Abstract
Clinical trials reveal that plasmid DNA (pDNA)–based gene delivery must be improved to realize its potential to treat human disease. Current pDNA platforms suffer from brief transgene expression, primarily due to the spread of transcriptionally repressive chromatin initially deposited on plasmid bacterial backbone sequences. Minicircle (MC) DNA lacks plasmid backbone sequences and correspondingly confers higher levels of sustained transgene expression upon delivery, accounting for its success in preclinical gene therapy models. In this study, we show for the first time that MC DNA also functions as a vaccine platform. We used a luciferase reporter transgene to demonstrate that intradermal delivery of MC DNA, relative to pDNA, resulted in significantly higher and persistent levels of luciferase expression in mouse skin. Next, we immunized mice intradermally with DNA encoding a peptide that, when presented by the appropriate major histocompatibility complex class I molecule, was recognized by endogenous CD8+ T cells. Finally, immunization with peptide-encoding MC DNA, but not the corresponding full-length (FL) pDNA, conferred significant protection in mice challenged with Listeria monocytogenes expressing the model peptide. Together, our results suggest intradermal delivery of MC DNA may prove more efficacious for prophylaxis than traditional pDNA vaccines.
Introduction
Plasmid DNA (pDNA) is an attractive platform for gene delivery in vivo because it is a non-viral, non-integrating vector that is safe, inexpensive, stable, and easily manipulated.1 As such, it has been evaluated in hundreds of clinical trials.2,3 Collectively, these trials reveal that although pDNA has promise, the major challenge to its widespread use for the prevention and treatment of human disease is poor protein production in situ. This results from both low transfection efficiency and low transgene expression.
Expression of the transgene cassette in pDNA vectors rapidly wanes because transcriptionally repressive proteins are initially deposited on extragenic DNA, the backbone pDNA located between the 5′ and 3′ ends of the transgene expression cassette.4,5,6 Chromatin deposition is independent of the sequence, CpG content, and methylation status of the extragenic pDNA but is dependent on its length.7,8,9,10 Chromatinization spreads in cis from the extragenic DNA to the transgene cassette, silencing its expression. This results in a brief burst of protein production in situ followed by its rapid loss.
Minicircle (MC) DNA is similar to pDNA as both contain expression cassettes that permit transgene products to be made at high levels shortly after delivery in vivo.11 They differ in that MC DNA is devoid of essentially all prokaryotic sequence elements (e.g., origin of replication and antibiotic-resistance genes). Removal of these backbone pDNA sequences is achieved via site-specific recombination in Escherichia coli before episomal DNA isolation.12,13 The lack of these prokaryotic sequence elements also reduces MC size relative to its parental full-length (FL) pDNA, leading to enhanced transfection efficiencies.14 The net result is that compared with their FL pDNA counterparts, MCs transfect more cells and permit sustained high level transgene expression upon delivery in vivo. Because of this, we hypothesized that immune responses elicited by MC vaccines would be more potent than responses to FL pDNA vaccines encoding the same antigens (Ags).
The two main routes for administering pDNA vaccines are intramuscular and intradermal. The intradermal route is preferred because the skin is a barrier organ relatively rich in immune sentinel cells that present Ag to the immune system.15,16 Keratinocytes are critical for maintaining the immunological barrier function of the skin by initiating inflammation via recognition of damage- and pathogen-associated molecular patterns by pattern recognition receptors.17 Dermal dendritic cells similarly initiate proinflammatory responses but they also can internalize and present Ag to B and T cells.15 Intradermal delivery methods for DNA include injection with a hypodermic needle, bombardment of the skin with DNA-coated gold particles ejected from a gene gun, topical application, electroporation, and tattooing.18,19,20,21,22 Tattooing has the advantage of being relatively inexpensive and rapid, and the infliction of thousands of perforations likely serves as an adjuvant. Work by the Haanen group demonstrated that pDNA delivery to the skin via tattooing elicited more rapid and robust immune responses specific for the pDNA-encoded Ags than did intramuscular delivery.23
We used tattooing to deliver FL pDNA and their derivative MC vaccines intradermally to mice. We found that the level, duration and immunogenicity of the MC transgene-encoded proteins were all significantly greater than those elicited by the FL pDNA vaccines, and that the primary and memory immune responses elicited by MC vaccines conferred significant protection against bacterial infection in a model of listeriosis. We discuss these results relative to future strategies involving pDNA-based vaccines.
Results
Efficient production of MC-based expression vectors
We constructed two series of expression vectors. One encoded enhanced firefly (eff) luciferase as a reporter to track expression. The other encoded a model peptide to monitor Ag-specific T-cell responses. We chose eff luciferase as a reporter because of its sensitivity; mouse cells expressing eff luciferase emit >100 times more light than cells expressing standard firefly luciferase.24 The model peptide was the chicken ovalbumin-derived peptide SIINFEKL (amino acid residues 257–264). When SIINFEKL is bound by the mouse major histocompatibility class I molecule H-2Kb, it forms an Ag recognized by mouse CD8+ T cells bearing the Vα2/Vβ5 OT-I transgenic T-cell receptor.25 Ag-specific OT-I responses are easily tracked after these cells are adoptively transferred into C57BL/6 (H-2b) recipients subsequently immunized with the SIINFEKL peptide plus adjuvant.26 Responses of Ag-specific endogenous CD8+ T cells are also readily tracked by flow cytometry using fluorophore-labeled SIINFEKL/Kb tetramers.27 Expression of the eff and Ag genes is controlled by the human ubiquitin C (hUbC) promoter, chosen because it efficiently drives transgene expression in many mouse tissues including skin.28
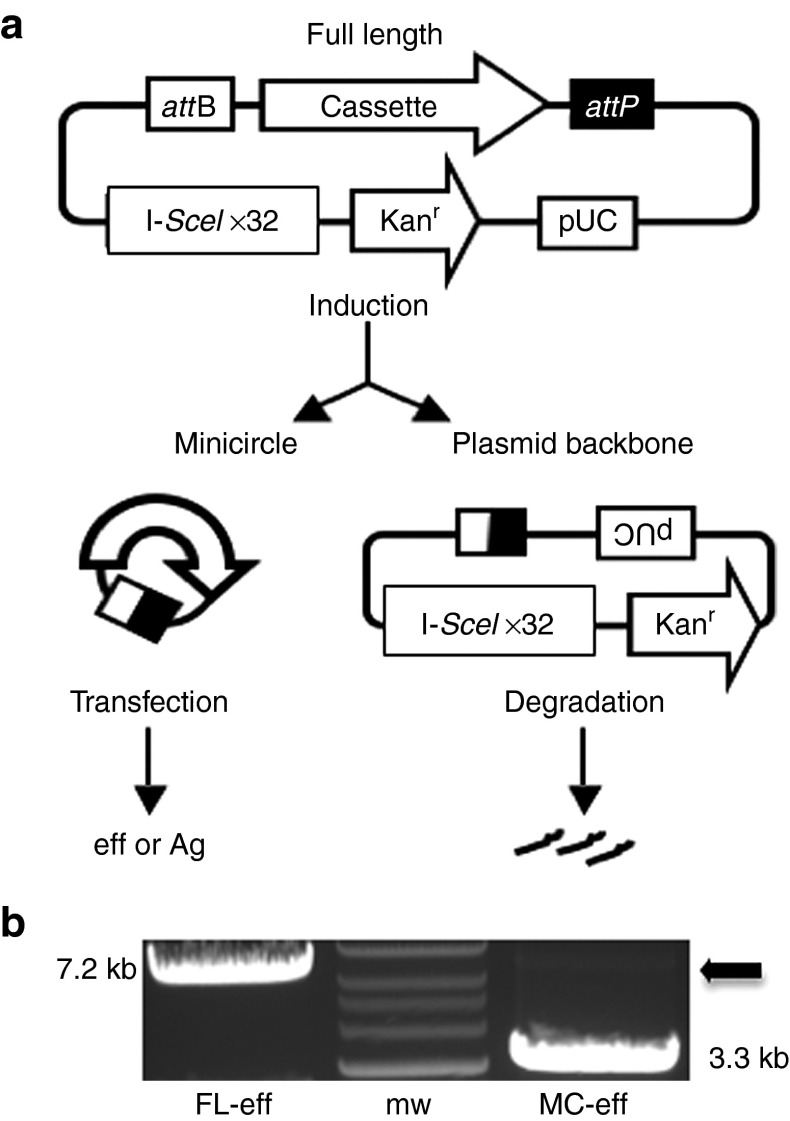
MC constructs encoding eff or Ag (MC-eff and MC-Ag, respectively) were generated from FL pDNA precursors (Figure 1a). Briefly, we cloned the hUbC-driven expression cassettes between the ϕC31 integrase recognition sites attB and attP in pMC.BESPX, the MC producer plasmid.13 Upstream of the attB site was a block of 32 tandem repeats of the I-SceI homing endonuclease recognition sequence.29 FL pDNA was transformed into an E. coli strain (ZYCY10P3S2T) engineered to express ϕC31 integrase and I-SceI endonuclease.13 Upon induction, ϕC31 integrase activity generated the MC and circular plasmid backbone; the latter was subsequently degraded by host bacterial exonucleases after linearization by the I-SceI endonuclease. Densitometry analyses of ethidium bromide-stained agarose gels revealed that less than 2% of the parental FL-eff pDNA remained in the purified MC-eff preparations (Figure 1b). MC-Ag preparations were comparably pure (data not shown).
Figure 1.
