0.917), or anaerobic (P = 0.683) viable counts with antibiotic treatment (Figure 2). Similarly, comparison of TVCs (Friedman test) for the 13 patients from whom 3 samples (start and end of antibiotic treatment, stable) were collected revealed no evidence of a difference in either aerobic (P = 0.097) or anaerobic (P = 0.869) counts between the 3 groups (Figure 2).
Figure 2.
Total viable counts per gram sputum of aerobic and anaerobic bacteria cultured from sputum samples collected from patients with bronchiectasis at the start of treatment (n = 14) and end of treatment (n = 14) for an exacerbation and when clinically stable after exacerbation (n = 13). Aerobic bacteria were present in significantly greater numbers than anaerobic bacteria at the end of antibiotic treatment (median difference in total viable count, 1.9 × 107 cfu/g [95% CI, 1.41 × 107, 5.15 × 107]; P = 0.001, Wilcoxon signed-rank test) and when the same patients were clinically stable (median difference in total viable count, 6.0 × 107 cfu/g [95% CI, 3.2 × 107, 15.0 × 107]; P = 0.001) but less so at the start of antibiotic treatment (median difference in total viable count, 4.3 × 106 cfu/g [95% CI, 1.2 × 107, 2.0 × 107]; P = 0.064). *Comparing two groups using Wilcoxon signed-rank test; ^comparing three groups using Friedman test.
Analysis of Lung Microbiota Composition by Pyrosequencing
The bacterial communities in 29 sputum samples (cross-sectional study, n = 10 samples; longitudinal study, n = 19 samples [start of treatment, n = 11; end of treatment, n = 5, stable after exacerbation, n = 3]) from 21 patients were profiled by pyrosequencing. In agreement with our culture results, a community of lung-resident bacteria was identifiable in all sputum samples. After quality control processing and removal of all sequences that were not at least classified to the kingdom “Bacteria,” a total of 159,621 high-quality reads were generated from these samples with an average of 5,701 (range, 3,200–8,352) reads per sample. After normalization, on the basis of the sample with the fewest reads (3,200), a total of 92,800 reads were included in the analysis. From these sequences, we identified 113 operational taxonomic units (OTUs) at 97% identity (see Table E4). The mean (range) number of OTUs per sample was 29 (9–57).
Taxa from multiple phyla including Proteobacteria (60%), Firmicutes (24%), Bacteroidetes (5%), and Fusobacteria (1%) were detected (see Table E4); for each phylum present in a sample, there were typically one or two dominant genera including Haemophilus (30.7% of sequences), Streptococcus (14.8%), Pseudomonas (12.6%), and Achromobacter (8.8%). Furthermore, of the 113 OTUs identified, 10 accounted for more than 90% of the total sequences generated (see Table E4), indicating that the majority of the observed community richness resulted from taxa present in low abundance.
The agreement between the presence or absence of bacteria detected by culture and pyrosequencing was relatively good for aerobic bacteria such as P. aeruginosa (κ = 0.84) and Achromobacter (κ = 0.87) but poor for H. influenzae (κ = 0.21), Staphylococcus (κ = 0.05), and anaerobic genera such as Prevotella (κ = 0.075) and Veillonella (κ = −0.070). Streptococcus were detected in 16 of 29 (55%) samples by culture, but as they were detected in all 29 samples by pyrosequencing, a κ coefficient could not be calculated. With the exception of Staphylococcus, where bacteria were detected in eight samples by culture but not pyrosequencing, lack of agreement between the methods was due to genera being detected by pyrosequencing but not culture (see Table E5).
Community Structure Dynamics
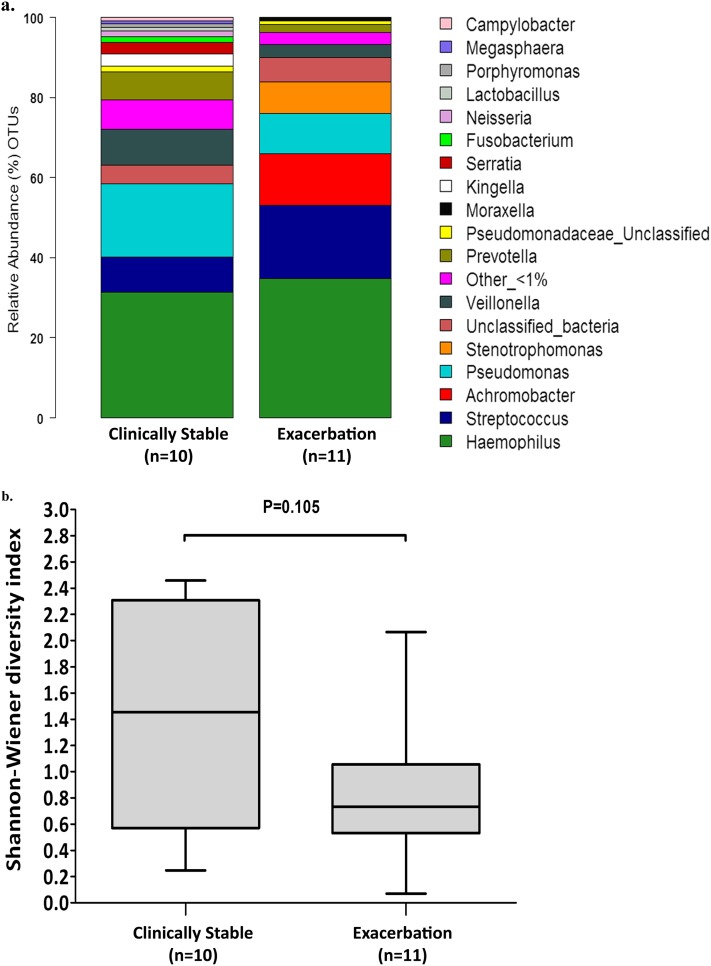
Community structure was compared in samples from clinically stable patients (n = 10 samples) and in patients at the onset of an exacerbation (n = 11 samples) to determine whether clinical status affected community structure. Similar patterns of phyla distribution were observed in both groups (Figure 3a) with no significant difference (P = 0.105, Mann-Whitney test) in microbial community diversity (SW index) apparent (Figure 3b). To enable further comparison, pairwise ecologic distances, calculated for all samples on the basis of the BC distance metric, were visualized by nMDS. There was considerable overlap between communities in samples from patients in each group, with differences in community composition not reflecting which cohort to which an individual patient/sample belonged (Figure 4). In addition, when we grouped all samples (n = 29), there was no correlation between lung function and microbial community diversity (ρ = 0.123, P = 0.549).
