Abstract
Islet transplantation is a promising therapy for type 1 diabetes, but graft function and survival are compromised by recurrent islet autoimmunity. Immunoprotection of islets will be required to improve clinical outcome. We engineered human β cells to express herpesvirus-encoded immune-evasion proteins, “immunevasins.” The capacity of immunevasins to protect β cells from autoreactive T-cell killing was evaluated in vitro and in vivo in humanized mice. Lentiviral vectors were used for efficient genetic modification of primary human β cells without impairing their function. Using a novel β-cell–specific reporter gene assay, we show that autoreactive cytotoxic CD8+ T-cell clones isolated from patients with recent onset diabetes selectively destroyed human β cells, and that coexpression of the human cytomegalovirus-encoded US2 protein and serine proteinase inhibitor 9 offers highly efficient protection in vitro. Moreover, coimplantation of these genetically modified pseudoislets with β-cell–specific cytotoxic T cells into immunodeficient mice achieves preserved human insulin production and C-peptide secretion. Collectively, our data provide proof of concept that human β cells can be efficiently genetically modified to provide protection from killing mediated by autoreactive T cells and retain their function in vitro and in vivo.
Introduction
Type 1 diabetes results from selective and progressive destruction of insulin-producing cells by autoreactive CD8+ T cells.1,2 The direct cell–cell contact initiated by T-cell receptors recognizing a β-cell–specific antigenic peptide presented by the major histocompatibility complex (MHC) class I (MHC-I) at the surface of the target cell appears to be critical for β-cell destruction in type 1 diabetes. In humans, during acute insulitis, β cells show hyper-expression of MHC-I and selective infiltration by islet-specific autoreactive CD8+ T cells.3 Also, mice lacking MHC-I expression do not develop diabetes,4,5,6 whereas adoptive spleen cell transfer from NOD mice to recipients selectively expressing MHC-I on β cells leads to β-cell destruction.7,8 In addition, the Fas-signaling pathway is not essential in the destruction of β cells,9,10 whereas disruption of the perforin gene delays the onset of autoimmune diabetes in NOD mice, pointing to the perforin/granzyme pathway as a key effector in β-cell destruction by cytotoxic T cells.11,12
To date, immunotherapies to cure, prevent, or delay disease onset in humans have been inefficient.13,14 Options for patients with type 1 diabetes to restore normoglycemia are limited to daily insulin injection, combined kidney-pancreas or experimental islet transplantation. However, numerous factors have been reported to potentially affect islet allograft function and survival.15 Importantly, the close correlation between loss of islet-graft function and frequencies of circulating autoreactive islet-specific T cells observed in transplanted patients, in particular when the donor and the recipient share the same HLA class I haplotypes, points to the importance of recurrent autoimmunity in islet-graft failure.16,17,18,19 Along the same line, it appears that, in case of autotransplantation, islet grafts are much less affected, suggesting that persistent attacks from autoimmunity, alloimmunity and possibly the toxicity of immunosuppressive treatments have a major responsibility in graft loss.20
An alternative could be the transplantation of genetically immunoprotected β cells. We have previously explored the capacity of herpesvirus-derived immunevasins to elude the host immune responses (reviewed in refs. 21,22,23). In this study, we aim to combine the US2 protein of human cytomegalovirus, known to interfere with antigenic peptide presentation by inducing proteasomal degradation of MHC-I molecules,24 and Serpin-9, a serine protease inhibitor specific for granzyme B activity.25
Furthermore, the identification of specific human β-cell protectants is hindered by the difficulty of studying the dialogue between human insulin-producing cells and the immune system. Even in vitro, the cellular heterogeneity of dissociated islet preparations makes the standard release-based assays imperfect to specifically assess β-cell cytotoxicity. Islet preparations contain a mixture of cell types, including β cells, α cells, δ cells, ε cells, duct cells, endothelial cells, stem cells, and leukocytes, that all may interfere with the assays to selective assess β-cell cytotoxicity.26
Here, we describe the development of a new strategy to measure β-cell cytotoxicity and protection from autoreactive T-cell–mediated killing. We report that lentivirus vectors can be used to genetically modify human islet cells. Moreover, using this powerful technology, we demonstrate that downregulation of MHC-I combined with the inhibition of granzyme B activity protects human β cells from acute recurrent islet autoimmunity both in vitro and in vivo.