Minicircle derivation. (a) Plasmid construct and minicircle generation. The expression cassette is cloned between the ϕC31 integrase recognition sites attB and attP in the minicircle producer plasmid pMC.BESPX, which also contains I-SceI homing endonuclease recognition sequences. Following transformation of the E. coli strain ZYCY10P3S2T with a full-length (FL) parental plasmid, ϕC31 integrase activity generates a minicircle (MC) containing the expression cassette and a circular plasmid backbone; the latter is subsequently degraded by host exonucleases following linearization by the I-SceI endonuclease. (b) Agarose gel electrophoresis analysis of linearized FL-eff and its MC derivative (MC-eff). The arrow indicates residual FL plasmid in the purified MC-eff DNA preparation. Sizes of the DNA species are noted next to each band. eff, enhanced firefly luciferase; FL-eff, full-length plasmid encoding eff; I-SceI x32, 32 tandem repeats of the I-SceI homing endonuclease recognition sequence; Kanr, kanamycin-resistance gene; MC-eff, minicircle-encoding eff; MW, molecular weight standards; pUC, plasmid origin of replication.
Enhanced expression of MC-encoded eff luciferase in vitro
To determine if the expression levels or transfection efficiencies of MC-encoded genes were enhanced as compared with FL plasmid-encoded genes, we transfected COS cells transiently with equimolar amounts of MC-eff or FL-eff DNA. Two days later, the cells were analyzed by flow cytometry for intracellular luciferase expression (Figure 2a). On average, ~14% of cells transfected with MC-eff expressed luciferase, more than twice the frequency (6%) of luciferase-expressing cells transfected with an equimolar amount of FL-eff (Figure 2b). However, the median fluorescent intensities of the two luciferase-expressing populations were comparable (P = 0.65). Together, these data suggest that under the conditions used, the smaller MC-eff (3348 bp) is superior to the larger FL-eff (7262 bp) in transfection frequency but not in gene expression level on a per cell basis as assessed 2 days after transfection.
Figure 2.
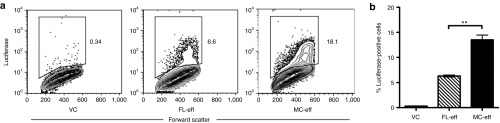
Enhanced expression of minicircle-encoded eff in vitro. COS cells were transiently transfected with equimolar amounts (1.1 pmol; 2.5 µg MC-eff) of each DNA species. A luciferase-specific antibody was used to determine the frequencies of luciferase-expressing cells 48 hours later. (a) Flow cytometric analysis of transfected COS cells. These are representative flow cytometry contour plots used to determine the frequencies of eff-positive COS cells shown in b. (b) Frequencies of luciferase-positive COS cell transfectants. Data are presented as means ± SEM (n = 3) (**P < 0.01) and represent three experiments. eff, enhanced firefly luciferase; FL-eff, full-length plasmid encoding eff; MC-Ag, minicircle encoding eff; VC, empty full-length plasmid vector control.
Enhanced expression of MC-encoded eff luciferase in skin
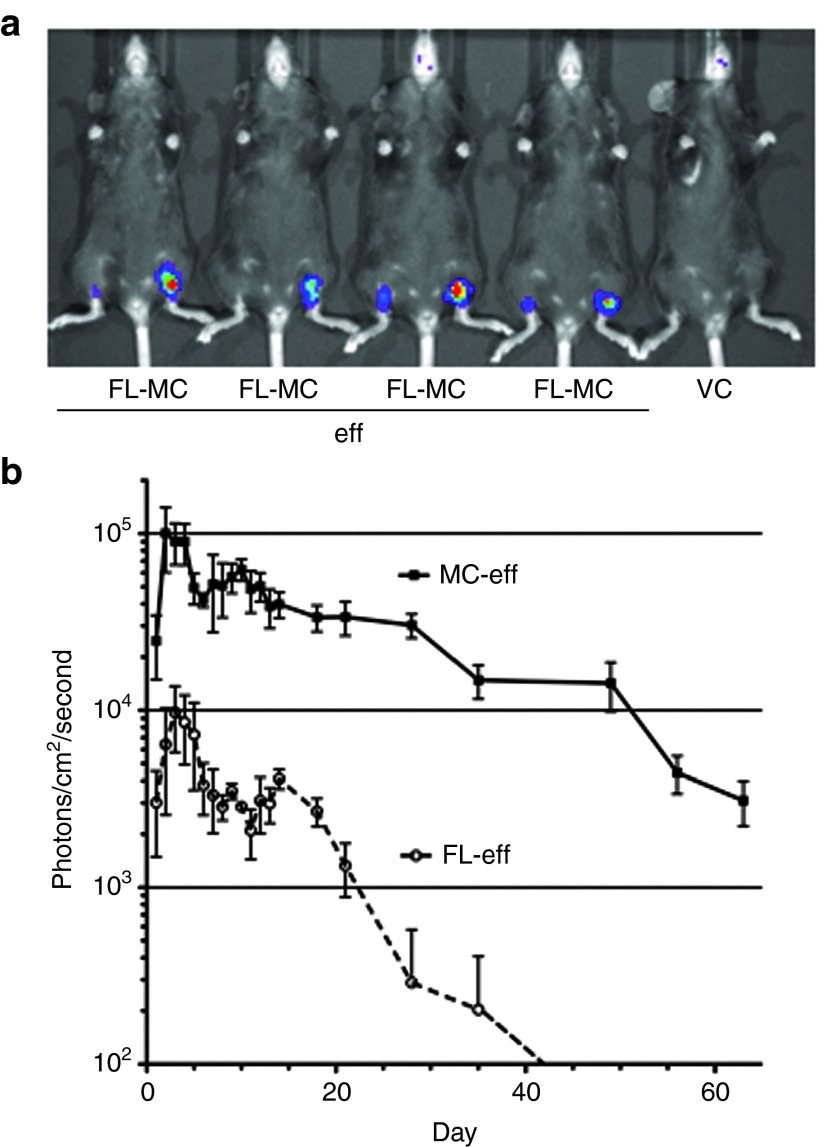
Because our goal is to develop MC-based vaccines, we next determined the intensity and duration of MC gene expression in vivo following intradermal delivery via tattooing. Each mouse in the experimental cohort was tattooed on the left and right thighs while lying supine with equimolar amounts of FL-eff and MC-eff DNA, respectively. Mice in a separate cohort were tattooed with equimolar amounts of FL pDNA lacking an expression cassette (empty vector control (VC)). The mice were then injected with saturating amounts of D-luciferin substrate on the indicated days and imaged for bioluminescence (Figure 3a). Expression peaked 2–3 days after delivery in both groups (Figure 3b).
Figure 3.
Enhanced intensity and duration of minicircle-encoded eff expression in the skin. The ventral skin on both thighs of C57BL/6 mice was tattooed with equimolar (9.2 pmol; 20 µg eff-encoding minicircle DNA) amounts of empty full-length plasmid VC, FL plasmid encoding eff luciferase, or the MC-eff derivative. (a) Xenogen IVIS imaging of in vivo luciferase activity 4 days after gene delivery. FL-eff and MC-eff luciferase activities are shown on the left and right thighs, respectively, of the first four mice as they lie in the supine position. (b) Quantitative long-term in vivo luciferase expression. The data for the FL-eff and MC-eff cohorts are presented as the mean ± SEM (n = 4), corrected for background bioluminescence. Values for the VC cohort (n = 4) never exceeded background and are not shown. Luciferase activity in the FL-eff and MC-eff cohorts was significantly different (P < 0.05) on each day analyzed except the first day (P < 0.06). This is representative of two similar experiments. eff, enhanced firefly luciferase; FL-eff, full-length plasmid encoding eff; MC-eff, minicircle-encoding eff; VC, vector control.
The groups differed, though, in that luciferase activity encoded by MC-eff was consistently and significantly higher than that of FL-eff. Luciferase activity decayed to background levels by day 42 in the FL-eff cohort but it remained detectable in the MC-eff cohort throughout the 63-day–observation period. It is noteworthy that the MC-eff luciferase activity on day 63 was comparable with the FL-eff peak response on day 3. It also took twice as long for luciferase activity to decay to levels significantly lower (P < 0.05; paired t-test) than the peak response in MC-eff (56 days) versus FL-eff (28 days) mice. Together, these data indicate that luciferase expression in the skin persists significantly longer and at significantly higher levels in mice tattooed with MC-eff relative to FL-eff.
