Abstract
Purpose
It is unclear why some children with falciparum malaria develop acute seizures and what determines the phenotype of seizures. We sought to determine if polymorphisms of malaria candidate genes are associated with acute seizures.
Methods
Logistic regression was used to investigate genetic associations with malaria-associated seizures (MAS) and complex MAS (repetitive, prolonged, or focal seizures) in four MalariaGEN African sites, namely: Blantyre, Malawi; Kilifi, Kenya; Kumasi, Ghana; and Muheza, Tanzania. The analysis was repeated for five inheritance models (dominant, heterozygous, recessive, additive, and general) and adjusted for potential confounders and multiple testing.
Key Findings
Complex phenotypes of seizures constituted 71% of all admissions with MAS across the sites. MAS were strongly associated with cluster of differentiation-ligand-rs3092945 in females in Kilifi (p = 0.00068) and interleukin (IL)-17 receptor E-rs708567 in the pooled analysis across the sites (p = 0.00709). Complex MAS were strongly associated with epidermal growth factor module-containing mucin-like hormone receptor (EMR)1-rs373533 in Kumasi (p = 0.00033), but none in the pooled analysis. Focal MAS were strongly associated with IL-20 receptor A-rs1555498 in Muheza (p = 0.00016), but none in the pooled analysis. Prolonged MAS were strongly associated with complement receptor 1-rs17047660 in Kilifi (p = 0.00121) and glucose-6-phosphate dehydrogenase-rs1050828 in females in the pooled analysis (p = 0.00155). Repetitive MAS were strongly associated with EMR1-rs373533 in Kumasi (p = 0.00003) and cystic fibrosis transmembrane conductance receptor-rs17140229 in the pooled analysis (p = 0.00543). MAS with coma/cerebral malaria were strongly associated with EMR1-rs373533 in Kumasi (p = 0.00019) and IL10-rs3024500 in the pooled analysis across the sites (p = 0.00064).
Significance
We have identified a number of genetic associations that may explain the risk of seizures in >2,000 cases admitted to hospitals with MAS across four sites in Africa. These associations differed according to phenotype of seizures and site.
Keywords: Africa, Children, Seizure phenotypes, Malaria-associated seizures, Genetic risk
Acute seizures are common in children admitted with falciparum malaria to hospitals in sub-Saharan Africa (Taylor et al., 2006; Idro et al., 2008). Some malarial seizures are focal, repetitive, or prolonged (complex), which are associated with the development of subsequent neurocognitive impairments or epilepsy (Carter et al., 2005; Birbeck et al., 2010). It is not clear if these seizures are febrile seizures or acute symptomatic seizures caused by the sequestration of the parasites within the brain (Idro et al., 2005). Furthermore, it is difficult to attribute seizures to malaria in a malaria-endemic area, since a proportion of children will have sequestered parasites and be asymptomatic, and malaria is the most common cause of fever in children aged 6 months to 6 years (Idro et al., 2005). We have determined that most seizures in children admitted to hospital with malaria parasitemia are attributable to malaria using logistic regression techniques (Kariuki et al., 2011), and that children admitted with seizures are more likely to have relatives with seizure disorders than controls (Versteeg et al., 2003).
There is a strong genetic predisposition for febrile seizures, investigated through twin studies, family history, and a number of genetic polymorphisms (DNA sequence variation occurring when a single nucleotide—A, T, C, or G—n the genome differs between members of a biologic species; Rich et al., 1987; Kugler & Johnson, 1998; Kjeldsen et al., 2002). The risk of febrile seizures has been associated with interleukin-1β (IL-1B; Virta et al., 2002; Kira et al., 2005) and interleukin-1 receptor antagonist (IL-1RA; Tsai, 1987). Furthermore, a number of polymorphisms associated with falciparum malaria infections have been identified, which may be protective for example, sickle cell trait (Williams et al., 2005), glucose-6-phosphate dehydrogenase (G6PD; Clark et al., 2009), interadhesion molecule (ICAM-1; Kun et al., 1999), and cluster of differentiation ligand (CD-40LG; Sabeti et al., 2002), or susceptible, for example, complement receptor (CR-1; Nagayasu et al., 2001) and IL-10, -12, and -18 (Chaisavaneeyakorn et al., 2003; Wilson et al., 2005). However, the role of these genes in the genesis of seizures has not been examined.
We examined a number of different polymorphisms associated with malaria-associated seizures (MAS), and the phenotypes of MAS using 4,472 children admitted to hospitals with falciparum malaria in four African countries (Ghana, Kenya, Malawi, and Tanzania) as part of a case–control study for MalariaGEN (Malaria Genetic Epidemiological Network) consortial project (MalariaGEN, 2008). The malaria candidate polymorphisms in the MalariaGEN consortial project (Table S1) were chosen for this analysis because they reflect a pathogenic mechanism for severe malaria that could be involved in the genesis of acute seizures in falciparum malaria. Malaria pathogenesis is considered in the context of malaria with impaired consciousness and/or respiratory distress (Marsh et al., 1996), although the former (of which cerebral malaria is the most important severe neurologic complication) is important as seizures occur in >80% of the cases (Idro et al., 2005).
Methods
Study sites, participants, and samples
The study participants for this analysis were recruited from four of the sites contributing date to the MalariaGEN study as described in detail elsewhere (MalariaGEN, 2008). The four sites were Queen Elizabeth Central Hospital, Blantyre, Malawi; Kilifi District Hospital on the coast of Kenya; Teule Hospital in Moshi, Tanzania; and Komfo Anokye Teaching Hospital in Kumasi, Ghana.
The entomologic inoculation rate (EIR) for P. falciparum in the city of Blantyre is estimated to be around one infective bite/person/year, but a high proportion of families make regular visits to nearby rural areas where the EIR is estimated to be >100 (Table S2). In Kilifi, the overall annual EIR has been estimated at 1–100, and recent years have seen a significant decline in the rate of transmission from mesoendemic in the 1990s to hypoendemic transmission today. In Kumasi, Annual Biting Rates and Annual Entomological Inoculation Rates have been reported to be 11,643 and 866, respectively. Muheza has an intense transmission of P. falciparum (50–700 infected bites/person/year), with two seasonal peaks and the community prevalence of P. falciparum in children aged 2–5 years in the study area recorded as high as 88.2% in 2002. Additional detailed description of the sites is provided in Table S2.
Detailed data were available for each study participant, including a range of demographic parameters. We defined cases as children admitted to hospital with severe malaria with a history of seizures during the acute illness, and controls as children admitted with severe malaria without a history of seizures during their life. Severe malaria was diagnosed as the presence of peripheral parasitemia in the blood of a child presenting with coma or impaired consciousness, respiratory distress, acidosis (base excess less than −8), hypoglycemia (whole blood glucose < 2.2 mm), and/or severe normocytic anemia (<5 g/dl), with exclusion of other coincidental causes of severe illnesses such as pneumonia (Marsh et al., 1995; World Health Organization, 2000). The blood was put on a slide stained with 10% Giemsa. The distribution of cases and controls, in that order, was 770 and 620 in Blantyre, 900 and 1,148 in Kilifi, 250 and 250 in Kumasi, and 175 and 359 in Muheza. Children in this study were treated appropriately according to the local national guidelines and protocols.
Case definitions of phenotypes of MAS
Children with MAS (whose DNA samples were available for genotyping) admitted to hospital between 1996 and 2001 in Blantyre, 2002 and 2008 in Kilifi, 2006 and 2009 in Kumasi, and 2006 and 2007 in Muheza were selected. Details about various seizure semiologies and or malaria were extracted from the clinical notes and databases at each site, and the notes were further examined in order to categorize cases and controls.
