Abstract
Transient receptor potential canonical (TRPC) channels are the canonical (C) subset of the TRP proteins, which are widely expressed in mammalian cells. They are thought to be primarily involved in determining calcium and sodium entry and have wide-ranging functions that include regulation of cell proliferation, motility and contraction. The channels are modulated by a multiplicity of factors, putatively existing as integrators in the plasma membrane. This review considers the sensitivities of TRPC channels to lipids that include diacylglycerols, phosphatidylinositol bisphosphate, lysophospholipids, oxidized phospholipids, arachidonic acid and its metabolites, sphingosine-1-phosphate, cholesterol and some steroidal derivatives and other lipid factors such as gangliosides. Promiscuous and selective lipid sensing have been detected. There appear to be close working relationships with lipids of the phospholipase C and A2 enzyme systems, which may enable integration with receptor signalling and membrane stretch. There are differences in the properties of each TRPC channel that are further complicated by TRPC heteromultimerization. The lipids modulate activity of the channels or insertion in the plasma membrane. Lipid microenvironments and intermediate sensing proteins have been described that include caveolae, G protein signalling, SEC14-like and spectrin-type domains 1 (SESTD1) and podocin. The data suggest that lipid sensing is an important aspect of TRPC channel biology enabling integration with other signalling systems.
Keywords: cation channel, lipid signalling, transient receptor potential
There are seven mammalian genes encoding transient receptor potential canonical (TRPC) proteins and all of them except TRPC2 are expressed in humans (Flockerzi 2007, Nilius 2007, Venkatachalam & Montell 2007, Yildirim & Birnbaumer 2007, Abramowitz & Birnbaumer 2008). Like voltage-gated K+ channels, they are thought to form channels by gathering as a group of four around a central ion pore either using the same type (homomultimeric channels) or a mixture of transient receptor potentials (TRPs) (heteromultimeric channels). Initial studies suggested that TRPC1/4/5 and TRPC3/6/7 multimerize as exclusive subgroups, consistent with the observation that these TRPCs cluster in amino acid sequence comparisons. However, subsequent studies have suggested more flexibility (Strubing et al. 2003) and that other subtypes of TRP (e.g. TRPP2, TRPV4) may be incorporated (Tsiokas et al. 1999, Bai et al. 2008, Ma et al. 2010). The exact compositions of native TRPC-containing channels remain important unsolved problems.
The TRPC channels are permeable to the cations Ca2+, Na+ and K+. They are not voltage gated (i.e. they do not require a change in membrane potential to open), but they are often voltage sensitive (i.e. their activity is modulated by voltage). They may display significant constitutive activity (Dietrich et al. 2003, Nichols et al. 2007, Xu et al. 2008), but are most often stimulated or inhibited by a range of chemical or protein factors (Abramowitz & Birnbaumer 2008). They are emerging as polymodal ion channels that are sensitive to a multiplicity of activators and inhibitors, suggesting that they may serve as integrative sensors of complex chemical signals (Zeng et al. 2004, Abramowitz & Birnbaumer 2008). Importantly, although different cells express different relative amounts of TRPC, it seems that all mammalian cell types express most (if not all) of the TRPCs, suggesting that TRPC channels serve generic cell functions. For example, several TRPCs have been linked positively or negatively to cell migration, including an interesting reciprocal relationship between TRPC5 and TRPC6 through Rac1 and RhoA proteins respectively (Greka et al. 2003, Xu et al. 2006, Chaudhuri et al. 2008, Fabian et al. 2008, Tian et al. 2010).
Numerous specific functions of TRPCs in physiology and disease are starting to emerge, with examples that include roles of: TRPC1 in neointimal hyperplasia, cardiac hypertrophy, angiogenesis and saliva secretion (Jho et al. 2005, Kumar et al. 2006, Liu et al. 2007b, Seth et al. 2009, Yu et al. 2010); TRPC2 in pheromone sensation (Yildirim & Birnbaumer 2007); TRPC3 in pancreatitis, heart failure and NFKB activation (Kim et al. 2009, Kiyonaka et al. 2009, Smedlund et al. 2010); TRPC4 in gastrointestinal motility and blood pressure regulation (Freichel et al. 2001, Tsvilovskyy et al. 2009); TRPC5 in fear responses, regulation of matrix metalloprotease secretion from fibroblast-like synoviocytes and degranulation of mast cells (Ma et al. 2008, Xu et al. 2008, Riccio et al. 2009); and TRPC6 in familial focal segmental glomerulosclerosis, hypoxic pulmonary vasoconstriction, pulmonary hypertension, oesophageal cancer and angiogenesis (Winn et al. 2005, Weissmann et al. 2006, Hamdollah Zadeh et al. 2008, Shi et al. 2009).
This review addresses the topic of TRPC channel sensitivities to lipids as components of specific membrane environments or as active intracellular or intercellular signalling molecules. It explores the hypothesis that a function of TRPCs is to serve as integrators of lipid environments and signalling. An abridged summary is provided (Fig. 1), but it is a simplification of the available information and hence should be considered alongside the text below. The reader is referred to published review articles for general background on lipid structures, classification and signalling pathways, bilayer structures and lipid relevance to disease (Fahy et al. 2005, Maxfield & Tabas 2005, Wymann & Schneiter 2008, Marrink et al. 2009, Fukami et al. 2010, Sanchez-Mejia & Mucke 2010).
Figure 1.
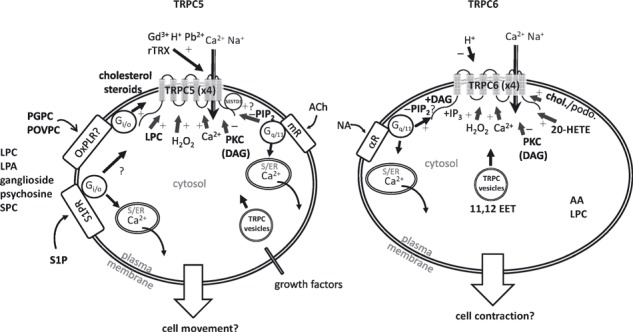
Abridged schematics for regulation of transient receptor potential canonical 5 (TRPC5) (left) and TRPC6 (right) channels by lipids in simplified mammalian cells. See the main text for details. Examples of regulation by other factors are included, but are not exhaustive. Abbreviations not provided in the main text are: Gd3+, gadolinium; Pb2+, lead; rTRX, reduced thioredoxin; OxPLR, putative oxidized phospholipid receptor (identity unknown); H2O2, hydrogen peroxide; chol., cholesterol; podo., podocin; AA, arachidonic acid. Gi/o and Gq/11 are different types of GTP-binding proteins. The integral phospholipase C (PLC) and phospholipase A2 (PLA2) enzymes are not shown. TRPC6 may also be stimulated through receptor tyrosine kinase (Hamdollah Zadeh et al. 2008, Ge et al. 2009). Tian et al. (2010) should be consulted for the proposed distinction of TRPC5 coupling to cell movement and TRPC6 to contraction. Vesicular trafficking of TRPC5 has been described in response to growth factors (Bezzerides et al. 2004).
Phosphatidylinositol (PI) phosphates
Phospholipase C (PLC) enzymes are stimulated by G proteins and receptor tyrosine kinases to generate key messengers such as inositol 1,4,5-trisphosphate (IP3) and diacyglycerols (DAGs) from phosphatidylinositol 4,5-bisphosphate (PIP2). PIP2 is not only the source of IP3 and DAGs, but also a key regulator of protein activity in its own right, as its local concentration depletes in response to receptor stimulation and target proteins contain PIP2-responsive elements. Phosphatidylinositol-3,4,5-trisphosphate (PIP3) is generated by the action of PI 3-kinase on PIP2, feeding into the Akt signalling pathway.