Enhanced presentation of MC-encoded Ag in vitro
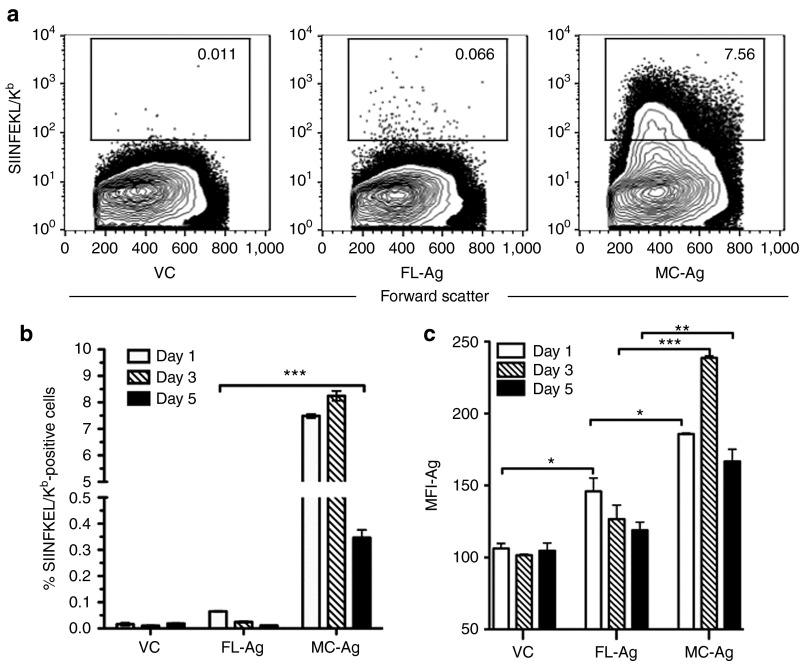
As a prelude to vaccination, we assessed Ag secretion and presentation in vitro by transfecting a dendritic cell line, DC2.4 (H-2b haplotype) with equimolar amounts of VC, FL-Ag or MC-Ag DNA. The Ag cassette encoded the chicken ovalbumin-derived peptide SIINFEKL linked to a secretion sequence and a histidine tag (Supplementary Figure S1). Western blot analysis of transiently transfected COS cells revealed the Ag was secreted (data not shown). To measure presentation of the SIINFEKL peptide, we analyzed DC2.4 cells at 1, 3, and 5 days after transfection for reactivity with 25-D1.16 (Figure 4a). This monoclonal antibody specifically binds SIINFEKL only when presented by H-2Kb.30 The frequency of cells bearing detectable SIINFEKL/H-2Kb complexes was significantly higher and persisted longer in the MC-Ag transfectants as compared with the FL-Ag transfectants (Figure 4b). These MC-Ag transfectants also expressed significantly higher levels of SIINFEKL/H-2Kb complexes than the FL-Ag transfectants, as measured by median fluorescent intensity (Figure 4c). This level of enhanced Ag expression in the MC-Ag versus the FL-Ag transfectants on a per cell basis contrasts with the comparable levels of eff expression level in the MC-eff and FL-eff transfectants noted above. We speculate that the SIINFEKL/H-2Kb complexes on the cell surface have a greater half-life than intracellular eff. An alternative, but not mutually exclusive, possibility is that the Ag-transgene encoded protein containing the SIINFEKL peptide and the eff protein are degraded at similar rates but the resultant pool of SIINFEKL peptides provides a reservoir for prolonged presentation by H-2Kb.
Figure 4.
Enhanced presentation of minicircle-encoded Ag by dendritic cells. Cells from the C57BL/6-derived dendritic cell line DC2.4 were transiently transfected with equimolar (1.8 pmol; 2.8 µg MC-Ag) amounts of empty full-length plasmid VC, full-length plasmid encoding the chicken ovalbumin-derived peptide SIINFEKL, or the SIINFEKL-encoding minicircle derivative of the full-length plasmid. The latter two samples are respectively abbreviated FL-Ag and MC-Ag, as SIINFEKL presented by the MHC class I molecule H-2Kb is an Ag recognized by specific CD8+ T cells. Cells were analyzed by flow cytometry for presentation of SIINFEKL by H-2Kb, as determined by reactivity with the monoclonal antibody 25.D1-16. (a) Flow cytometric analysis of transfected DC2.4 cells. These are representative flow cytometry contour plots (day 3) used to determine the frequencies of Ag-positive DC2.4 cells shown in b. (b) Frequencies of Ag-positive DC2.4 cells. Data are presented as the means ± SEM (n = 3) (***P < 0.001) and represent three experiments. (c) Median fluorescent intensities (MFIs) of Ag-positive DC2.4 cells. Data are presented as the MFI ± SD (n = 3) (*P < 0.05; **P < 0.01; ***P < 0.001) and represent three experiments. Ag, antigen (SIINFEKL/H-2Kb); FL-Ag, full-length plasmid encoding SIINFEKL; MC-Ag, minicircle-encoding SIINFEKL; VC, vector control.
Enhanced immunogenicity of MC-encoded Ag in vivo in an adoptive transfer model
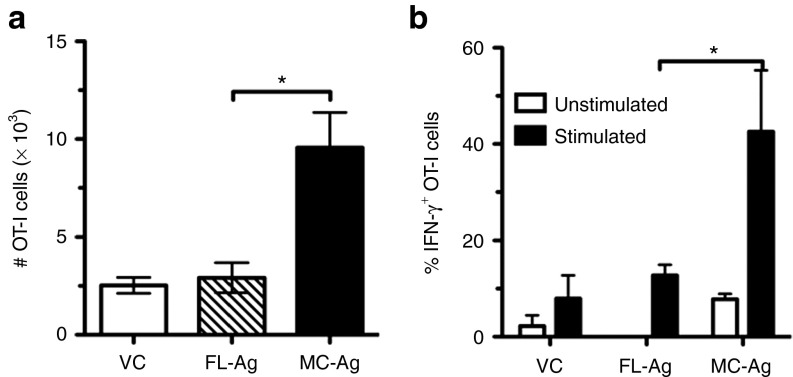
To determine if the enhanced presentation of MC-encoded Ag observed in vitro corresponded to increased immunogenicity in vivo, we adoptively transferred equal numbers of CD8+ OT-I (Thy1.1+) cells into congenic C57BL/6 (Thy1.2+) mice and tracked the OT-I cell responses following DNA immunization. One day after OT-I cell adoptive transfers, recipients were tattooed with equimolar amounts of VC, FL-Ag or MC-Ag DNA. At the peak of the response 7 days later, flow cytometry was used to identify OT-I cells by their expression of CD8 and the allelic marker Thy1.1. The proliferative responses of OT-I cells, as measured by an increase in absolute OT-1 cell numbers, in MC-Ag–immunized mice were significantly greater than the responses in mice immunized with FL-Ag or VC. This was true in the spleen (Figure 5a) and draining lymph nodes (data not shown). To assess their functionality, OT-I cells from immunized mice were stimulated briefly in vitro with cognate Ag (syngeneic H-2b splenocytes pulsed with SIINFEKL peptide) and examined for interferon-γ (IFN-γ) expression. IFN-γ was expressed by significantly more OT-I cells from mice immunized with MC-Ag than from mice immunized with FL-Ag (Figure 5b). Moreover, the levels of intracellular IFN-γ tended to be higher in OT-I cells from MC-Ag–immunized mice than in FL-Ag–immunized mice (median fluorescent intensities of 370 versus 235, respectively).
Figure 5.
Enhanced activation of Ag-specific CD8+ T cells in vivo following immunization with MC-Ag. C57BL/6 mice were adoptively transferred with OT-I cells and 24 hours later tattooed with equimolar (13.8 pmol; 20 µg MC-Ag) amounts of VC, FL-Ag, or MC-Ag. Mice were killed 1 week later and their splenocytes analyzed by flow cytometry. (a) OT-I cell quantification. OT-I cells were identified by their expression of CD8 and Thy1.1 (CD57BL/6 endogneous CD8+ T cells express Thy1.2). (b) Frequencies of interferon-γ+ (IFN-γ+) OT-I cells. Splenocytes from adoptively transferred, immunized mice were incubated with or without SIINFEKL peptide in vitro before analysis by flow cytometry for intracellular IFN-γ expression. Data are presented as the means ± SEM (n = 3) and represent three experiments. (*P < 0.05). FL-Ag, full-length plasmid encoding SIINFEKL; MC-Ag, minicircle-encoding SIINFEKL; VC, vector control.
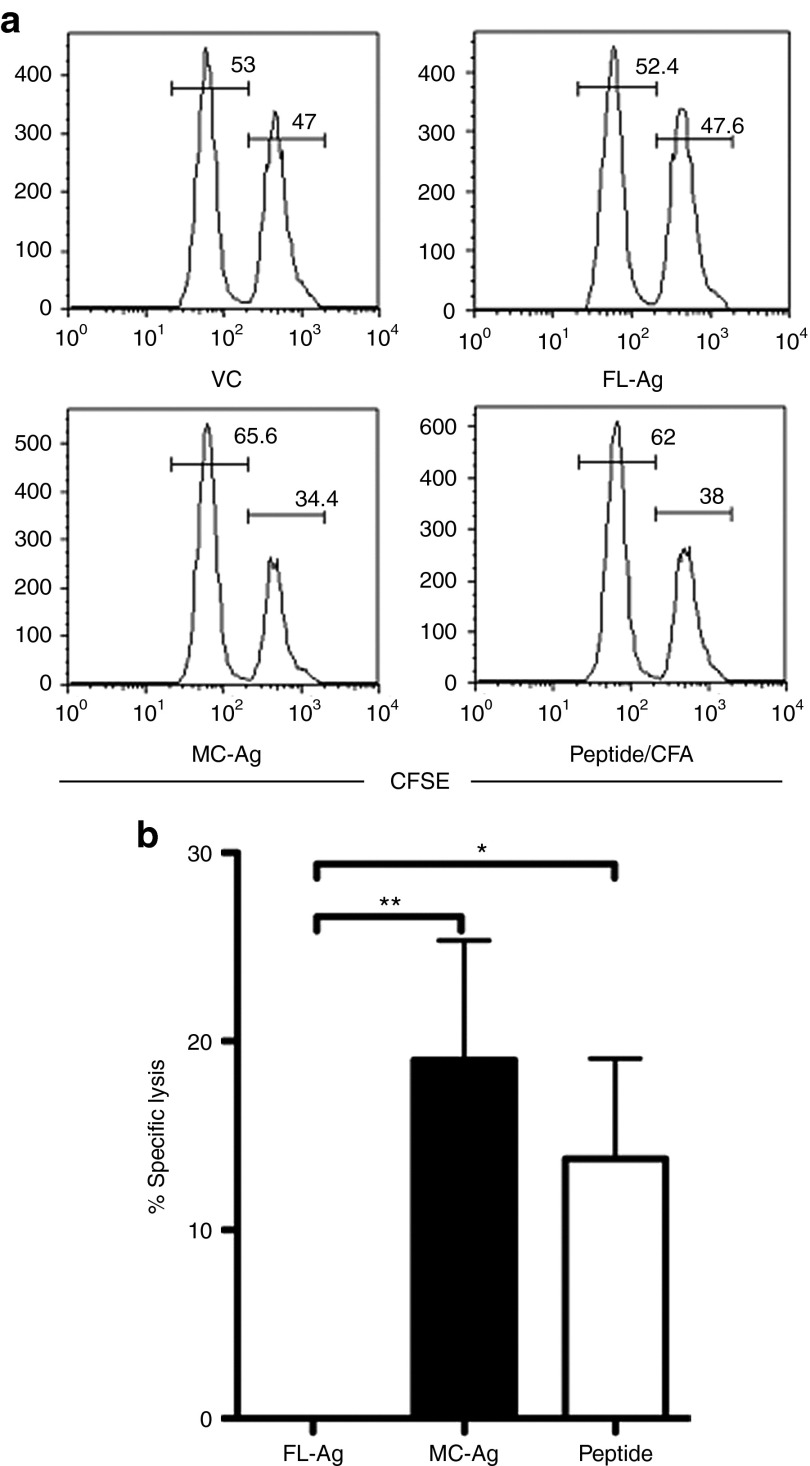
To assess in vivo cytotoxicity, C57BL/6 mice were adoptively transferred with OT-I cells and the next day immunized with equimolar amounts of VC, MC-Ag, or FL-Ag DNA via tattooing. One week later, these mice were injected with equal numbers of SIINFEKL-pulsed syngeneic splenocytes labeled with 5 µmol/l 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFSEhigh) and unpulsed syngeneic splenocytes labeled with tenfold less CFSE (CFSElow). The loss of CFSEhigh (Ag-positive) relative to CFSElow (Ag-negative) cells provided a measure of OT-I cytolytic activity in vivo (Figure 6a).31 Ag-specific cytolytic activity in MC-Ag–immunized mice was significantly greater than that in FL-Ag–immunized mice (Figure 6b). OT-I cytolytic activity was comparable between mice immunized with MC-Ag DNA and SIINFEKL peptide plus complete Freund's adjuvant (positive control; Figure 6b). The latter is remarkable given the 3,700 molar excess of peptide (52 nmol) relative to MC-Ag DNA (14 pmol), and that pDNA transfection efficiencies in vivo are poor.22 Together, these data indicate MC-Ag immunization elicits a significantly greater OT-I response than does immunization with FL-Ag pDNA.