We classified MAS into two major phenotypes namely: simple and complex, with complex further subdivided into focal MAS, repetitive MAS, and prolonged MAS (Table 1), based upon the International League Against Epilepsy definitions for acute/febrile seizures (ILAE, 1989, 1993). Focal MAS were defined as convulsions, twitches, or subtle seizures (cycling or suckling movements) starting in one part of the body or associated with lateralized asymmetric body weakness (Todd's paresis). Repetitive seizures were defined as two or more seizures occurring within 24 h of an illness or during the same illness. Seizures occurring in the ward were considered prolonged if they were lasting ≥15 min as documented by a clinician or a nurse, or phenytoin or phenobarbital was needed to stop uncontrolled seizures, or if seizures on admission did not stop after the first dose of diazepam (Sadarangani et al., 2008). Seizures were also considered prolonged if the histories from parents or caretakers, who rarely have watches, suggested that they lasted for ≥15 min, that is, seizures lasting the period of the journey to the hospital for those living more than a kilometer away, or seizures lasting more than the period of boiling a pot of maize, milking a dairy cow, or lasting more than the news broadcast on the radio. The “all focal,” “all repetitive” or “all prolonged” included any complex phenotype and were not mutually exclusive (Gwer et al., 2012). Simple seizures included those that were short (<15 min or if parental history as explained above suggested so), generalized and occurred once during the episode of malaria.
Table 1.
Case definition characterizing the phenotypes of malaria-associated seizures
| Category of seizures | Case definition characterizing the seizure phenotype |
|---|---|
| Repetitive seizures | Two or more seizures within 24 h during the same illness |
| Prolonged seizures | Seizures lasting ≥15 min and documented by a clinician or a nurse |
| Convulsing on admission and does not stop after first dose of diazepam | |
| Use of phenytoin or phenobarbital to stop uncontrolled seizures | |
| Parental history suggesting of seizures lasting ≥15 min, for example, a child convulsing all way to the hospital for those living more than a kilometer away, seizures lasting more than the period of boiling a pot of maize, milking a dairy cow, or lasting more than the news broadcast on the radio | |
| Focal seizures | Convulsions localized to or starting from one part of the body |
| Rolling of eye, cycling movements, or twitching of mouth localized to one body side | |
| Lateralized asymmetric body weakness postictally (Todd's paralysis) | |
| Motor deficits of the limbs of one side postseizure event |
MAS in cerebral malaria (defined as impaired consciousness [Blantyre Coma Score ≤ 2] in a child with peripheral malaria parasitemia in whom other causes of encephalopathy such as hypoglycemia and bacterial meningitis were excluded) are in this study referred to as “MAS with coma” because the occurrence of prolonged seizures may have contributed to the depressed conscious level in some children.
The genetic associations with MAS were investigated for six phenotypes, namely, all MAS, all focal MAS, all prolonged MAS, all repetitive MAS, all complex MAS, and all MAS with coma. In addition to being common in malaria, complex MAS are thought by some authors to form a more homogeneous group for genetic studies (Baulac et al., 2004).
Genetic data
Venous blood collected at admission from study subjects was separated by centrifugation, and the packed cells were stored at −80°C prior to subsequent extraction of DNA using commercially available kits according to the manufacturer's instructions. Genomic DNA samples then underwent whole genome amplification through either Primer Extension Pre-amplification (PEP; Zhang et al., 1992) or Multiple Displacement Amplification (MDA; Gonzalez et al., 2005), before genotyping on a Sequenom® Mass Array genotyping platform (Ross et al., 1998). Details of the malaria candidate polymorphisms genotyped, including the frequencies of major alleles (“wild type alleles” that are dominant and most frequent) and minor alleles (“mutant alleles” that are less dominant and rare), are shown in Table S1.
Statistical analysis
All statistical analysis was performed using STATA (version 11; Stata Corp, College Station, Texas, U.S.A.). Quality control procedures were applied to the genotype data across both individuals and polymorphisms. For example, polymorphisms with >10% genotypes missing duplicated records and individuals with excessive missing genotypes (>10%) were removed. We did not exclude polymorphisms on the basis of Hardy-Weinberg equilibrium deviation because both cases and controls were children admitted to hospital.
Logistic regression models were used to measure polymorphism's association with MAS or complex MAS (odds ratios). The model could not run the associations for pure focal seizures in all sites and pure prolonged seizures in Muheza because of lack of power due to small numbers. Models included the polymorphism of interest assuming several related genotypic mechanisms (additive, dominant, recessive, heterozygous advantage) and general models (mutant homozygous and heterozygous gene against wild homozygous gene). Based on an a priori assumption of potentially confounding effects of country ethnic groups in each site (accounts for between site differences), age, sex, and adverse perinatal events (defined as delay in breathing, crying and breastfeeding after birth) on the genetic susceptibility for seizures in malaria, we adjusted for these factors in the genotypic logistic regression models. We report the minimum p-value from the correlated genotypic tests or models. However, significant models with very large odds ratios (>100) were not reported as this could have introduced sparse-data bias due to infrequent genes in some categories.
Pooled estimates for genotypic tests were computed by performing a logistic regression on combined raw data across the four sites. We chose the raw data method after establishing that its findings were similar to the individual site estimates combined using the meta-analysis method that utilizes both fixed effects (inverse-variance weighting) and random effects models (where heterogeneity across the sites is evident; DerSimonian & Laird, 1986). Meta-analysis was used to compute Cochran's Q statistic for estimating genotypic tests' heterogeneity across the sites.
Because performing multiple statistical tests leads to inflation in the occurrence of false positives, we adjusted for multiple testing using a permutation approach that accounted for correlation between markers and tests (Gao et al., 2008). The permutation approach was preferred to Bonferroni correction because the polymorphisms in this analysis were dependent on each other. A derived p-value of 0.009 was estimated to be significant for all genotypic tests, and was based on both permutation adjustment (Gao et al., 2008) and authors' judgment that it was neither too liberal to include false-positive nor too conservative to exclude true-positive associations (Fig. S1).
All DNA samples were collected and genotyped after approval was provided by the relevant research ethics committees and written informed consent was provided by the participants.
Results
The distribution of phenotypes of MAS
Of the 2,095 cases of MAS across the sites, and the proportion of complex MAS pooled from across the sites was 70.7%. Blantyre and Kilifi had the highest proportion of complex MAS (543 [70.5%] and 652 [70.4%], respectively; Fig. 1).
Figure 1.
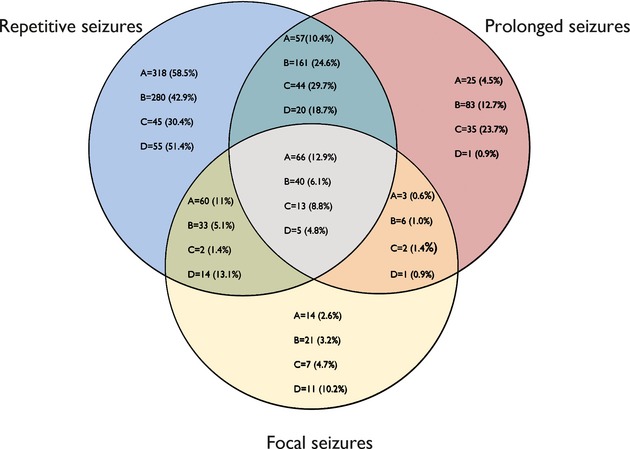
Proportion of complex seizures in each site. A: Blantyre, Malawi (N = 543); B: Kilifi, Kenya (N = 652); C: Kumasi, Ghana (N = 148); D: Muheza, Tanzania (N = 107). Percentages were computed using denominators as numbers for each site.
