Abstract
Cell therapy in animal models of Parkinson's disease (PD) is effective after intrastriatal grafting of dopamine (DA) neurons, whereas intranigral transplantation of dopaminergic cells does not cause consistent behavioral recovery. One strategy to promote axonal growth of dopaminergic neurons from the substantia nigra (SN) to the striatum is degradation of inhibitory components such as chondroitin sulphate proteoglycans (CSPG). An alternative is the guidance of DA axons by chemotropic agents. Semaphorins 3A and 3C enhance axonal growth of embryonic stem (ES) cell–derived dopaminergic neurons in vitro, while Semaphorin 3C also attracts them. We asked whether intranigral transplantation of DA neurons, combined with either degradation of CSPG or with grafts of Semaphorin 3–expressing cells, towards the striatum, is effective in establishing a new nigrostriatal dopaminergic pathway in rats with unilateral depletion of DA neurons. We found depolarization-induced DA release in dorsal striatum, DA axonal projections from SN to striatum, and concomitant behavioral improvement in Semaphorin 3–treated animals. These effects were absent in animals that received intranigral transplants combined with Chondroitinase ABC treatment, although partial degradation of CSPG was observed. These results are evidence that Semaphorin 3–directed long-distance axonal growth of dopaminergic neurons, resulting in behavioral improvement, is possible in adult diseased brains.
Introduction
Parkinson's disease (PD) is produced after degeneration of substantia nigra (SN) dopamine (DA) neurons (A9 group) with concomitant dopaminergic denervation of the dorsal striatum. Ectopic grafts of human DA neurons isolated from developing ventral mesencephalon into the caudate-putamen (striatum) benefit patients under certain conditions.1,2 In animal models of PD, intrastriatal grafting of DA neurons differentiated from wild-type and transgenic embryonic stem (ES) cells as well as inducible pluripotent stem cells, improves motor performance for extended time periods,3,4,5,6 restores DA release together with DA transporter binding and suppresses DA receptor super-sensitivity.4 Although transplanted DA neurons extend processes beyond the graft core and establish synaptic contacts with the host striatum,3 DA somata are outside the SN, thus precluding their regulation by neural circuits in the midbrain. Grafting of DA neurons in the adult SN did not improve motor alterations in parkinsonian rats because axons were unable to reach the dorsolateral striatal region.7,8,9
The adult mammalian brain is largely inhibitory for axonal growth due to the presence of inhibitors such as chondroitin sulphate proteoglycans (CSPG) and other extracellular matrix or myelin components.10 One strategy to allow axonal extension of grafted neurons is degradation of such inhibitors; an alternative is to use chemotropic molecules that specifically attract the axons of DA neurons. During establishment of the dopaminergic nigrostriatal pathway, some class 3 Semaphorins (Sema3) are expressed in regions traversed by DA axons en route to reach the striatum.11,12,13,14
Sema3 proteins are secreted and their actions are mediated through activation of receptors containing Neuropilins (Nrp) and plexins.15,16 Sema3A was initially described as a chemo-repellent that also causes axonal growth cone collapse of sensory neurons.17,18 Knockout mice for Sema3A show abnormal sensory innervation and defective cerebral cortex.19 In developing cerebro-cortical explants, Sema3A is repulsive for axons,20,21 but attractive to apical dendrites.21 Such opposing effects are explained by differential local signaling in dendrites21 and the axon.22 Sema3C is attractive for cortical axons20 and has been involved in hemisphere crossing of commissural axons through the corpus callosum.23
In midbrain explants growing in collagen gels, Sema3-transfected human embryonic kidney (HEK) 293 cells had differential effects on DA neurons: Sema3A and Sema3C induced axonal growth, whereas Sema3C attracted DA axons as well.11 These responses to Sema3 are also present in axons of dissociated DA neurons isolated from developing ventral mesencephalon or differentiated in vitro from mouse ES cells in collagen gel assays.24 Seventy-seven percent of DA neurons differentiated in vitro from mouse ES cells express Nrp1, whereas Nrp2 is present in 48% of Tyrosine Hydroxylase (TH)-positive neurons; these proportions of Nrp1+ and Nrp+ neurons are very similar to those found in dopaminergic neurons isolated from the developing midbrain.24 The effects of Sema3 on DA axons in this in vitro system are mediated by Nrp receptors, because only Nrp+ axons were responsive. Furthermore, in ES cell–derived DA neurons, Sema3 effects were blocked by incubation with Nrp-neutralizing antibodies.24
In this study, we report that cografting of DA neurons in the SN with Sema3C-expressing cells along a straight trajectory to the striatum produced significant behavioral recovery in rats with unilateral depletion of DA neurons, similar to DA neuron striatal grafting. The observed improvement after cografting was concomitant with striatal DA release and the establishment of new synaptic contacts between the SN and the dorsal striatum, evidenced by immunohistochemistry and retrograde labeling.
Results
Transplantation of ES cell–derived DA neurons in the striatum causes behavioral recovery
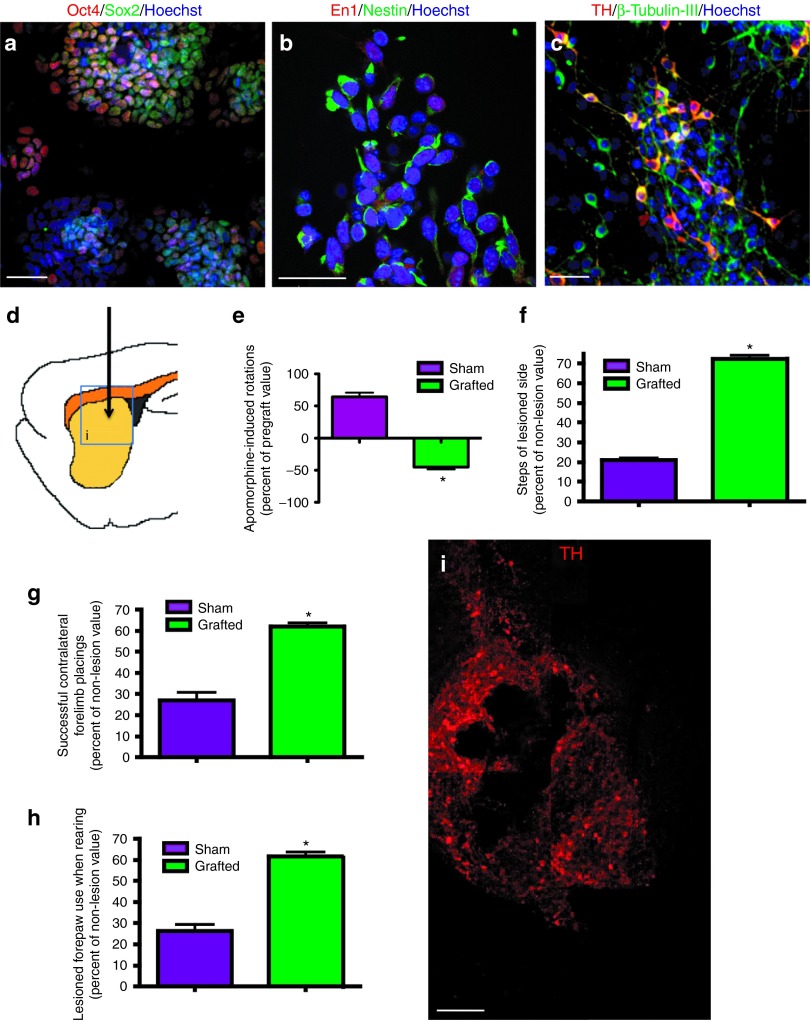
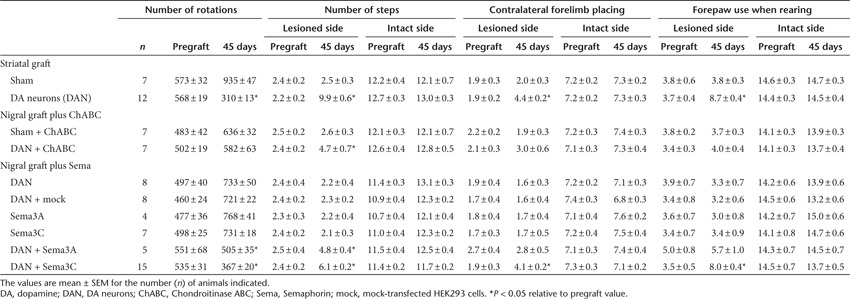
We differentiated mouse wild-type ES cells to DA neurons by a protocol described previously3,4,25 (Figure 1a–c). The effect of grafts in behavioral recovery of parkinsonian traits was assessed in adult young rats lesioned with 6-hydroxyDA (6-OHDA) in one cerebral hemisphere (Figure 1d). Injection of 6-OHDA causes an acute depletion of striatal DA. This PD animal model has been widely used to test several strategies aimed to restore DA levels and behavioral alterations, but lacks the aging component associated to development of sporadic Parkinson in humans. As reported,3,4 striatal transplantation of 5 × 105 ES-derived neurons (in which close to 30% are dopaminergic) induced behavioral improvement in apomorphine-induced rotations and in non-pharmacological motor tests (Figure 1e–h; Table 1). The observed improvement in these behavioral assessments was used as reference for intranigral grafting. TH-positive neurons were present within the graft 45 days after transplantation (Figure 1i).