Figure 6.
Enhanced cytolytic activity of Ag-specific CD8+ T cells following immunization with MC-Ag. C57BL/6 mice were adoptively transferred with OT-I cells and 24 hours later tattooed with equimolar (13.8 pmol; 20 µg MC-Ag) amounts of VC, FL-Ag, or the MC-Ag. Control mice were immunized subcutaneously with 50 µg SIINFEKL peptide emulsified in complete Freund's adjuvant (CFA). Eight days later, mice were adoptively transferred with equal numbers of syngeneic splenocytes pulsed with SIINFEKL peptide (labeled with 5 µmol/l CFSE) and unpulsed splenocytes (labeled with 0.5 µmol/l CFSE). Mice were killed 14 hours later and their splenocytes analyzed by flow cytometry for the frequencies of CFSElow (unpulsed) and CFSEhigh (peptide-pulsed) cells. (a) Representative histograms of CFSElow and CFSEhigh cells. The numbers show the frequencies of CFSElow and CFSEhigh cells in the CFSE+ populations in immunized mice. (b) Ag-specific in vivo cytolytic activity. The frequency of unpulsed cells (CFSElow) was divided by the frequency of peptide-pulsed cells (CFSEhigh) to determine the ratio of Ag-negative to Ag-positive target cells in each mouse. These ratios were then used to calculate the percent Ag-specific cytolytic activity by the equation: [1-(VC ratio/FL-Ag or MC-Ag ratio)] × 100]. Data are presented as means ± SEM (n = 5) (*P < 0.05; **P < 0.01) and represent three replicate experiments. CFA, complete Freund's adjuvant; CFSE, 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester; FL-Ag, full-length plasmid-encoding SIINFEKL; MC-Ag, minicircle-encoding SIINFEKL; VC, empty full-length plasmid vector control.
Superior protection from a MC vaccine in model of listeriosis
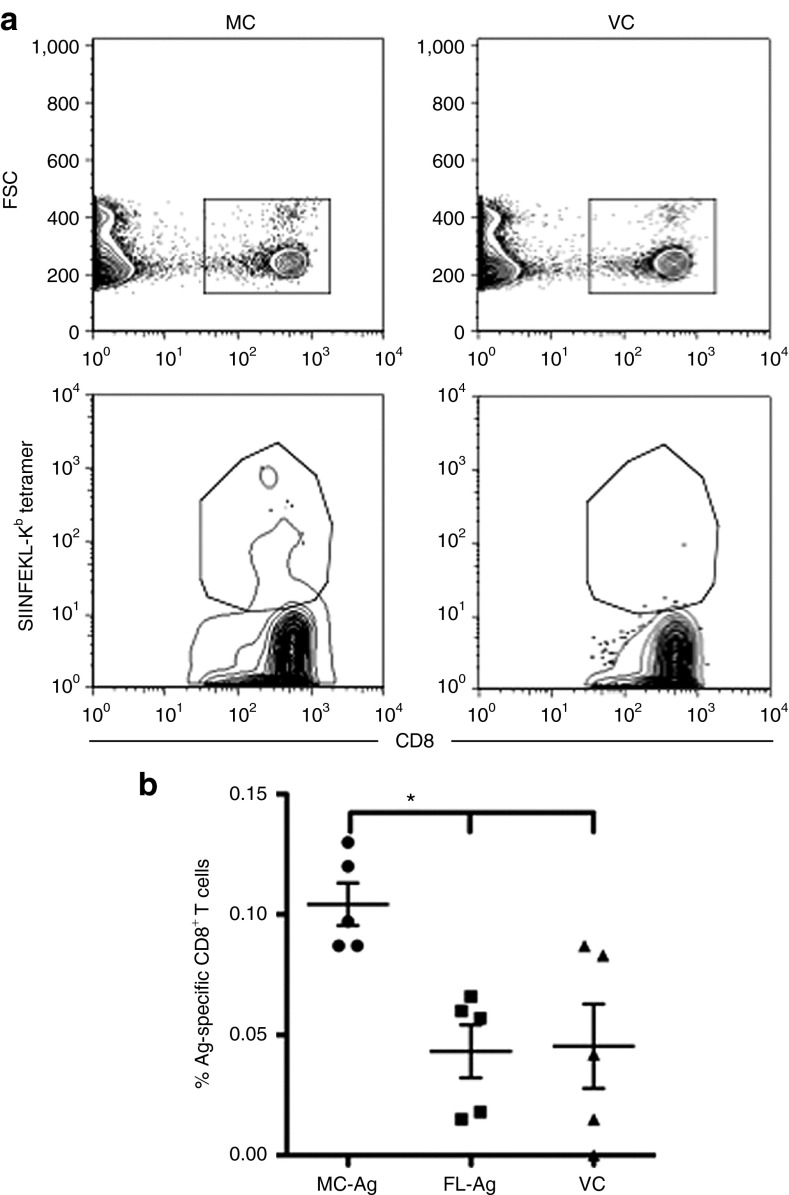
Vaccine efficacy is measured by the ability of endogenous Ag-specific immune cells to protect a vaccinated animal from a subsequent pathogen challenge. To determine if MC immunization enhanced the responses of endogenous Ag-specific CD8+ T cells, we first tracked the frequencies of SIINFEKL/Kb tetramer-binding CD8+ T cells in immunized C57BL/6 mice that had not been adoptively transferred with OT-I cells. Bins et al. showed that three intradermal immunizations 3 days apart were required for an optimal immune response to a pDNA-encoded Ag which peaked 5 days after the third immunization.23 We replicated this immunization scheme by tattooing mice three times, 3 days apart (on days 0, 3, and 6) with equimolar amounts of VC, MC-Ag, or FL-Ag DNA. One day after each immunization, mice were injected subcutaneously at the tattoo site with polyIC, a toll-like receptor 3 agonist that serves as an adjuvant by eliciting type I interferons.32 The peak response in our immunized mice also occurred 5 days after the third immunization (on day 11), as measured by the frequencies of SIINFEKL/Kb tetramer-binding CD8+ T cells in the peripheral blood taken over time (data not shown). Mice immunized with MC-Ag had significantly higher frequencies of tetramer-binding CD8+ T cells on day 11 than mice immunized with either the FL-Ag or VC pDNA (Figure 7).
Figure 7.
Enhanced expansion of endogenous Ag-specific CD8+ T cells in vivo following immunization with MC-Ag. C57BL/6 mice were immunized via tattooing three times, 3 days apart (days 0, 3, and 6) with equimolar (13.8 pmol per immunization; 20 µg MC-Ag) amounts of VC, FL-Ag, or MC-Ag. Twenty-four hours after each immunization, mice were injected subcutaneously on the ventral portion of the tattooed thigh with 10 µg of polyIC. Five days following the last immunization (day 11), the mice were bled via the facial vein. (a) Representative flow cytometry plots showing the gates set on CD8+ SIINFEKL/Kb tetramer-binding subsets of lymphocytes. Cells were identified by reactivity with anti-CD8-allophycocyanin monoclonal antibody and SIINFEKL/Kb-PE tetramer. (b) Frequency of antigen-specific CD8+ T cells. Data are presented as the mean ± SEM (n = 5) (*P < 0.05; Kruskal–Wallis test corrected with a Dunn's multiple comparison test) and represent three experiments. FL-Ag, full-length plasmid encoding SIINFEKL; MC-Ag, minicircle-encoding SIINFEKL; VC, vector control.