Results
Primary human islet cells can be transduced efficiently by lentiviral vectors
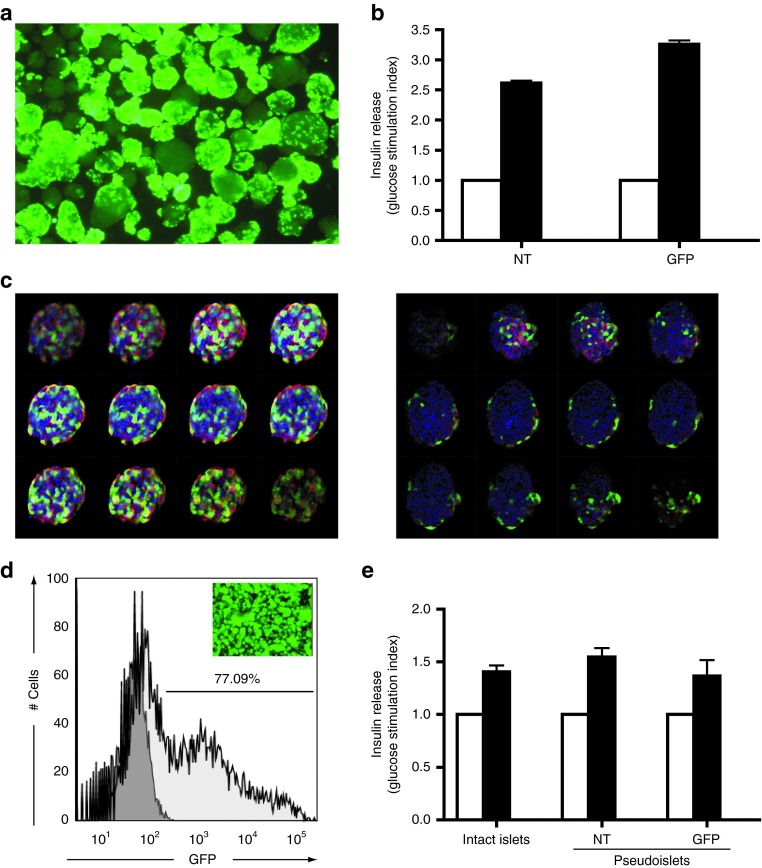
Lentiviral vectors are the gene transfer system of choice for stable genetic modification of primary cells. To evaluate their capacity to genetically modify freshly isolated human islets, a vector carrying an enhanced green fluorescent protein (GFP) reporter gene under the control of the human cytomegalovirus immediate early promoter was used (LV-CMV-GFP). Seventy-two hours after transduction, islets were inspected for reporter gene expression (Figure 1a). A multiplicity of infection of 5 was well tolerated without any apparent signs of virus-induced toxicity. In addition, exposure to the virus did not significantly affect islet function, as exemplified by their capacity to secrete insulin upon glucose stimulation (Figure 1b). Yet, GFP expression within the islet structures was heterogeneous. Following insulin/DNA staining, whole mount confocal microscopy confirmed that in most islets only cells at the rim of the islets were efficiently transduced, demonstrating that in intact human islets only a limited number of cells are permissive to lentiviral vector transduction (Figure 1c). To increase the transduction efficiency, human islets were dispersed, and the resulting single-cell suspension was exposed to the lentivirus vector LV-CMV-GFP. Subsequently, pseudoislets were formed by self-aggregation of the transduced cells. Seven days after reaggregation, the 3D structures were macroscopically similar to islets. The majority of the cells expressed GFP as determined by flow cytometry (Figure 1d). To evaluate the functionality of genetically modified pseudoislet structures, a static glucose-stimulated insulin-secretion test was performed and glucose responsiveness was compared with non-transduced and intact islets. Neither dispersion nor transduction hampered islet functionality, because no differences in insulin release were detected after glucose stimulation (Figure 1e).