Figure 1.
Intrastriatal grafting of mouse embryonic stem (ES) cells differentiated to dopamine (DA) neurons cause behavioral recovery in pharmacological and non-pharmacological tests. (a) Undifferentiated ES cells express the pluripotency markers Oct4 and Sox2. (b) ES cell–derived neural precursors are positive for Nestin and Engrailed1 (En1). (c) During stage 5, neurons labeled with β-tubulin-III are present, and 27 ± 3% of these are tyrosine hydroxylase (TH)-positive, indicating differentiation to DA neurons. Nuclear staining with Hoechst 33258 is shown in a–c. Scale bar = 30 µm. (d) Half a million ES cells–differentiated in vitro to DA neurons were grafted in the lesioned dorsal striatum of 6-OHDA–injected hemiparkinsonian adult rats as shown in the scheme. (e–h) Behavioral recovery was monitored for 45 days. Pharmacological and non-pharmacological behavioral tests showed, as previously reported, that grafted animals improved significantly. (e) Apomorphine-induced rotations decreased in grafted animals 45 days after grafting compared with the sham group. Accordingly, (f) adjusting step, (g) forelimb placing, and (h) cylinder tests showed a significant recovery in the grafted group 45 days after grafting. *P < 0.05. (i) Representative composition of a graft 45 days after transplantation decorated with anti-TH antibodies in the area depicted in a. Scale bar = 100 μm.
Table 1. Summary of behavioral evaluations of hemiparkinsonian rats, before and after grafting.
Partial degradation of CSPG is not sufficient to allow growth of DA axons from the SN to the striatum
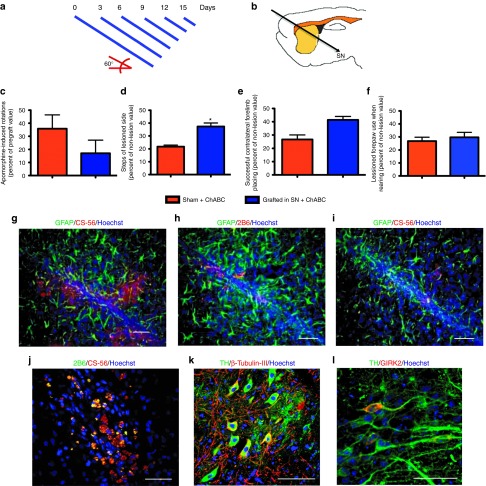
We asked whether DA neuron transplantation in the SN, combined with degradation of CSPG, is sufficient to direct dopaminergic axonal growth to the striatum. Degradation of CSPG by Chondroitinase ABC (ChABC) has allowed axonal regeneration and plasticity in the spinal cord.26,27 To test whether degradation of CSPG permits DA axons to grow from SN to striatum, we performed transplantation of lesioned animals with 3.3 × 105 ES cell–differentiated DA neurons (to use the same cell suspension for striatal grafting, but in a smaller volume) in a 60° angle to reach the SN pars compacta, followed by application of ChABC along a straight line towards the striatum (encompassing cerebral cortex, striatum, internal capsule, thalamus, and zona incerta), either as a single bolus (data not shown) or in repeated injections at different days (Figure 2a,b).
Figure 2.
Partial degradation of chondroitin sulphate proteoglycans (CSPG) through a nigrostriatal trajectory does not provide functional recovery after dopamine (DA) neuron transplantation in the substantia nigra (SN). (a) Diagram of the stylets of decreasing length used for injection of Chondroitinase ABC (ChABC) at the indicated days. (b) Schematic representation of the trajectory for transplantation and further administration of ChABC. The head of animals was tilted 60° and grafting was performed with mouse ES cell–derived DA neurons in the SN. On days 0, 3, 6, 9, 12 and 15 after transplantation, 0.03 U of ChABC divided in six applications going towards the striatum at 0.5 mm intervals, were made. After grafting and ChABC treatment, animals did not recover when tested in the (c) rotational (e) forelimb placing, or (f) cylinder tests when compared with the control group. (d) A significant (*P < 0.05) recovery is observed in the adjusting step test. (g) As a control, injections of ChABC vehicle were made, and the brains of these animals were analyzed after 45 days. We found the presence of full forms of CSPG recognized by antibody CS-56 associated with Glial Fibrillary Acidic Protein (GFAP) immunoreactivity at the injection trajectory. In contrast, administration of ChABC in the described scheme caused partial digestion of CSPG, as evidenced by the appearance of labeling with the 2B6 antibody, that recognize (h) hydrolyzed CSPG forms, and (i) a parallel decrease in full-CSPG forms. Furthermore, in ChABC-treated animals, (j) full and partially hydrolyzed forms of CSPG are colocalized. Scale bar (g–j) = 50 µm. After 45 days of grafting, (k) tyrosine hydroxylase (TH)-positive neurons were present in the SN and (l) these neurons express the A9 dopaminergic marker Girk2. Nuclei were stained with Hoechst 33258. Scale bar (k–l) = 30 μm.
On the day of surgery, ChABC was injected at 0.01 U/μl in 6 applications of 0.5 μl each, retracting the injector 0.5 mm every time. At days 3, 6, 9, 12, and 15 after grafting, six additional ChABC injections were made per session, but since the injector was 0.5 mm shorter every time, these applications started closer to the striatum. Every injection at the indicated days consisted of 3 μl of ChABC solution encompassing 2.5 mm. Behavioral evaluations were performed during 45 days. Neither axonal growth nor overt behavioral recovery was detected, although a significant difference in the stepping test was observed (Figure 2c–f; Table 1). As a control, ChABC application was made in the absence of nigral grafts (sham + ChABC). Additional controls included injections of ChABC vehicle, in the absence of enzyme or grafts, to analyze the expression of CSPG at the injection trajectory from the nigra to the striatum. One week after vehicle administration, we found that reactivity for Glial Fibrillary Acidic Protein is closely associated with the presence of full forms of CSPG detected with antibody CS-56 (data not shown). After 45 days, CS-56 immunoreactivity is still present in animals that did not receive ChABC (Figure 2g).
Animals that received ChABC, with or without graft, were sacrificed and their brains analyzed at 7 weeks after surgery. Administration of ChABC in the described scheme, regardless of DA neuron grafting, caused partial digestion of CSPG as evidenced by the appearance of labeling with the 2B6 antibody that recognizes hydrolyzed CSPG forms (Figure 2h), and a parallel decrease in full-CSPG forms (Figure 2i). Furthermore, in ChABC-treated animals, full and partially hydrolyzed forms of CSPG are colocalized, indicating that ChABC degraded CSPG, albeit not completely (Figure 2j). After 45 days of grafting, dopaminergic TH-positive neurons were present (Figure 2k) and express the specific marker for A9 DA neurons GIRK2 (Figure 2l). Although intranigral grafts contained DA neurons, and ChABC degraded CSPG, at least partially, no recovery was observed. We did not find any GIRK2-positive cells in the lesioned side of sham animals, ruling out a regenerative effect of ChABC on DA neurons.
Transfected HEK293 cells secrete Sema3 proteins when grafted in rat brains
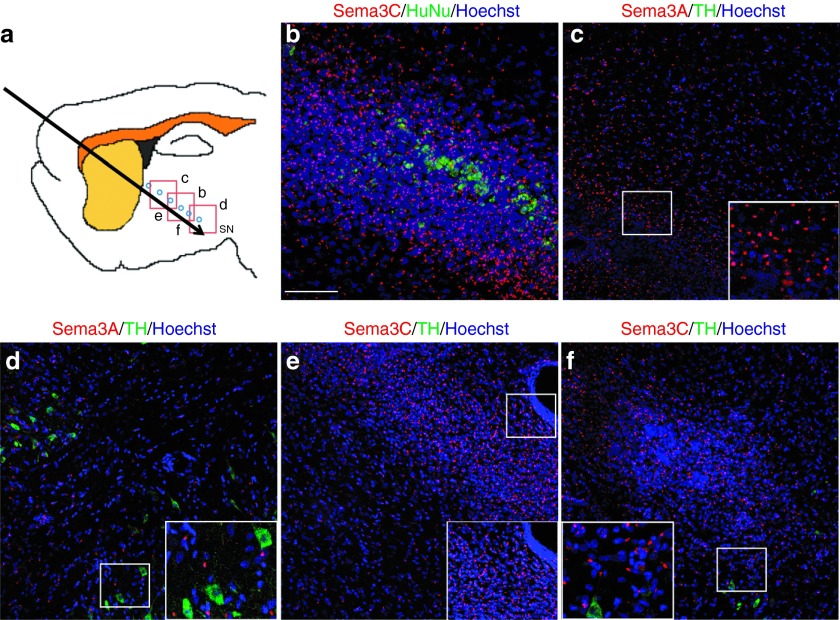
A similar paradigm, but substituting ChABC treatment with grafts of HEK293 cells transfected with expression vectors for Sema3A or Sema3C,24 according to National Institutes of Health guidelines for recombinant DNA, was then tested (Figure 3a). After intranigral grafting of DA neurons, HEK293 cells were transplanted in six deposits (7000 cells each) separated by 500 µm as shown in Figure 3a. To identify grafted HEK293 cells in the rodent brain, we used antihuman nuclei (HuNu) antibodies (Figure 3b), which also labeled cultured transfected HEK293 cells and mock-transfected control cells in the brain (Supplementary Figure S1). One week after cografting transfected cells and DA neurons, specific immunostaining with antibodies for either Sema3A or Sema3C was apparent within HEK cells and in surrounding areas close to transfected cells (Figure 3b–f), whereas the brains of lesioned rats without transplantation (data not shown), or grafted with mock-transfected HEK293 cells were devoid of Sema3A and Sema3C staining (Supplementary Figure S1). Sema3C and Sema3A detection with this punctate pattern is consistent with previous reports.28,29 Only a few transplanted dopaminergic neurons are shown in these images (Figure 3d,f).