In heterologous expression studies, TRPC6 was found to be stimulated by PIP2 or PIP3, with PIP3 showing the highest affinity (Tseng et al. 2004, Kwon et al. 2007). It bound phosphoinositides directly at a site in the C terminus, competing with calmodulin (Kwon et al. 2007). Mutations in TRPC6 that decreased PIP3 binding suppressed channel activity, as did an Akt-PH domain that acted as a PIP3 sponge (Kwon et al. 2007). Other TRPC proteins were also found to bind phosphoinositides, especially TRPC1 (Kwon et al. 2007). PIP2 or PIP3 were subsequently found to stimulate endogenous channels containing TRPC1 in vascular smooth muscle cells (Saleh et al. 2009), whereas PIP2 inhibited endogenous TRPC6 or TRPC6/7 channels (Ju et al. 2010). Optimal receptor activation of TRPC6 was suggested to occur after depletion of PIP2 and generation of DAG (LarGe et al. 2009). The phosphatase and tensin homologue deleted on chromosome 10 (PTEN) phosphatase, which generates PIP2 from PIP3, was found to regulate TRPC6 surface expression independent of its phosphatase activity (Kini et al. 2010).
Phosphatidylinositol 4,5-bisphosphate inhibited TRPC4α but had no effect on TRPC4β channel (Otsuguro et al. 2008). Several other phosphoinositides had no effect, or stimulated TRPC4α. Evidence was provided for PIP2 interacting with the C terminus of TRPC4α and effects depended on the actin cytoskeleton and the postsynaptic density protein Drosophila disc large tumour suppressor and zona occludens-1 (PDZ)-binding motif of TRPC4 (Otsuguro et al. 2008). In one study, it was observed that PIP2 stimulated TRPC5 channel activity in excised inside-out patches, yet agents that caused PIP2 depletion had stimulatory effects on whole TRPC5 channel currents, as if PIP2 normally inhibited TRPC5 (Trebak et al. 2009). In another study, it was observed that PIP2 had no effect other than to slow the rate of TRPC5 channel desensitization following receptor activation (Kim et al. 2008).
A screen of a human aorta cDNA library revealed SESTD1 as a binding partner of TRPC4 and TRPC5 (Miehe et al. 2010). SESTD1 is a previously unrecognized protein that binds PI mono- and di-phosphates and phosphatidic acid, but not phosphatidylcholine, phosphatidylserine or PI (Miehe et al. 2010). Binding of PIP2 was shown to be Ca2+ dependent. SESTD1 was associated with the channels at the calmodulin/IP3 binding domain and suggested to be required for efficient receptor activation of the channels.
The data suggest that there are divergent and complex effects of PIP2 on TRPC channels. In several cases, the functional consequences require clarification and this situation is made more difficult by a evidence that TRPC heteromultimerization in native cells complicates the net effect of PIP2. Effects may occur through direct binding or intermediate proteins such as SESTD1.
Diacyglycerols
Diacyglycerols are composed of two covalently linked fatty acids and may be formed from various sources, one of which is PIP2. Early studies searching for activators of TRPC channels identified DAGs as activators of the TRPC3/6/7 subgroup of TRPCs (Hofmann et al. 1999). TRPC2 was also activated by DAGs (Lucas et al. 2003). DAGs are now being used routinely as activators of these subclasses of TRPC channels. Various DAGs have been found to be effective, including 1-stearoyl-2-arachidonyl-sn-glycerol and the related 2,4-diacylphloroglucinols (Hofmann et al. 1999, Aires et al. 2007, Leuner et al. 2010). DAG activation of TRPC6 was not prevented by protein kinase C inhibitors, suggesting that it was independent of protein kinase C and had a relatively direct effect on the channel (Hofmann et al. 1999). On the basis of the computational analysis of amino acid sequences and mutagenesis studies, an N-terminal section of TRPC3/6/7 has been proposed as a DAG-sensing domain, although as a regulator vesicle fusion (van Rossum et al. 2008).
The concentrations of exogenous DAGs required to stimulate the channels are relatively high, but the effects are suggested to be relevant to endogenous DAGs because there is also activation by DAG lipase inhibitors (Hofmann et al. 1999). There is, nevertheless, evidence of synergism with IP3, potentially conferring greater sensitivity to DAG (Albert & Large 2003). Intriguingly, the effect of PIP2 on TRPC6/7, but not on TRPC6 channels was overcome by IP3 (Ju et al. 2010). The receptor activation of TRPC3/6/7 channels by agonists at G protein-coupled receptors is an effect that therefore arises, at least in part, because of G protein or receptor tyrosine kinase stimulation of PLCβ/γ leading to degradation and thus depletion of PIP2 and generation of DAGs and IP3, all of which impinge on the channels to varying degrees.
TRPC1 is not thought to be directly activated by DAGs, although TRPC is difficult to study on its own because trafficking to the plasma membrane is poor in the absence of other co-expressed factors (e.g. other TRP proteins). It has been activated by DAGs when co-expressed with TRPC3 (Lintschinger et al. 2000). It is, however, also described that TRPC1 was phosphorylated via protein kinase C (which is activated by DAGs) and that endogenous TRPC1-containing channels were stimulated, as a consequence (Ahmmed et al. 2004, Saleh et al. 2008). TRPC4 and 5 readily traffic to the plasma membrane but, in contrast to TRPC3/6/7, are not activated by DAG (Hofmann et al. 1999, Venkatachalam et al. 2003). There is, nevertheless, a suggestion that TRPC5 forms part of a DAG-activated channel with TRPC3 (Liu et al. 2007a). Furthermore, desensitization following receptor activation of TRPC4/5 occurred via protein kinase C-dependent phosphorylation (Venkatachalam et al. 2003, Zhu et al. 2005). Similarly, protein kinase C-inhibited TRPC3 (Trebak et al. 2005) and TRPC6 (Bousquet et al. 2010) suggest that DAGs have stimulatory (direct) and inhibitory (indirect via protein kinase C) effects on these channels.
Therefore, DAGs acutely and directly stimulate some of the TRPCs, but they also activate or inhibit TRPCs by triggering protein kinase C-dependent phosphorylation.
Lysophospholipids
Lysophospholipids such as lysophosphatidylcholine (LPC) are generated by the enzymatic action of phospholipase A2 (PLA2) enzymes on phosphatidylcholine and other related substrates. LPC was first shown to be a stimulator of TRPC5 (Flemming et al. 2006). There is a partial insight into the mechanism of this effect. Chemically, the effect lacked head-group specificity because replacement of choline with inositol (to generate LPI) did not affect activity (Flemming et al. 2006). In contrast, the length of the carbon side chain was important, suggesting necessity of solubilization of the lysophospholipid in the lipid bilayer. As a result of this solubilization property, exogenous LPC has detergent effects on the lipid bilayers (hence ‘lyso’ indicating cell lysis). However, activation of TRPC5 occurred at low (subdetergent) concentrations of LPC and it was characterized by the distinctive current–voltage relationship (I–V) of TRPC5, showing that the effect reflected TRPC5 channel activity rather than non-specific bilayer disturbance.
Lysophosphatidylcholine is a ligand at certain G protein-coupled receptors (Ishii et al. 2004), but stimulation of TRPC5 by LPC did not require G protein signalling (Flemming et al. 2006). Furthermore, LPC activated TRPC5 in excised outside-out membrane patches in the absence of guanosine triphosphate (GTP), suggesting that it acted relatively directly at the channel. Consistent with negative data from convex membrane curvature experiments (Beech et al. 2009), LPC applied to the inner face of the lipid bilayer also activated TRPC5 (Flemming et al. 2006); that is, the effect of LPC on TRPC5 lacked polarity – acting similarly whether applied to the outside or inside of the membrane. This result is consistent with a model where membrane-spanning elements of TRPC5 contain a lipid interaction site that is accessible from either side of the membrane, conferring on the channel sensitivity to changes in lipid composition of the bilayer. Alternatively, TRPC5 activity may be influenced by specific membrane fluidity changes that occur with the introduction of LPC.