We next asked if vaccination with MC-Ag DNA protected mice from a challenge of Listeria monocytogenes engineered to express the model Ag, chicken ovalbumin (LM-OVA).33 Listeria monocytogenes is a gram-positive intracellular bacterium that can cause fatal listeriosis in neonates, pregnant women, and immunosuppressed individuals.34 LM-OVA is particularly useful because it provides a well-defined T cell Ag (e.g., SIINFEKL peptide presented by H-2Kb) and because CD8+ T cells are necessary for induction of protective immunity to the pathogen.35 Mice were immunized as described above except that polyIC was replaced with polyICLC because of the latter's enhanced pharmacokinetics.36 At the peak of the immune response 5 days after the last immunization (day 11), all mice were challenged intravenously with LM-OVA bacteria. Mice were euthanized 5 days after bacterial challenge (day 16) and the frequencies of peripheral blood and splenic CD8+ T cells binding the SIINFKEL/Kb tetramer were determined by flow cytometry. Bacterial burden was measured as the number of colony-forming units of LM-OVA in the spleen.
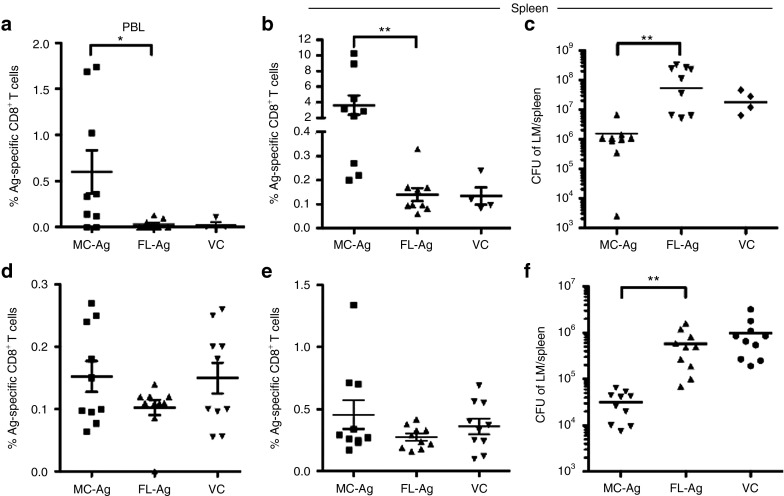
Consistent with the day 11 results, on day 16 there were significantly higher frequencies of Ag-specific CD8+ T cells in the peripheral blood and spleens of mice immunized with MC-Ag than in mice immunized with FL-Ag or VC DNA (Figures 8a and 8b, respectively; gating strategies shown in Supplementary Figure S2). Vaccination with MC-Ag DNA correspondingly conferred significant protection to challenge with LM-OVA. Bacterial burden was significantly reduced in mice immunized with MC-Ag as compared with FL-Ag or VC (Figure 8c). Although the numbers of colony-forming units were not significantly different between the FL-Ag and VC groups, challenge with a lower dose of LM-OVA showed a protective effect of FL-Ag immunization (Supplementary Figure S3).
Figure 8.
Enhanced protection from challenge with Listeria monocytogenes following immunization with MC-Ag. C57BL/6 mice were immunized via tattooing three times, 3 days apart (days 0, 3, and 6) with equimolar (13.8 pmol per immunization; 20 µg MC-Ag) amounts of VC, FL-Ag, or MC-Ag. Twenty-four hours after each immunization, mice were injected subcutaneously on the ventral portion of the tattooed thigh with 10 µg of polyICLC. Five or 30 days following the last immunization (days 11 or 36), mice were infected with LM-OVA intravenously in the tail vein. Five days following infection (days 16 or 41), mice were bled by the facial vein, killed, and their spleens harvested. Ag-specific T cells were identified by staining with anti-CD8-allophycocyanin and SIINFEKL/Kb-PE tetramer. Bacterial burden was measured as the number of Listeria colony-forming units (CFUs) in the spleen. Frequencies of antigen-specific CD8+ T cells in the peripheral blood and spleen on days (a,b) 16 and (d,e) 41. Data are presented as the mean ± SEM (n = 10) (*P < 0.05; **P < 0.01; Kruskal–Wallis test corrected with a Dunn's multiple comparison test) and represent three experiments. (c) Bacterial burden in the spleen on day 16. Mice were infected intravenously with 6 × 104 CFUs of LM-OVA on day 11 and bacterial burden was measured 5 days later (day 16). (**P < 0.01; Kruskal–Wallis test corrected with a Dunn's multiple comparison test). (f) Bacterial burden in the spleen on day 41. Mice were infected intravenously with 4 × 104 CFUs of LM-OVA on day 36 and bacterial burden was measured 5 days later (day 41). Data are presented as the mean ± SEM (n = 10) (*P < 0.05; Kruskal–Wallis test corrected with a Dunn's multiple comparison test). These are pooled data from two experiments. FL-Ag, full-length plasmid encoding SIINFEKL; MC-Ag, minicircle-encoding SIINFEKL; VC, vector control.
To test whether the memory response elicited MC-Ag DNA was also superior to that elicited by FL-Ag DNA, mice were challenged with LM-OVA on day 36, 30 days after the last DNA immunization. All mice were euthanized 5 days after bacterial challenge (day 41) to measure bacterial burden in their spleens and the frequencies of peripheral blood and splenic CD8+ T cells binding the SIINFKEL/Kb tetramer. Although the frequencies of Ag-specific CD8+ T cells in the peripheral blood (Figure 8d) and spleens (Figure 8e) of mice immunized with MC-Ag, FL-Ag, or VC DNA were not significantly different, vaccination with MC-Ag DNA 30 days before LM-OVA challenge conferred significant protection (Figure 8f). We speculate that infection leads to priming of naïve SIINFEKL-specific cells in all groups, which mask the proliferation of memory cells in immunized mice. Together, these data suggest vaccination with MC DNA encoding a pathogen-associated Ag can provide protection from a subsequent challenge with the corresponding pathogen.
Discussion
Our data indicate MC DNAs delivered via tattooing are superior to their FL counterparts in terms of the expression levels and duration, as well as the immunogenicity, of the proteins they encode. The enhanced levels and duration of protein expression we observe in the skin corroborate studies of MC DNA delivered to various tissues via other routes for gene therapy.12,37,38 The novelty of our report lies in the enhanced Ag-specific CD8+ T-cell response to an epitope encoded by a MC delivered intradermally. This enhanced response conferred significant protection to mice challenged with Listeria monocytogenes expressing the MC-encoded Ag.
Standard pDNA has two components: the bacterial backbone required for plasmid propagation in bacteria and the transcription cassette for expression in mammalian cells. Removal of the bacterial backbone reduced the size of MC DNA relative to pDNA by ~50%. pDNA size is inversely related to transfection efficiency14 and correspondingly, the transfection efficiency and expression levels of MC are higher than pDNA levels.11,38,39 Accordingly, we showed transfection of cells in vitro with MC resulted in higher rates of gene transfer (Figure 2a). This was also true in vivo, as evidenced by the ≥10-fold levels of luciferase activity in mice tattooed with MC-eff compared with FL-eff at all time points measured (Figure 3b).
Similarly, the significantly higher frequencies of effector Ag-specific CD8+ T cells measured 5 days following MC-Ag DNA immunization most likely reflected higher transfection efficiencies relative to FL-Ag DNA. We speculate that the Ag provided by the MC DNA and the adjuvant effect resulting from the thousands of needle pricks inflicted by the tattoo device combine to produce a local microenvironment conducive for naïve OT-I CD8+ T-cell activation. It is unlikely anything other than inflammation induced by the needle pricks serves as an adjuvant because the needles are sterile, the tattoo site is wiped with ethanol to minimize introduction of skin commensals, and the DNA preparations have exceedingly low endotoxin levels (≤0.1 pg/µg DNA). However, the adjuvanticity of the needle pricks was insufficient to elicit significant numbers of endogenous Ag-specific CD8+ T cells (data not shown), necessitating the use of polyICLC. This need for a greater immunostimulatory microenvironment may reflect the much lower precursor frequency of endogenous SIINFKEL/Kb specific CD8+ T cells (100–200 cells/mouse)40 as compared with the frequency of OT-I cells in adoptively transferred mice.
Because the majority of cells transfected by tattooing pDNA are likely keratinocytes,22 we included a secretory signal in the Ag expression cassette. Our rationale was that secreted Ag by transfected cells could drain to the regional lymph node for presentation or be internalized by resident Ag-presenting cells in the skin. These cells could then migrate to the draining lymph node and cross-present SIINFKEL/Kb to naïve CD8+ T cells. This is consistent with reports that dermal dendritic cells are required to stimulate naïve CD8+ T cells upon intradermal immunization with pDNA.41 Studies of pDNA-encoded Ags delivered intradermally suggest, though, that transfection of Ag-presenting cells themselves is the key event required to elicit specific CD8+ T-cell responses. Ag expression in keratinocytes failed to elicit specific CD8+ T-cell responses upon intradermal delivery of pDNA containing a K14 promoter, whereas Ag expression in dermal dendritic cells (via a CD11c promoter) did.42 Moreover, depletion of langerin+ dermal dendritic cells ablated the response of CD8+ T cells to Ag encoded by pDNA delivered intradermally, suggesting that specific delivery of MC to dermal dendritic cells should further enhance immunogenicity upon tattooing. It remains to be determined if differential cellular uptake of MC versus FL DNA impacts both the length of expression and the nature of the immune response.
