To the editor:
Adeno-associated virus serotype 9 (AAV9) has shown remarkable efficiency in transducing organs in vivo including the heart, liver, and brain.1,2,3,4,5,6 Recently, AAV9 has gained renewed interest due to its ability in crossing the blood–brain barrier upon intravenous injection.7,8,9,10,11,12 In adult mice, AAV9 was shown to preferentially transduce endothelial cells, astrocytes, and neurons after intravenous injection.10 The implications of these findings are enormous: a noninvasive route of administration and widespread gene delivery to the brain. Complete characterization of the gene transfer properties of AAV9, including kinetics of expression, immunogenicity, and biodistribution in different species, will be necessary for clinical translation. Here we show that intravenous injection of single-stranded AAV9 encoding the reporter genes firefly luciferase (Fluc) and green fluorescent protein (GFP) yielded higher transgene expression in the brain of females compared with male mice. This observation was consistent in two strains of mice (nude and C57BL/6) and was correlated with an increased number of AAV genomes in the female brains. These findings stress the importance of proper matching of mice gender in different experimental groups when using systemic injection of AAV9.
Age-matched (6–8 weeks) male and female athymic NU/NU nude mice and C57BL/6N mice were injected intravenously (through the tail vein) with 1.5 × 1012 gene copies (g.c.)/kg of single-stranded AAV9 vector encoding Fluc, driven by the ubiquitously active cytomegalovirus/chicken beta-actin (CBA) hybrid promoter (AAV9-Fluc). The transduction efficiency of AAV9 in the brain and abdomen was monitored 7 and 14 days later using bioluminescence imaging (BLI). We observed a higher bioluminescent signal in the head region of females compared with males in both strains of mice and at both time points (Figure 1a,b). In contrast to the bioluminescent signal in the head region, and in support of previous reports,13,14,15 the Fluc signal from the abdomen (most likely liver) was lower in female mice than in males (Supplementary Figure S1).
Figure 1.
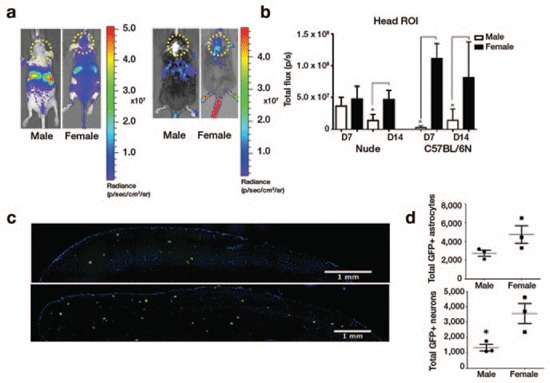
Systemic injection of AAV9 yields higher transduction of the brain of female compared to male mice. (a,b) Male and female nude and C57BL/6 mice were injected via the tail vein with 1.5 × 1012 g.c./kg of AAV9-Fluc vector. Mice were injected 7 and 14 days later with D-luciferin and imaged for Fluc-associated light emission. Representative mice from each group (n = 5 per group) at day 14 are shown in a. Total flux was calculated following data collection by selecting a region of interest (ROI) around the head (b). (c,d) C57BL/6 mice were injected via the tail vein with 5 × 1013 g.c./kg of AAV9-GFP and 2 weeks later killed for histological analysis for GFP expression (n = 3 per group). Representative images of GFP immunohistochemistry from male (top) and female (bottom) mouse brain cortex are shown in c. Quantification of GFP-positive astrocytes and neurons revealed higher numbers of both cell types in females (d). Experiments in a and b were repeated twice, and similar results were obtained. *P < 0.05.
In another set of mice, we performed a kinetic analysis of luciferase expression in the head and liver in both male and female nude and C57BL/6N mice over a 4-week period. We observed a stable luminescence expression in the head of nude and C57BL/6N mice, whereas the abdomen values showed more variability over time in both strains (Supplementary Figure S2). Next we confirmed the BLI results by direct analysis of Fluc enzymatic activity in the brain regions from dead animals, ex vivo. Consistent with BLI, the Fluc levels in the cortex, cerebellum, and olfactory bulbs of female mice were significantly higher (*P < 0.05) compared with the brain of male mice in both strains (Supplementary Figure S3).
We next sought to determine the transduction profile of AAV9 expressing GFP under CBA promoter (AAV9-GFP) in the brains of male and female mice. C57BL/6N mice were injected intravenously with 5 × 1013 g.c./kg of AAV9-GFP. A higher dose was utilized in this experiment to allow visualization of GFP, which is a less sensitive reporter than the Fluc enzyme. At 2 weeks after injection, these mice were killed, then perfused with phosphate-buffered saline, and their brains and livers harvested. Immunostaining with an antibody specific for GFP revealed diffuse expression of almost exclusively neurons and astrocytes throughout the brain (Figure 1c and Supplementary Figure S4). A count of the GFP-positive neurons and astrocytes per square millimeter in the cortex revealed a 1.7-fold (P = 0.111) and 2.7-fold (P = 0.032) increase, respectively, in the female vs. the male brains (Figure 1d). Interestingly, the ratio of GFP-positive astrocytes to neurons was 2.1 and 1.333 for males and females, respectively, which may indicate a slight difference in AAV tropism in the brain between mice genders.