Figure 3.
Transfected HEK293 cells secrete Sema3C or Sema3A in vivo. (a) Diagram showing the transplantation sites for HEK293 cells (blue circles) and the location of subsequent panels. (b) In animals that received Sema3C-transfected grafts, HEK293 cells were identified with the antihuman nuclei (HuNu) antibody, and Sema3C immunoreactivity was detected 1 week after grafting. (c–f) Detection of (c,d) Sema3A and (e,f) Sema3C in the (c,e) injection path, and close to the substantia nigra (SN), where (d,f) grafted TH-positive cells were present 1 week after transplantation. The non-transplanted contralateral hemispheres of these rats did not show any labeling with Sema 3A nor 3C antibodies (data not shown), and Sema3C was absent in rats grafted with mock-transfected HEK293 cells (Supplementary Figure S1). Nuclei were marked with Hoechst 33258. Scale bar in b applies to b–f = 50 µm.
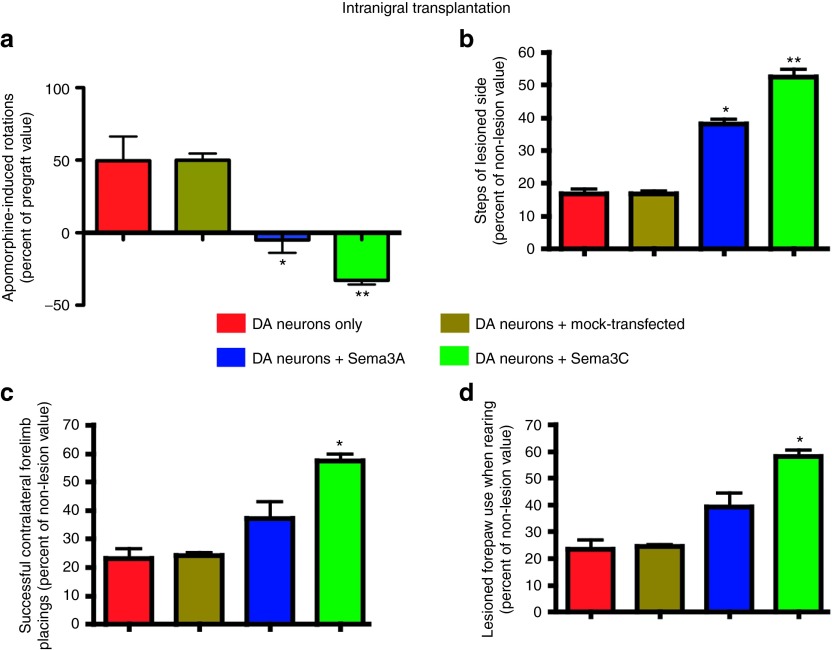
Cografting of transfected HEK cells with intranigral transplantation of DA neurons results in behavioral recovery of hemiparkinsonian rats
Behavioral and histological evaluations were performed on lesioned rats 45 days after intranigral transplantation of ES cell–derived DA neurons, either alone or in combination with Sema3- or mock-transfected HEK293 cells as shown in Figure 3a. DA neuron transplantation alone did not induce behavioral improvement, nor did the combination of mock-transfected HEK293 cells with DA neurons (Figure 4a–d; Table 1). In contrast, intranigral DA neuron grafting together with Sema3A, but especially with Sema3C, produced significant benefit in pharmacological and spontaneous motor tasks (Figure 4a–d; Table 1). Additional controls consisting of transplantation of HEK293 cells transfected with either Sema3A or Sema3C, in the absence of DA neurons, had no effect on the motor deficits of lesioned animals (Supplementary Figure S2; Table 1).
Figure 4.
Cotransplantation of dopamine (DA) neurons with Semaphorin 3–expressing cells elicits functional recovery. (a) Cografts of DA neurons in the SN with transfected HEK293 cells caused significant behavioral recovery after apomorphine challenge. (b) In keeping with this, the adjusting step test showed significant improvement when either Sema3A or Sema3C were combined with intranigral DA neuron grafts. (c) Forelimb placing and (d) cylinder tests revealed that Sema3C, together with DA neurons, were effective in reverting motor deficits in hemiparkinsonian animals. *P < 0.05 and **P < 0.01 versus DA neurons only and also versus DA neurons plus mock-transfected HEK293 cells.
Cografting of Sema3-expressing cells with intranigral DA neurons causes DA release in the dorsal striatum
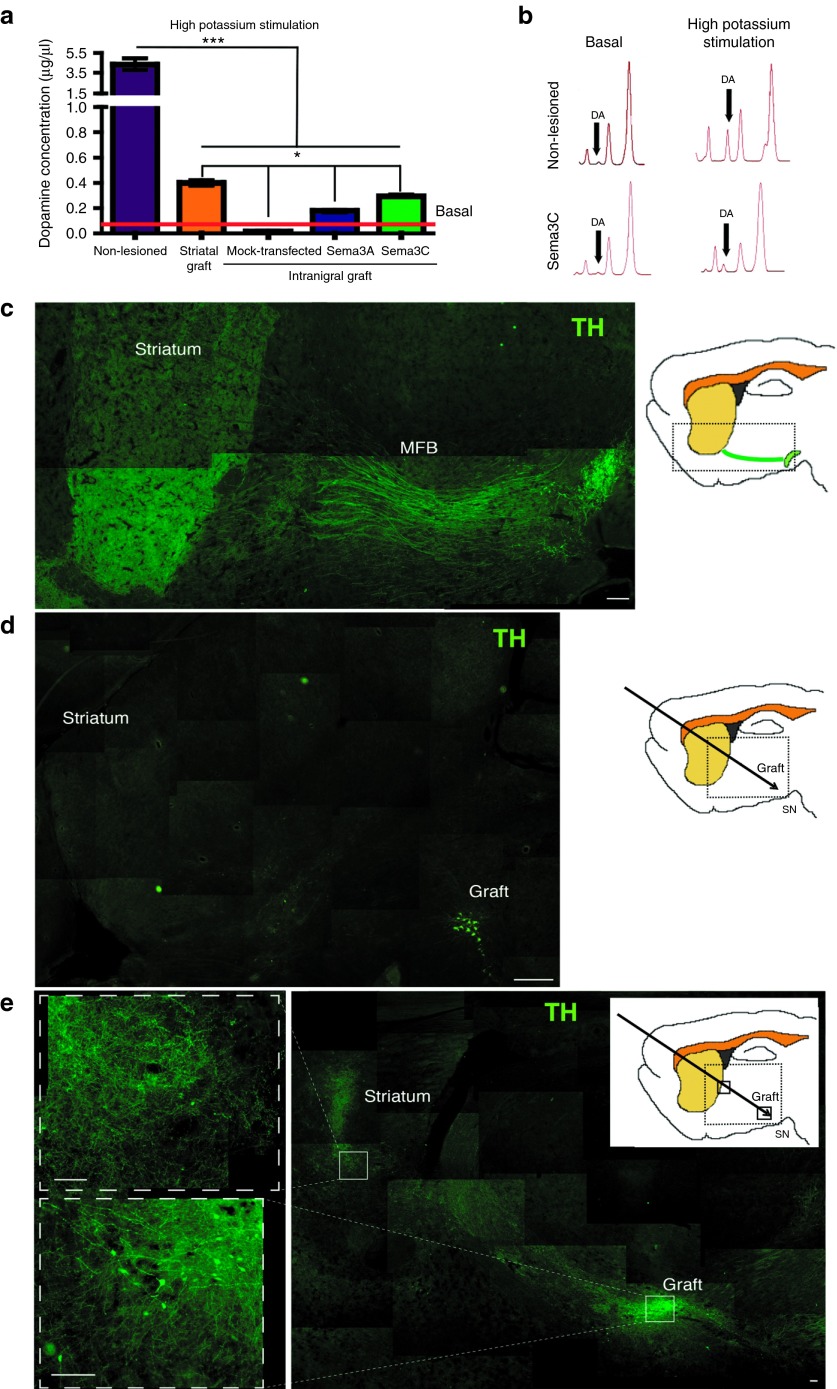
Intrastriatal grafting of DA neurons into hemiparkinsonian rats causes depolarization-induced DA release in the dorsolateral striatum in vivo.4 We measured striatal extracellular DA concentrations by bilateral microdialysis in parkinsonian animals, after receiving striatal grafts of DA cells, or nigral cotransplants of DA neurons and HEK293 cells. The non-lesioned striatum of all animals showed a significant potassium-stimulated DA release when compared with basal levels (Figure 5a). Consistent with previous work,4 DA release was detected after striatal grafting of ES cell–derived DA neurons, albeit at significantly lower levels than those observed in non-lesioned conditions. Intranigral transplantation of DA cells together with Sema3A- or Sema3C-transfected cells resulted in DA release above basal concentrations, but below intrastriatal graft levels (Figure 5a,b). For the lesioned hemisphere that received intranigral DA neurons and mock-transfected HEK293 cells, depolarization-induced striatal DA release was undetectable (Figure 5a).