Figure 1.
Genetic modification of human islets in vitro. (a) Fluorescent microscopy pictures of freshly isolated human islets transduced with LV-CMV-GFP (MOI = 5). GFP expression was assessed by fluorescent microscopy (200× original magnification). (b) Comparative glucose responsiveness of NT islets and GFP-modified islets (GFP) determined by glucose-stimulated insulin-secretion test. Insulin release data are shown as glucose stimulating index (i.e., insulin release at low glucose—white bars—is used as reference and set to 1 and used as reference for high glucose (black bars) induction. (c) Series of optical sections of two representative islets (2 µm) taken by confocal microscopy. Insulin is depicted in red, GFP in green and nuclei are visualized by DAPI (blue) staining. Although some GFP expression could be observed in the central core of the islet (left panel), in most islets GFP expression was limited to the outer rim of the islet (right panel). (d) Fluorescent microscopy picture of GFP-modified pseudoislets (MOI = 2) and quantification of the GFP positive cells by flow cytometry. Light gray histogram shows GFP-transduced cells and non-transduced dispersed cells are shown in dark gray histogram. Experiments are performed 6 days after transduction. (e) Glucose responsiveness of pseudoislets or genetically modified pseudoislets compared with intact islets from the same donor. Similar to b, insulin release data are shown as glucose stimulating index. Low glucose (white bars) concentration is set to 1 and used as reference for high glucose (black bars) induction. GFP, green fluorescent protein; MOI, multiplicity of infection; NT, non-transduced.
Genetically modified pseudoislets are functional in vivo
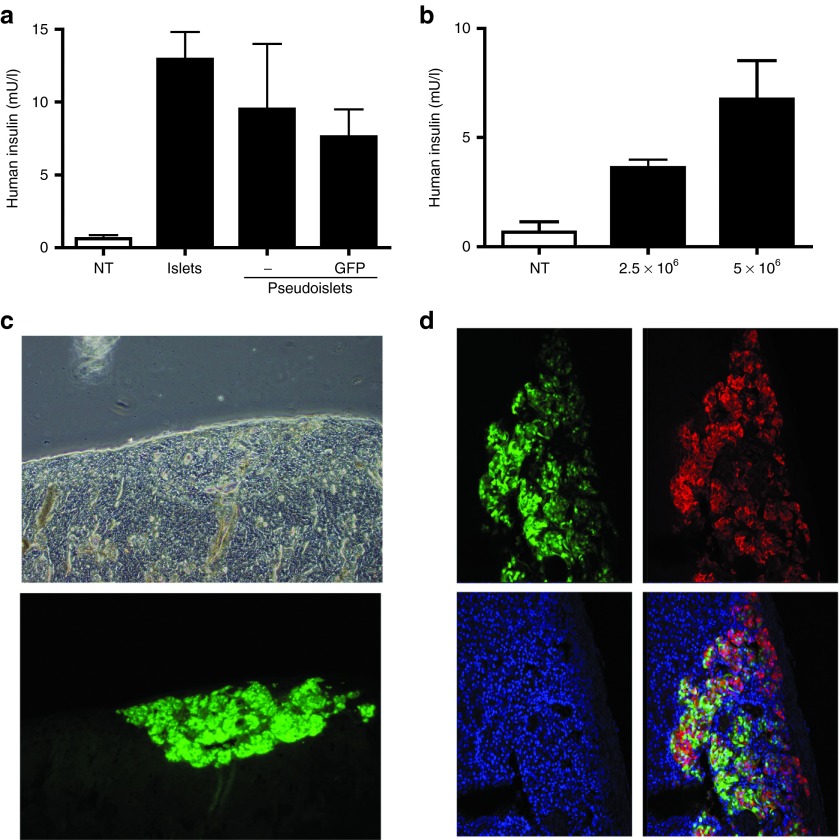