Figure 3.
(a) Comparison of the percent abundance of the major identified phyla in pooled samples collected from patients when clinically stable (cross-sectional study, n = 10) and at the start of treatment for an exacerbation (longitudinal study, n = 11). Similar patterns of phyla distribution were observed in both groups. OTUs = operational taxonomic units. (b) Box plot comparison of microbial diversity (Shannon-Wiener diversity index) in samples from patients when clinically stable (cross-sectional study, n = 10) and at the start of treatment for an exacerbation (longitudinal study, n = 11), where higher values correspond to higher diversity. The top and bottom boundaries of each box indicate 75th and 25th quartile values, respectively, with the black line inside each box representing the median (50th quartile). The ends of the whiskers indicate the range. No significant difference (P = 0.105, Mann-Whitney test) in microbial community diversity is apparent between the two groups.
Figure 4.
Pairwise comparison of Bray-Curtis distances for 29 sputum samples from 21 patients with bronchiectasis, using nonmetric multidimensional scaling (nMDS) analysis. Considerable overlap between communities in samples from patients in each group can be discerned, with differences in community composition not reflecting to which cohort an individual patient/sample belonged. A limited shift in community composition within individuals after antibiotic treatment was also apparent, with samples from individual patients clustering closely together. Clinically stable samples (C), n = 10; exacerbation samples, n = 19 (start of treatment [SOT], n = 11; end of treatment [EOT], n = 5; stable after exacerbation [ST], n = 3). Red boxes group together samples collected from the same patient at various time points.
To further assess changes in microbial community structure after antibiotic treatment, we compared, by pyrosequencing, samples from five patients from whom sufficient sample was collected at both the start and end of treatment of an exacerbation. In general, abundance of the predominant bacteria decreased slightly after treatment, with a similar small increase in the abundance of unclassifiable bacteria and obligate anaerobes such as Veillonella and Prevotella (Figure 5). This limited shift in community composition within individuals after antibiotic treatment was confirmed when we compared BC distance metrics with samples from individual patients clustering closely together (Figure 4). To compare sequence similarities in samples collected from these 5 patients at various time points, we analyzed OTU clusters containing more than 10 sequences. In general, OTUs for the same sequence types were detected in matched samples from patients at different time points (see Table E6).
Figure 5.
Comparison of the percent abundance of the major identified phyla in samples collected from patients at the start of treatment (SOT, n = 5) and end of treatment (EOT, n = 5) of an acute infective exacerbation and when clinically stable after exacerbation (ST, n = 3). Abundance of the predominant bacteria decreased slightly after treatment, with a similar small increase in the abundance of unclassifiable bacteria and obligate anaerobes such as Veillonella and Prevotella.
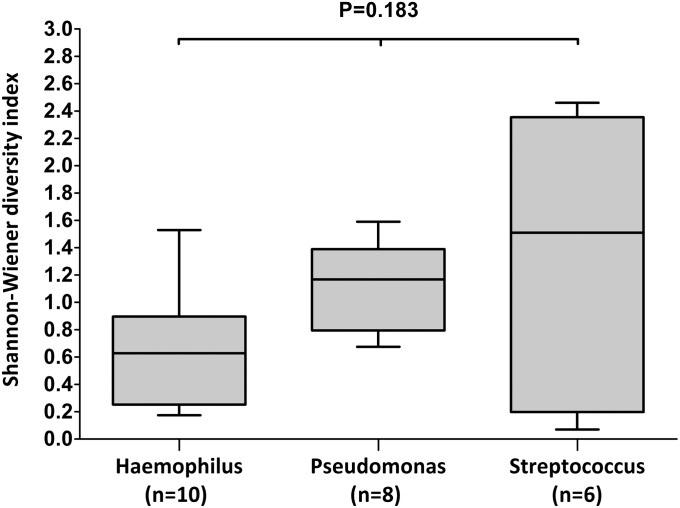
In our patient cohort, communities were most frequently dominated by Haemophilus (n = 10 samples), Pseudomonas (n = 8 samples), and Streptococcus (n = 6 samples), with these genera present as either a single dominant organism or sharing the majority of sequences with other genera. However, there was no significant difference (P = 0.183, Kruskal-Wallis test) in community diversity in samples within these three groups (Figure 6).