Figure 5.
Striatal dopamine (DA) release and formation of a new dopaminergic pathway between the substantia nigra (SN) and the striatum after intranigral grafting combined with Sema3C-expressing cells. (a) In vivo DA release measured by microdialysis. Non-lesioned striatum presented DA concentrations significantly above baseline (red) after stimulation with high potassium. Intrastriatal grafts as well as intranigral DA neuron grafts combined with transfected cells (Sema3A or Sema3C) also showed DA release after depolarization. Grafts of DA neurons plus mock-transfected HEK293 cells did not release DA. (b) Representative electrofluorograms for basal and depolarization-induced levels in non-lesioned conditions, and after DA neuron + Sema3C-transfected HEK293 cografts. (c) Composition showing the intact nigrostriatal pathway, including the medial forebrain bundle (MFB). Scale bar = 100 µm. (d) Composite photograph showing a representative lesioned rat that received DA neurons in the SN and mock-transfected cells. Note the drastic decrease in tyrosine hydroxylase (TH) reactivity in the striatal area and the loss of staining in the MFB. Scale bar = 200 µm. (e) Low-power composition of a rat that received DA neurons and Sema3C-expressing cells, analyzed by TH staining 45 days after grafting. Dopaminergic somata are localized in the SN and absent from the striatal area. TH+ processes can be observed close to the striatum in the upper inset; this condition also increased striatal TH immunoreactivity. Scale bar = 50 µm. *P < 0.05 and ***P < 0.001.
Establishment of new dopaminergic synaptic communication assessed by retrograde tracing in Sema3C-grafted animals
To observe DA processes, we performed TH staining in lesioned rats grafted intranigrally in combination with Sema3C-expressing cells, and compared them with the normal nigrostriatal pathway present in non-lesioned conditions and with control animals receiving intranigral grafts plus mock-transfected cells. In sagittal sections, the typical morphology of SN and the medial forebrain bundle was apparent (Figure 5c). In sharp contrast, lesioned rats that received DA neurons together with mock-transfected cells (Figure 5d) had some TH-positive neurons in the SN, but presented dramatic decreases of TH immunoreactivity in the striatal area, as well as in the medial forebrain bundle. After cografting of DA neurons and Sema3C-transfected cells, we found dopaminergic somata in the SN and TH+ neuronal processes extending towards the striatal region (amplified in the superior inset of Figure 5e). The presence of TH in a straight line from the SN to the striatum in the Sema3C group strongly suggests re-innervation rather than regeneration or protection of the natural nigrostriatal pathway. This brain section is shown because re-innervation of the striatum was more evident, although at this plane of section there were fewer grafted dopaminergic somata in the SN (compare the graft region of Figure 5e with the density of DA neurons in Figure 7b,c,e). Importantly, the observed recovery after intranigral grafting with Sema3C-transfected cells was independent of the presence of dopaminergic somata in the striatum, ruling out migration of TH-positive neurons from the SN to the striatal area (Figure 5e). To estimate the degree of striatal re-innervation, we measured TH immunoreactivity in the groups represented by Figure 5d,e and related them to the non-lesioned control (Figure 5c), set arbitrarily as 100%. Following normalization, the intranigral graft combined with mock-transfected cells resulted in a significant decrease to 12.4 ± 0.6% of control (mean ± SEM, n = 3; P < 0.0001). Intranigral transplantation of DA neurons and Sema3C-transfected cells resulted in a significant increase in TH reactivity in the striatum to 55.8 ± 2.8% of control (mean ± SEM, n = 3; P < 0.0001 relative to intranigral grafting plus mock-transfected cells).
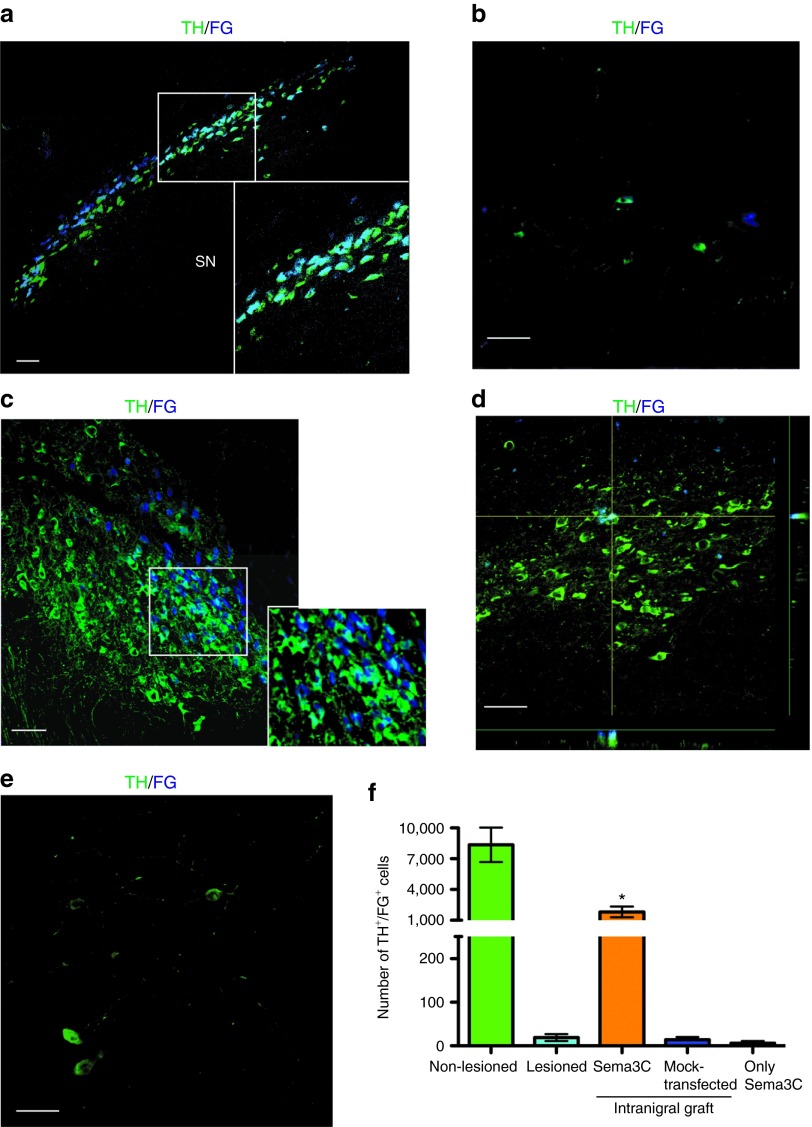
Synaptic contacts can be studied with retrograde tracers that are taken up by the axon and transported to the soma of the neuron. Fluoro-gold (FG) has been extensively used because is preserved after fixation and does not interfere with immunohistochemistry.30 FG does not penetrate intact axons and has been used to detect monosynaptic contacts between the SN and striatum after DA neuron grafting.31 To establish whether intranigrally transplanted DA neurons extended axons to reach the dorsal striatum, we performed injections of FG in two striatal sites. In non-lesioned hemispheres of hemiparkinsonian rats, TH+ somata in the SN labeled with this retrograde marker were detected 3 days after injection (Figure 6a). In lesioned animals without grafted DA neurons, the number of TH+ cells that were also FG+ decreased notably (Figure 6b). In animals with nigral grafts of DA neurons combined with Sema3C-transfected cells, we found a considerable number of TH+/FG+ neurons in the SN (Figure 6c), indicating that axons of transplanted DA neurons had projected to the dorsal striatum. The distribution of TH immunoreactivity in the neuronal soma is not homogeneous and stains preferentially the contours of the cells, but the vast majority of FG+ cells are dopaminergic, as shown in the inset of Figure 6c. In sharp contrast, animals that received DA neurons + mock-transfected HEK cells (Figure 6d) or Sema3C-transfected HEK cells in the absence of DA neurons (Figure 6e) have very few TH+/FG+ neurons. Stereological quantification of TH+/FG+ cells in the SN revealed significant differences between the DA neuron + Sema3C condition, in comparison with the remaining experimental groups (Figure 6f). The number of FG+ DA neurons in the non-lesioned condition is in agreement with previous studies.32
Figure 6.