TRPC6-containing channels were found to be stimulated by LPC in endothelial cells (Chaudhuri et al. 2008). The I–V of the stimulated current lacked the distinctive rectification of TRPC channels, but responses were reduced when TRPC6 expression was suppressed or prevented, suggesting TRPC6 was involved but not alone. Compelling biochemical evidence was presented for forward trafficking of TRPC5 in response to LPC-evoked TRPC6-dependent Ca2+ entry. In these cells, TRPC5 expression at the plasma membrane was initially low, which might explain why there was no obvious stimulation of TRPC5 in the absence of TRPC6.
Stimulation of TRPC channels by LPC has biological importance in endothelial cell migration (Chaudhuri et al. 2008). It may also have wider importance. Human monocytes, for example, showed Ca2+ entry in response to LPC that was independent of G protein and PLC signalling and dependent on LPC carbon chain length (Yun et al. 2004). Human monocytes express TRPC5 and other TRPCs (Liu et al. 2007a). The pharmacological profile of the LPC-activated current in monocytes was similar to that of TRPC6 (Schilling & Eder 2009). A role of TRPC activation by LPC has been suggested in erectile dysfunction (So et al. 2005). Moreover, LPC is a major component of oxidized low-density lipoprotein (oxLDL), which may explain the Ca2+ influx and apoptosis induced by oxLDL in vascular smooth muscle cells (Ingueneau et al. 2008). Various endogenous non-selective cationic channels have been found to be stimulated by LPC, but it is not clear whether they are explained by TRPC channels (Smani et al. 2004, Schilling & Eder 2009).
Therefore, lysophospholipids stimulate TRPC channels, apparently relatively directly. The effects are relevant to endogenous concentrations of lysophospholipids and may be important in wide-ranging biological phenomena, both in terms of intracellular and extracellular signalling.
Oxidized phospholipids
1-Palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine is a common component of cell membranes. Its susceptibility to oxidation leads to bioactive oxidation products called oxidized phospholipids, which include 1-palmitoyl-2-oxovaleroyl-phosphatidylcholine (POVPC) and 1-palmitoyl-2-glutaroyl-phosphatidylcholine (PGPC). These lipids constitute a diverse family of signalling lipids that accumulate during oxidative stress, apoptosis and necrosis, and are often associated with inflammatory conditions such as rheumatoid arthritis and atherosclerosis. There are also suggestions of physiological roles for oxidized phospholipids that include pattern recognition in innate immunity. Although the importance of oxidized phospholipids is increasingly established, the initial reception and signalling mechanisms have been poorly understood. A recent study revealed that these lipid factors are stimulators of TRPC5 or TRPC5-containing channels (Al-Shawaf et al. 2010).
Low micromolar concentrations of PGPC and POVPC stimulated TRPC5 exogenously expressed in human embryonic kidney (HEK) 293 cells (Al-Shawaf et al. 2010). Relevance to endogenous TRPC5-containing channels was found in vascular smooth muscle cells where the oxidized phospholipids evoked TRPC1/5 channel activity without causing Ca2+ release. The effect was functionally relevant to cell migration. Surprisingly, given the chemical similarity to LPC, the actions of PGPC and POVPC depended almost completely on G-protein (Gi/o) signalling (Al-Shawaf et al. 2010). Previously identified G protein-coupled receptors for oxidized phospholipids were not involved, suggesting that the effects occurred via a previously unrecognized receptor or independently of receptors, but nevertheless requiring G protein function. In our experience, these oxidized phospholipids are amongst the best TRPC5 (or TRPC1/5) activators, having an advantage that the activation occurs without complications from Ca2+ release (Al-Shawaf et al. 2010).
Arachidonic acid and its metabolites
Arachidonic acid is a polyunsaturated fatty acid of lipid bilayers that is generated by phospholipase enzymes and is the precursor for many active metabolites. There are reports that TRPC channels are modulated by arachidonic acid and some of its metabolites. Basora et al. (2003) reported direct activation of TRPC6 by arachidonic acid and its metabolite 20-hydroxyeicosatetraenoic acid (20-HETE). The I–V of the stimulated current resembled that of TRPC6 only at high 20-HETE concentrations and, surprisingly, no Ca2+ entry was evoked by 20-HETE despite the fact that current was observed (Basora et al. 2003). Inoue et al. (2009) also reported TRPC6 stimulation by 20-HETE, in this case with a distinct TRPC6 I–V. Furthermore, dependence of hypotonic- or 2,4,6-trinitrophenol-stimulated TRPC6 on cytosolic PLA2 activity was identified (Inoue et al. 2009). Relationships of TRPC channels with arachidonic acid metabolites have also been suggested by other studies. Ben-Amor et al. (2006) reported block by anti-TRPC1 antibody of Ca2+ entry evoked by 5,6-epoxyeicosatrienoic acid (5,6-EET) in human platelets and Fleming et al. (2007) reported surface trafficking of TRPC6 in response to 11,12-EET and pulmonary vasoconstriction evoked by 11,12-EET was less in lungs from TRPC6 gene-disrupted mice. A stable urea EET analogue has been suggested to act through TRPC channel modulation (Liu et al. 2011) and 15-HETE has been observed to stimulate TRPC1 expression (Li et al. 2010).
Wu et al. (2002) suggested contribution of endogenous TRPC4 to arachidonic acid-evoked Ca2+ entry in HEK 293 cells. However, we have found no activation by arachidonic acid of the related TRPC5 channel overexpressed in HEK 293 cells (Flemming et al. 2006, Beech et al. 2009). TRPC5 is stimulated by the arachidonic acid metabolite prostaglandin E2, but acts through the E-type prostaglandin-1 (EP1) G protein-coupled receptor (Tabata et al. 2002). TRPC7 has been suggested to be required for induction of apoptosis by prostaglandin E2 in leukaemia cells (Foller et al. 2006).
In summary, arachidonic acid metabolites have importance as stimulators of TRPC channels, most notably of TRPC6 channels.
Sphingosine-1-phosphate
Sphingosine-1-phosphate (S1P) is generated from sphingosine, which derives from sphingomyelin, a constituent lipid of microdomains in the plasma membrane. TRPC5 was stimulated by S1P (Xu et al. 2006). S1P applied to the intracellular surface stimulated TRPC5 in inside-out membrane patches. TRPC5 was, therefore, suggested to be an intracellular target for S1P but without known physiological importance. Potentially related is the observation that S1P bound to a putative TRPC3-PLCγ1 intermolecular domain that also interacted with PI phosphates, although the functional relevance of this binding was not determined (van Rossum et al. 2005). Unlike for LPC, the extracellular effect of S1P on TRPC5 occurred via a G-protein (Gi/o) signalling pathway (Xu et al. 2006), further illustrating the significance of TRPC activation via receptors that have lipids as their ligands. S1P receptors are widely expressed, including HEK 293 cells often used for TRPC5 overexpression. S1P had no effect on TRPC5 studied in excised outside-out patches without GTP in the pipette, showing that S1P (unlike LPC) had no direct extracellular effect on TRPC5. The extracellular S1P effect on TRPC5 was found to be functionally important in cell motility (Xu et al. 2006). Therefore, S1P is an example of a lipid factor that activates TRPC channels via a G-protein signalling pathway (Xu et al. 2006), in some ways similar to the action of oxidized phospholipids (Al-Shawaf et al. 2010), but involving Ca2+ release also. Suggested intracellular actions of S1P could be biologically important, but remain relatively little explored.