Figure 6.
Box plot comparison of microbial diversity (Shannon-Wiener diversity index) in samples dominated by the genera Haemophilus (n = 10), Pseudomonas (n = 8), and Streptococcus (n = 6), where higher values correspond to higher diversity. The top and bottom boundaries of each box indicate 75th and 25th quartile values, respectively, with the black line inside each box representing the median (50th quartile). The ends of the whiskers indicate the range. There was no significant difference in diversity between the three groups (median Shannon-Wiener diversity index [interquartile range]: Haemophilus, 0.63 [0.25, 0.90]; Pseudomonas, 1.17 [0.79, 1.40]; Streptococcus, 1.51 [0.19, 2.35]; P = 0.183, Kruskal-Wallis test).
Discussion
In this study we used both quantitative aerobic/anaerobic culture and pyrosequencing to provide a comprehensive analysis of lung microbiota composition in patients with bronchiectasis when clinically stable and at initiation and completion of antibiotic treatment of acute pulmonary exacerbations. Previous studies of bronchiectasis have focused on patients in a stable state and used only routine aerobic selective culture. In the present study, bacteria such as H. influenzae, P. aeruginosa, and S. pneumoniae were detected by culture at similar rates to those reported in these studies (8–10, 21–25). However, additional aerobic and anaerobic bacteria from a wide range of genera were also detected in high abundance, highlighting that complex polymicrobial communities exist within the lungs of these patients. Aerobic genera detected included Achromobacter, Stenotrophomonas, and streptococci from the Streptococcus milleri group (SMG), which have been reported to contribute to the onset of exacerbations in patients with CF (26). Although present in lower numbers than aerobes, anaerobes from genera including Prevotella, Veillonella, and Actinomyces were also detected by culture in more than 80% of samples. Only one previous study has reported detection of anaerobic bacteria in sputum from patients with bronchiectasis, with “anaerobic organisms” detected in only 2 of 123 (1.6%) samples and no details provided as to their identification (7). Similar aerobic and anaerobic genera have been detected, using both culture and molecular methods, in the lower airways of patients with a range of respiratory diseases including CF (11, 12, 19, 27–31), chronic obstructive pulmonary disease (COPD) (32, 33), and asthma (34) and also in healthy volunteers (32, 35). However, the densities detected here were more similar to that seen in CF samples.
High-throughput pyrosequencing revealed considerably greater bacterial diversity within sputa than detected by culture with multiple taxa from phyla including Proteobacteria, Firmicutes, and Bacteroidetes identified. Agreement between culture and pyrosequencing was relatively good for detection of abundant aerobic pathogens such as P. aeruginosa but was poor for detection of other genera including anaerobes present in lower abundance but greater diversity. In general, lack of agreement was due to bacteria being detected by culture-independent pyrosequencing but not culture, as has been previously reported for detection of bacteria in CF sputum (12). Culture was more sensitive in detecting Staphylococcus than was pyrosequencing, a finding similar to that reported in a study examining CF respiratory specimens by a species-specific quantitative PCR assay (36). One study has raised concerns with respect to the efficient extraction of Staphylococcus DNA from sputum samples, using standard bacterial cell lysis buffer, similar to that employed in the present study, with more efficient extraction achieved by the addition of either lysostaphin or lysozyme (37). Our results suggests that pyrosequencing enables more comprehensive characterization of airway microbial community composition and is required to detect the presence of less abundant and potentially more difficult-to-culture bacterial genera. As has previously been described for CF, results from culture-independent pyrosequencing analysis could be potentially used to inform changes to culture protocols, thereby optimizing their sensitivity to detect difficult-to-culture bacteria (38).
Our results also support the observations in CF, that airway microbial communities can be partitioned into two groups (19, 39–41). One group is composed of a relatively small number of taxa that dominate the environment and in our patients with bronchiectasis, communities were most frequently dominated by Haemophilus, Pseudomonas, and Streptococcus. The second group consists of less commonly found taxa present in low abundance, which account for the majority of the observed community richness. Furthermore, individual patients had unique microbiomes, with the composition of airway communities differing among patients. This finding of patient-specific community structures dominated by a single or few genera is consistent with studies of the lung microbiome in other respiratory diseases, which have shown a distinctive individual profile that is maintained over time (19, 32, 33).
Concerns have been expressed regarding contamination of sputum from sources outside the lung including the mouth, sinuses, and pharynx. However, a number of studies in CF have shown that sputum samples are not contaminated to a significant effect by bacterial species found within the oral cavity (11) and that anaerobic bacteria cultured from CF sputum samples were derived from the lungs and not the oral cavity (42). Furthermore, our finding that microbial communities were dominated by a single or few genera is similar to that of a study in CF that sampled secretions directly from the lobar airways of CF lungs, removed at the time of transplantation, and reported that lungs were dominated by one to three typical CF pathogens (43).