Focal MAS occurred in 306 children (14.6%) overall, and the proportion was highest in Blantyre (143 [18.6%]; Fig. 1). Prolonged MAS occurred in 579 children (27.6%) overall, and the proportion was highest (94 [37.6%]) in Kumasi. Repetitive seizures occurred in 1,239 (59.1%) children overall, and the proportion was highest in Blantyre (501 [65.1%]).
The proportion of complex MAS that was focal was highest in Muheza (31 [29.0%]) (Fig. 1). The proportion of complex MAS that was prolonged was highest in Kumasi 94 (63.5%). The proportion of complex MAS that was repetitive was highest in Blantyre 501 (92.3%). There was considerable overlap between the complex phenotypes and the distribution differed across the sites (Fig. 1).
The proportion of cases who met the WHO definition of cerebral malaria (also referred to as “MAS with coma” was 1,267/2,095 [60.5%] for all sites, 713/770 [92.6%] for Blantyre, 410/900 [45.6%] for Kilifi, 73/250 [29.2%] for Kumasi, and 71/175 [40.6%] for Muheza). The proportion of cases with a previous history of seizures was 320/2,095 (15.3%) for all sites, 190/770 (24.7%) for Blantyre, 100/900 (11.1%) for Kilifi, 29/250 (11.6%) for Kumasi, and 1/175 (0.6%) for Moshi.
Minor allele frequencies and genotypic tests
The minor allele frequencies for the polymorphisms considered in the final analysis are summarized in Table S1. Generally there was little heterogeneity of minor allele frequencies between cases and controls either within or between each site. Following filtering of the single nucleotide polymorphisms (SNPs) as described in Methods, analysis for association with MAS was undertaken using several genetic polymorphisms (Tables 2–7). Results for SNPs where the p-values from the analysis were <0.009 (to allow for multiple testing [see Methods]) were identified and are shown with the data from across all sites.
Table 2.
Polymorphisms associated with all malaria-associated seizures. (A) Site-specific and (B) pooled analysis across sites
| (A) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blantyre, Malawi | Kilifi, Kenya | Kumasi, Ghana | Muheza, Tanzania | |||||||||
| Polymorphism | Odds ratio | p-value | Model | Odds ratio | p-value | Model | Odds ratio | p-value | Model | Odds ratio | p-value | Model |
| IL10 rs1800896 | 0.37 (0.18–0.74) | 0.00531 | rec | 0.85 (0.62–1.16) | 0.29979 | rec | 0.53 (0.33–0.85) | 0.00739 | het | 1.22 (0.64–2.34) | 0.53950 | rec |
| EMR1 rs373533 | 4.96 (1.78–13.81) | 0.00219 | add | 1.18 (0.92–1.52) | 0.19266 | gen | 0.52 (0.33–0.81) | 0.00360 | het | 0.58 (0.35–0.99) | 0.04401 | rec |
| EMR1 rs461645 | 0.26 (0.10–0.68) | 0.00678 | add | 1.16 (0.94–1.42) | 0.15859 | het | 0.55 (0.36–0.86) | 0.00820 | het | 1.43 (0.87–2.36) | 0.16016 | dom |
| G6PD rs1050828 (male) | 0.85 (0.44–1.64) | 0.63689 | rec | 0.67 (0.46–0.99) | 0.043059 | rec | 0.26 (0.11–0.60) | 0.00155 | dom | 1.40 (0.33–8.51) | 0.51996 | gen |
| G6PD rs1050829 (male) | 1.66 (0.93–3.00) | 0.08877 | gen | 0.66 (0.16–2.74) | 0.56976 | add | 0.27 (0.11–0.62) | 0.00230 | dom | 0.58 (0.15–2.26) | 0.42957 | het |
| CD40LG rs3092945 (female) | 8.07 (1.65–37.25) | 0.00953 | het | 0.43 (0.26–0.69) | 0.00068 | rec | 1.48 (0.82–2.68) | 0.19521 | dom | 0.32 (0.10–1.21) | 0.094107 | rec |
| CR1 rs17047660 | 1.58 (0.87–2.91) | 0.13540 | het | 2.74 (1.44–5.20) | 0.00212 | rec | 0.83 (0.53–1.30) | 0.42305 | dom | 0.68 (0.43–1.06) | 0.09166 | het |
| TNF rs3093662 | 0.68 (0.42–1.09) | 0.10847 | het | 1.46 (1.14–1.88) | 0.00274 | het | 0.54 (0.16–1.87) | 0.3359 | het | 7.22 (0.78–67.13) | 0.08220 | gen |
| CR1 rs17047661 | 1.55 (0.75–3.22) | 0.23483 | add | 1.14 (0.80–1.64) | 0.46397 | gen | 0.49 (0.21–1.17) | 0.10869 | gen | 0.37 (0.19–0.72) | 0.00354 | gen |
| (B) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pooled analysis across the four sites | ||||||||||||
| Gene | Odds ratio (95% CI) | p-value | Model | |||||||||
| IL10 rs3024500 | 1.37 (1.09–1.71) | 0.00846 | gen | |||||||||
| IL17RE rs708567 | 0.78 (0.65–0.94) | 0.00709 | rec | |||||||||
Genotypic tests with a p-value ≤ 0.009 are reported, with comparisons from other sites (on adjustment for multiple testing, a p-value of ≤0.009 was considered to represent true genotypic tests). The logistic model was adjusted for age, sex, ethnicity, and history of adverse perinatal events. Model denotes the inheritance pattern of interest: add = additive, dom = dominant, het = heterozygous, and rec = recessive. Polymorphisms are represented as HUGO gene symbol and rs number.
Table 7.
Polymorphisms associated with malaria-associated seizures with coma. (A) Site specific and (B) pooled analysis across sites
| (A) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blantyre, Malawi | Kilifi, Kenya | Kumasi, Ghana | Muheza, Tanzania | |||||||||
| Polymorphism | Odds ratio | p-value | Model | Odds ratio | p-value | Model | Odds ratio | p-value | Model | Odds ratio | p-value | Model |
| IL10 rs1800896 | 0.35 (0.17–0.73) | 0.00475 | rec | 0.71 (0.45–1.13) | 0.14675 | rec | 0.39 (0.18–0.87) | 0.02062 | gen | 1.17 (0.65–2.13) | 0.60259 | dom |
| EMR1 rs373533 | 4.58 (1.62–19.6) | 0.00405 | add | 1.29 (0.97–1.72) | 0.07953 | het | 0.26 (0.13–0.53) | 0.00019 | het | 0.43 (0.18–1.03) | 0.05695 | add |
| EMR1rs461645 | 0.26 (0.10–0.73) | 0.01021 | gen | 1.35 (1.02–1.80) | 0.03569 | het | 2.95 (1.49–5.90) | 0.00198 | rec | 2.01 (0.87–4.65) | 0.10129 | add |
| CD40LG rs1126535 (female) | 0.41 (0.21–0.81) | 0.00988 | het | 1.43 (0.93–2.20) | 0.10370 | dom | 1.96 (0.34–11.5) | 0.45469 | het | 3.53 (1.42–8.79) | 0.00659 | het |
| CD40LG rs3092945 (female) | 8.27 (1.74–39.21) | 0.00781 | rec | 0.62 (0.35–1.09) | 0.10165 | rec | 0.65 (0.16–2.74) | 0.56353 | gen | 0.26 (0.10–2.13) | 0.21076 | rec |
| CR1rs17047660 | 1.81 (0.96–3.42) | 0.06569 | het | 3.47 (1.51–7.99) | 0.00341 | gen | 1.17 (0.59–2.37) | 0.64389 | het | 0.25 (0.11–0.60) | 0.00177 | add |
| (B) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pooled analysis across the four sites | ||||||||||||
| Gene | Odds ratio (95% CI) | p-value | Model | |||||||||
| IL13 rs20541 | 0.72 (0.52–0.91) | 0.00601 | dom | |||||||||
| IL10 rs3024500 | 1.55 (1.21–2.00) | 0.00064 | dom | |||||||||
Genotypic tests with a p-value ≤ 0.009 are reported, with comparisons from other sites (on adjustment for multiple testing, a p-value of ≤0.009 was considered to represent true genotypic tests). The logistic model was adjusted for age, sex, ethnicity, and adverse perinatal events. Model denotes the inheritance pattern of interest: add = additive, dom = dominant, het = heterozygous, and rec = recessive. The cells with missing data (–) represents assays that had missing data or poor fidelity. Polymorphisms are represented as HUGO gene symbol and rs number.