Cholesterol and derivatives (steroids)
Cholesterol is a constituent sterol lipid of the plasma membrane. Its depletion with methyl-β-cyclodextrin has been shown to suppress store-operated Ca2+ signals and ionic current linked to TRPC1 (Bergdahl et al. 2003, Kannan et al. 2007, Alicia et al. 2008). Similarly, cholesterol loading of cells was found to have a positive effect on signals relating to TRPC3 (Graziani et al. 2006). TRPC1 has been associated with cholesterol-containing caveolae and other lipid rafts (Lockwich et al. 2000) and suggested to function as a component of store-operated channels only when linked to stromal interaction molecule-1 (STIM1) in lipid rafts (Alicia et al. 2008). Several studies have linked TRPC1 with caveolins (Lockwich et al. 2000, Bergdahl et al. 2003, Remillard & Yuan 2006, Ingueneau et al. 2008). An elegant study (Huber et al. 2006) showed enhancement of TRPC6 by the cholesterol-binding protein podocin, dependent on cholesterol binding by podocin which localizes specifically to the slit diaphragm of the kidney and is present at the inner leaflet of the bilayer. Cholesterol depletion with methyl-β-cyclodextrin inhibited the effect of podocin on TRPC6.
Cholesterol is the precursor for steroid hormones such as the neuroactive steroids, which are synthesized in the brain, adrenal glands and gonads (Compagnone & Mellon 2000). Examples of neuroactive steroids are pregnenolone sulphate and allopregnanolone. Specific types of neuroactive steroid have inhibitory actions in TRPC5, strengthening the emerging idea that TRP channels have unique steroid-sensing capabilities (Wagner et al. 2008, Majeed et al. 2010). TRPC5 was found to be negatively modulated via a rapid non-genomic mechanism (Majeed et al. 2011). The channels were inhibited by pregnenolone sulphate, pregnanolone (or its β-sulphated form), progesterone or dihydrotestosterone. There was a small effect of 17β-oestradiol, but no effect of pregnenolone, allopregnanolone or cortisol. Rapid and reversible effects of progesterone were shown in excised membrane patches. Sensitivity to pregnanolone, but not its stereo-isomer allopregnanolone, suggested the existence of a specific binding site. Endogenous TRPC1/5 channels were also inhibited by progesterone, albeit at a relatively high concentration. A prior study suggested that TRPC2, which is not expressed in humans, is activated by sulphated steroids from the urine, with importance for odour sensation of rodents (Nodari et al. 2008).
The data suggest dependence of TRPC channels on cholesterol and modulation of TRPC function by localization to lipid rafts. Furthermore, it is emerging that TRPC channels show highly specific and potentially unique steroid-sensing capability leading to inhibition of channel function.
Gangliosides and other lipid factors
Using an intracellular Ca2+ assay for HEK 293 cells conditionally overexpressing TRPC5, we investigated additional lipid factors as potential acute activators (Beech et al. 2009). Several lysophospholipids were activators, including the important signalling lipid lysophosphatidic acid, but not lysophosphatidylethanolamine or phosphatidylcholine. Platelet-activating factor (PAF) and lyso-PAF (which is inactive at PAF receptors) were activators at 3 μm concentration; both are chemically similar to LPC. Sphingosine, sphingomyelin, ceramide and ceramide-1-phosphate (C1P) were not stimulators of the channels but sphingosylphosphorylcholine was, by contrast, a strong activator. Cerebrosides, sulphatides and anandamide (an arachidonic acid metabolite) failed to activate but gangliosides and psychosine were modest activators. Gangliosides are glycosphingolipids containing sialic acid. It was found that crosslinking of the monosialosyl of gangliotetraose (GM1) ganglioside with multivalent ligands stimulated endogenous TRPC5-containing channels via α5β1 integrin (Wu et al. 2007); the effect was important in neuronal growth cone formation. We did not find a stimulatory effect of C1P on TRPC5, but it was recently reported that ceramide kinase and TRPC1 colocalize in cavealae (Hinkovska-Galcheva et al. 2008), raising the possibility that endogenous TRPC complexes are sensitive to C1P. Furthermore, in a human leukaemia T-cell line, Ca2+ entry evoked by Δ9-tetrahydrocannabinoid (a lipid-soluble plant-derived cannabinoid) was suppressed when TRPC1 was downregulated by RNA interference (Rao & Kaminski 2006); the effect occurred through cannabinoid G protein-coupled receptors. Therefore, there is an emerging breadth to the spectrum of lipids that modulate TRPC channels but also evidence of specificity.
Relationships to receptor agonists, membrane stretch and anaesthetics
As indicated above, common downstream effects of agonist binding to receptors are activation of PLC and PLA2 enzymes, which affect local concentrations of PIP2, DAGs, arachidonic acid metabolites, etc. Therefore, a consequence of lipid sensitivity of TRPC channels is that they are modulated, often positively, by a plethora of G protein-coupled or tyrosine kinase receptor agonists. Related to such effects may be the reported stimulatory effects of membrane deformation or stretch on the TRPC channels and the suggested relevance to myogenic tone in arteries (Welsh et al. 2002, Maroto et al. 2005, Gomis et al. 2008). Myogenic tone, for example, is associated with elevated levels of DAG, arachidonic acid metabolites and oxidative stress factors such as hydrogen peroxide (Hill et al. 2009). Stretch activation of TRPC6 has been suggested to arise because of sensitivity of G protein-coupled receptors and associated signalling pathways to membrane deformation, leading to downstream effects on TRPC6 activity (Mederos y Schnitzler et al. 2008, Inoue et al. 2009).
In part, anaesthetics modulate ion channel function by disturbing the lipid bilayer. Therefore, lipid sensitivity of TRPC channels may confer sensitivity to anaesthetics, as occurs with other ion channels. TRPC5 was found to be sensitive to general anaesthetics with the dominant net effect being inhibition of channel function (Bahnasi et al. 2008). The study included the surprising finding that TRPC5 stimulated by LPC was resistant to the intravenous anaesthetic propofol, whereas TRPC5 stimulated by gadolinium was strongly inhibited. It was suggested that propofol may not directly inhibit TRPC5, but instead compromised a signalling pathway that was necessary for TRPC5 activation by the lanthanide (Bahnasi et al. 2008). The data suggest a complex relationship between anaesthetics and TRPC5. It is not known if other TRPC channels are sensitive to anaesthetics.
Summary and conclusions
Transient receptor potential canonicals (TRPCs) have emerged as a class of proteins that form lipid-sensing cationic channels. Understanding remains elementary but, in some instances, we may start to consider them as lipid ionotropic receptors or lipid sensors through intermediate proteins. It should be recognized, nevertheless, that TRPC channels also exhibit constitutive activity (Dietrich et al. 2003, Nichols et al. 2007, Xu et al. 2008) and can be modulated by non-lipid factors that include extracellular acid, toxic metal ions, intracellular Ca2+, hydrogen peroxide and redox proteins (Schaefer et al. 2000, Strubing et al. 2001, Shi et al. 2004, Zeng et al. 2004, Hui et al. 2006, Semtner et al. 2007, Xu et al. 2008, Graham et al. 2010, Naylor et al. 2011). Although TRPC channels are responsive to lipid factors, they may not depend on them.
Figure 1 gives an abridged summary of knowledge of lipids and additional factors that modulate TRPC5 or TRPC6, providing comparisons for these two examples of TRPCs that have been studied relatively intensely. However, it should be recognized that the diagrams overly simplify the situation and hence they should be studied alongside the main text of this article and the original research publications. Although other TRPCs may show similar characteristics (e.g. TRPC4 like TRPC5, TRPC3 like TRPC6), there are also important differences, and incorporation of TRPC1 and other heteromultimeric assemblies may have a significant impact. Nevertheless, some impressions can be gained from the diagrams: the clearest distinctions between the channels are the activation of TRPC6 but not TRPC5 by DAG and arachidonic acid metabolites; in both cases there is evidence of promiscuity but also selectivity; the lipid profiles are consistent with intricate relationships of TRPCs with PLC and PLA2 enzymes; and, although not absolute, it is emerging that TRPC5 may be more associated with cell migration and proliferation, whereas TRPC6 is more associated with cell contraction and stability.