In patients with CF, it has also been suggested that decreased microbial diversity is associated with poorer lung function (13). However, in the patients with bronchiectasis included in this study, there was no correlation between diversity and lung function. This may be because patients with bronchiectasis do not receive the same intensive antibiotic therapy that has been shown to be the primary driver of decreasing diversity in patients with CF (31). A study by Sze and colleagues has reported a similar finding in that bacterial diversity in patients with very severe COPD (GOLD 4) was the same as that in nonsmoker and smoker control groups (33).
Pulmonary exacerbations are the single most important cause of morbidity in patients with bronchiectasis and are associated with disease progression (5, 6, 44), with bacterial colonization predicting future exacerbation risk (25). Although it is not clear what causes exacerbations, it has been suggested that onset may be triggered by changes in airway bacterial community composition (40), emergence of new strains or species (45), or spread of infection by the same species to new regions of the lung (19). Previous studies have shown that acquisition of a new strain of organisms including H. influenzae and P. aeruginosa is strongly associated with the occurrence of an exacerbation in COPD (46, 47). Similar to the reports for patients with CF (12, 31, 48, 49), we found by culture that total bacterial density did not increase significantly at the onset of exacerbation in patients with bronchiectasis, suggesting that an increase in bacterial load was unlikely to be a cause of exacerbation. However, there was an increase in anaerobic load relative to aerobic load and it is possible that this change may be a factor contributing to the onset of exacerbations. The majority of patients were treated with broad-spectrum antibiotics for 2 weeks and some effect on sputum bacterial density would have been expected. However, bacterial load did not decrease significantly with treatment. Sputum represents an average of inflammatory exudates produced in the lower respiratory tract and it is conceivable that heavily infected exudates produced during an infective exacerbation may remain trapped in the lower airways. Consequently, new airway infection caused by organisms present in low abundance may not always be detected or may be underrepresented in sputum collected from the mouth, resulting in no significant change in bacterial load with treatment. Furthermore, there may be modest fluctuations in bacterial load over time that would not be detected by processing a single sputum sample. However, because of the technical challenges of collecting and immediately processing sputum samples under strict anaerobic conditions, it was not feasible to process 24-hour sputum samples in the current study.
A limitation of our study is that we had only a small number of patients (n = 5) from whom sufficient sputum was collected before and after antibiotic treatment to allow for pyrosequencing in addition to culture analysis. However, results from this data set provide further supportive evidence that microbial community composition remained relatively stable within individual patients, with only a limited reduction in abundance of the most abundant genera. Furthermore, OTUs for the same sequence types were determined in matched samples from patients at different time points. There are inherent limitations associated with the methods used to assign taxonomic ranking to data originating from pyrosequencing in that they can normally allow reliable taxon assignment only at the family or genus level (50). However, despite these limitations, our data illustrate that for the majority of genera, similar sequences were present pre- and postantibiotic treatment. Although changes in microbial community composition were not detected by culture and pyrosequencing, it is possible that exacerbations were driven by changes and/or adaptation in strains that would not be detected by pyrosequencing. For example, genomic differences have been shown to be associated with the ability of H. influenzae strains to cause exacerbations of COPD (51). Furthermore, adaptation within a species, such as mucoidy in P. aeruginosa, could also drive exacerbation (52) but would not be detected by sequence analysis. Further analysis of isolates, using typing methods such as pulsed-field gel electrophoresis and multilocus sequence typing, would be required to fully address this question.
Exacerbations are associated with increased airway inflammation, with the highest levels of inflammation associated with the acquisition of new bacterial pathogens (53). Similarly, in patients with COPD, it has been shown that disease progression is associated with an adaptive immune response that accounts for the increase in lymphocytes and their organization into lymphoid follicles (54). Therefore, it is possible that a similar type of immune response is present in the lungs of patients with bronchiectasis potentially driven by changes and/or adaptation in strains within the microbiome that would not be detected by sequence analysis. Determination of immune responses to bacteria is important in understanding the pathogenesis of infectious exacerbations. However, given the diverse polymicrobial communities present in the lungs of patients with bronchiectasis, it would be difficult to study the immune response to organisms by analyzing sputum directly. This further emphasizes the importance of culturing individual bacterial strains from all genera present, which can then be used to determine antibody response.
In four patients, P. aeruginosa was detected by culture before but not after antibiotic treatment; there was no significant change in total sputum bacterial density in these patients, suggesting that the reduction in some bacteria with antibiotic treatment may be offset by an increase in other bacteria. A previous study has reported that despite aggressive antibiotic treatment, only 1 of 15 patients with chronic P. aeruginosa colonization had the organism eradicated for more than 6 months (2). Therefore, further longitudinal samples would be required to determine whether P. aeruginosa had been truly eradicated in these patients or whether P. aeruginosa load had been transiently reduced to levels that could not be detected.
All patients with an exacerbation improved clinically with treatment, as shown by a decrease in markers of inflammation such as CRP and WCC. However, similar to other studies that have examined the effect of long-term inhaled antibiotic therapy in bronchiectasis (55, 56), there was no improvement in lung function after treatment of an infective exacerbation. This is in contrast to the marked improvements reported in lung function for patients with CF after both long-term inhaled antibiotic treatment (57, 58) and antibiotic treatment of acute infective exacerbations (12, 27).