Establishment of synaptic contacts between the dopaminergic somata in the substantia nigra (SN) and the striatal region, assessed by Fluoro-gold (FG) injection in Sema3C animals. Two striatal FG injections were made 3 days before euthanasia, and FG/TH detection was performed. (a) Composition showing TH+ somata in SN after injection of FG in the non-lesioned striatum. The inset shows a magnification of dopaminergic cell bodies. (b) A lesioned non-grafted animal showed a significant decrease in numbers of TH/FG double-positive cells in the SN. (c) TH/FG detection 45 days after intranigral grafting combined with Sema3C-transfected cells. The inset shows a magnification of dopaminergic cell bodies positive for FG. The vast majority of FG-positive cells are also dopaminergic, although TH immunostaining marks preferentially the contours of cells. (d) Only a few neurons are double-positive in a rat the received intranigral transplantation + mock-transfected HEK cells. The orthogonal projections show colocalization of TH and FG. (e) Representative image of a rat that received Sema3C-transfected cells in the absence of dopamine (DA) neurons (only Sema3C). Note that the number of TH-positive neurons is smaller than in c and d. (f) Quantification of TH+/FG+ neurons in the SN of (a) non-lesioned, (b) lesioned, (c) intranigral graft of DA neurons + Sema3C-transfected cells, (d) intranigral grafting + mock-transfected, and (e) only Sema3C-transfected HEK groups. Scale bar (a–e) = 30 µm. *P < 0.05 compared with remaining groups. TH, tyrosine hydroxylase.
Intranigral grafts contain midbrain DA neurons and its survival rate is similar to that found in striatal grafts
To confirm the identity of DA neurons grafted in the SN, we detected the expression of mesencephalic (Pitx3) and A9 (GIRK2) DA neuron markers among the surviving TH+ cells in the SN. Differentiated DA neurons express Pitx3 in vitro (Figure 7a) and are positive for both Pitx3 and the potassium channel GIRK2 after intranigral grafting (Figure 7b–d). An example of surviving grafted dopaminergic neurons in the SN is shown in Figure 7e. Stereological counting of intranigrally or intrastriatally grafted dopaminergic neurons was performed. The number of surviving TH+ neurons in the striatum (see Figure 1i) was 43% higher than that found in intranigral grafts (Figure 7f), consistent with the 51% higher number of implanted DA neurons in the former type of graft, showing similar survival rates in both regions. We did not find uncontrolled growth of HEK293 cells in agreement with previous work.33 No teratomas were present in striatal or nigral DA neuron grafts, in line with previous studies, where animals that received ES cell–derived DA cells in the striatum were free of teratomas 32 weeks after grafting.4
Figure 7.
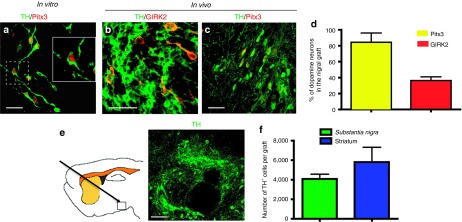
Expression of dopaminergic markers before and after intranigral transplantation, and quantification of surviving dopamine (DA) neurons in nigral and striatal grafts after 45 days. (a) DA neurons at day 6 of stage 5 express tyrosine hydroxylase (TH) and Pitx3. (b) Coexpression of TH and GIRK2 in DA neurons in the substantia nigra (SN) of a rat that received cografts of ES cell–derived neurons and Sema3C-transfected cells. (c) Intranigrally grafted TH-positive neurons also expresses Pitx3. Scale bar (a–c) = 30 µm. (d) Quantification of the proportion of TH+ neurons that express Pitx3 or GIRK2 in the SN, 45 days after transplantation. (e) Localization and representative image of nigral grafts stained with TH. Scale bar = 50 µm. (f) The number of surviving DA neurons after 45 days was quantified by stereological counting in the SN after intranigral grafts, or in the striatum after intrastriatal transplantation (see Figure 1i). No significant differences were found in intranigral dopaminergic transplantation combined with either Sema3A or Sema3C, and therefore these groups were pooled. Note that striatal grafts received 5 × 105 cells, whereas nigral grafts consisted of 3.3 × 105 cells, and therefore similar survival rates of DA neurons were found in both regions.
Discussion
Our results show that DA axon guidance can be achieved using class 3 Semaphorins in the lesioned adult mammalian brain, with behavioral improvement comparable with that of the current gold-standard, intrastriatal transplantation of DA cells. Intranigral transplantation of DA neurons combined with secreted Sema3 proteins caused significant recovery in pharmacological and spontaneous motor tests, a finding that correlated with DA release in the dorsal striatum and with anatomical connections of TH+ axons from the SN to the striatum in hemiparkinsonian rats. Recovery was observed only when DA neurons were cografted with Sema3-expressing cells, as neither type of graft by itself was effective; DA neurons cografted with mock-transfected cells did not elicit behavioral recovery, thus ruling out non-specific effects by HEK cells.
Our first approximation was to degrade CSPG by ChABC administration after nigral transplantation. Application of ChABC has been shown to promote plasticity and regeneration of axons27 and functional restoration of respiration, when combined with a peripheral nerve auto graft, in the damaged spinal cord.26 Unfortunately, axons of grafted DA neurons did not follow the path of hydrolyzed CSPG in hemiparkinsonian rats, although only partial degradation of CSPG was detected along the needle tract 45 days after grafting. No overt behavioral recovery was achieved by this combinatorial strategy but we found a significant recovery in the number of steps of the lesioned forelimb. It is noteworthy that systemic apomorphine by itself has been reported to improve the stepping test in lesioned rats.9 Although we do not know the reason for the aforementioned recovery, we speculate that repeated testing of apomorphine-induced rotational behavior might be causing some benefit, but only when combining intranigral grafting with ChABC, because intranigral neurons alone did not improve the stepping test (Figure 4b).
Interestingly, interactions of Semaphorins with different components of extracellular matrix can have opposing effects: membrane-anchored Sema5A is attractive when interacting with heparan sulphate proteoglycans to guide diencephalic axons; in contrast, when Sema5A interacts with CSPG, a repulsive effect is observed. Repulsion of such axons by Sema3F is unaffected after Heparinase treatment, suggesting that interaction of soluble class 3 Sema with heparan sulphate is not required for repulsion.34
In the combinatorial strategy described here, we did not find TH-positive somata in the striatum, and therefore the recovery cannot be ascribed to DA neuron migration from the SN. In agreement, DA neurons exposed to Sema3-transfected HEK293 aggregates in vitro did not migrate, and only showed axonal responses.24 Sema3 proteins have been shown to influence migration of adult oligodendrocyte precursor cells: Sema3F attracted these cells in vitro and caused mobilization of precursors to the demyelinating areas caused by lysophosphatidylcholine administration in the spinal cord in vivo. Sema3A, on the other hand, was shown to be repulsive for pre-oligodendrocytes in vivo and Nrp1Sema− mice, which possess a modified Nrp1 receptor that cannot be activated by Sema3A, showed increased levels of precursors after demyelination, suggesting a negative role of Sema3A upon oligodendrocyte precursors recruitment. These effects were independent of cell proliferation and apoptotic cell death.35 Regarding the effects of Sema3A on neuronal populations different from mesencephalic DA neurons, it has been reported that is repellent to several types of developing motor axons,36 and interferes with regeneration of axons after adult spinal cord transection.37
Recently, several groups described the regeneration of the nigrostriatal pathway in hemiparkinsonian rodents. Rats partially lesioned and treated with a D3 DA receptor agonist had increased numbers of newborn TH+ neurons in the SN. Behavioral recovery in rotational and paw reaching tests were reported; although this treatment increased retrograde labeling from striatum to SN, no evidence of newborn TH+ neurons labeled with FG in the SN was provided.38 In two similar articles, mice were injected unilaterally with 6-OHDA in the SN, and grafted in the same region with fetal mesencephalic tissue. These animals showed anatomical reconstitution of dopaminergic meso-striatal pathway, and recovery in drug-induced rotational tests,39,40 which are not as stringent as non-pharmacological evaluations. In one case, DA lesions were partial and the innervation was observed mostly in ventral striatum; furthermore, over expression of GDNF enhanced TH+ outgrowth to the thalamus and sprouting to the globus pallidus, and it was only under such conditions that behavior was significantly improved.40 The main differences between these studies and our results are that we used ES cells–derived DA neurons, evaluated both pharmacological and spontaneous motor test to monitor recovery, detected DA release in a large area of the striatum through microdialysis, and found synaptic connections by FG labeling. The evidence from all these tests reveals DA innervation of the striatum from the SN.