To assess the fate of these pseudoislets in vivo, non-transduced and LV-CMV-GFP–transduced human pseudoislets were transplanted under the kidney capsule of NSG mice. The release of human insulin after intraperitoneal glucose administration was measured. Assuming that one islet would contain ~1,000 to 1,500 cells,27 ~3,000 islet equivalents were transplanted and compared with 5 × 106 dispersed/reaggregated cells. Pseudoislets efficiently released insulin upon glucose challenge, because no significant differences were observed when compared with intact islets (Figure 2a). To define the limitation of the insulin assay used, we transplanted NSG mice with 2.5 × 106 cells (~1,500 pseudoislets) or 5 × 106 cells (~3,000 pseudoislets) and monitored human insulin release. As anticipated, the amount of insulin secreted correlated with the number of transplanted cells (Figure 2b). Similar results were obtained with a human C-peptide assay (data not shown). To confirm the survival of the insulin-producing cells, the graft was removed 19 days after transplantation and analyzed by confocal imaging for GFP fluorescence and, after immunostaining, for insulin reactivity. Macroscopically, a strong and sustained GFP expression was detected at the site of reimplantation (Figure 2c). Insulin immunostaining demonstrated a large proportion of GFP positive β cells (Figure 2d). From these data, we conclude that lentivirus-mediated gene transfer into cells from freshly isolated human islets is feasible, and that the cells can be reaggregated to form functional pseudoislets.
Figure 2.
Function of genetically modified pseudoislets in vivo. (a) Intraperitoneal glucose-tolerance test performed on mice transplanted with 3,000 intact islets (n = 1) or 5 × 106 GFP-modified pseudoislets (n = 4) or non-modified islets (n = 2). N represents the number of transplanted mice. Results are represented as average of 3 different time points at 4, 11, and 19 days after transplantation. (b) Similar experiment performed with GFP-modified pseudoislets formed with 2.5 × 106 cells or 5 × 106 cells (n = 2). Non-transplanted mice were used as negative control. (c) Fluorescent microscopy of the kidney performed after nephrectomy 19 days after transplantation of pseudoislets containing 5 × 106 cells. (d) Immunostaining of the graft. Insulin is shown in red, GFP in green and nuclei are stained by DAPI in blue. Sections were analyzed by confocal microscopy. GFP, green fluorescent protein; NT, non-transduced.