Studies have also reported that treatment with long-term azithromycin decreases infective exacerbations in bronchiectasis and suppresses airway pathogens including P. aeruginosa (59, 60). Within our clinically stable patient cohort, lung function was significantly less in patients prescribed azithromycin; however, there was some evidence that bacterial density in sputum was less in these patients, suggesting that the antibacterial, antiinflammatory, and immunomodulatory effects of azithromycin had some effect on microbial load (40, 61). Furthermore, there was no relationship between bacterial load and lung function, with no difference in total viable counts across GOLD categories. Therefore, despite progression of lung disease, sputum bacterial density remains relatively stable as has been reported for patients with CF (31).
In summary, this study describes the composition of the lung microbiota in patients with bronchiectasis when clinically stable and during acute infective exacerbations and clearly shows that classical microbial culture techniques underestimate the richness and diversity of airway infection in patients with bronchiectasis. Although an array of bacterial genera including anaerobes was detected, communities were dominated by a small number of genera. Significant changes in sputum bacterial density and community diversity were not observed before and after treatment of pulmonary exacerbations, suggesting that changes in lung microbiota composition do not account for exacerbations in patients with bronchiectasis. Further studies of this complex microbial ecology are required to better determine the relationship between the airway microbiome in bronchiectasis and severity and progression of lung disease. Improved understanding of this relationship could enable more targeted use of antibiotics, particularly during exacerbations, which has the potential to enhance clinical efficacy and improve outcomes for patients.
Acknowledgments
Acknowledgment
The authors acknowledge the technical assistance of Mr. Gerry McGrillen (School of Pharmacy, Queen’s University Belfast).
Footnotes
Supported by a Northern Ireland Chest Heart Stroke grant (20081080). M.M.T. was supported by a Health and Social Care Research and Development, Public Health Agency, Northern Ireland–funded UK National Institute for Health Research Career Scientist Award. M.M.T., G.G.E., and J.S.E. were supported by HSC Research and Development, Public Health Agency, Northern Ireland and the Medical Research Council through a United States–Ireland Partnership Grant. E.R.K., R.C.B., and M.C.W. were supported by a grant from the U.S. National Institutes of Health (HL092964).
Author Contributions: M.M.T., M.E., and J.S.E. conceived and designed research; L.W. and M.D. collected samples and clinical data; G.G.E., L.W., M.D., and E.R.K. performed research; M.M.T., G.G.E., C.C., and M.C.W. analyzed data; G.G.E. performed bioinformatic analysis; M.E. and R.C.B. made intellectual contributions; and M.M.T., G.G.E., M.C.W., and J.S.E. wrote the paper.
Data Deposition: All 454-FLX Titanium sequence data have been submitted to the EBI Sequence Read Archive (SRA) under the study accession number ERP002060. http://www.ebi.ac.uk/ena/data/view/ERP002060
Originally Published in Press as DOI: 10.1164/rccm.201210-1937OC on January 24, 2013
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Barker AF. Bronchiectasis. N Engl J Med. 2002;346:1383–1393. doi: 10.1056/NEJMra012519. [DOI] [PubMed] [Google Scholar]
- 2.Martínez-García MA, Soler-Cataluña J-J, Perpiñá-Tordera M, Román-Sánchez P, Soriano J. Factors associated with lung function decline in adult patients with stable non–cystic fibrosis bronchiectasis. Chest. 2007;132:1565–1572. doi: 10.1378/chest.07-0490. [DOI] [PubMed] [Google Scholar]
- 3.Evans DJ, Greenstone M. Long-term antibiotics in the management of non-CF bronchiectasis—do they improve outcome? Respir Med. 2003;97:851–858. doi: 10.1016/s0954-6111(03)00063-5. [DOI] [PubMed] [Google Scholar]
- 4.Martínez-García MA, Perpiñá-Tordera M, Román-Sánchez P, Soler-Cataluña JJ. Quality-of-life determinants in patients with clinically stable bronchiectasis. Chest. 2005;128:739–745. doi: 10.1378/chest.128.2.739. [DOI] [PubMed] [Google Scholar]