To investigate whether the increase in transduction of the female brain was linked to higher transport of the vector to this organ, quantitative polymerase chain reaction for AAV genome was performed on tissue homogenates from the cortex of mice. In both strains a significantly higher number of AAV genome copies (*P < 0.05) was detected in the brain tissue of females compared with male mice (Supplementary Figure S5).
This study is the first to provide a comparative analysis of transgene expression in the brain of male and female mice upon systemic injection of a single-stranded AAV9 vector. Previous studies using this vector serotype used either male or female mice exclusively11,13 or mixed littermates were used,10 with no direct comparison between different genders. Although the exact mechanism of increased transgene expression in the brain of female mice remains unknown, our findings are important for future AAV9 vector research, specifically when the systemic injection route is chosen. Of immediate application, our results suggest that equal distribution of mouse gender between groups or the use of one gender for a given study is crucial for accurate data interpretation. Additionally, this study warrants further investigation of AAV9-mediated brain transduction of genders of other species (e.g., rats, dogs, nonhuman primates) for possible translation of these findings to human gene therapy.
SUPPLEMENTARY MATERIAL Supplementary Methods Figure S1. Bioluminescent signal in the abdomen region of male and female mice injected systemically with AAV9-Fluc. Figure S2. Bioluminescence kinetic analysis in the head and abdomen region of mice injected with AAV9-Fluc vector. Figure S3. Fluc activity in brain tissue homogenates in male and female mice injected intravenously with AAV9-Fluc. Figure S4. AAV9 transduces primarily neurons and astrocytes in male and female mice upon systemic injection. Figure S5. Quantitation of AAV9 genome copies in the brain of male and female mice after systemic vector injection.
Acknowledgments
This work was supported by grants NIH/NINDS R01NS064983 (to B.A.T.) and R21 NS081374–01 (to C.A.M.). C.A.M. is supported by a Fellowship from the American Brain Tumor Association. C.A.M. has a financial interest in Exosome Diagnostics, Inc. C.A.M.'s interests were reviewed and are managed by the Massachusetts General Hospital and Partners HealthCare in accordance with their conflict-of-interest policies. M.H.W.C. is supported by KWF Kankerbestrijding (Dutch Cancer Society), Stichting Bekker-la Bastide-fonds, the VU University, and VU University Medical Center funds.
We acknowledge the Nucleic Acid Quantitation Core and Vector Development Core (supported by P30NS045776) at Massachusetts General Hospital Neuroscience Center for quantitative polymerase chain reaction analysis and vector development.
Supplementary Material
References
- Bish LT, Morine K, Sleeper MM, Sanmiguel J, Wu D, Gao G.et al. (2008Adeno-associated virus (AAV) serotype 9 provides global cardiac gene transfer superior to AAV1, AAV6, AAV7, and AAV8 in the mouse and rat Hum Gene Ther 191359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X.et al. (2004Clades of adeno-associated viruses are widely disseminated in human tissues J Virol 786381–6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki K, Fuess S, Storm TA, Gibson GA, McTiernan CF, Kay MA.et al. (2006Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8 Mol Ther 1445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Dayton RD, Tatom JB, Henderson KM., and, Henning PP. AAV8, 9, Rh10, Rh43 vector gene transfer in the rat brain: effects of serotype, promoter and purification method. Mol Ther. 2008;16:89–96. doi: 10.1038/sj.mt.6300331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak CA, Mah CS, Thattaliyath BD, Conlon TJ, Lewis MA, Cloutier DE.et al. (2006Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo Circ Res 99e3–9. [DOI] [PubMed] [Google Scholar]
- Zincarelli C, Soltys S, Rengo G., and, Rabinowitz JE. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- Bevan AK, Duque S, Foust KD, Morales PR, Braun L, Schmelzer L.et al. (2011Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders Mol Ther 191971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayton RD, Wang DB., and, Klein RL. The advent of AAV9 expands applications for brain and spinal cord gene delivery. Expert Opin Biol Ther. 2012;12:757–766. doi: 10.1517/14712598.2012.681463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque S, Joussemet B, Riviere C, Marais T, Dubreil L, Douar AM.et al. (2009Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons Mol Ther 171187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SJ, Matagne V, Bachaboina L, Yadav S, Ojeda SR., and, Samulski RJ. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol Ther. 2011;19:1058–1069. doi: 10.1038/mt.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yang B, Mu X, Ahmed SS, Su Q, He R.et al. (2011Several rAAV vectors efficiently cross the blood–brain barrier and transduce neurons and astrocytes in the neonatal mouse central nervous system Mol Ther 191440–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff AM, Ng CY, Zhou J, Spence Y., and, Nathwani AC. Sex significantly influences transduction of murine liver by recombinant adeno-associated viral vectors through an androgen-dependent pathway. Blood. 2003;102:480–488. doi: 10.1182/blood-2002-09-2889. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Cochrane M, McIntosh J, Ng CY, Zhou J, Gray JT.et al. (2009Enhancing transduction of the liver by adeno-associated viral vectors Gene Ther 1660–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paneda A, Vanrell L, Mauleon I, Crettaz JS, Berraondo P, Timmermans EJ.et al. (2009Effect of adeno-associated virus serotype and genomic structure on liver transduction and biodistribution in mice of both genders Hum Gene Ther 20908–917. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