The human insulin promoter drives β-cell–specific expression in human islet cells
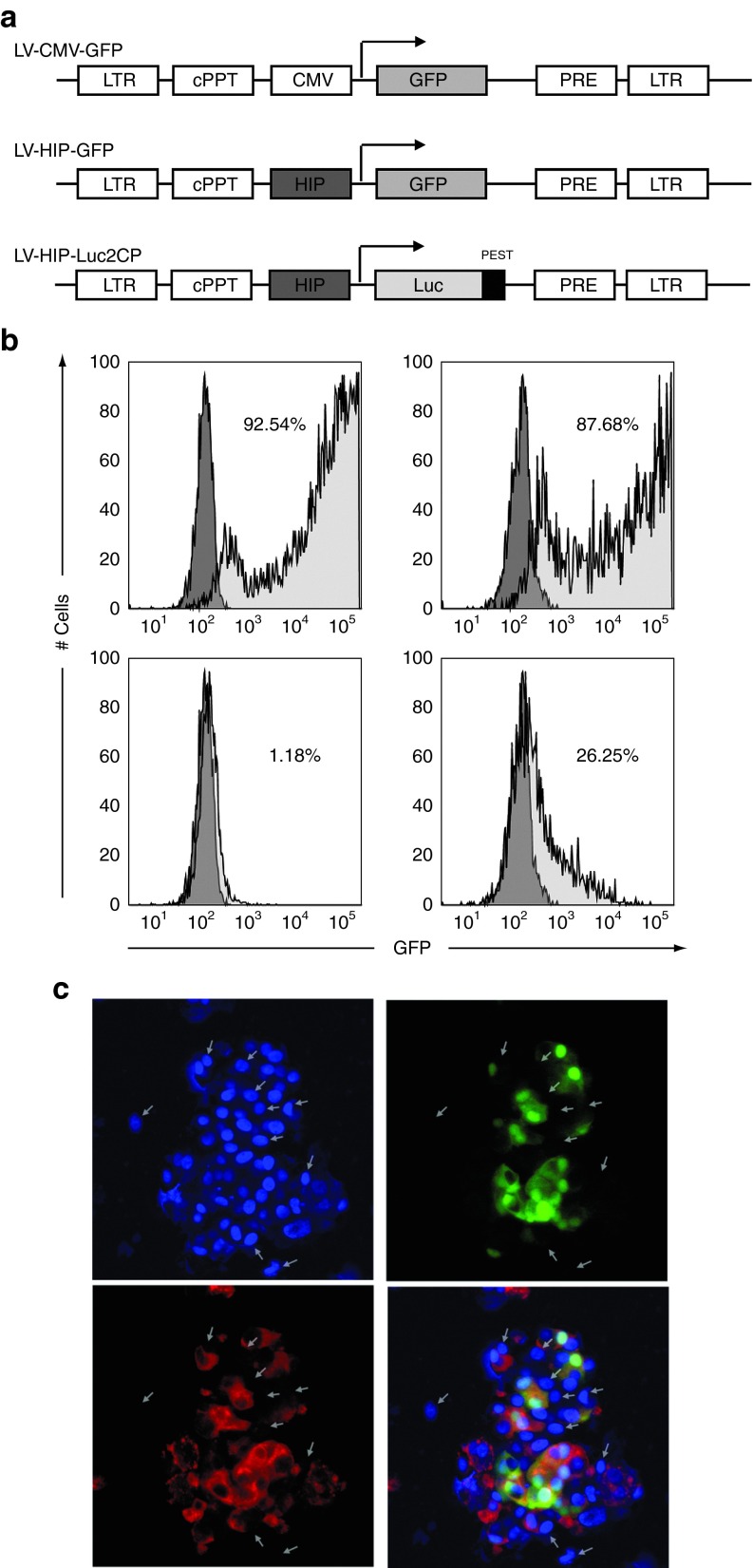
Next, to acquire specific expression of the gene of choice in β cells, the CMV promoter was replaced by the human insulin promoter (HIP) (Figure 3a). To assess HIP promoter specificity, we first compared CMV-GFP lentivirus transduction efficiency in human embryonic kidney (HEK) cells or rat insulinoma cell lines (INS-1E) and confirmed that both cell types can be efficiently modified by lentiviruses (Figure 3b, upper panel). Second, we performed similar experiments using the HIP-GFP lentivirus and detected only few GFP positive HEK cells whereas 25% of the INS-1E expressed GFP (Figure 3b, lower panel). Finally, we verified HIP specificity and efficiency in human primary cells. One week after transduction, HIP-GFP human pseudoislets were analyzed for GFP expression using confocal microscopy (Figure 3c). Altogether, these data demonstrate that the HIP promoter facilitates efficient transgene expression and limits this expression to β cells.
Figure 3.
HIP specificity. (a) Schematic representation of the lentivirus constructs used: LV-CMV-GFP; LV-HIP-GFP; LV-HIP-Luc2CP (the arrow indicates the transcription initiation). (b) Comparative GFP expression as determined by flow cytometry in HEK 293T cells (left column) and INS-1E cells (right column) after transduction with LV-CMV-GFP (MOI = 1) (upper panel) or LV-HIP-GFP (MOI = 1) (lower panel). Non-transduced cells were used as negative control and shown in dark gray histogram. (c) Whole mount immunostaining using anti-insulin antibody (red) performed on HIP-GFP–transduced pseudoislets. Nuclei were stained by DAPI in blue. White arrows indicate the insulin negative cells. cPPT, central polypurine tract; GFP, green fluorescent protein; HEK, human embryonic kidney; HIP, human insulin promoter; LTR, Long terminal repeat; MOI, multiplicity of infection; PRE, posttranscriptional regulatory E.