It seems clear that TRPC channels are capable of sensing various important lipids, enabling them to respond to these lipids as part of signalling events or to integrate with dynamic lipid environments of physiological or pathological contexts. Despite the technical difficulties of such studies, further investigation of TRPC modulation by lipids will be important. In many cases, knowledge of the lipid-sensing profile of a TRPC channel is limited or there is information only about a TRPC overexpressed in a cell line rather than endogenously as a heteromultimeric complex. In most cases, the mechanism of action of the lipid is unknown or superficially understood. The potential for synergy between actions of lipids and other factors has been underexplored.
Acknowledgments
Funded by the Wellcome Trust.
Conflict of interest
None.
References
- Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2008;23:297–328. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmmed GU, Mehta D, Vogel S, Holinstat M, Paria BC, Tiruppathi C, Malik AB. Protein kinase C alpha phosphorylates the TRPC1 channel and regulates store-operated Ca2+ entry in endothelial cells. J Biol Chem. 2004;279:20941–20949. doi: 10.1074/jbc.M313975200. [DOI] [PubMed] [Google Scholar]
- Aires V, Hichami A, Boulay G, Khan NA. Activation of TRPC6 calcium channels by diacylglycerol (DAG)-containing arachidonic acid: a comparative study with DAG-containing docosahexaenoic acid. Biochimie. 2007;89:926–937. doi: 10.1016/j.biochi.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Albert AP, Large WA. Synergism between inositol phosphates and diacylglycerol on native TRPC6-like channels in rabbit portal vein myocytes. J Physiol. 2003;552:789–795. doi: 10.1113/jphysiol.2003.052977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alicia S, Angelica Z, Carlos S, Alfonso S, Vaca L. STIM1 converts TRPC1 from a receptor-operated to a store-operated channel: moving TRPC1 in and out of lipid rafts. Cell Calcium. 2008;44:479–491. doi: 10.1016/j.ceca.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Al-Shawaf E, Naylor J, Taylor H, Riches K, Milligan CJ, O’Regan D, Porter KE, Li J, Beech DJ. Short-term stimulation of calcium-permeable transient receptor potential canonical 5-containing channels by oxidized phospholipids. Arterioscler Thromb Vasc Biol. 2010;30:1453–1459. doi: 10.1161/ATVBAHA.110.205666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahnasi YM, Wright HM, Milligan CJ, Dedman AM, Zeng F, Hopkins PM, Bateson AN, Beech DJ. Modulation of TRPC5 cation channels by halothane, chloroform and propofol. Br J Pharmacol. 2008;153:1505–1512. doi: 10.1038/sj.bjp.0707689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai CX, Giamarchi A, Rodat-Despoix L, Padilla F, Downs T, Tsiokas L, Delmas P. Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO Rep. 2008;9:472–479. doi: 10.1038/embor.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basora N, Boulay G, Bilodeau L, Rousseau E, Payet MD. 20-Hydroxyeicosatetraenoic acid (20-HETE) activates mouse TRPC6 channels expressed in HEK293 cells. J Biol Chem. 2003;278:31709–31716. doi: 10.1074/jbc.M304437200. [DOI] [PubMed] [Google Scholar]
- Beech DJ, Bahnasi YM, Dedman AM, Al-Shawaf E. TRPC channel lipid specificity and mechanisms of lipid regulation. Cell Calcium. 2009;45:583–588. doi: 10.1016/j.ceca.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Amor N, Redondo PC, Bartegi A, Pariente JA, Salido GM, Rosado JA. A role for 5,6-epoxyeicosatrienoic acid in calcium entry by de novo conformational coupling in human platelets. J Physiol. 2006;570:309–323. doi: 10.1113/jphysiol.2005.100800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergdahl A, Gomez MF, Dreja K, Xu SZ, Adner M, Beech DJ, Broman J, Hellstrand P, Sward K. Cholesterol depletion impairs vascular reactivity to endothelin-1 by reducing store-operated Ca2+ entry dependent on TRPC1. Circ Res. 2003;93:839–847. doi: 10.1161/01.RES.0000100367.45446.A3. [DOI] [PubMed] [Google Scholar]
- Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- Bousquet SM, Monet M, Boulay G. Protein kinase C-dependent phosphorylation of transient receptor potential canonical 6 (TRPC6) on serine 448 causes channel inhibition. J Biol Chem. 2010;285:40534–40543. doi: 10.1074/jbc.M110.160051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri P, Colles SM, Bhat M, Van Wagoner DR, Birnbaumer L, Graham LM. Elucidation of a TRPC6-TRPC5 channel cascade that restricts endothelial cell movement. Mol Biol Cell. 2008;19:3203–3211. doi: 10.1091/mbc.E07-08-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Mederos y Schnitzler M, Emmel J, Kalwa H, Hofmann T, Gudermann T. N-linked protein glycosylation is a major determinant for basal TRPC3 and TRPC6 channel activity. J Biol Chem. 2003;278:47842–47852. doi: 10.1074/jbc.M302983200. [DOI] [PubMed] [Google Scholar]
- Fabian A, Fortmann T, Dieterich P, Riethmuller C, Schon P, Mally S, Nilius B, Schwab A. TRPC1 channels regulate directionality of migrating cells. Pflugers Arch. 2008;457:475–484. doi: 10.1007/s00424-008-0515-4. [DOI] [PubMed] [Google Scholar]
- Fahy E, Subramaniam S, Brown HA, Glass CK, Merrill AH, Jr, Murphy RC, Raetz CR, Russell DW, Seyama Y, Shaw W, et al. A comprehensive classification system for lipids. J Lipid Res. 2005;46:839–861. doi: 10.1194/jlr.E400004-JLR200. [DOI] [PubMed] [Google Scholar]
- Fleming I, Rueben A, Popp R, Fisslthaler B, Schrodt S, Sander A, Haendeler J, Falck JR, Morisseau C, Hammock BD, Busse R. Epoxyeicosatrienoic acids regulate Trp channel dependent Ca2+ signaling and hyperpolarization in endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27:2612–2618. doi: 10.1161/ATVBAHA.107.152074. [DOI] [PubMed] [Google Scholar]
- Flemming PK, Dedman AM, Xu SZ, Li J, Zeng F, Naylor J, Benham CD, Bateson AN, Muraki K, Beech DJ. Sensing of lysophospholipids by TRPC5 calcium channel. J Biol Chem. 2006;281:4977–4982. doi: 10.1074/jbc.M510301200. [DOI] [PubMed] [Google Scholar]
- Flockerzi V. An introduction on TRP channels. Handb Exp Pharmacol. 2007;179:1–19. doi: 10.1007/978-3-540-34891-7_1. [DOI] [PubMed] [Google Scholar]
- Foller M, Kasinathan RS, Duranton C, Wieder T, Huber SM, Lang F. PGE2-induced apoptotic cell death in K562 human leukaemia cells. Cell Physiol Biochem. 2006;17:201–210. doi: 10.1159/000094125. [DOI] [PubMed] [Google Scholar]
- Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G, Hofmann F, Flockerzi V, Nilius B. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4-/- mice. Nat Cell Biol. 2001;3:121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- Fukami K, Inanobe S, Kanemaru K, Nakamura Y. Phospholipase C is a key enzyme regulating intracellular calcium and modulating the phosphoinositide balance. Prog Lipid Res. 2010;49:429–437. doi: 10.1016/j.plipres.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Ge R, Tai Y, Sun Y, Zhou K, Yang S, Cheng T, Zou Q, Shen F, Wang Y. Critical role of TRPC6 channels in VEGF-mediated angiogenesis. Cancer Lett. 2009;283:43–51. doi: 10.1016/j.canlet.2009.03.023. [DOI] [PubMed] [Google Scholar]