- 5.Wilson CB, Jones PW, O’Leary CJ, Hansell DM, Cole PJ, Wilson R. Effect of sputum bacteriology on the quality of life of patients with bronchiectasis. Eur Respir J. 1997;10:1754–1760. doi: 10.1183/09031936.97.10081754. [DOI] [PubMed] [Google Scholar]
- 6.Roberts ME, Lowndes L, Milne DG, Wong CA. Socioeconomic deprivation, readmissions, mortality and acute exacerbations of bronchiectasis. Intern Med J. 2012;42:e129–e136. doi: 10.1111/j.1445-5994.2011.02444.x. [DOI] [PubMed] [Google Scholar]
- 7.Nicotra MB, Rivera M, Dale AM, Shepherd R, Carter R. Clinical, pathophysiologic, and microbiologic characterization of bronchiectasis in an aging cohort. Chest. 1995;108:955–961. doi: 10.1378/chest.108.4.955. [DOI] [PubMed] [Google Scholar]
- 8.Pasteur MC, Helliwell SM, Houghton SJ, Webb SC, Foweraker JE, Coulden RA, Flower CD, Bilton D, Keogan MT. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med. 2000;162:1277–1284. doi: 10.1164/ajrccm.162.4.9906120. [DOI] [PubMed] [Google Scholar]
- 9.Angrill J, Agustí C, de Celis R, Rañó A, Gonzalez J, Solé T, Xaubet A, Rodriguez-Roisin R, Torres A. Bacterial colonisation in patients with bronchiectasis: microbiological pattern and risk factors. Thorax. 2002;57:15–19. doi: 10.1136/thorax.57.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King PT, Holdsworth SR, Freezer NJ, Villanueva E, Holmes PW. Microbiologic follow-up study in adult bronchiectasis. Respir Med. 2007;101:1633–1638. doi: 10.1016/j.rmed.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Tunney MM, Field TR, Moriarty TF, Patrick S, Doering G, Muhlebach MS, Wolfgang MC, Boucher R, Gilpin DF, McDowell A, et al. Detection of anaerobic bacteria in high numbers in sputum from patients with cystic fibrosis. Am J Respir Crit Care Med. 2008;177:995–1001. doi: 10.1164/rccm.200708-1151OC. [DOI] [PubMed] [Google Scholar]
- 12.Tunney MM, Klem ER, Fodor AA, Gilpin DF, Moriarty TF, McGrath SJ, Muhlebach MS, Boucher RC, Cardwell C, Doering G, et al. Use of culture and molecular analysis to determine the effect of antibiotic treatment on microbial community diversity and abundance during exacerbation in patients with cystic fibrosis. Thorax. 2011;66:579–584. doi: 10.1136/thx.2010.137281. [DOI] [PubMed] [Google Scholar]
- 13.Cox MJ, Allgaier M, Taylor B, Baek MS, Huang YJ, Daly RA, Karaoz U, Andersen GL, Brown R, Fujimura KE, et al. Airway microbiota and pathogen abundance in age-stratified cystic fibrosis patients. PLoS One. 2010;5:e11044. doi: 10.1371/journal.pone.0011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filkins LM, Hampton TH, Gifford AH, Gross MJ, Hogan DA, Sogin ML, Morrison HG, Paster BJ, O’Toole GA. Prevalence of streptococci and increased polymicrobial diversity associated with cystic fibrosis patient stability. J Bacteriol. 2012;194:4709–4717. doi: 10.1128/JB.00566-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blainey PC, Milla CE, Cornfield DN, Quake SR. Quantitative analysis of the human airway microbial ecology reveals a pervasive signature for cystic fibrosis. Sci Transl Med. 2012;4:153ra130. doi: 10.1126/scitranslmed.3004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasteur MC, Bilton D, Hill AT British Thoracic Society Bronchiectasis Non-CF Guideline Group. British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010;65:i1–i58. doi: 10.1136/thx.2010.142778. [DOI] [PubMed] [Google Scholar]
- 17.Einarsson GG, Klem E, Fodor AA, Wei L, Drain M, Wolfgang MC, Elborn JS, Tunney MM. Microbial community composition in the lungs of patients with cystic fibrosis and non-CF bronchiectasis [abstract] Thorax. 2011;66:A48. doi: 10.1136/thx.2010.137281. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, Rosenstein BJ, Smith AL, Wohl ME Pulmozyme Study Group. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. N Engl J Med. 1994;331:637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 19.Fodor AA, Klem ER, Gilpin DF, Elborn JS, Boucher RC, Tunney MM, Wolfgang MC. The adult cystic fibrosis airway microbiota is stable over time and infection type, and highly resilient to antibiotic treatment of exacerbations. PLoS One. 2012;7:e45001. doi: 10.1371/journal.pone.0045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (revised 2011). Global Initiative for Chronic Obstructive Lung Disease; 2011 [accessed 2012 Sep]. Available from: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html.