Autoreactive HLA-A2–restricted preproinsulin-directed cytotoxic T lymphocyte clones kill HLA-A2 human islet cells in vitro
To quantify β-cell death, the GFP cassette was replaced by a destabilized luciferase reporter gene, allowing short half-life luciferase expression specifically in β cells (Figure 3a). To validate this method, we designed an in vitro killing assay by incubating HIP-Luc2CP–modified human islet cells with autoreactive CD8+ T cells isolated from a recent onset patient with type 1 diabetes and directed against an epitope located in the signal peptide of the preproinsulin (PPI) molecule.28 cytotoxic T lymphocyte (CTL) killing capacity was validated in a standard chromium release assay using K562 surrogate β cells (Supplementary Figure S1 and Supplementary Data). Using fractions of different purities from the same donor, killing assays were performed with different target/effector ratios (corrected for purity of the fraction). These experiments demonstrate that the luciferase assay is not affected by the quality of the isolated islet fraction (Figure 4a). Similarly, killing assays performed in parallel with HLA-A2–restricted PPI-directed CTL, incubated with HIP-Luc2CP islet cells from HLA-A3 and HLA-A2 donors, demonstrated that PPI-directed CTL were able to specifically kill HLA-A2 β cells, as seen by a massive drop in luciferase activity. When HLA-A3 donor cells were used as targets, no significant decrease in light emission was observed (Figure 4b). Moreover, when using HLA-A2–restricted pp65CMV-specific CTL, the viability of the HLA-A2 positive β cells was not affected (Figure 4c), which is consistent with the absence of pp65CMV-target epitope on human β cells. This demonstrates that β-cell death is dependent on the presence of the PPI-specific CTL.
Figure 4.
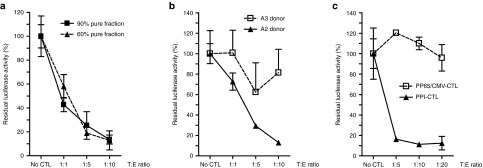
Autoreactive HLA-A2–specific PPI-directed CTL clones kill HLA-A2 human islet cells in vitro. (a) Luciferase killing assay performed on 60% purity (triangle) or 90% pure β-cell preparations (square) HIP-Luc2CP HLA-A2 human islet cells using PPI-directed CTLs with increasing target:effector (T:E) ratios. Results are shown as residual luciferase activity 48 hours after coculture. (b) Luciferase killing assay performed on HLA mismatched (HLA-A3; open square) or matched (HLA-A2; triangle) LV-HIP-Luc2CP–transduced human islet cells using PPI-directed CTLs (with increasing target:effector ratios). (c) Luciferase killing assay performed on HLA-A2 LV-HIP-Luc2CP–transduced human islet cells using increasing target:effector ratios of pp65CMV-directed CTLs (open squares) or PPI-directed CTLs (triangles). Experiments have been performed in triplicates, normalized to luciferase activity without CTL (set to 100%) and were shown as means of triplicate measurements (±SD). Matched killing assay have been performed and confirmed on HLA-A2 islet cells from six different donors. CTL, cytotoxic T lymphocyte; HIP, human insulin promoter; PPI, peptide of the preproinsulin.