In addition to enhancing transfection efficiency, a second critical benefit to the removal of the plasmid backbone elements relates to their role in gene silencing. We have shown that the deposition of heterochromatin on the bacterial sequences causes the loss of expression that is independent of DNA methylation, CpG content, immune cell clearance, and plasmid copy number.7,8,9,43 More recent studies confirm silencing is sequence independent but unexpectedly is dependent on sequence length.10 Silencing requires the pDNA separating the 5′ and 3′ ends of the transgene expression cassette to be ≥1 kb; silencing does not occur when this extragenic DNA is <0.5 kb. In our MC constructs, the extragenic DNA is <40 bp. It remains unclear why transgene silencing does not occur with shorter extragenic DNA.
The near complete absence of plasmid backbone elements most likely allowed expression of the eff transgene encoded by MC DNA to persist throughout the duration of the 9-week observation period. It is noteworthy that MC-eff expression at 7 weeks was comparable with that at 1 day. This was unexpected because 33–44% of C57BL/6 keratinocytes are replaced weekly and keratinocytes are the predominant cell type in the skin.44 Moreover, van den Berg et al. reported that 99% of human skin cells transfected by pDNA delivered via tattooing were keratinocytes.22 The persistence of eff luciferase expression we observe is consistent with the transfection of longer-lived progenitor cells in the skin, such as hair follicle-associated dermal precursors.45
The duration of Ag expression following intradermal immunization with pDNA affects CD8+ T-cell responses to the encoded Ag.46 Hovav et al. reported that Ag-presenting cell activity slowly increased over a 2-week period following injection of pDNA into the ear pinnae of mice.47 Removal of the ear pinnae at various times after immunization diminished this activity and the resultant primary specific CD8+ T-cell responses. These data suggest a depot of Ag controls CD8+ T-cell expansion at the level of Ag presentation. A persistent Ag depot afforded by MC immunization arguably could permit sustained CD8+ T-cell responses through the initial activation of naïve CD8+ T cells and the persistent reactivation of resultant memory cells or through modification of the kinetics of memory CD8+ T-cell differentiation, as Hovav et al. suggest.47 Our data showing enhanced protection to LM-OVA challenge 30 days after immunization with MC-Ag DNA is consistent with this possibility. Alternatively, higher Ag-transgene expression following MC-Ag DNA immunization could have generated greater numbers of Ag-specific CD8+ T cells which in turn, yielded higher frequencies of Ag-specific memory CD8+ T cells. Regardless, the combination of Ag and coadministered proinflammatory cytokines/chemokine all encoded by MC might establish a long-lived immunostimulatory microenvironment in the skin, leading to efficacious anti-pathogen and antitumor immunity.
In conclusion, we show for the first time the applicability of MC DNA to mediate long-term expression of transgenes when delivered intradermally. Although the immunogenicity of the MC-encoded Ags undoubtedly needs to be increased for the MC platform to serve as a potent vaccine, this clinically relevant delivery method and the inherent safety benefit of the non-integrating MC DNA represents a novel approach toward DNA-based therapies.
Materials and Methods
DNA constructs. The plasmid backbone for the FL constructs (pMC.BESPX.MCS1) contains the requisite sequences for bacterial propagation (e.g., the pUC origin of replication), the kanamycin-resistance gene, the ϕC31 integrase recognition sites attB and attP, and a block of 32 tandem repeats of the recognition sequence for the I-SceI homing endonuclease.13
To generate the FL-eff construct, the eff gene was amplified from the pUltra Bright eff luciferase+ plasmid24 and inserted into a vector containing the bovine growth hormone polyadenylation signal (BpA). The luciferase-BpA fragment was then PCR amplified. In a parallel PCR reaction, the hUbC promoter (a generous gift from Michael Kyba, University of Minnesota, Minneapolis, MN) was amplified. The hUbC amplicon contains a 5′ overlap region with the pMC.BESPX plasmid upstream of the EcoRI site and an overlapping region with the eff 5′ sequence. The luciferase/BpA amplicon contains an overlap region with the pMC.BESPX plasmid downstream of the EcoRV site. The two PCR products and the EcoRI/EcoRV digested pMC.BESPX were subjected to a one-step isothermal DNA assembly protocol allowing for seamless joining of the overlapping fragments.48
A similar strategy was utilized to generate the FL-Ag construct containing the hUbC promoter-driven Ag cassette. The cassette encodes a signal sequence, a 10× histidine tag, a linker and a region that includes the chicken ovalbumin-derived peptide SIINFEKL (Supplementary Figure S1). It was amplified from a codon-optimized synthetic gene (GenScript, Piscataway, NJ) and inserted upstream of BpA. Overlapping PCR products for cloning hUbC-Ag-BpA were generated and isothermally assembled into pMC.BESPX. Sequences of all amplifying primers are available upon request from the authors.
All PCR reactions were performed using Phusion High-Fidelity DNA Polymerase (New England BioLabs, Ipswich, MA) under the following conditions: 98 °C × 30 seconds, followed by 35 cycles of 98 °C × 10 seconds, 60 °C × 30 seconds, and 72 °C × 120 seconds. Plasmids containing the eff or Ag cassettes were then transformed into the ZYCY10P3S2T bacterial strain. ZYCY10P3S2T bears ten copies of the ϕC31 integrase gene, three copies of the I-Sce1 homing endonuclease gene, and the araE and LacY arabinose transporter genes that constitutively express proteins importing arabinose to induce ϕC31 integrase and I-Sce1 endonuclease expression.13 For FL pDNA preparation, transformed bacteria were grown in LB broth (Invitrogen, Carlsbad, CA) supplemented with kanamycin. pDNA was isolated using the GenElute Endtoxin-free Plasmid Maxiprep Kit (Sigma-Aldrich, St Louis, MO) according to the manufacturer's instructions. For MC generation, bacteria transformed with FL pDNA were grown overnight in Terrific Broth supplemented with kanamycin (Invitrogen). The following day MC induction media (fresh LB broth containing 0.04 volumes of 1 N NaOH and 0.02% L-arabinose (Sigma-Aldrich, St Louis, MO)) were added and the culture temperature was decreased from 37 °C to 32 °C for 5–8 hours. The culture was centrifuged and MC DNA was purified with the GenElute Endtoxin-free Plasmid Maxiprep Kit (Sigma-Aldrich) by increasing the recommended volumes of buffers six-fold and using four columns. Following elution in water, the DNA was concentrated to 4–6 µg/µl by ethanol precipitation, resuspended in water and stored at −20 °C.
Sequence analyses verified the genes encoded by the FL pDNAs and their derivative MCs were identical. The amounts of residual FL pDNA in the MC DNA preparations were evaluated by KpnI (FL pDNA) or StuI (MC DNA) restriction endonuclease-mediated linearization, followed by agarose gel electrophoresis and DNA visualization using ethidium bromide (Figure 1b). For the GenElute Endtoxin-free Plasmid Maxiprep Kits, the manufacturer defines “endotoxin-free” as ≤0.1 endotoxin unit/µg DNA. The levels of endotoxin in MC and FL pDNA preparations were measured by the ToxinSensor Chromogenic LAL Endotoxin Assay Kit (GenScript, Piscataway, NJ) and were all found to be <0.001 endotoxin unit/µg DNA (<0.1 pg endotoxin/µg DNA).
Cell lines and transfections. COS cells were maintained in complete Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen) and DC2.4 cells were maintained in complete RPMI 1640 (Invitrogen) supplemented with 10% fetal bovine serum at 37 °C with 5% CO2. Adherent cells were dissociated from tissue culture flasks with TrypLE Express (Invitrogen) and plated in six-well tissue culture-treated plates (BD Biosciences, San Jose, CA) at equal numbers (2–5 × 105 cells) per well without antibiotics. Cells were transfected the next day using Lipofectamine LTX with PLUS Reagent (Invitrogen) according to the manufacturer's protocol. COS cells were transfected with 2.5 µg (1.1 pmol) per well of MC-eff or equimolar amounts of VC or FL-eff and were harvested 2 days later for flow cytometry analysis. DC2.4 cells were transfected with 2.6 µg (1.8 pmol) of MC-Ag per well or equimolar amounts of VC or FL-Ag and were harvested 1, 3, and 5 days later for flow cytometry analysis.
Measurements of eff and Ag expression by flow cytometry. Staining for intracellular eff luciferase in COS cells was carried out using the BD Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's protocol. Fixed and permeabilized cells were incubated with an anti-luciferase-fluorescein isothiocyanate conjugate (Lifespan Biosciences, Seattle, WA) for 20 minutes. The DC2.4 cells were analyzed for cell surface Ag presentation using a 25-D1.16-allophycocyanin conjugate (eBioscience, San Diego, CA). 25-D1.16 is an antibody specific for the SIINFEKL/H-2Kb complex.30 Cells were stained at 4 °C for 30 minutes. Data were acquired on a BD FACSCalibur flow cytometer (BD Biosciences) using BD CellQuest Pro software (BD Biosciences) and analyzed with FlowJo software (Tree Star, Ashland, OR). Data are reported as the percentage and median fluorescent intensity of cells in a defined gated population.
Mice. Four- to eight-week-old, female C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME) or the National Cancer Institute (Frederick, MD). C57BL/6-Tg (TcraTcrb) 1100Mjb/J (OT-I) Thy1.1+/Thy1.2+ mice were a generous gift from Stephen Jameson at the University of Minnesota (Minneapolis, MN). All mice were housed under specific pathogen free conditions at the University of Minnesota. All animal procedures were carried out according to protocols approved by the Institutional Animal Care and Use Committee.