Polymorphisms associated with all MAS
All-MAS encompasses all MAS phenotypes and two genes were significantly associated with all-MAS in at least two sites, IL10-rs1800896 in Blantyre (odds ratio [OR] = 0.37 [95% confidence interval (CI), 0.18–0.74], p = 0.00531, recessive model) and Kumasi (OR = 0.53 [95% CI, 0.33–0.84], p = 0.00739, heterozygous model) and epidermal growth factor module-containing mucin-like hormone receptor (EMR)1-rs373533 in Blantyre (4.96 [95% CI, 1.78–13.81], p = 0.00219, additive model) and Kumasi (OR = 0.52 [95% CI, 0.33–0.81], p = 0.00360, heterozygous model). A second SNP (rs461645) in EMR1 was also significant in Blantyre and Kumasi due to high linkage disequilibrium with EMR1-rs373533 in all the ethnic groups (r2 > 0.9, data not shown). Others shown in Table 2 were significantly associated with MAS in one site only.
Most polymorphisms were not significant in analysis pooled across-site analysis except for IL10-rs3024500 (OR = 1.36 [95% CI, 1.08–1.71], p = 0.00651, general model) and IL-17 receptor E-rs708567 (OR = 0.78 [95% CI, 0.65–0.94], p = 0.00709, recessive model). We also looked for associations in the sub-phenotypes of MAS to reduce heterogeneity of the phenotype, reported below, although there would be some loss of power in the sample sizes. Meta-analysis of association estimates of IL10-rs1800896 in the all MAS phenotype showed that there was considerable heterogeneity across the sites (68.7% [95% CI 34.8–93.3%]).
Polymorphisms associated with all-complex MAS
This phenotype comprises the prolonged, focal, and repetitive phenotypes that together define the majority of the malaria-associated seizures. We analyzed them together, since they have some degree of overlap (Fig. 1). Several genes showed a significant association in at least one site (Table 3), two of which were significant in all-MAS above, namely IL10-rs1800896 (OR = 0.43 [95% CI, 0.24–0.75], p = 0.00309, heterozygous model) in Kumasi and EMR1-rs373533 in Blantyre (OR = 4.43 [95% CI, 1.47–13.34], p = 0.00813, heterozygous model) and Kumasi (OR = 0.38 [95% CI, 0.23–0.65], p = 0.00033, heterozygous model). None of these SNPs were associated with complex MAS in a pooled analysis.
Table 3.
Polymorphisms associations with combined complex malaria-associated seizures. (A) Site specific and (B) pooled analysis across sites
| (A) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blantyre, Malawi | Kilifi, Kenya | Kumasi, Ghana | Muheza, Tanzania | |||||||||
| Gene | Odds ratio | p-value | Model | Odds ratio | p-value | Model | Odds ratio | p-value | Model | Odds ratio | p-value | Model |
| IL10 rs1800896 | 0.43 (0.20–0.92) | 0.02894 | rec | 0.77 (0.53–1.12) | 0.17754 | gen | 0.43 (0.24–0.75) | 0.00309 | het | 1.29 (0.55–3.01) | 0.56062 | gen |
| IL4 rs2243250 | 0.55 (0.18–0.73) | 0.00960 | rec | 1.10 (0.86–1.40) | 0.43910 | het | 0.86 (0.50–1.49) | 0.61018 | het | 0.87 (0.51–1.49) | 0.61917 | het |
| EMR1 rs373533 | 4.43 (1.47–13.34) | 0.00813 | het | 1.23 (0.97–1.55) | 0.08112 | het | 0.38 (0.23–0.65) | 0.00033 | het | 1.40 (0.84–2.33) | 0.193088 | het |
| EMR1 rs461645 | 0.27 (0.10–0.80) | 0.01831 | gen | 1.27 (1.02–1.61) | 0.03680 | het | 0.41 (0.25–0.68) | 0.00055 | het | 1.36 (0.82–2.24) | 0.22393 | het |
| CR1 rs17047660 | 0.24 (0.03–2.09) | 0.19451 | gen | 2.94 (1.49–5.80) | 0.00183 | rec | 0.83 (0.49–1.42) | 0.49958 | het | 1.85 (0.59–5.78) | 0.28809 | rec |
| CTL4 rs2242665 | 1.29 (0.65–2.56) | 0.46678 | rec | 1.38 (0.87–2.19) | 0.16543 | gen | 0.61 (0.25–1.53) | 0.29522 | het | 1.98 (1.19–3.30) | 0.00833 | het |
| ICAM1 rs5498 | 1.90 (1.17–3.08) | 0.00902 | het | 0.69 (0.51–0.93) | 0.015872 | gen | 0.73 (0.38–1.41) | 0.35340 | het | 1.16 (0.66–2.04) | 0.60455 | dom |
| IL17RE rs708567 | 0.70 (0.41–1.21) | 0.205212 | rec | 0.65 (0.47–0.90) | 0.00889 | add | 0.69 (0.36–1.36) | 0.29232 | rec | 1.69 (0.78–3.68) | 0.18357 | gen |
| (B) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pooled analysis across the four sites | ||||||||||||
| Gene | Odds ratio (95% CI) | p-value | Model | |||||||||
| – | – | – | – | |||||||||
Genotypic tests with a p-value ≤ 0.009 are reported, with comparisons from other sites (on adjustment for multiple testing, a p-value of ≤0.009 was considered to represent true genotypic tests). The logistic model was adjusted for age, sex, ethnicity, and history of adverse perinatal events. Model denotes the inheritance pattern of interest: add = additive, dom = dominant, het = heterozygous, and rec = recessive. The cells with missing data (–) represents genotypic tests, which did not reach significant levels in the pooled analysis. Polymorphisms are represented as HUGO gene symbol and rs number.
Polymorphisms associated with focal-MAS, prolonged-MAS, and repetitive-MAS
Several polymorphisms, none of which appeared in MAS and complex MAS, were significantly associated with focal MAS, particularly IL-20 receptor A-rs1555498 (OR = 3.86 [95% CI, 1.67–8.97], p = 0.00158, recessive model) in Moshi and CD36-rs3211938 (OR = 10.16 [95% CI, 1.79–57.83], p = 0.00894, recessive model) for Kumasi. However, there was no significant association with focal MAS in a pooled analysis across the sites.
Three polymorphisms that appeared significant in MAS and/or complex MAS were associated with prolonged MAS (Tables 3 and 4), EMR1-rs461645 (OR = 0.41 [95% CI, 0.23–0.74], p = 0.00336, heterozygous model) in Kumasi and CR1-rs1704660 (OR = 3.92 [95% CI, 1.71–8.99], p = 0.00121, general model) and IL17RE-rs708567 (OR = 0.55 [95% CI, 0.35–0.86], p = 0.00917, additive model) in Kilifi. Only CRI-rs1704660 from these two polymorphisms was significant in a pooled analysis across the sites (Table 5).