Combined US2/Serpin-9 expression protects surrogate β cells and human primary β cells from PPI-directed CTL killing in vitro
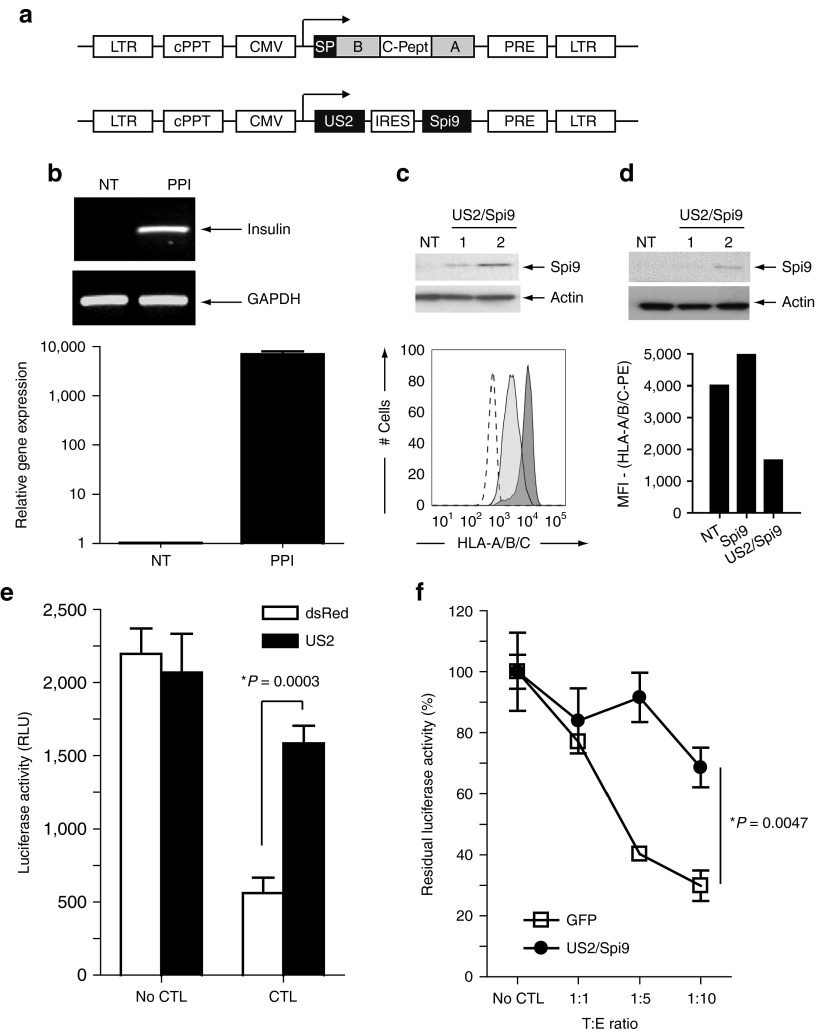
To explore the protective effect of combined MHC-I downregulation and granzyme B inactivation, we generated a bicistronic lentivirus vector encoding the immune-evasion gene US2 and the serine protease inhibitor specific for granzyme B activity (Figure 5a). First, the protective effect was evaluated on surrogate β cells generated by overexpression of PPI in K562-A2 cells and then transpose to primary human islets. Insulin mRNA level in surrogate β cells was verified by reverse-transcription PCR and quantitative PCR (Figure 5b). Transduction of K562-A2 with a US2-containing lentivirus vector led to decreased HLA class I expression as quantified by flow cytometry (Figure 5c, lower panel). After transduction of HEK 293T cells at a multiplicity of infection of 1 or 2, the expression of Serpin-9 was verified by western blotting (Figure 5c, upper panel). Similar results were obtained in primary human islets (Figure 5d). Following cotransduction of surrogate β cells with CMV-Luc2CP and CMV-DsRed or CMV-US2 lentiviruses, a killing assay was performed with CTLs against PPI. After 48 hours of coculture, massive cell death of DsRed-expressing cells was observed. HLA-A/B/C downregulation mediated by US2 led to protection of the surrogate β cells from PPI-specific CTL killing (Figure 5e). Importantly, US2/Serpin-9 coexpression in primary human islets had no effect on insulin release upon glucose stimulation (Supplementary Figure S2a) and protected human β cells from autoreactive CD8+ T cells as seen by residual luciferase activity after coculture when compared with GFP-modified islets (Figure 5f).
Figure 5.