OT-I adoptive cell transfer. All work was carried out under asceptic conditions using sterile reagents. OT-I mice were euthanized and lymph nodes were harvested. Single cell suspensions were prepared and cells were washed in phosphate-buffered saline supplemented with 2% (v/v) fetal bovine serum (Invitrogen). Cells were counted and analyzed by flow cytometry to determine the percentage and number of OT-I cells with the following antibody-fluorochrome conjugates: CD44-fluorescein isothiocyanate, B220-phycoerythrin, and CD8-allophycocyanin (eBioscience) and Thy1.1- Peridinin Chlorophyll Protein Complex (PerCP) (BD Pharmingen, San Diego, CA). The OT-I phenotype was B220−CD8+Thy1.1+. In addition, the OT-I cells were CD44low, indicating they were naïve at the time of transfer. To determine the cell count, PKH26 Reference Beads (Sigma-Aldrich) were mixed with cells; 5,000 bead events were collected on the flow cytometer. The following equation was used to determine the number of lymphocytes: Number of cells/ml = (number of cells acquired × dilution factor of cells × number of singlet beads/ml)/(number of beads acquired × dilution factor beads). Cell counts were verified manually using a hemocytometer. Data were acquired on a BD FACSCalibur flow cytometer (BD Biosciences) using BD CellQuest Pro software (BD Biosciences) and analyzed with FlowJo software (Tree Star). Cells were washed in phosphate-buffered saline with 1% (v/v) fetal bovine serum and then in phosphate-buffered saline before being resuspended at 106 cells/ml in phosphate-buffered saline. 105 cells were transferred into each anesthetized mouse via retro-orbital injection using 1 ml tuberculin syringes and 27 gauge needles.
DNA immunizations. Mice were anesthetized and their inner hind legs were shaved and wiped with 70% ethanol. On the basis of pilot experiments, mice were immunized with 20 µg of MC DNA in 10–15 µl volumes. This mass converts to 9.2 and 13.8 pmol for MC-eff and MC-Ag, respectively. Equimolar amounts of VC and the corresponding FL pDNA samples were diluted in sterile water to the same volumes. All DNA was delivered intradermally over an area of ~1 cm2 for 30 seconds using a Cheyenne Hawk PU II tattoo device (Unimax Supply, New York, NY) set at 110 Hz using 9-point needles adjusted to a depth of 0.5 mm. Mice were immunized once for adoptive transfer experiments. For endogenous T-cell tracking and LM-OVA challenge experiments, mice were immunized three times, 3 days apart and 10 µg of polyIC (Sigma-Aldrich) or polyICLC (Oncovir) adjuvant were administered subcutaneously 24 hours after each immunization in the same inner hind leg as the DNA.
Bioluminescence measured in vivo. Mice were imaged for bioluminescence using a Xenogen IVIS Imaging System (Caliper Life Sciences, Hopkinton, MA). Animals were imaged daily for 18 days and then weekly from days 21 to 63. Mice were injected intraperitoneally with 100 µl of 30 mg/ml D-luciferin and anesthetized with isoflurane. Bioluminescence was measured for 5 minutes. Data were analyzed using Living Image 2.5 Software (Caliper Life Sciences).
CD8+ T-cell proliferation and function assays. Mice were adoptively transferred with 105 OT-I cells and the next day immunized with DNA using a tattoo device as described above. Splenocytes and the draining inguinal lymph nodes were harvested 7 days later and analyzed by flow cytometry for percentages of OT-I cells. Functionality was assessed by IFN-γ production following a brief exposure of cells to Ag in vitro. Splenocytes were incubated with or without 1 µmol/l SIINFEKL for 4 hours at 37 °C and then examined for extracellular expression of CD8 and Thy1.1 (to identify OT-I cells) and intracellular expression of IFN-γ using BD Cytofix/Cytoperm kit (BD Biosciences). Data were acquired on a BD FACSCalibur flow cytometer (BD Biosciences) using BD CellQuest Pro software (BD Biosciences) and analyzed with FlowJo software (Tree Star).
In vivo cytotoxicity. C57BL/6 mice were adoptively transferred with 106 OT-I cells and were immunized the next day with equimolar amounts of DNA using the tattoo device as described above. Eight days later these mice were adoptively transferred with equal numbers (107 each) of syngeneic splenocytes that had been pulsed or not with SIINFEKL peptide (1 µg peptide per 2.5 × 107 cells for one hour at 37 °C). To distinguish these cells from one another and from host cells, peptide-pulsed and unpulsed splenocytes were respectively labeled with 5 and 0.5 µmol/l CFSE. Fourteen hours later, the mice were euthanized and their splenocytes harvested and analyzed by flow cytometry for percentages of CFSEhigh (peptide-pulsed) and CFSElow (unpulsed control) cells. The percent specific killing was determined using the following formula: [1-(ratio in VC-immunized mouse/ratio in FL-Ag or MC-Ag–immunized mouse)] × 100.31
Endogenous T-cell analysis. Peripheral blood samples taken from the facial vein and single cell suspensions prepared from harvested spleens were incubated with CD44-fluorescein isothiocyanate, CD8-allophycocyanin (eBioscience), and SIINFEKL/H-2Kb tetramer formed with streptavidin coupled to phycoerythrin. Splenocytes were counted manually using a hemocytometer. Data were acquired on a BD FACSCalibur flow cytometer (BD Biosciences) using BD CellQuest Pro software (BD Biosciences) and analyzed with FlowJo software (Tree Star).
Infection with LM-OVA. Mice were infected with 4–6 × 104 colony-forming unit intravenously in the tail vein 5 days after the last immunization. Bacteria were grown in tryptic soy broth with 50 µg/ml of streptomycin to an absorbance at 600 nm of about 0.1. Actual numbers of colony-forming unit injected were determined for each experiment by plate count. Five days following infection, mice were bled from the facial vein, killed, and their spleens harvested. Half of the cells from each spleen were used for flow cytometric analysis and the other half were serially diluted and plated on tryptic soy agar plates containing streptomycin. Bacterial colonies were counted after plate incubation for 24 hours at 37 °C.
Statistical analysis. Data were graphed and analyzed using Prism v5.0c (GraphPad Software, La Jolla, CA). Statistical analyses were performed by the unpaired two-tailed Student's t-test unless noted otherwise.
SUPPLEMENTARY MATERIAL Figure S1. Single letter amino acid sequence of Ag insert. Figure S2. Gating strategy for detecting Ag-specific CD8+ T cells in mice challenged with LM-OVA. Figure S3. Protection against lower dose LM-OVA challenge elicited by MC-Ag or FL-Ag immunization.
Acknowledgments
We thank Stephen Jameson for the OT-I mice, the SIINFEKL/Kb-PE tetramer, and critical reading of this manuscript, and Michael Kyba for the hUbC promoter (both of whom are at University of Minnesota). We also thank the staff of the University of Minnesota Masonic Cancer Center Flow Cytometry Core their for assistance. This work was supported by a kind and generous gift to the University of Minnesota Masonic Cancer Center from Rosella Qualey, the Minnesota Futures Grant Program and grants from the NIH (R01 CA72669 and CA113576). W.M.D. was supported in part by a 3M Science and Technology Fellowship. N.E.S. was supported in part by the National Institutes of Health Medical Scientist Training Program Grant (T32 GM008244) and the Graduate School at the University of Minnesota (through the Wilfred Wetzel Fellowship, Warren J Warwick and Henriette Holm Warwick Fellowship, and the Doctoral Dissertation Fellowship). This work was done in Minneapolis, Minnesota, USA. The authors declared no conflict of interest.