- 21.Li AM, Sonnappa S, Lex C, Wong E, Zacharasiewicz A, Bush A, Jaffe A. Non-CF bronchiectasis: does knowing the aetiology lead to changes in management? Eur Respir J. 2005;26:8–14. doi: 10.1183/09031936.05.00127704. [DOI] [PubMed] [Google Scholar]
- 22.Hare KM, Grimwood K, Leach AJ, Smith-Vaughan H, Torzillo PJ, Morris PS, Chang AB. Respiratory bacterial pathogens in the nasopharynx and lower airways of Australian indigenous children with bronchiectasis. J Pediatr. 2010;157:1001–1005. doi: 10.1016/j.jpeds.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Eastham KM, Fall AJ, Mitchell L, Spencer DA. The need to redefine non–cystic fibrosis bronchiectasis in childhood. Thorax. 2004;59:324–327. doi: 10.1136/thx.2003.011577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill AT, Welham S, Reid K, Bucknall CE British Thoracic Society. British Thoracic Society national bronchiectasis audit 2010 and 2011. Thorax. 2012;67:928–930. doi: 10.1136/thoraxjnl-2012-201983. [DOI] [PubMed] [Google Scholar]
- 25.Chalmers JD, Smith MP, McHugh BJ, Doherty C, Govan JR, Hill AT. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non–cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2012;186:657–665. doi: 10.1164/rccm.201203-0487OC. [DOI] [PubMed] [Google Scholar]
- 26.Sibley CD, Parkins MD, Rabin HR, Duan K, Norgaard JC, Surette MG. A polymicrobial perspective of pulmonary infections exposes an enigmatic pathogen in cystic fibrosis patients. Proc Natl Acad Sci USA. 2008;105:15070–15075. doi: 10.1073/pnas.0804326105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Worlitzsch D, Rintelen C, Böhm K, Wollschläger B, Merkel N, Borneff-Lipp M, Döring G. Antibiotic-resistant obligate anaerobes during exacerbations of cystic fibrosis patients. Clin Microbiol Infect. 2009;15:454–460. doi: 10.1111/j.1469-0691.2008.02659.x. [DOI] [PubMed] [Google Scholar]
- 28.Harris JK, De Groote MA, Sagel SD, Zemanick ET, Kapsner R, Penvari C, Kaess H, Deterding RR, Accurso FJ, Pace NR. Molecular identification of bacteria in bronchoalveolar lavage fluid from children with cystic fibrosis. Proc Natl Acad Sci USA. 2007;104:20529–20533. doi: 10.1073/pnas.0709804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers GB, Daniels TW, Tuck A, Carroll MP, Connett GJ, David GJ, Bruce KD. Studying bacteria in respiratory specimens by using conventional and molecular microbiological approaches. BMC Pulm Med. 2009;9:14. doi: 10.1186/1471-2466-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guss AM, Roeselers G, Newton IL, Young CR, Klepac-Ceraj V, Lory S, Cavanaugh CM. Phylogenetic and metabolic diversity of bacteria associated with cystic fibrosis. ISME J. 2011;5:20–29. doi: 10.1038/ismej.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao J, Schloss PD, Kalikin LM, Carmody LA, Foster BK, Petrosino JF, Cavalcoli JD, VanDevanter DR, Murray S, Li JZ, et al. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc Natl Acad Sci USA. 2012;109:5809–5814. doi: 10.1073/pnas.1120577109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erb-Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA, Young VB, Toews GB, Curtis JL, Sundaram B, et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS One. 2011;6:e16384. doi: 10.1371/journal.pone.0016384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sze MA, Dimitriu PA, Hayashi S, Elliott WM, McDonough JE, Gosselink JV, Cooper J, Sin DD, Mohn WW, Hogg JC. The lung tissue microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185:1073–1080. doi: 10.1164/rccm.201111-2075OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charlson ES, Bittinger K, Haas AR, Fitzgerald AS, Frank I, Yadav A, Bushman FD, Collman RG. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med. 2011;184:957–963. doi: 10.1164/rccm.201104-0655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zemanick ET, Wagner BD, Sagel SD, Stevens MJ, Accurso FJ, Harris JK. Reliability of quantitative real-time PCR for bacterial detection in cystic fibrosis airway specimens. PLoS One. 2010;5:e15101. doi: 10.1371/journal.pone.0015101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao J, Carmody LA, Kalikin LM, Li J, Petrosino JF, Schloss PD, Young VB, LiPuma JJ. Impact of enhanced Staphylococcus DNA extraction on microbial community measures in cystic fibrosis sputum. PLoS One. 2012;7:e33127. doi: 10.1371/journal.pone.0033127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sibley CD, Grinwis ME, Field TR, Eshaghurshan CS, Faria MM, Dowd SE, Parkins MD, Rabin HR, Surette MG. Culture enriched molecular profiling of the cystic fibrosis airway microbiome. PLoS One. 2011;6:e22702. doi: 10.1371/journal.pone.0022702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Gast CJ, Walker AW, Stressmann FA, Rogers GB, Scott P, Daniels TW, Carroll MP, Parkhill J, Bruce KD. Partitioning core and satellite taxa from within cystic fibrosis lung bacterial communities. ISME J. 2011;5:780–791. doi: 10.1038/ismej.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han MK, Huang YJ, Lipuma JJ, Boushey HA, Boucher RC, Cookson WO, Curtis JL, Erb-Downward J, Lynch SV, Sethi S, et al. Significance of the microbiome in obstructive lung disease. Thorax. 2012;67:456–463. doi: 10.1136/thoraxjnl-2011-201183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madan JC, Koestler DC, Stanton BA, Davidson L, Moulton LA, Housman ML, Moore JH, Guill MF, Morrison HG, Sogin ML, et al. Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: interaction between intestinal and respiratory tracts and impact of nutritional exposures. MBio. 2012;3:e00251–12. doi: 10.1128/mBio.00251-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogers GB, Carroll MP, Serisier DJ, Hockey PM, Jones G, Kehagia V, Connett GJ, Bruce KD. Use of 16S rRNA gene profiling by terminal restriction fragment length polymorphism analysis to compare bacterial communities in sputum and mouthwash samples from patients with cystic fibrosis. J Clin Microbiol. 2006;44:2601–2604. doi: 10.1128/JCM.02282-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goddard AF, Staudinger BJ, Dowd SE, Joshi-Datar A, Wolcott RD, Aitken ML, Fligner CL, Singh PK. Direct sampling of cystic fibrosis lungs indicates that DNA-based analyses of upper-airway specimens can misrepresent lung microbiota. Proc Natl Acad Sci USA. 2012;109:13769–13774. doi: 10.1073/pnas.1107435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seitz AE, Olivier KN, Steiner CA, Montes de Oca R, Holland SM, Prevots DR. Trends and burden of bronchiectasis-associated hospitalizations in the United States, 1993–2006. Chest. 2010;138:944–949. doi: 10.1378/chest.10-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 46.Sethi S, Sethi R, Eschberger K, Lobbins P, Cai X, Grant BJ, Murphy TF. Airway bacterial concentrations and exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:356–361. doi: 10.1164/rccm.200703-417OC. [DOI] [PubMed] [Google Scholar]
- 47.Murphy TF, Brauer AL, Eschberger K, Lobbins P, Grove L, Cai X, Sethi S. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:853–860. doi: 10.1164/rccm.200709-1413OC. [DOI] [PubMed] [Google Scholar]
- 48.Stressmann FA, Rogers GB, van der Gast CJ, Marsh P, Vermeer LS, Carroll MP, Hoffman L, Daniels TW, Patel N, Forbes B, et al. Long-term cultivation-independent microbial diversity analysis demonstrates that bacterial communities infecting the adult cystic fibrosis lung show stability and resilience. Thorax. 2012;67:867–873. doi: 10.1136/thoraxjnl-2011-200932. [DOI] [PubMed] [Google Scholar]
- 49.Stressmann FA, Rogers GB, Marsh P, Lilley AK, Daniels TW, Carroll MP, Hoffman LR, Jones G, Allen CE, Patel N, et al. Does bacterial density in cystic fibrosis sputum increase prior to pulmonary exacerbation? J Cyst Fibros. 2011;10:357–365. doi: 10.1016/j.jcf.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Dowd SE, Wolcott RD, Sun Y, McKeehan T, Smith E, Rhoads D. Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP) PLoS One. 2008;3:e3326. doi: 10.1371/journal.pone.0003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fernaays MM, Lesse AJ, Sethi S, Cai X, Murphy TF. Differential genome contents of nontypeable Haemophilus influenzae strains from adults with chronic obstructive pulmonary disease. Infect Immun. 2006;74:3366–3374. doi: 10.1128/IAI.01904-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chotirmall SH, Smith SG, Gunaratnam C, Cosgrove S, Dimitrov BD, O’Neill SJ, Harvey BJ, Greene CM, McElvaney NG. Effect of estrogen on Pseudomonas mucoidy and exacerbations in cystic fibrosis. N Engl J Med. 2012;366:1978–1986. doi: 10.1056/NEJMoa1106126. [DOI] [PubMed] [Google Scholar]
- 53.Sethi S, Wrona C, Eschberger K, Lobbins P, Cai X, Murphy TF. Inflammatory profile of new bacterial strain exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:491–497. doi: 10.1164/rccm.200708-1234OC. [DOI] [PubMed] [Google Scholar]
- 54.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 55.Barker AF, Couch L, Fiel SB, Gotfried MH, Ilowite J, Meyer KC, O’Donnell A, Sahn SA, Smith LJ, Stewart JO, et al. Tobramycin solution for inhalation reduces sputum Pseudomonas aeruginosa density in bronchiectasis. Am J Respir Crit Care Med. 2000;162:481–485. doi: 10.1164/ajrccm.162.2.9910086. [DOI] [PubMed] [Google Scholar]
- 56.Drobnic ME, Suñé P, Montoro JB, Ferrer A, Orriols R. Inhaled tobramycin in non–cystic fibrosis patients with bronchiectasis and chronic bronchial infection with Pseudomonas aeruginosa. Ann Pharmacother. 2005;39:39–44. doi: 10.1345/aph.1E099. [DOI] [PubMed] [Google Scholar]
- 57.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, Vasiljev-K M, Borowitz D, Bowman CM, Marshall BC, et al. Cystic Fibrosis Inhaled Tobramycin Study Group. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med. 1999;340:23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 58.McCoy KS, Quittner AL, Oermann CM, Gibson RL, Retsch-Bogart GZ, Montgomery AB. Inhaled aztreonam lysine for chronic airway Pseudomonas aeruginosa in cystic fibrosis. Am J Respir Crit Care Med. 2008;178:921–928. doi: 10.1164/rccm.200712-1804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anwar GA, Bourke SC, Afolabi G, Middleton P, Ward C, Rutherford RM. Effects of long-term low-dose azithromycin in patients with non-CF bronchiectasis. Respir Med. 2008;102:1494–1496. doi: 10.1016/j.rmed.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 60.Wong C, Jayaram L, Karalus N, Eaton T, Tong C, Hockey H, Milne D, Fergusson W, Tuffery C, Sexton P, et al. Azithromycin for prevention of exacerbations in non–cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet. 2012;380:660–667. doi: 10.1016/S0140-6736(12)60953-2. [DOI] [PubMed] [Google Scholar]
- 61.Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010;23:590–615. doi: 10.1128/CMR.00078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]