- Gomis A, Soriano S, Belmonte C, Viana F. Hypoosmotic- and pressure-induced membrane stretch activate TRPC5 channels. J Physiol. 2008;586:5633–5649. doi: 10.1113/jphysiol.2008.161257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham S, Ding M, Ding Y, Sours-Brothers S, Luchowski R, Gryczynski Z, Yorio T, Ma H, Ma R. Canonical transient receptor potential 6 (TRPC6), a redox-regulated cation channel. J Biol Chem. 2010;285:23466–23476. doi: 10.1074/jbc.M109.093500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziani A, Rosker C, Kohlwein SD, Zhu MX, Romanin C, Sattler W, Groschner K, Poteser M. Cellular cholesterol controls TRPC3 function: evidence from a novel dominant-negative knockdown strategy. Biochem J. 2006;396:147–155. doi: 10.1042/BJ20051246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greka A, Navarro B, Oancea E, Duggan A, Clapham DE. TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci. 2003;6:837–845. doi: 10.1038/nn1092. [DOI] [PubMed] [Google Scholar]
- Hamdollah Zadeh MA, Glass CA, Magnussen A, Hancox JC, Bates DO. VEGF-mediated elevated intracellular calcium and angiogenesis in human microvascular endothelial cells in vitro are inhibited by dominant negative TRPC6. Microcirculation. 2008;15:605–614. doi: 10.1080/10739680802220323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MA, Meininger GA, Davis MJ, Laher I. Therapeutic potential of pharmacologically targeting arteriolar myogenic tone. Trends Pharmacol Sci. 2009;30:363–374. doi: 10.1016/j.tips.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Hinkovska-Galcheva V, Clark A, VanWay S, Huang JB, Hiraoka M, Abe A, Borofsky M, Kunkel RG, Shanley T, Shayman JA, Lanni F, Petty HR, Boxer LA. Ceramide kinase promotes Ca2+ signaling near IgG-opsonized targets and enhances phagolysosomal fusion in COS-1 cells. J Lipid Res. 2008;49:531–542. doi: 10.1194/jlr.M700442-JLR200. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Huber TB, Schermer B, Muller RU, Hohne M, Bartram M, Calixto A, Hagmann H, Reinhardt C, Koos F, Kunzelmann K, et al. Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc Natl Acad Sci USA. 2006;103:17079–17086. doi: 10.1073/pnas.0607465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui H, McHugh D, Hannan M, Zeng F, Xu SZ, Khan SU, Levenson R, Beech DJ, Weiss JL. Calcium-sensing mechanism in TRPC5 channels contributing to retardation of neurite outgrowth. J Physiol. 2006;572:165–172. doi: 10.1113/jphysiol.2005.102889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingueneau C, Huynh-Do U, Marcheix B, Athias A, Gambert P, Negre-Salvayre A, Salvayre R, Vindis C. TRPC1 is regulated by caveolin-1 and is involved in oxidized LDL-induced apoptosis of vascular smooth muscle cells. J Cell Mol Med. 2008;13:1620–1631. doi: 10.1111/j.1582-4934.2008.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R, Jensen LJ, Jian Z, Shi J, Hai L, Lurie AI, Henriksen FH, Salomonsson M, Morita H, Kawarabayashi Y, Mori M, Mori Y, Ito Y. Synergistic activation of vascular TRPC6 channel by receptor and mechanical stimulation via phospholipase C/diacylglycerol and phospholipase A2/omega-hydroxylase/20-HETE pathways. Circ Res. 2009;104:1399–1409. doi: 10.1161/CIRCRESAHA.108.193227. [DOI] [PubMed] [Google Scholar]
- Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signaling and biology. Annu Rev Biochem. 2004;73:321–354. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- Jho D, Mehta D, Ahmmed G, Gao XP, Tiruppathi C, Broman M, Malik AB. Angiopoietin-1 opposes VEGF-induced increase in endothelial permeability by inhibiting TRPC1-dependent Ca2+ influx. Circ Res. 2005;96:1282–1290. doi: 10.1161/01.RES.0000171894.03801.03. [DOI] [PubMed] [Google Scholar]
- Ju M, Shi J, Saleh SN, Albert AP, Large WA. Ins(1,4,5)P3 interacts with PIP2 to regulate activation of TRPC6/C7 channels by diacylglycerol in native vascular myocytes. J Physiol. 2010;588:1419–1433. doi: 10.1113/jphysiol.2009.185256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan KB, Barlos D, Hauser CJ. Free cholesterol alters lipid raft structure and function regulating neutrophil Ca2+ entry and respiratory burst: correlations with calcium channel raft trafficking. J Immunol. 2007;178:5253–5261. doi: 10.4049/jimmunol.178.8.5253. [DOI] [PubMed] [Google Scholar]
- Kim BJ, Kim MT, Jeon JH, Kim SJ, So I. Involvement of phosphatidylinositol 4,5-bisphosphate in the desensitization of canonical transient receptor potential 5. Biol Pharm Bull. 2008;31:1733–1738. doi: 10.1248/bpb.31.1733. [DOI] [PubMed] [Google Scholar]
- Kim MS, Hong JH, Li Q, Shin DM, Abramowitz J, Birnbaumer L, Muallem S. Deletion of TRPC3 in mice reduces store-operated Ca2+ influx and the severity of acute pancreatitis. Gastroenterology. 2009;137:1509–1517. doi: 10.1053/j.gastro.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kini V, Chavez A, Mehta D. A new role for PTEN in regulating transient receptor potential canonical channel 6-mediated Ca2+ entry, endothelial permeability, and angiogenesis. J Biol Chem. 2010;285:33082–33091. doi: 10.1074/jbc.M110.142034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyonaka S, Kato K, Nishida M, Mio K, Numaga T, Sawaguchi Y, Yoshida T, Wakamori M, Mori E, Numata T, et al. Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound. Proc Natl Acad Sci USA. 2009;106:5400–5405. doi: 10.1073/pnas.0808793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B, Dreja K, Shah SS, Cheong A, Xu SZ, Sukumar P, Naylor J, Forte A, Cipollaro M, McHugh D, et al. Upregulated TRPC1 channel in vascular injury in vivo and its role in human neointimal hyperplasia. Circ Res. 2006;98:557–563. doi: 10.1161/01.RES.0000204724.29685.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Hofmann T, Montell C. Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Mol Cell. 2007;25:491–503. doi: 10.1016/j.molcel.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large WA, Saleh SN, Albert AP. Role of phosphoinositol 4,5-bisphosphate and diacylglycerol in regulating native TRPC channel proteins in vascular smooth muscle. Cell Calcium. 2009;45:574–582. doi: 10.1016/j.ceca.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Leuner K, Heiser JH, Derksen S, Mladenov MI, Fehske CJ, Schubert R, Gollasch M, Schneider G, Harteneck C, Chatterjee SS, Muller WE. Simple 2,4-diacylphloroglucinols as classic transient receptor potential-6 activators – identification of a novel pharmacophore. Mol Pharmacol. 2010;77:368–377. doi: 10.1124/mol.109.057513. [DOI] [PubMed] [Google Scholar]
- Li S, Ran Y, Zheng X, Pang X, Wang Z, Zhang R, Zhu D. 15-HETE mediates sub-acute hypoxia-induced TRPC1 expression and enhanced capacitative calcium entry in rat distal pulmonary arterial myocytes. Prostaglandins Other Lipid Mediat. 2010;93:60–74. doi: 10.1016/j.prostaglandins.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Lintschinger B, Balzer-Geldsetzer M, Baskaran T, Graier WF, Romanin C, Zhu MX, Groschner K. Coassembly of Trp1 and Trp3 proteins generates diacylglycerol- and Ca2+-sensitive cation channels. J Biol Chem. 2000;275:27799–27805. doi: 10.1074/jbc.M002705200. [DOI] [PubMed] [Google Scholar]