Supplementary Material
References
- Faurez F, Dory D, Le Moigne V, Gravier R, Jestin A. Biosafety of DNA vaccines: New generation of DNA vectors and current knowledge on the fate of plasmids after injection. Vaccine. 2010;28:3888–3895. doi: 10.1016/j.vaccine.2010.03.040. [DOI] [PubMed] [Google Scholar]
- Abdulhaqq SA, Weiner DB. DNA vaccines: developing new strategies to enhance immune responses. Immunol Res. 2008;42:219–232. doi: 10.1007/s12026-008-8076-3. [DOI] [PubMed] [Google Scholar]
- Ferraro B, Morrow MP, Hutnick NA, Shin TH, Lucke CE, Weiner DB. Clinical applications of DNA vaccines: current progress. Clin Infect Dis. 2011;53:296–302. doi: 10.1093/cid/cir334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, He CY, Ehrhardt A, Kay MA. Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Mol Ther. 2003;8:495–500. doi: 10.1016/s1525-0016(03)00168-0. [DOI] [PubMed] [Google Scholar]
- Chen ZY, He CY, Meuse L, Kay MA. Silencing of episomal transgene expression by plasmid bacterial DNA elements in vivo. Gene Ther. 2004;11:856–864. doi: 10.1038/sj.gt.3302231. [DOI] [PubMed] [Google Scholar]
- Gracey Maniar LE, Maniar JM, Chen ZY, Lu J, Fire AZ, Kay MA. Minicircle DNA vectors achieve sustained expression reflected by active chromatin and transcriptional level. Mol Ther. 2013;21:131–138. doi: 10.1038/mt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Riu E, He CY, Xu H, Kay MA. Silencing of episomal transgene expression in liver by plasmid bacterial backbone DNA is independent of CpG methylation. Mol Ther. 2008;16:548–556. doi: 10.1038/sj.mt.6300399. [DOI] [PubMed] [Google Scholar]
- Riu E, Chen ZY, Xu H, He CY, Kay MA. Histone modifications are associated with the persistence or silencing of vector-mediated transgene expression in vivo. Mol Ther. 2007;15:1348–1355. doi: 10.1038/sj.mt.6300177. [DOI] [PubMed] [Google Scholar]
- Riu E, Grimm D, Huang Z, Kay MA. Increased maintenance and persistence of transgenes by excision of expression cassettes from plasmid sequences in vivo. Hum Gene Ther. 2005;16:558–570. doi: 10.1089/hum.2005.16.558. [DOI] [PubMed] [Google Scholar]
- Lu J, Zhang F, Xu S, Fire AZ, Kay MA. The extragenic spacer length between the 5' and 3' ends of the transgene expression cassette affects transgene silencing from plasmid-based vectors. Mol Ther. 2012;20:2111–2119. doi: 10.1038/mt.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darquet AM, Cameron B, Wils P, Scherman D, Crouzet J. A new DNA vehicle for nonviral gene delivery: supercoiled minicircle. Gene Ther. 1997;4:1341–1349. doi: 10.1038/sj.gt.3300540. [DOI] [PubMed] [Google Scholar]
- Chen ZY, He CY, Kay MA. Improved production and purification of minicircle DNA vector free of plasmid bacterial sequences and capable of persistent transgene expression in vivo. Hum Gene Ther. 2005;16:126–131. doi: 10.1089/hum.2005.16.126. [DOI] [PubMed] [Google Scholar]
- Kay MA, He CY, Chen ZY. A robust system for production of minicircle DNA vectors. Nat Biotechnol. 2010;28:1287–1289. doi: 10.1038/nbt.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar MJ, Gilbert R, Lu Y, Liu AB, Guo A, Larochelle N, et al. Factors influencing the efficacy, longevity, and safety of electroporation-assisted plasmid-based gene transfer into mouse muscles. Mol Ther. 2004;10:447–455. doi: 10.1016/j.ymthe.2004.06.642. [DOI] [PubMed] [Google Scholar]
- Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani N, Clausen BE, Stoitzner P. Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunol Rev. 2010;234:120–141. doi: 10.1111/j.0105-2896.2009.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning HD, Rodijk-Olthuis D, van Vlijmen-Willems IM, Joosten LA, Netea MG, Schalkwijk J, et al. A comprehensive analysis of pattern recognition receptors in normal and inflamed human epidermis: upregulation of dectin-1 in psoriasis. J Invest Dermatol. 2010;130:2611–2620. doi: 10.1038/jid.2010.196. [DOI] [PubMed] [Google Scholar]
- Barry MA, Johnston SA. Biological features of genetic immunization. Vaccine. 1997;15:788–791. doi: 10.1016/s0264-410x(96)00265-4. [DOI] [PubMed] [Google Scholar]
- Kang MJ, Kim CK, Kim MY, Hwang TS, Kang SY, Kim WK, et al. Skin permeation, biodistribution, and expression of topically applied plasmid DNA. J Gene Med. 2004;6:1238–1246. doi: 10.1002/jgm.620. [DOI] [PubMed] [Google Scholar]
- Meykadeh N, Mirmohammadsadegh A, Wang Z, Basner-Tschakarjan E, Hengge UR. Topical application of plasmid DNA to mouse and human skin. J Mol Med. 2005;83:897–903. doi: 10.1007/s00109-005-0669-x. [DOI] [PubMed] [Google Scholar]
- Gothelf A, Eriksen J, Hojman P, Gehl J. Duration and level of transgene expression after gene electrotransfer to skin in mice. Gene Ther. 2010;17:839–845. doi: 10.1038/gt.2010.35. [DOI] [PubMed] [Google Scholar]
- van den Berg JH, Nujien B, Beijnen JH, Vincent A, van Tinteren H, Kluge J, et al. Optimization of intradermal vaccination by DNA tattooing in human skin. Hum Gene Ther. 2009;20:181–189. doi: 10.1089/hum.2008.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bins AD, Jorritsma A, Wolkers MC, Hung CF, Wu TC, Schumacher TN, et al. A rapid and potent DNA vaccination strategy defined by in vivo monitoring of antigen expression. Nat Med. 2005;11:899–904. doi: 10.1038/nm1264. [DOI] [PubMed] [Google Scholar]
- Rabinovich BA, Ye Y, Etto T, Chen JQ, Levitsky HI, Overwijk WW, et al. Visualizing fewer than 10 mouse T cells with an enhanced firefly luciferase in immunocompetent mouse models of cancer. Proc Natl Acad Sci USA. 2008;105:14342–14346. doi: 10.1073/pnas.0804105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- Schorpp M, Jäger R, Schellander K, Schenkel J, Wagner EF, Weiher H, et al. The human ubiquitin C promoter directs high ubiquitous expression of transgenes in mice. Nucleic Acids Res. 1996;24:1787–1788. doi: 10.1093/nar/24.9.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellaiche Y, Mogila V, Perrimon N. I-SceI endonuclease, a new tool for studying DNA double-strand break repair mechanisms in Drosophila. Genetics. 1999;152:1037–1044. doi: 10.1093/genetics/152.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- Ingulli E. Tracing tolerance and immunity in vivo by CFSE-labeling of administered cells. Methods Mol Biol. 2007;380:365–376. doi: 10.1007/978-1-59745-395-0_23. [DOI] [PubMed] [Google Scholar]
- Caskey M, Lefebvre F, Filali-Mouhim A, Cameron MJ, Goulet JP, Haddad EK, et al. Synthetic double-stranded RNA induces innate immune responses similar to a live viral vaccine in humans. J Exp Med. 2011;208:2357–2366. doi: 10.1084/jem.20111171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudani R, Chapdelaine Y, Faassen Hv Hv, Smith DK, Shen H, Krishnan L, et al. Multiple mechanisms compensate to enhance tumor-protective CD8(+) T cell response in the long-term despite poor CD8(+) T cell priming initially: comparison between an acute versus a chronic intracellular bacterium expressing a model antigen. J Immunol. 2002;168:5737–5745. doi: 10.4049/jimmunol.168.11.5737. [DOI] [PubMed] [Google Scholar]
- Paul ML, Dwyer DE, Chow C, Robson J, Chambers I, Eagles G, et al. Listeriosis–a review of eighty-four cases. Med J Aust. 1994;160:489–493. [PubMed] [Google Scholar]
- Kägi D, Ledermann B, Bürki K, Hengartner H, Zinkernagel RM. CD8+ T cell-mediated protection against an intracellular bacterium by perforin-dependent cytotoxicity. Eur J Immunol. 1994;24:3068–3072. doi: 10.1002/eji.1830241223. [DOI] [PubMed] [Google Scholar]
- Chirigos MA, Schlick E, Ruffmann R, Budzynski W, Sinibaldi P, Gruys E. Pharmacokinetic and therapeutic activity of polyinosinic-polycytidylic acid stabilized with poly-L-lysine in carboxymethylcellulose [poly(I,C)-LC]. J Biol Response Mod. 1985;4:621–627. [PubMed] [Google Scholar]
- Stenler S, Andersson A, Simonson OE, Lundin KE, Chen ZY, Kay MA, et al. Gene transfer to mouse heart and skeletal muscles using a minicircle expressing human vascular endothelial growth factor. J Cardiovasc Pharmacol. 2009;53:18–23. doi: 10.1097/FJC.0b013e318194234e. [DOI] [PubMed] [Google Scholar]
- Zhang X, Epperly MW, Kay MA, Chen ZY, Dixon T, Franicola D, et al. Radioprotection in vitro and in vivo by minicircle plasmid carrying the human manganese superoxide dismutase transgene. Hum Gene Ther. 2008;19:820–826. doi: 10.1089/hum.2007.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darquet AM, Rangara R, Kreiss P, Schwartz B, Naimi S, Delaère P, et al. Minicircle: an improved DNA molecule for in vitro and in vivo gene transfer. Gene Ther. 1999;6:209–218. doi: 10.1038/sj.gt.3300816. [DOI] [PubMed] [Google Scholar]
- Obar JJ, Khanna KM, Lefrançois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoui S, Davey GM, Lew AM, Heath WR. Equivalent stimulation of naive and memory CD8 T cells by DNA vaccination: a dendritic cell-dependent process. Immunol Cell Biol. 2009;87:255–259. doi: 10.1038/icb.2008.105. [DOI] [PubMed] [Google Scholar]
- Elnekave M, Furmanov K, Nudel I, Arizon M, Clausen BE, Hovav AH. Directly transfected langerin+ dermal dendritic cells potentiate CD8+ T cell responses following intradermal plasmid DNA immunization. J Immunol. 2010;185:3463–3471. doi: 10.4049/jimmunol.1001825. [DOI] [PubMed] [Google Scholar]
- Osborn MJ, McElmurry RT, Lees CJ, DeFeo AP, Chen ZY, Kay MA, et al. Minicircle DNA-based gene therapy coupled with immune modulation permits long-term expression of a-L-iduronidase in mice with mucopolysaccharidosis type I. Mol Ther. 2011;19:450–460. doi: 10.1038/mt.2010.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh EA, Chai CM, de Lumen BO, Neese RA, Hellerstein MK. Dynamics of keratinocytes in vivo using HO labeling: a sensitive marker of epidermal proliferation state. J Invest Dermatol. 2004;123:530–536. doi: 10.1111/j.0022-202X.2004.23303.x. [DOI] [PubMed] [Google Scholar]
- Biernaskie J, Paris M, Morozova O, Fagan BM, Marra M, Pevny L, et al. SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell. 2009;5:610–623. doi: 10.1016/j.stem.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bins AD, Wolkers MC, van den Boom MD, Haanen JB, Schumacher TN. In vivo antigen stability affects DNA vaccine immunogenicity. J Immunol. 2007;179:2126–2133. doi: 10.4049/jimmunol.179.4.2126. [DOI] [PubMed] [Google Scholar]
- Hovav AH, Panas MW, Rahman S, Sircar P, Gillard G, Cayabyab MJ, et al. Duration of antigen expression in vivo following DNA immunization modifies the magnitude, contraction, and secondary responses of CD8+ T lymphocytes. J Immunol. 2007;179:6725–6733. doi: 10.4049/jimmunol.179.10.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, 3rd, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009;6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.