Table 4.
Polymorphisms associations with focal malaria-associated seizures. (A) Site-specific and (B) pooled analysis across sites
| (A) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blantyre, Malawi | Kilifi, Kenya | Kumasi, Ghana | Muheza, Tanzania | |||||||||
| Polymorphism | Odds ratio | p-value | Model | Odds ratio | p-value | Model | Odds ratio | p-value | Model | Odds ratio | p-value | Model |
| IL20RA rs1555498 | 0.50 (0.15–1.70) | 0.27119 | add | 0.93 (0.48–1.82) | 0.83213 | gen | 0.26 (0.03–2.17) | 0.211485 | add | 3.86 (1.67–8.93) | 0.00168 | rec |
| CD36 rs3211938 | 0.65 (0.17–2.44) | 0.524159 | dom | 1.47 (0.81–2.70) | 0.20875 | het | 10.16 (1.79–57.83) | 0.00894 | het | 1.53 (0.56–4.22) | 0.402252 | het |
| (B) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pooled analysis across the four sites | ||||||||||||
| Gene | Odds ratio (95% CI) | p-value | Model | |||||||||
| – | – | – | – | |||||||||
Genotypic tests with a p-value ≤ 0.009 are reported, with comparisons from other sites (on adjustment for multiple testing, a p-value of ≤0.009 was considered to represent true genotypic tests). The logistic model was adjusted for age, sex, ethnicity, and history of adverse perinatal events. Model denotes the inheritance pattern of interest: add = additive, dom = dominant, het = heterozygous, and rec = recessive. The cells with missing data (–) represents genotypic tests, which did not reach significant levels in the pooled analysis. Polymorphisms are represented as HUGO gene symbol and rs number.
Table 5.
Polymorphisms associations with prolonged malaria-associated seizures. (A) Site-specific and (B) pooled analysis across sites
| (A) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blantyre, Malawi | Kilifi, Kenya | Kumasi, Ghana | Muheza, Tanzania | |||||||||
| Polymorphism | Odds ratio | p-value | Model | Odds ratio | p-value | Model | Odds ratio | p-value | Model | Odds ratio | p-value | Model |
| EMR1373533 | 2.32 (0.57–9.42) | 0.23843 | het | 1.44 (1.05–1.99) | 0.02510 | het | 0.41 (0.23–0.74) | 0.00033 | het | 0.19 (0.04–0.95) | 0.043112 | add |
| EMR1 rs461645 | 2.61 (0.66–10.33) | 0.17128 | het | 1.50 (1.09–2.07) | 0.01254 | het | 0.40 (0.28–0.7) | 0.00147 | het | 2.88 (0.54–18.36) | 0.20131 | add |
| CR1 rs17047660 | 0.39 (0.10–1.62) | 0.19522 | gen | 3.92 (1.71–8.99) | 0.00121 | gen | 1.51 (0.52–4.42) | 0.44375 | rec | 3.15 (0.54–18.36) | 0.20131 | gen |
| IL17RE rs708567 | 1.24 (0.49–3.12) | 0.64466 | gen | 0.55 (0.35–0.86) | 0.00917 | add | 1.30 (0.74–2.32) | 0.35336 | het | 2.66 (0.59–12.01) | 0.201649 | dom |
| (B) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pooled analysis across the four sites | ||||||||||||
| Gene | Odds ratio (95% CI) | p-value | Model | |||||||||
| CR1 rs17047660 | 2.56 (1.41–4.67) | 0.00194 | rec | |||||||||
| G6PD rs1050828 | 0.45 (0.27–0.74) | 0.00155 | dom | |||||||||
Genotypic tests with a p-value ≤ 0.009 are reported, with comparisons from other sites (on adjustment for multiple testing, a p-value of ≤0.009 was considered to represent true genotypic tests). The logistic model was adjusted for age, sex, ethnicity, and history of adverse perinatal events. Model denotes the inheritance pattern of interest: add = additive, dom = dominant, het = heterozygous, and rec = recessive. Polymorphisms are represented as HUGO gene symbol and rs number.
Two polymorphisms identified as significant in MAS, complex MAS, or prolonged MAS (Tables 3 and 4), were associated with repetitive MAS, EMRI-rs373533 in Blantyre (OR = 5.73 [95% CI, 1.67–19.65], p = 0.00545, general model) and Kumasi (OR = 0.27 [95% CI, 0.14–0.50], p = 0.00003, heterozygous model) and CR1-rs17047660 (OR = 3.04 [95% CI, 1.51–6.12], p = 0.00180, recessive model) in Kilifi. Of these two polymorphisms only CR1-rs17047660 (OR = 1.97 [95% CI, 1.22–3.22], p = 0.00547, recessive model) was associated with repetitive MAS in the pooled analysis, and others were as well (Table 6).
Table 6.
Polymorphisms associated with repetitive malaria-associated seizures. (A) Site specific and (B) pooled analysis across sites
| (A) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blantyre, Malawi | Kilifi, Kenya | Kumasi, Ghana | Muheza, Tanzania | |||||||||
| Polymorphism | Odds ratio | p-value | Model | Odds ratio | p-value | Model | Odds ratio | p-value | Model | Odds ratio | p-value | Model |
| CR1 rs17047660 | 0.27 (0.03–3.49) | 0.25132 | gen | 3.04 (1.51–6.12) | 0.00180 | rec | 2.17 (0.70–6.75) | 0.17981 | gen | 2.24 (0.68–7.33) | 0.18336 | gen |
| IL4 rs2243250 | 0.29 (0.11–0.73) | 0.00884 | gen | 1.12 (0.87–1.40) | 0.37876 | het | 0.66 (0.18–2.43) | 0.53585 | gen | 0.71 (0.40–1.26) | 0.24413 | het |
| EMR1 rs373533 | 5.74 (1.67–19.6) | 0.00545 | gen | 1.27 (0.94–1.72) | 0.11501 | gen | 0.27 (0.14–0.50) | 0.00003 | het | 0.74 (0.39–1.40) | 0.35779 | rec |
| EMR1 rs461645 | 0.23 (0.07–0.73) | 0.01243 | gen | 1.21 (0.95–1.55) | 0.121345 | het | 0.38 (0.21–0.69) | 0.00127 | het | 1.24 (0.65–2.37) | 0.52182 | add |
| ICAM1 rs5498 | 1.97 (1.21–3.21) | 0.00676 | het | 0.70 (0.51–0.97) | 0.03135 | gen | 0.60 (0.27–1.34) | 0.20767 | het | 1.11 (0.61–2.01) | 0.73366 | add |
| CD40LG rs3092945 (female) | 10.90 (1.92–62.05) | 0.00706 | gen | 0.49 (0.28–0.86) | 0.012395 | rec | 1.06 (0.38–2.95) | 0.90875 | het | 0.61 (0.16–2.42) | 0.48705 | add |
| IL17RE rs708567 | 0.65 (0.37–1.15) | 0.13993 | rec | 0.64 (0.46–0.90) | 0.00951 | rec | 1.10 (0.62–1.97) | 0.72768 | het | 1.63 (0.73–3.69) | 0.23548 | gen |
| ABO rs8176746 | 0.74 (0.46–1.19) | 0.21816 | dom | 2.27 (1.24–4.17) | 0.00803 | rec | 0.69 (0.38–1.24) | 0.21205 | het | 0.25 (0.03–2.03) | 0.19634 | rec |
| IL10 rs1800896 | 1.62 (1.03–2.55) | 0.03673 | het | 0.81 (0.55–1.20) | 0.30182 | gen | 0.42 (0.22–0.81) | 0.00934 | het | 1.54 (0.65–3.65) | 0.32719 | gen |
| (B) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pooled analysis across the four sites | ||||||||||||
| Gene | Odds ratio (95% CI) | p-value | Model | |||||||||
| CR1 rs17047660 | 1.97 (1.22–3.22) | 0.00547 | rec | |||||||||
| CFTR rs171140229 | 0.60 (0.41–0.86) | 0.00543 | het | |||||||||
| G6PD rs1050829 (males) | 0.29 (0.11–0.72) | 0.00817 | gen | |||||||||
Genotypic tests with a p-value ≤ 0.009 are reported, with comparisons from other sites (on adjustment for multiple testing, a p-value of ≤0.009 was considered to represent true genotypic tests). The logistic model was adjusted for age, sex, ethnicity, and history of adverse perinatal events. Model denotes the inheritance pattern of interest: add = additive, dom = dominant, het = heterozygous, and rec = recessive. Polymorphisms are represented as HUGO gene symbol and rs number.