There are two major subgroups of midbrain dopaminergic neurons: A9 neurons in the SN pars compacta and A10 neurons in the ventral tegmental area. In addition to their different anatomical localization, their axons project to distinct targets: A9 neurons innervate the dorsolateral striatum and A10 cells contact the ventral striatum, nucleus accumbens and cortical areas. Pitx3 knockout mice lack A9 (i.e., GIRK2+) DA neurons in the mesencephalon41 and suspensions of differentiated ES cells enriched in Pitx3-expressing neurons induce recovery of parkinsonian rats after intrastriatal grafting.42 Recent experiments show that A9 DA neurons are required to elicit behavioral improvement and efficient re-innervation of the dorsolateral striatum with TH+ fibers in hemiparkinsonian rats.43 Interestingly, in animals that recovered after ectopic striatal transplantation, GIRK2+ neurons were found predominantly in the periphery of the grafts,44 suggesting a higher ability of these DA neurons to establish synaptic contacts with the host tissue. Our results show the presence of DA neurons with mesencephalic (Pitx3-positive) and a lower proportion of A9 (GIRK2+) dopaminergic cells in the SN after transplantation; these DA neurons re-innervate the dorsolateral region of the lesioned striatum, the region normally innervated by endogenous DA neurons.
The use of mesencephalic fetal donor tissue has a fundamental limitation for the clinical use of DA neuron grafts in PD due to the scarcity of DA neurons from donors and to the inability of neural precursors from this brain area to preserve dopaminergic potential after expansion.45 ES cells are regarded as promising in regenerative medicine as they overcome the limitations of cell number and stable DA differentiation potential. In addition, these cells have the important advantage of being more amenable to genetic manipulation. The clinical use of human ES cell–derived neurons, however, will require not only the optimization of DA neuron enrichment protocols and the complete elimination of teratoma-forming cells prior to grafting, but also the development of efficient strategies to guide axons of intranigrally grafted neurons to the striatum. Our study constitutes an important step in that direction although preferable protocols are expected to use cell-free systems to deliver the guidance cues to the grafted brain. Moreover, further studies involving different approaches to those used here, are required in this line of research to assess the effective integration of grafted DA neurons into functional circuits to ensure that they are also innervated correctly in the midbrain and are thus capable of physiologically regulated DA release.
In conclusion, we describe conditions to promote DA axonal growth after intranigral grafting that allowed the establishment of new terminals in the host striatum, resulting in behavioral recovery of adult hemiparkinsonian rats. Taken together, our results constitute proof-of-principle that directed long-distance axonal growth of grafted DA neurons, combined with neurotropic molecules is possible. These findings reinforce the idea that stem cell–based strategies can be effective to restore input to striatal neurons, normalizing information flow through a damaged motor circuit.
Materials and Methods
In vitro differentiation of ES cells to DA neurons. We used R1 mouse ES cells, which have been proved to produce DA neurons.3,4,25 The differentiation procedure was performed as reported.46 Briefly, undifferentiated ES cells expressing the pluripotent markers Oct4 and Sox2 (stage 1; Figure 1a) were cultured on gelatin-coated tissue culture plates in the presence of 1000 U/ml of leukemia inhibitory factor (Merck Millipore, Billerica, MA) in Knockout DMEM medium (Gibco, Carlsbad, CA) supplemented with 15% ES cell–tested fetal bovine serum (Wisent, Quebec, Canada). To induce formation of floating embryoid bodies (EB's, stage 2), cells were dissociated with Trypsin solution (Gibco) and plated onto bacterial dishes in the presence of leukemia inhibitory factor. EB's were cultured for 4 days and then seeded onto adherent tissue culture plates. Enrichment of Nestin-positive cells (stage 3) was initiated in serum-free Insulin-Transferrin-Selenite medium (Gibco) supplemented with 5 µg/ml Fibronectin (Invitrogen, Carlsbad, CA). After 9–11 days of culture, cells were dissociated with Trypsin and seeded on dishes or glass coverslips precoated with 15 µg/ml poly-l-ornithine (Sigma, St Louis, MO) and 1 µg/ml Fibronectin in N2 medium (Gibco) containing 10 ng/ml fibroblast growth factor 2, 100 ng/ml fibroblast growth factor 8 and 100 ng/ml of human Sonic Hedgehog (growth factors from R&D Systems, Minneapolis, MN) during 4 days to form DA precursors (stage 4). At this stage, a high proportion of cells (>93%) express the neural stem cell marker Nestin (Figure 1b). Differentiation (stage 5) was induced by growth factor withdrawal and feeding with N2 medium with 200 µmol/l ascorbic acid for 3 days for grafting or for 6–8 days for terminal differentiation analysis. The yield of DA neurons with this procedure was 27 ± 3%, in agreement with previous work.25,46
Immunocytochemistry and immunohistochemistry. Standard immunocytochemical procedures were carried out using described protocols.25 The procedure, antibody distributors, and used dilutions are listed in Supplementary Materials and Methods.
6-OHDA lesion, apomorphine-induced rotational test and striatal transplantation. Lesion, striatal transplantation, and behavioral evaluations were made as described.3,4 Surgical procedures were approved by the local Animal Care and Use Committee and complied local (NOM-062-ZOO-1999) and international guidelines (Animal Welfare Assurance A5281-01). Adult female Wistar rats that were at least 2 months old (230–250 g) were housed with a 12-hour light–dark cycle and room temperature at 22 ± 2 °C with free access to water and food. Briefly, rats were placed in an airtight anesthesia chamber supplied with 3% sevoflurane (Abbot Laboratories, Abbott Park, IL) in 95% O2–5% CO2 gas mixture. To minimize stress, rats were minimally handled and maintained with inhaled anesthetic (0.5–1.5% sevoflurane). Rats were injected with 8 µg of 6-hydroxyDA (6-OHDA; Sigma) in the left medial forebrain bundle with the following stereotactic coordinates relative to bregma: antero-posterior (AP), −1.0 mm; lateral (L), 1.5 mm; ventral (V), −7.5 mm. Fifteen days after injection, apomorphine (1 mg/kg, s.c.)-induced rotations were quantified over 60 minutes, and animals were classified as lesioned when they had more than seven contralateral turns per minute. Lesioned animals that received sham surgery showed an increase in apomorphine-induced rotations after 45 days, in agreement with previous work.47 Hemiparkinsonian rats were grafted in the dorsal striatum with mouse ES cells–derived TH-positive neurons differentiated as described above. At day 3 of stage 5, cells were trypsinized and resuspended at a density of 167,000 viable cells per µl. Three microliter of cell suspension containing 0.5 × 106 viable cells were grafted into the lesioned dorsolateral striatum with the following coordinates: AP, 0.0 mm; L, 3.0 mm; V, −5.5 mm. Cell suspension was injected in three deposits separated by 0.5 mm (n = 12). In sham animals (n = 7), 3 µl of N2 medium was injected with the same coordinates. All animals were immunosuppressed daily with cyclosporine A (10 mg/kg; Neoral, Novartis, East Hanover, NJ) starting 24 hours before grafting. An experimenter blind to the treatment of each animal evaluated apomorphine-induced rotations every 2 weeks, although only results 45 days after grafting are shown. Results are represented as percentage of pregraft values in all rotational graphs. The mean values ± SEM for the rotational test before normalization, are presented in Table 1.
Non-pharmacological behavioral tests. To assess more natural behavior, we conducted the following tests; all these evaluations were made blinded to the experimental treatment of animals.
Adjusting step test: This evaluation was performed as described.9 The rat is held with one hand, holding and lifting the hindlimbs, and then moved laterally (0.9 m in 5 seconds) close to the surface of a table. Each forelimb was independently evaluated by counting the number of adjusting steps on the table surface. The value of the lesioned forelimb was normalized with the number of steps registered for the non-lesioned paw in these trials, and represented in the graphs as percent of non-lesion value for each group. Table 1 includes the average number of steps for each forelimb before percentage calculation, before and after grafting.
Forelimb placing test: The rat was held in the air over a table, leaving the forelimbs free. The animal was then lowered allowing the tactile whiskers of each side independently to touch gently the table edge. The number of contacts of each upper limb with the table after the sensory stimulation was quantified in 10 trials, and these were considered successful responses. The percentage of successful contralateral (lesioned) use is represented relative to non-lesion side, which served as the baseline activity.48 Pre- and postgraft mean values for both forelimbs are listed in Table 1.
Cylinder test: The exploratory activity was analyzed by filming each animal in a transparent acrylic cylinder (35 cm diameter by 40 cm high) for 7–10 minutes, depending on the frequency of vertical movements. Contacts of each forelimb with cylinder walls when rearing were recorded, and the percentage of lesioned side use, relative to the non-lesioned limb was calculated.3 The mean (± SEM) number of contacts for both forelimbs before and after grafting is summarized in Table 1.