- Liu DY, Thilo F, Scholze A, Wittstock A, Zhao ZG, Harteneck C, Zidek W, Zhu ZM, Tepel M. Increased store-operated and 1-oleoyl-2-acetyl-sn-glycerol-induced calcium influx in monocytes is mediated by transient receptor potential canonical channels in human essential hypertension. J Hypertens. 2007a;25:799–808. doi: 10.1097/HJH.0b013e32803cae2b. [DOI] [PubMed] [Google Scholar]
- Liu X, Cheng KT, Bandyopadhyay BC, Pani B, Dietrich A, Paria BC, Swaim WD, Beech D, Yildrim E, Singh BB, Birnbaumer L, Ambudkar IS. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(-/-) mice. Proc Natl Acad Sci USA. 2007b;104:17542–17547. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang R, Li J, Rao J, Li W, Falck JR, Manthati VL, Medhora M, Jacobs ER, Zhu D. Stable EET urea agonist and soluble epoxide hydrolase inhibitor regulate rat pulmonary arteries through TRPCs. Hypertens Res. 2011 doi: 10.1038/hr.2011.5. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwich TP, Liu X, Singh BB, Jadlowiec J, Weiland S, Ambudkar IS. Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J Biol Chem. 2000;275:11934–11942. doi: 10.1074/jbc.275.16.11934. [DOI] [PubMed] [Google Scholar]
- Lucas P, Ukhanov K, Leinders-Zufall T, Zufall F. A diacylglycerol-gated cation channel in vomeronasal neuron dendrites is impaired in TRPC2 mutant mice: mechanism of pheromone transduction. Neuron. 2003;40:551–561. doi: 10.1016/s0896-6273(03)00675-5. [DOI] [PubMed] [Google Scholar]
- Ma HT, Peng Z, Hiragun T, Iwaki S, Gilfillan AM, Beaven MA. Canonical transient receptor potential 5 channel in conjunction with Orai1 and STIM1 allows Sr2+ entry, optimal influx of Ca2+, and degranulation in a rat mast cell line. J Immunol. 2008;180:2233–2239. doi: 10.4049/jimmunol.180.4.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Cao J, Luo J, Nilius B, Huang Y, Ambudkar IS, Yao X. Depletion of intracellular Ca2+ stores stimulates the translocation of vanilloid transient receptor potential 4-c1 heteromeric channels to the plasma membrane. Arterioscler Thromb Vasc Biol. 2010;30:2249–2255. doi: 10.1161/ATVBAHA.110.212084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed Y, Agarwal AK, Naylor J, Seymour VA, Jiang S, Muraki K, Fishwick CW, Beech DJ. Cis-isomerism and other chemical requirements of steroidal agonists and partial agonists acting at TRPM3 channels. Br J Pharmacol. 2010;161:430–441. doi: 10.1111/j.1476-5381.2010.00892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed Y, Amer M, Agarwal A, McKeown L, Porter K, O’Regan D, Naylor J, Fishwick C, Muraki K, Beech D. Stereo-selective inhibition of transient receptor potential TRPC5 cation channels by neuroactive steroids. Br J Pharmacol. 2011;162:1509–1520. doi: 10.1111/j.1476-5381.2010.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005;7:179–185. doi: 10.1038/ncb1218. [DOI] [PubMed] [Google Scholar]
- Marrink SJ, de Vries AH, Tieleman DP. Lipids on the move: simulations of membrane pores, domains, stalks and curves. Biochim Biophys Acta. 2009;1788:149–168. doi: 10.1016/j.bbamem.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 2008;27:3092–3103. doi: 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miehe S, Bieberstein A, Arnould I, Ihdene O, Rutten H, Strubing C. The phospholipid-binding protein SESTD1 is a novel regulator of the transient receptor potential channels TRPC4 and TRPC5. J Biol Chem. 2010;285:12426–12434. doi: 10.1074/jbc.M109.068304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor J, Al-Shawaf E, McKeown L, Manna PT, Porter KE, O’Regan D, Muraki K, Beech DJ. TRPC5 channel sensitivities to antioxidants and hydroxylated stilbenes. J Biol Chem. 2011;286:5078–5086. doi: 10.1074/jbc.M110.196956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols RA, Dengler AF, Nakagawa EM, Bashkin M, Paul BT, Wu J, Khan GM. A constitutive, transient receptor potential-like Ca2+ influx pathway in presynaptic nerve endings independent of voltage-gated Ca2+ channels and Na+/Ca2+ exchange. J Biol Chem. 2007;282:36102–36111. doi: 10.1074/jbc.M706002200. [DOI] [PubMed] [Google Scholar]
- Nilius B. TRP channels in disease. Biochim Biophys Acta. 2007;1772:805–812. doi: 10.1016/j.bbadis.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Nodari F, Hsu FF, Fu X, Holekamp TF, Kao LF, Turk J, Holy TE. Sulfated steroids as natural ligands of mouse pheromone-sensing neurons. J Neurosci. 2008;28:6407–6418. doi: 10.1523/JNEUROSCI.1425-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuguro K, Tang J, Tang Y, Xiao R, Freichel M, Tsvilovskyy V, Ito S, Flockerzi V, Zhu MX, Zholos AV. Isoform-specific inhibition of TRPC4 channel by phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2008;283:10026–10036. doi: 10.1074/jbc.M707306200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao GK, Kaminski NE. Induction of intracellular calcium elevation by Delta9-tetrahydrocannabinol in T cells involves TRPC1 channels. J Leukoc Biol. 2006;79:202–213. doi: 10.1189/jlb.0505274. [DOI] [PubMed] [Google Scholar]
- Remillard CV, Yuan JX. Transient receptor potential channels and caveolin-1: good friends in tight spaces. Mol Pharmacol. 2006;70:1151–1154. doi: 10.1124/mol.106.029280. [DOI] [PubMed] [Google Scholar]
- Riccio A, Li Y, Moon J, Kim KS, Smith KS, Rudolph U, Gapon S, Yao GL, Tsvetkov E, Rodig SJ, Van’t Veer A, Meloni EG, Carlezon WA, Jr, Bolshakov VY, Clapham DE. Essential role for TRPC5 in amygdala function and fear-related behavior. Cell. 2009;137:761–772. doi: 10.1016/j.cell.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rossum DB, Patterson RL, Sharma S, Barrow RK, Kornberg M, Gill DL, Snyder SH. Phospholipase Cgamma1 controls surface expression of TRPC3 through an intermolecular PH domain. Nature. 2005;434:99–104. doi: 10.1038/nature03340. [DOI] [PubMed] [Google Scholar]
- van Rossum DB, Oberdick D, Rbaibi Y, Bhardwaj G, Barrow RK, Nikolaidis N, Snyder SH, Kiselyov K, Patterson RL. TRP_2, a lipid/trafficking domain that mediates diacylglycerol-induced vesicle fusion. J Biol Chem. 2008;283:34384–34392. doi: 10.1074/jbc.M804707200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh SN, Albert AP, Peppiatt-Wildman CM, Large WA. Diverse properties of store-operated TRPC channels activated by protein kinase C in vascular myocytes. J Physiol. 2008;586:2463–2476. doi: 10.1113/jphysiol.2008.152157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh SN, Albert AP, Large WA. Activation of native TRPC1/C5/C6 channels by endothelin-1 is mediated by both PIP3 and PIP2 in rabbit coronary artery myocytes. J Physiol. 2009;587:5361–5375. doi: 10.1113/jphysiol.2009.180331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Mejia RO, Mucke L. Phospholipase A2 and arachidonic acid in Alzheimer’s disease. Biochim Biophys Acta. 2010;1801:784–790. doi: 10.1016/j.bbalip.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M, Plant TD, Obukhov AG, Hofmann T, Gudermann T, Schultz G. Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J Biol Chem. 2000;275:17517–17526. doi: 10.1074/jbc.275.23.17517. [DOI] [PubMed] [Google Scholar]
- Schilling T, Eder C. Non-selective cation channel activity is required for lysophosphatidylcholine-induced monocyte migration. J Cell Physiol. 2009;221:325–334. doi: 10.1002/jcp.21857. [DOI] [PubMed] [Google Scholar]