Polymorphisms association with MAS with coma/cerebral malaria
Two polymorphisms associated with most of the above phenotypes (Tables 2–6) were also significant for MAS with coma, EMRI-rs373533 in Blantyre (OR = 4.58 [95% CI, 1.62–12.93], p = 0.00405, additive model) and Kumasi (OR = 0.26 [95% CI, 0.13–0.53], p = 0.00019, dominant model) and CR1-rs17047660 in Kilifi (OR = 3.47 [95% CI, 1.51–7.99], p = 0.00341, general model), Kilifi and Moshi (OR = 0.25 [95% CI, 0.11–0.60], p = 0.00177, additive model). CD40-rs1126935 too was associated with MAS with coma in more than one site (Table 7).
Discussion
This is the first study to investigate the effect of candidate genes for malaria infection on the risk of acute seizures across multiple sites in Africa. Using established definitions for seizure phenotypes (Table 1), our data show that a significant proportion (>70%) of children admitted to hospitals in sub-Saharan Africa with severe malaria exhibit seizures with complex features (prolonged, focal, or repetitive), but the proportions of the different phenotypes vary between sites. A number of different polymorphisms (including the X-chromosome) were associated with the risk of the main phenotypes of MAS at each site, and in a pooled analysis. Polymorphisms of four genes were identified as significant in most of the subgroups and sites (CR1-rs1704660, IL10-rs1800896, and EMR1-rs373533/rs461645 and CD40-rs3092945 [females]), although only CR1 showed an overall significant association across the sites in the prolonged and repetitive MAS phenotypes. Other polymorphisms did show positive association but mostly for one phenotype and site (Table 3). Of the positive association polymorphisms several are thought to code for molecules that could have a putative role in the pathogenesis of seizures in falciparum malaria.
The classification of seizures is largely based on phenotypes found in febrile seizures (Baram & Shinnar, 2002), but some cases may have been misclassified, since generalized seizures may have had a focal origin with rapid secondary generalization (Gwer et al., 2012). We were not able to determine relatedness of the study participants, which may affect the associations, but adjusting for ethnicity did in part account for this. Electroencephalography and neuroimaging were not routinely performed at the time of the study due to logistic reasons. The strength of our study lies in the large sample size from combining studies across four countries, which allowed us to use a control group that comprised the severe malaria cases without seizures, thus ensuring that any associations identified represent the risk of developing acute seizures in malaria. The genotypic tests p-values are reported as they were generated from the analyzing software, so are easier to interpret.
Polymorphisms demonstrated heterogeneities in associations with MAS across the sites. These heterogeneities may be ascribed to sample size differences and the resulting power (Muheza had the smallest samples), possible differences in documenting the three phenotypes of complex MAS, haplotypic (polymorphisms on one or several loci that may be associated) differences between populations, and/or the effects of population structure. We tried to minimize the latter by adjusting for ethnicity within sites. Some of the heterogeneous associations in the sites may be due to linkage disequilibrium. Because we adjusted for multiple testing, such site-specific associations could be due to strong linkage disequilibrium with actual causal polymorphisms not investigated in this study. The finding that a gene was protective in one site and increased risk in another can be explained by the different inheritance models in the affected sites, or could suggest that the associations are related to a gene region rather than the effect of the particular polymorphism.
In these associations, we studied polymorphisms that were genotyped as part of MalariaGEN consortium project (MalariaGEN, 2008), and could not investigate some polymorphisms associated with febrile seizures such as the six susceptibility febrile seizure loci (FEB)1–6, voltage-gated sodium channel genes (SCN1A, SCN1B, or SCN2A), γ-amino-butyric acids genes (GABA(A) or GABRG2) (Nakayama & Arinami, 2006). We did, however, include polymorphisms of the interleukin family (IL-1A, 1L-1B, 1L-4, 1L-20RA), some of which have been previously investigated in febrile seizures studies (Tsai, 1987; Virta et al., 2002; Kira et al., 2005; Ishizaki et al., 2009).
Some polymorphisms had stronger associations with acute seizures with complex phenotypes, suggesting that these three phenotypes may provide insights into the epileptogenesis of malaria. The finding that specific polymorphisms were replicated across the different complex phenotypes supports the role of these genes in the epileptogenesis of malaria. The complex phenotypes are particularly associated with malaria (Kariuki et al., 2011) and are also important risk factors for development of epilepsy following severe malaria (Birbeck et al., 2010). Those polymorphisms that are found in more than one site are likely to be linked to genes that may play a significant role in the pathogenesis of acute seizures in these children.
The biologic functions of some polymorphisms have not been fully investigated, and can only be speculated. EMR1 is sometimes elevated in placental malaria (Muehlenbachs et al., 2007; Sevastianova et al., 2008), which is characterized by sequestration of parasites, similar to that found in the brain. EMR1 is a membrane receptor that also activates G protein receptor subunit alpha (GNAS) (Auburn et al., 2008), whose subclass C receptors bind glutamate and GABA, the two important neurotransmitters of epileptogenesis. GNAS did not reach the 0.009 significance level in this study but reached the 0.05 level. In addition, EMR1 is the human homologue of F4/80 in mice that defines macrophages, whose elevation in malaria infection precedes leucocyte sequestration into the brain (Wozencraft et al., 1984).
Many of the polymorphisms genotyped in this study have previous published associations with severe malaria, and we have analyzed them in the context of association with seizures. CR1 is postulated to be involved in the adhesion of P. falciparum infected erythrocytes to uninfected erythrocytes (rosetting; Nagayasu et al., 2001; Idro et al., 2005) and is therefore thought to play a role in the pathogenesis of seizures in severe malaria. Polymorphisms of the ABO blood system are associated with rosetting of erythrocytes among people of blood types A and B (Cserti & Dzik, 2007), resulting in impaired tissue perfusion that could increase the risk for seizures. Similarly, the association between ICAM1 polymorphisms seizures with severe malaria (Kun et al., 1999) could be explained by the binding of ICAM1 to P. falciparum–infected erythrocytes onto brain microvasculature (Berendt et al., 1992; Kun et al., 1999) causing sequestration. CD36 facilitates this sequestration (Cserti & Dzik, 2007). Interleukins are usually increased in falciparum malaria infections, and may directly precipitate seizures because they are inflammatory and pyrogenic in nature (Haysa et al., 1999; Chaisavaneeyakorn et al., 2003; Wilson et al., 2005). G6PD enhances parasite clearance by phagocytosis, a process that can culminate in release of inflammatory molecules often associated with seizures (Sabeti et al., 2002; Wilson et al., 2005). Activation of microglial CD40 results in inflammation-induced seizures (Sun et al., 2008) and underpins the association between this polymorphism and MAS in coma/cerebral malaria.