Chondroitinase ABC administration in the trajectory from SN to the striatum. A strategy to avoid axonal growth inhibition was tested trying to promote DA axon extension from the SN to the striatum by transplanting DA neurons in the nigra and implanting a cannula in the skull of lesioned rats to sequentially perform injections of Chondroitinase ABC (ChABC) solution to degrade CSPG, with stylets of decreasing length (each one being smaller 0.5 mm from the previous) at days 0, 3, 6, 9, 12, and 15 after grafting. With this scheme, ChABC was applied closer to the striatum in the last injection. The heads of hemiparkinsonian rats were tilted 60° to graft 2 µl containing 0.33 × 106 differentiated ES cells per animal in the SN of the lesioned side, as represented in Figure 2a, using the following stereotactic coordinates relative to bregma: AP, 3.5 mm; L, 2.4 mm; V, −13 mm. These coordinates reached the SN pars compacta. In the same surgery, a stent was implanted in the skull to allow injections of ChABC on the following days. Seven control (Sham + ChABC) and 7 experimental (intranigral grafting + ChABC) rats were studied.
Cografting of DA neurons with cells expressing Sema3A or Sema3C. To express Sema3A or Sema3C, HEK293 cells were transfected with FuGene reagent (Roche, Indianapolis, IN) with 1 μg/μl of expression vectors containing the mouse coding sequence of these Semaphorins; cells were then cultured for 24 hours, detached from the culture plate with Trypsin, counted and grafted alone or together with differentiated ES cells. DA neurons were dissociated at days 2–3 of stage 5 and resuspended at 1.67 × 105 cells/μl. HEK293 cells were loaded in the injection needle and then DA neurons were taken up separated from HEK cells by a small air bubble. A single deposit (2 μl) of 3.3 × 105 ES cell–derived DA neurons was made in the SN of the lesioned hemisphere of parkinsonian animals; DA cells were allowed to settle for 5 minutes and then six deposits of 7 × 103 HEK293 cells (0.5 μl each) were made, starting at −12.5 mm ventral, and retracting the needle 0.5 mm every time, covering 2.5 mm in direction to the striatum, as depicted by the blue circles in Figure 3a. We decided to use this number of HEK293 cells because we previously observed that such amount has optimal effects on DA axon outgrowth in vitro.24 Consistent with previous work, grafts of up to 1 × 105 HEK cells in the brain did not produce adverse effects.33 Functionality of these grafts was monitored by pharmacological and non-pharmacological tests. DA neurons alone in the SN, DA neurons plus mock-transfected HEK cells, or Sema-transfected HEK cells in the absence of ES cell–derived neurons did not cause recovery (see Figure 4 and Supplementary Figure S2). As shown in Table 1, pregraft values of lesioned animals did not differ between all analyzed groups. We analyzed the following number of animals: intranigral grafting without HEK293 cells (n = 8), DA neurons plus mock-transfected HEK cells (n = 8), Sema3A-transfected HEK cells without DA neurons (n = 4), Sema3C-transfected HEK without DA neurons (n = 7), DA neurons plus Sema3A-transfected cells (n = 5), and DA neurons plus Sema3C-transfected cells (n = 15).
Microdialysis and DA quantification. Microdialysis experiments were performed at 7 weeks after transplantation to measure DA release in the dorsal striatal region4 of animals grafted in the striatum or cografted with DA neurons in the SN and Sema-transfected cells. The sample was analyzed to calculate the concentration of DA, according to a standard of known concentration as described.49 After 1 hour of probe stabilization with Hanks solution, three basal samples were collected and DA release was stimulated with isosmotic Hanks balanced salt solution containing 56 mmol/l KCl (high potassium) through a 4-mm microdialysis membrane. DA quantification was made using a capillary electrophoresis system (Beckman Coulter, Brea, CA; P/ACE MDQ with laser-induced fluorescence; argon lamp of 488 nm and interference filters for 590 and 520 nm). The fluorogenic derivatization reagent, 3-(2-furoyl)quinoline-2-carboxaldehide (FQ) was from Molecular probes (Eugene, OR). For storage, FQ (10 mmol/l) was dissolved in methanol, aliquots were dispensed, and solvent was then removed under vacuum for 1 hour at room temperature. The dried FQ was stored at −20 °C and was directly used in the derivatization reaction without further treatment. This derivatized sample (5 µl) was injected and the resulting electropherogram was analyzed to calculate the concentration of DA. Basal levels in the groups did not differ and therefore, all of them were pooled to represent the baseline shown in Figure 4a.
Analysis of striatal TH expression. The intensity of TH immunoreactivity in the striatal area was quantified using Image J 1.40 software (Research Services Branch, National Institute of Mental Health, Bethesda, MD). The striata of 3 non-lesioned animals were used to establish the control value. Three rats that received intranigral dopaminergic neurons and mock-transfected cells were assessed and the results are expressed as percentage of control. A group of three animals that received intranigral grafts combined with Sema3C-transfected cells was also measured.
Stereological counting. Animals were perfused with 0.9% saline and then with 4% paraformaldehyde in phosphate-buffered saline. Brains were recovered and cryo-protected with 30% sucrose. Slices of 40 µm were obtained in a cryostat and immunostained with anti-TH antibodies for DA neuron counting. Every 5th section was quantified, and the total number of DA neurons was calculated by multiplying the number of TH+ neurons per slice by the number of slices that contained grafted cells.3,4,50
Retrograde tracing. Animals received bilateral intrastriatal injections of the retrograde fluorescent tracer FG (Fluorochrome, Denver, CO) into two sites in the dorsolateral striatum (2% solution in 0.9% saline; 0.1 µl/site) at a rate of 0.1 µl/minute, with the aid of a quintessential stereotaxic injector (Stoelting, Wood Dale, IL). The coordinates were AP, 1.3 mm; L, 2.5 mm; V, −5.5 mm for site 1, and AP, −0.9 mm; L, 3.5 mm; V, −5.5 for the second injection spot. Three days later, the rats were euthanized and their brains recovered for TH immunohistochemistry and FG detection.
Statistical analysis. Results are expressed as mean ± SEM. Statistical analysis was made by ANOVA followed by Fisher's test. GB-STAT Version 7.0 program (Dynamic Microsystems, Silver Spring, MD) was used for calculation of probability values.
SUPPLEMENTARY MATERIAL Figure S1. HEK293 cells can be identified by expression of human nuclei (HuNu) antigen both in vitro and in vivo. Figure S2. Transplantation of Sema3-transfected cells in the absence of DA neurons had no effect on behavioral recovery. Materials and Methods.
Acknowledgments
This work was supported by the National Institutes of Health (NINDS/FIC, NS 057850 to I.V.), CONACYT-México (14285, 50715, and 131281 to I.V.; 101433 to A.V.-E.), and IMPULSA 02-Universidad Nacional Autónoma de México (Stem cell group to A.V.-E.). N.E.D.-M. and C.M.G.-P. received graduate fellowships from CONACYT. N.F.D. was supported by a postdoctoral fellowship from NIH. This study was performed in partial fulfillment of the requirements for the Ph.D. degree in Biomedical Sciences of N.E.D.-M. at Universidad Nacional Autónoma de México. We acknowledge technical support from Kioko Guzmán-Ramos, Federico Bermúdez-Rattoni, Anayansi Molina-Hernández, Teresa Neri, and personnel from the microscopy unit and animal housing at Instituto de Fisiología Celular. The anti-Pitx3 antibody was a kind donation from Marten Smidt. We thank Raja Kittappa for advice and suggestions. The authors declare no conflict of interest.