- Semtner M, Schaefer M, Pinkenburg O, Plant TD. Potentiation of TRPC5 by protons. J Biol Chem. 2007;282:33868–33878. doi: 10.1074/jbc.M702577200. [DOI] [PubMed] [Google Scholar]
- Seth M, Zhang ZS, Mao L, Graham V, Burch J, Stiber J, Tsiokas L, Winn M, Abramowitz J, Rockman HA, Birnbaumer L, Rosenberg P. TRPC1 channels are critical for hypertrophic signaling in the heart. Circ Res. 2009;105:1023–1030. doi: 10.1161/CIRCRESAHA.109.206581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Mori E, Mori Y, Mori M, Li J, Ito Y, Inoue R. Multiple regulation by calcium of murine homologues of transient receptor potential proteins TRPC6 and TRPC7 expressed in HEK293 cells. J Physiol. 2004;561:415–432. doi: 10.1113/jphysiol.2004.075051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Ding X, He ZH, Zhou KC, Wang Q, Wang YZ. Critical role of TRPC6 channels in G2 phase transition and the development of human oesophageal cancer. Gut. 2009;58:1443–1450. doi: 10.1136/gut.2009.181735. [DOI] [PubMed] [Google Scholar]
- Smani T, Zakharov SI, Csutora P, Leno E, Trepakova ES, Bolotina VM. A novel mechanism for the store-operated calcium influx pathway. Nat Cell Biol. 2004;6:113–120. doi: 10.1038/ncb1089. [DOI] [PubMed] [Google Scholar]
- Smedlund K, Tano JY, Vazquez G. The constitutive function of native TRPC3 channels modulates vascular cell adhesion molecule-1 expression in coronary endothelial cells through nuclear factor kappaB signaling. Circ Res. 2010;106:1479–1488. doi: 10.1161/CIRCRESAHA.109.213314. [DOI] [PubMed] [Google Scholar]
- So I, Chae MR, Kim SJ, Lee SW. Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces the change of calcium mobilization via TRPC ion channels in cultured human corporal smooth muscle cells. Int J Impot Res. 2005;17:475–483. doi: 10.1038/sj.ijir.3901356. [DOI] [PubMed] [Google Scholar]
- Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem. 2003;278:39014–39019. doi: 10.1074/jbc.M306705200. [DOI] [PubMed] [Google Scholar]
- Tabata H, Tanaka S, Sugimoto Y, Kanki H, Kaneko S, Ichikawa A. Possible coupling of prostaglandin E receptor EP(1) to TRP5 expressed in Xenopus laevis oocytes. Biochem Biophys Res Commun. 2002;298:398–402. doi: 10.1016/s0006-291x(02)02455-5. [DOI] [PubMed] [Google Scholar]
- Tian D, Jacobo SM, Billing D, Rozkalne A, Gage SD, Anagnostou T, Pavenstadt H, Hsu HH, Schlondorff J, Ramos A, Greka A. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci Signal. 2010;3:ra77. doi: 10.1126/scisignal.2001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebak M, Hempel N, Wedel BJ, Smyth JT, Bird GS, Putney JW., Jr Negative regulation of TRPC3 channels by protein kinase C-mediated phosphorylation of serine 712. Mol Pharmacol. 2005;67:558–563. doi: 10.1124/mol.104.007252. [DOI] [PubMed] [Google Scholar]
- Trebak M, Lemonnier L, DeHaven WI, Wedel BJ, Bird GS, Putney JW., Jr Complex functions of phosphatidylinositol 4,5-bisphosphate in regulation of TRPC5 cation channels. Pflugers Arch. 2009;457:757–769. doi: 10.1007/s00424-008-0550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng PH, Lin HP, Hu H, Wang C, Zhu MX, Chen CS. The canonical transient receptor potential 6 channel as a putative phosphatidylinositol 3,4,5-trisphosphate-sensitive calcium entry system. Biochemistry. 2004;43:11701–11708. doi: 10.1021/bi049349f. [DOI] [PubMed] [Google Scholar]
- Tsiokas L, Arnould T, Zhu C, Kim E, Walz G, Sukhatme VP. Specific association of the gene product of PKD2 with the TRPC1 channel. Proc Natl Acad Sci USA. 1999;96:3934–3939. doi: 10.1073/pnas.96.7.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvilovskyy VV, Zholos AV, Aberle T, Philipp SE, Dietrich A, Zhu MX, Birnbaumer L, Freichel M, Flockerzi V. Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology. 2009;137:1415–1424. doi: 10.1053/j.gastro.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Zheng F, Gill DL. Regulation of canonical transient receptor potential (TRPC) channel function by diacylglycerol and protein kinase C. J Biol Chem. 2003;278:29031–29040. doi: 10.1074/jbc.M302751200. [DOI] [PubMed] [Google Scholar]
- Wagner TF, Loch S, Lambert S, Straub I, Mannebach S, Mathar I, Dufer M, Lis A, Flockerzi V, Philipp SE, Oberwinkler J. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat Cell Biol. 2008;10:1421–1430. doi: 10.1038/ncb1801. [DOI] [PubMed] [Google Scholar]
- Weissmann N, Dietrich A, Fuchs B, Kalwa H, Ay M, Dumitrascu R, Olschewski A, Storch U, Mederos y Schnitzler M, Ghofrani HA, Schermuly RT, Pinkenburg O, Seeger W, Grimminger F, Gudermann T. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci USA. 2006;103:19093–19098. doi: 10.1073/pnas.0606728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res. 2002;90:248–250. doi: 10.1161/hh0302.105662. [DOI] [PubMed] [Google Scholar]
- Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- Wu X, Babnigg G, Zagranichnaya T, Villereal ML. The role of endogenous human Trp4 in regulating carbachol-induced calcium oscillations in HEK-293 cells. J Biol Chem. 2002;277:13597–13608. doi: 10.1074/jbc.M110881200. [DOI] [PubMed] [Google Scholar]
- Wu G, Lu ZH, Obukhov AG, Nowycky MC, Ledeen RW. Induction of calcium influx through TRPC5 channels by cross-linking of GM1 ganglioside associated with alpha5beta1 integrin initiates neurite outgrowth. J Neurosci. 2007;27:7447–7458. doi: 10.1523/JNEUROSCI.4266-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- Xu SZ, Muraki K, Zeng F, Li J, Sukumar P, Shah S, Dedman AM, Flemming PK, McHugh D, Naylor J, Cheong A, Bateson AN, Munsch CM, Porter KE, Beech DJ. A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circ Res. 2006;98:1381–1389. doi: 10.1161/01.RES.0000225284.36490.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SZ, Sukumar P, Zeng F, Li J, Jairaman A, English A, Naylor J, Ciurtin C, Majeed Y, Milligan CJ, et al. TRPC channel activation by extracellular thioredoxin. Nature. 2008;451:69–72. doi: 10.1038/nature06414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim E, Birnbaumer L. TRPC2: molecular biology and functional importance. Handb Exp Pharmacol. 2007;179:53–75. doi: 10.1007/978-3-540-34891-7_3. [DOI] [PubMed] [Google Scholar]
- Yu PC, Gu SY, Bu JW, Du JL. TRPC1 is essential for in vivo angiogenesis in zebrafish. Circ Res. 2010;106:1221–1232. doi: 10.1161/CIRCRESAHA.109.207670. [DOI] [PubMed] [Google Scholar]
- Yun MR, Okajima F, Im DS. The action mode of lysophosphatidylcholine in human monocytes. J Pharmacol Sci. 2004;94:45–50. doi: 10.1254/jphs.94.45. [DOI] [PubMed] [Google Scholar]
- Zeng F, Xu SZ, Jackson PK, McHugh D, Kumar B, Fountain SJ, Beech DJ. Human TRPC5 channel activated by a multiplicity of signals in a single cell. J Physiol. 2004;559:739–750. doi: 10.1113/jphysiol.2004.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MH, Chae M, Kim HJ, Lee YM, Kim MJ, Jin NG, Yang DK, So I, Kim KW. Desensitization of canonical transient receptor potential channel 5 by protein kinase C. Am J Physiol Cell Physiol. 2005;289:C591–C600. doi: 10.1152/ajpcell.00440.2004. [DOI] [PubMed] [Google Scholar]