Our findings have demonstrated that children admitted with falciparum malaria may have a genetic predisposition to acute seizures, but the polymorphisms associated with seizures in malaria vary with the phenotype and sites across Africa. These polymorphisms were particularly associated with the three complex phenotypes of MAS, which could also be important in epileptogenesis of severe malaria. Studies from other malaria-endemic settings are required to confirm these findings, and genome-wide association studies are required to study if other seizures-specific genes determine the risk of acute seizures.
Acknowledgments
We would like to thank the all the study participants and their families for their help with this study. Carolyne Ndila, George Mtove, and Tony Enimil were invaluable in assisting with the clinical database and/or the clinical notes. This study was supported by Wellcome Trust through a Senior Clinical Fellowship awarded to CRJCN (083744) and a Research Training Fellowship (099782/Z/12/Z) awarded to SMK. TNW, HR, and TET are MalariaGEN principal investigators and SMK is a MalariaGEN data fellow. TNW is funded by the Wellcome Trust and the European Union EVIMalR Network 7 Programme.
The MalariaGEN Project is supported by the Wellcome Trust (WT077383/Z/05/Z) and the Bill and Melinda Gates Foundation through the Foundations of the National Institutes of Health (566) as part of the Grand Challenges in Global Health Initiative. This research was supported by the Medical Research Council (G0600230). The Wellcome Trust also provides core awards to Wellcome Trust Centre for Human Genetics (075491/Z/04; 090532/Z/09/Z) and the Wellcome Trust Sanger Institute (077012/Z/05/Z).
This work was presented as a poster in the Genomics of Malaria Conference, Cambridge, United Kingdom in June 2010 and in the 29th International Epilepsy Congress, Rome, Italy in August, 2011.
Disclosure
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
The cut-off for significant genotypic tests based on both permutation tests and authors' judgment as both not too conservative and too liberal.
Table S1. Minor allele frequencies (MAF) and Hardy-Weinberg equilibrium (HWE) test in the controls for polymorphisms included in the final analysis.
Table S2. Details of malaria transmission in the study areas across the four sites in Africa.
References
- Auburn S, Diakite M, Fry AE, Ghansah A, Campino S, Richardson A, Jallow M, Sisay-Joof F, Pinder M, Griffiths MJ, Peshu N, Williams TN, Marsh K, Molyneux ME, Taylor TE, Koram KA, Oduro AR, Rogers WO, Rockett KA, Haldar K, Kwiatkowski DP. Association of the GNAS locus with severe malaria. Hum Genet. 2008;124:499–506. doi: 10.1007/s00439-008-0575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Shinnar S. Febrile seizures. San Diego, CA: Academic Press; 2002. [Google Scholar]
- Baulac S, Gourfinkel-An I, Nabbout R, Huberfeld G, Serratosa J, Leguern E, Baulac M. Fever, genes, and epilepsy. Lancet Neurol. 2004;3:421–430. doi: 10.1016/S1474-4422(04)00808-7. [DOI] [PubMed] [Google Scholar]
- Berendt AR, McDowall A, Craig AG, Bates PA, Sternberg MJ, Marsh K, Newbold CI, Hogg N. The binding site on ICAM-1 for Plasmodium falciparum-infected erythrocytes overlaps, but is distinct from, the LFA-1-binding site. Cell. 1992;68:71–81. doi: 10.1016/0092-8674(92)90207-s. [DOI] [PubMed] [Google Scholar]
- Birbeck GL, Molyneux ME, Kaplan PW, Seydel KB, Chimalizeni YF, Kawaza K, Taylor TE. Blantyre Malaria Project Epilepsy Study (BMPES) of neurological outcomes in retinopathy-positive paediatric cerebral malaria survivors: a prospective cohort study. Lancet Neurol. 2010;9:1173–1181. doi: 10.1016/S1474-4422(10)70270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JA, Ross AJ, Neville BG, Obiero E, Katana K, Mung'ala-Odera V, Lees JA, Newton CR. Developmental impairments following severe falciparum malaria in children. Trop Med Int Health. 2005;10:3–10. doi: 10.1111/j.1365-3156.2004.01345.x. [DOI] [PubMed] [Google Scholar]
- Chaisavaneeyakorn S, Othoro C, Shi YP, Otieno J, Chaiyaroj SC, Lal AA, Udhayakumar V. Relationship between plasma Interleukin-12 (IL-12) and IL-18 levels and severe malarial anemia in an area of holoendemicity in western Kenya. Clin Diagn Lab Immunol. 2003;10:362–366. doi: 10.1128/CDLI.10.3.362-366.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark TG, Fry AE, Auburn S, Campino S, Diakite M, Green A, Richardson A, Teo YY, Small K, Wilson J, Jallow M, Sisay-Joof F, Pinder M, Sabeti P, Kwiatkowski DP, Rockett K. Allelic heterogeneity of G6PD deficiency in West Africa and severe malaria susceptibility. Eur J Hum Genet. 2009;17:1080–1085. doi: 10.1038/ejhg.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserti CM, Dzik WH. The ABO blood group system and Plasmodium falciparum malaria. Blood. 2007;110:2250–2258. doi: 10.1182/blood-2007-03-077602. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Gao X, Starmer J, Martin ER. A multiple testing correction method for genetic association studies using correlated single nucleotide polymorphisms. Genet Epidemiol. 2008;32:361–369. doi: 10.1002/gepi.20310. [DOI] [PubMed] [Google Scholar]
- Gonzalez JM, Portillo MC, Saiz-Jimenez C. Multiple displacement amplification as a pre-polymerase chain reaction (pre-PCR) to process difficult to amplify samples and low copy number sequences from natural environments. Environ Microbiol. 2005;7:1024–1028. doi: 10.1111/j.1462-2920.2005.00779.x. [DOI] [PubMed] [Google Scholar]
- Gwer S, Idro R, Fegan G, Chengo E, Garrashi H, White S, Kirkham FJ, Newton CR. Continuous EEG monitoring in Kenyan children with non-traumatic coma. Arch Dis Child. 2012;97:343–349. doi: 10.1136/archdischild-2011-300935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haysa TC, Szymusiakb RS, McGintyc D. GABA-A receptor modulation of temperature sensitive neurons in the diagonal band of Broca in vitro. Brain Res. 1999;845:215–223. doi: 10.1016/s0006-8993(99)01947-2. [DOI] [PubMed] [Google Scholar]
- Idro R, Jenkins NE, Newton CR. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol. 2005;4:827–840. doi: 10.1016/S1474-4422(05)70247-7. [DOI] [PubMed] [Google Scholar]
- Idro R, Gwer S, Kahindi M, Gatakaa H, Kazungu T, Ndiritu M, Maitland K, Neville BG, Kager PA, Newton CR. The incidence, aetiology and outcome of acute seizures in children admitted to a rural Kenyan district hospital. BMC Pediatr. 2008;8:5. doi: 10.1186/1471-2431-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ILAE. Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- ILAE. Guidelines for epidemiologic studies on epilepsy. Commission on Epidemiology and Prognosis, International League Against Epilepsy. Epilepsia. 1993;34:592–596. doi: 10.1111/j.1528-1157.1993.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Ishizaki Y, Kira R, Fukuda M, Torisu H, Sakai Y, Sanefuji M, Yukaya N, Hara T. Interleukin-10 is associated with resistance to febrile seizures: Genetic association and experimental animal studies. Epilepsia. 2009;50:761–767. doi: 10.1111/j.1528-1167.2008.01861.x. [DOI] [PubMed] [Google Scholar]