Supplementary Material
References
- Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- Kim JH, Auerbach JM, Rodríguez-Gómez JA, Velasco I, Gavin D, Lumelsky N, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418:50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Gómez JA, Lu JQ, Velasco I, Rivera S, Zoghbi SS, Liow JS, et al. Persistent dopamine functions of neurons derived from embryonic stem cells in a rodent model of Parkinson disease. Stem Cells. 2007;25:918–928. doi: 10.1634/stemcells.2006-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci USA. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez I, Sadi D, Hong M. Reconstruction of the nigrostriatal pathway by simultaneous intrastriatal and intranigral dopaminergic transplants. J Neurosci. 1996;16:7216–7227. doi: 10.1523/JNEUROSCI.16-22-07216.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhida K, Baker KA, Sadi D, Mendez I. Enhancement of sensorimotor behavioral recovery in hemiparkinsonian rats with intrastriatal, intranigral, and intrasubthalamic nucleus dopaminergic transplants. J Neurosci. 2001;21:3521–3530. doi: 10.1523/JNEUROSCI.21-10-03521.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M, Nikkhah G, Bentlage C, Björklund A. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci. 1995;15 5 Pt 2:3863–3875. doi: 10.1523/JNEUROSCI.15-05-03863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giger RJ, Hollis ER, 2nd, Tuszynski MH. Guidance molecules in axon regeneration. Cold Spring Harb Perspect Biol. 2010;2:a001867. doi: 10.1101/cshperspect.a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Montiel HL, Tamariz E, Sandoval-Minero MT, Varela-Echavarría A. Semaphorins 3A, 3C, and 3F in mesencephalic dopaminergic axon pathfinding. J Comp Neurol. 2008;506:387–397. doi: 10.1002/cne.21503. [DOI] [PubMed] [Google Scholar]
- Kolk SM, Gunput RA, Tran TS, van den Heuvel DM, Prasad AA, Hellemons AJ, et al. Semaphorin 3F is a bifunctional guidance cue for dopaminergic axons and controls their fasciculation, channeling, rostral growth, and intracortical targeting. J Neurosci. 2009;29:12542–12557. doi: 10.1523/JNEUROSCI.2521-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre ER, Gutekunst CA, Gross RE. Expression by midbrain dopamine neurons of Sema3A and 3F receptors is associated with chemorepulsion in vitro but a mild in vivo phenotype. Mol Cell Neurosci. 2010;44:135–153. doi: 10.1016/j.mcn.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi K, Mizushima S, Tamada A, Yamamoto N, Takashima S, Murakami F. FGF8 signaling regulates growth of midbrain dopaminergic axons by inducing semaphorin 3F. J Neurosci. 2009;29:4044–4055. doi: 10.1523/JNEUROSCI.4794-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, et al. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99:59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- Messersmith EK, Leonardo ED, Shatz CJ, Tessier-Lavigne M, Goodman CS, Kolodkin AL. Semaphorin III can function as a selective chemorepellent to pattern sensory projections in the spinal cord. Neuron. 1995;14:949–959. doi: 10.1016/0896-6273(95)90333-x. [DOI] [PubMed] [Google Scholar]
- Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature. 1996;383:525–528. doi: 10.1038/383525a0. [DOI] [PubMed] [Google Scholar]
- Bagnard D, Lohrum M, Uziel D, Püschel AW, Bolz J. Semaphorins act as attractive and repulsive guidance signals during the development of cortical projections. Development. 1998;125:5043–5053. doi: 10.1242/dev.125.24.5043. [DOI] [PubMed] [Google Scholar]
- Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404:567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- Wu KY, Hengst U, Cox LJ, Macosko EZ, Jeromin A, Urquhart ER, et al. Local translation of RhoA regulates growth cone collapse. Nature. 2005;436:1020–1024. doi: 10.1038/nature03885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niquille M, Garel S, Mann F, Hornung JP, Otsmane B, Chevalley S, et al. Transient neuronal populations are required to guide callosal axons: a role for semaphorin 3C. PLoS Biol. 2009;7:e1000230. doi: 10.1371/journal.pbio.1000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamariz E, Díaz-Martínez NE, Díaz NF, García-Peña CM, Velasco I, Varela-Echavarría A. Axon responses of embryonic stem cell-derived dopaminergic neurons to semaphorins 3A and 3C. J Neurosci Res. 2010;88:971–980. doi: 10.1002/jnr.22268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz NF, Díaz-Martínez NE, Camacho-Arroyo I, Velasco I. Estradiol promotes proliferation of dopaminergic precursors resulting in a higher proportion of dopamine neurons derived from mouse embryonic stem cells. Int J Dev Neurosci. 2009;27:493–500. doi: 10.1016/j.ijdevneu.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alilain WJ, Horn KP, Hu H, Dick TE, Silver J. Functional regeneration of respiratory pathways after spinal cord injury. Nature. 2011;475:196–200. doi: 10.1038/nature10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Minor KH, Bournat JC, Toscano N, Giger RJ, Davies SJ. Decorin, erythroblastic leukaemia viral oncogene homologue B4 and signal transducer and activator of transcription 3 regulation of semaphorin 3A in central nervous system scar tissue. Brain. 2011;134 Pt 4:1140–1155. doi: 10.1093/brain/awq304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio SE, Martínez A, Chauvet S, Mann F, Soriano E, Pascual M. Semaphorin 3C is not required for the establishment and target specificity of the GABAergic septohippocampal pathway in vitro. Eur J Neurosci. 2011;34:1923–1933. doi: 10.1111/j.1460-9568.2011.07906.x. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Fallon JH. Fluoro-Gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res. 1986;377:147–154. doi: 10.1016/0006-8993(86)91199-6. [DOI] [PubMed] [Google Scholar]
- Mendez I, Baker KA, Hong M. Simultaneous intrastriatal and intranigral grafting (double grafts) in the rat model of Parkinson's disease. Brain Res Brain Res Rev. 2000;32:328–339. doi: 10.1016/s0165-0173(99)00091-0. [DOI] [PubMed] [Google Scholar]
- Bukhatwa S, Iravani MM, Zeng BY, Cooper JD, Rose S, Jenner P. An immunohistochemical and stereological analysis of PSI-induced nigral neuronal degeneration in the rat. J Neurochem. 2009;109:52–59. doi: 10.1111/j.1471-4159.2009.05956.x. [DOI] [PubMed] [Google Scholar]
- Courtès S, Vernerey J, Pujadas L, Magalon K, Cremer H, Soriano E, et al. Reelin controls progenitor cell migration in the healthy and pathological adult mouse brain. PLoS ONE. 2011;6:e20430. doi: 10.1371/journal.pone.0020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, et al. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron. 2004;44:961–975. doi: 10.1016/j.neuron.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Piaton G, Aigrot MS, Williams A, Moyon S, Tepavcevic V, Moutkine I, et al. Class 3 semaphorins influence oligodendrocyte precursor recruitment and remyelination in adult central nervous system. Brain. 2011;134 Pt 4:1156–1167. doi: 10.1093/brain/awr022. [DOI] [PubMed] [Google Scholar]
- Varela-Echavarría A, Tucker A, Püschel AW, Guthrie S. Motor axon subpopulations respond differentially to the chemorepellents netrin-1 and semaphorin D. Neuron. 1997;18:193–207. doi: 10.1016/s0896-6273(00)80261-5. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Iwanami A, Nakamura M, Kishino A, Kikuchi K, Shibata S, et al. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med. 2006;12:1380–1389. doi: 10.1038/nm1505. [DOI] [PubMed] [Google Scholar]
- Van Kampen JM, Eckman CB. Dopamine D3 receptor agonist delivery to a model of Parkinson's disease restores the nigrostriatal pathway and improves locomotor behavior. J Neurosci. 2006;26:7272–7280. doi: 10.1523/JNEUROSCI.0837-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard A, Decressac M, Frappé I, Fernagut PO, Prestoz L, Besnard S, et al. Anatomical and functional reconstruction of the nigrostriatal pathway by intranigral transplants. Neurobiol Dis. 2009;35:477–488. doi: 10.1016/j.nbd.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Thompson LH, Grealish S, Kirik D, Björklund A. Reconstruction of the nigrostriatal dopamine pathway in the adult mouse brain. Eur J Neurosci. 2009;30:625–638. doi: 10.1111/j.1460-9568.2009.06878.x. [DOI] [PubMed] [Google Scholar]
- Zhao S, Maxwell S, Jimenez-Beristain A, Vives J, Kuehner E, Zhao J, et al. Generation of embryonic stem cells and transgenic mice expressing green fluorescence protein in midbrain dopaminergic neurons. Eur J Neurosci. 2004;19:1133–1140. doi: 10.1111/j.1460-9568.2004.03206.x. [DOI] [PubMed] [Google Scholar]
- Hedlund E, Pruszak J, Lardaro T, Ludwig W, Viñuela A, Kim KS, et al. Embryonic stem cell-derived Pitx3-enhanced green fluorescent protein midbrain dopamine neurons survive enrichment by fluorescence-activated cell sorting and function in an animal model of Parkinson's disease. Stem Cells. 2008;26:1526–1536. doi: 10.1634/stemcells.2007-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grealish S, Jönsson ME, Li M, Kirik D, Björklund A, Thompson LH. The A9 dopamine neuron component in grafts of ventral mesencephalon is an important determinant for recovery of motor function in a rat model of Parkinson's disease. Brain. 2010;133 Pt 2:482–495. doi: 10.1093/brain/awp328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keeffe FE, Scott SA, Tyers P, O'Keeffe GW, Dalley JW, Zufferey R, et al. Induction of A9 dopaminergic neurons from neural stem cells improves motor function in an animal model of Parkinson's disease. Brain. 2008;131 Pt 3:630–641. doi: 10.1093/brain/awm340. [DOI] [PubMed] [Google Scholar]
- Studer L, Tabar V, McKay RD. Transplantation of expanded mesencephalic precursors leads to recovery in parkinsonian rats. Nat Neurosci. 1998;1:290–295. doi: 10.1038/1105. [DOI] [PubMed] [Google Scholar]
- Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000;18:675–679. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- Liu CQ, Hu DN, Liu FX, Chen Z, Luo JH. Apomorphine-induced turning behavior in 6-hydroxydopamine lesioned rats is increased by histidine and decreased by histidine decarboxylase, histamine H1 and H2 receptor antagonists, and an H3 receptor agonist. Pharmacol Biochem Behav. 2008;90:325–330. doi: 10.1016/j.pbb.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Guzmán-Ramos K, Osorio-Gómez D, Moreno-Castilla P, Bermúdez-Rattoni F. Off-line concomitant release of dopamine and glutamate involvement in taste memory consolidation. J Neurochem. 2010;114:226–236. doi: 10.1111/j.1471-4159.2010.06758.x. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.