- Kariuki SM, Ikumi M, Ojal J, Sadarangani M, Idro R, Olotu A, Bejon P, Berkley JA, Marsh K, Newton CR. Acute seizures attributable to falciparum malaria in an endemic area on the Kenyan coast. Brain. 2011;134:1519–1528. doi: 10.1093/brain/awr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kira R, Torisu H, Takemoto M, Nomura A, Sakai Y, Sanefuji M, Sakamoto K, Matsumoto S, Gondo K, Hara T. Genetic susceptibility to simple febrile seizures: interleukin-1beta promoter polymorphisms are associated with sporadic cases. Neurosci Lett. 2005;384:239–244. doi: 10.1016/j.neulet.2005.04.097. [DOI] [PubMed] [Google Scholar]
- Kjeldsen MJ, Kyvic KO, Friis ML, Christensen K. Genetic and environmetal factors in febrile seizures: a Danish population-based twin study. Epilepsy Res. 2002;51:167–177. doi: 10.1016/s0920-1211(02)00121-3. [DOI] [PubMed] [Google Scholar]
- Kugler SL, Johnson WG. Genetics of the febrile seizure susceptibility trait. Brain Dev. 1998;20:265–274. doi: 10.1016/s0387-7604(98)00020-5. [DOI] [PubMed] [Google Scholar]
- Kun JFJ, Klabunde J, Lell B, Luckner D, Alpers M, May J, Meyer C, Kremesner PG. Association of the ICAM-1Kilifi mutations with protection against severe malaria in Lambare′ne′, Gabon. Am J Trop Med Hyg. 1999;61:776–779. doi: 10.4269/ajtmh.1999.61.776. [DOI] [PubMed] [Google Scholar]
- MalariaGEN. A global network for investigating the genomic epidemiology of malaria. Nature. 2008;456:732–737. doi: 10.1038/nature07632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, Newton C, Winstanley P, Warn P, Peshu N, Pasvol G, Snow R. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- Marsh K, English M, Crawley J, Peshu N. The pathogenesis of severe malaria in African children. Ann Trop Med Parasitol. 1996;90:395–402. doi: 10.1080/00034983.1996.11813068. [DOI] [PubMed] [Google Scholar]
- Muehlenbachs A, Fried M, Lachowitzer J, Mutabingwa TK, Duffy PE. Genome-wide expression analysis of placental malaria reveals features of lymphoid neogenesis during chronic infection. J Immunol. 2007;179:557–565. doi: 10.4049/jimmunol.179.1.557. [DOI] [PubMed] [Google Scholar]
- Nagayasu E, Ito M, Akaki M, Nakano Y, Kimura M, Looareesuwan S, Aikawa M. CR1 density polymorphism on erythrocytes of falciparum malaria patients in Thailand. Am J Trop Med Hyg. 2001;64:1–5. doi: 10.4269/ajtmh.2001.64.1.11425154. [DOI] [PubMed] [Google Scholar]
- Nakayama J, Arinami T. Molecular genetics of seizures. Epilepsy Res. 2006;70:S190–S198. doi: 10.1016/j.eplepsyres.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Rich SS, Annegers JF, Hauser WA, Anderson VE. Complex segregation analysis of febrile convulsions. Am J Hum Genet. 1987;41:249–257. [PMC free article] [PubMed] [Google Scholar]
- Ross P, Hall L, Smirnov I, Haff L. High level multiplex genotyping by MALDI-TOF mass spectrometry. Nat Biotechnol. 1998;16:1347–1351. doi: 10.1038/4328. [DOI] [PubMed] [Google Scholar]
- Sabeti P, Usen S, Farhadian S, Jallow M, Doherty T, Newport M, Pinder M, Ward R, Kwiatkowski D. CD40L association with protection from severe malaria. Genes Immun. 2002;3:286–291. doi: 10.1038/sj.gene.6363877. [DOI] [PubMed] [Google Scholar]
- Sadarangani M, Seaton C, Scott JA, Ogutu B, Edwards T, Prins A, Gatakaa H, Idro R, Berkley JA, Peshu N, Neville BG, Newton CR. Incidence and outcome of convulsive status epilepticus in Kenyan children: a cohort study. Lancet Neurol. 2008;7:145–150. doi: 10.1016/S1474-4422(07)70331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevastianova K, Sutinen J, Kannisto K, Hamsten A, Ristola M, Yki-Järvinen H. Adipose tissue inflammation and liver fat in patients with highly active antiretroviral therapy-associated lipodystrophy. Am J Physiol Endocrinol Metab. 2008;295:E85–E91. doi: 10.1152/ajpendo.90224.2008. [DOI] [PubMed] [Google Scholar]
- Sun H, Huang Y, Yu X, Li Y, Yang J, Li R, Deng Y, Zhao G. Peroxisome proliferator-activated receptor gamma agonist, rosiglitazone, suppresses CD40 expression and attenuates inflammatory responses after lithium pilocarpine-induced status epilepticus in rats. Int J Dev Neurosci. 2008;26:505–515. doi: 10.1016/j.ijdevneu.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Taylor T, Olola C, Valim C, Agbenyega T, Kremsner P, Krishna S, Kwiatkowski D, Newton CR, Missinou M, Pinder M, Wypij D Network. obotS. Standardized data collection for multi-centre clinical studies of severe malaria in African children: establishing the SMAC network. Trans R Soc Trop Med Hyg. 2006;100:615–622. doi: 10.1016/j.trstmh.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai T. Genetic analysis of febrile convulsions: twin and family studies. Hum Genet. 1987;75:7–14. doi: 10.1007/BF00273830. [DOI] [PubMed] [Google Scholar]
- Versteeg AC, Carter JA, Dzombo J, Neville BG, Newton CRJC. Seizure disorders among relatives of Kenyan children with severe falciparum malaria. Trop Med Int Health. 2003;8:12–16. doi: 10.1046/j.1365-3156.2003.00965.x. [DOI] [PubMed] [Google Scholar]
- Virta M, Hurme M, Helminen M. Increased frequency of interleukin-1beta (-511) allele 2 in febrile seizures. Pediatr Neurol. 2002;26:192–195. doi: 10.1016/s0887-8994(01)00380-0. [DOI] [PubMed] [Google Scholar]
- Williams TN, Mwangi TW, Wambua S, Alexander ND, Kortok M, Snow RW, Marsh K. Sickle cell trait and the risk of Plasmodium falciparum malaria and other childhood diseases. J Infect Dis. 2005;192:178–186. doi: 10.1086/430744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JN, Rockett K, Jallow M, Pinder M, Sisay-Joof F, Newport M, Newton J, Kwiatkowski D. Analysis of IL10 haplotypic associations with severe malaria. Genes Immun. 2005;6:462–466. doi: 10.1038/sj.gene.6364227. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94:S1–S90. [PubMed] [Google Scholar]
- Wozencraft AO, Dockrell HM, Taverne J, Targett GA, Playfair JH. Killing of human malaria parasites by macrophage secretory products. Infect Immun. 1984;43:664–669. doi: 10.1128/iai.43.2.664-669.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Cui X, Schmitt K, Hubert R, Navidi W, Arnheim N. Whole genome amplification from a single cell: implications for genetic analysis. Proc Natl Acad Sci USA. 1992;89:5847–5851. doi: 10.1073/pnas.89.13.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The cut-off for significant genotypic tests based on both permutation tests and authors' judgment as both not too conservative and too liberal.
Table S1. Minor allele frequencies (MAF) and Hardy-Weinberg equilibrium (HWE) test in the controls for polymorphisms included in the final analysis.
Table S2. Details of malaria transmission in the study areas across the four sites in Africa.