Combined US2/Serpin-9 expression protects surrogate β cells from PPI-directed CTL killing. (a) Schematic representation of the lentivirus constructs used: LV-CMV-PPI and LV-CMV-US2-bc-Spi9. (b) Reverse-transcription PCR (upper panel) and reverse-transcription quantitative PCR (lower panel) validating insulin-gene expression in LV-CMV-PPI–transduced A2/K562 cells. The GAPDH housekeeping gene was used as internal control. (c) Serpin-9 expression assessed by western blot using Spi9 antibody following LV-CMV-US2-bc-Spi9 transduction (MOI = 1 or MOI = 2; NT cells were used as control) of HEK 293T cells (upper panel) and HLA-ABC expression in US2/Spi9 A2/K562 cells assessed by flow cytometry analysis (lower panel). Unstained A2/K562 cells (dashed line) and HLA-A/B/C–stained K562 (dark gray area) were used as controls, HLA-A/B/C surface expression of US2/Spi9-modified A2/K562 cells is shown in light gray area. (d) Serpin-9 expression and US2 inhibitory effect on HLA-ABC level in human pseudoislets. Following LV-CMV-US2-bc-Spi9 transduction of primary human islet cells (MOI = 1 or MOI = 2; NT cells were used as control), Serpin-9 expression was assessed by western blot (upper panel). Effect of US2 on HLA-ABC expression in islet cells was determined, following US2/Spi9 transduction MOI = 2, by median fluorescent intensity of the HLA-ABC-PE antibody used and compared with NT islets cells or Serpin-9–transduced islets (Spi9) (lower panel). (e) Luciferase killing assay performed on DsRed LV-CMV-Luc2CP A2/K562 (white bars) or US2/Spi9 LV-CMV-luc2CP–transduced A2/K562 (black bars) using PPI-directed CTLs (target:effector 1:5). (f) Luciferase killing assay performed on LV-HIP-Luc2CP–transduced HLA-A2 human islet cells modified by DsRed (opened square) or US2/Spi9 (closed circle) using PPI-directed CTLs. Experiments have been performed in triplicates, normalized to luciferase activity without CTL (set to 100%). Data were shown as means of triplicate measurements (±SD). P value has been calculated by unpaired Student's t-test relative to non-protected control. CTL, cytotoxic T lymphocyte; HEK, human embryonic kidney; HIP, human insulin promoter; MOI, multiplicity of infection; NT, non-transduced; PPI, peptide of the preproinsulin.
Primary human β cells can be efficiently immunoprotected from PPI-directed CTL killing in vivo
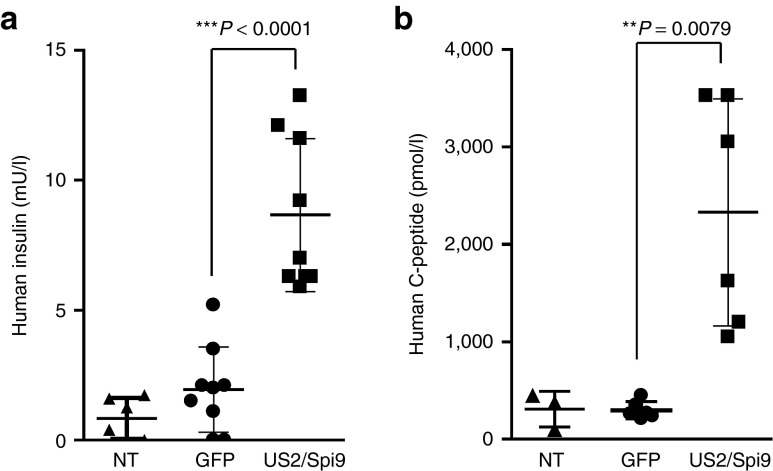
As an in vivo proof of concept, equal amounts of GFP- or US2/Serpin-9–modified pseudoislets (~3,000) and PPI-directed CTLs (E:T ratio 1:100) were transplanted under the kidney capsule of NSG mice and human insulin and C-peptide were monitored following intraperitoneal glucose-tolerance tests (Figure 6a,b). In agreement with the in vitro results, human insulin or C-peptide secretion by GFP pseudoislets was low following cotransplantation with autoreactive CTL. US2/Spi9 expression had no effect on islets functionality (Supplementary Figure S2b) and US2/Serpin-9 expressing cells maintained both insulin (and C-peptide) secretion, to a level similar to the one measured in absence of autoreactive T cells (Figure 2a,b), indicating that US2/Serpin-9 expression does not impact on islet viability in vivo and protects β cells from autoimmune CTL attack.
Figure 6.
Immune protection of human islet cells. (a,b) Intraperitoneal glucose-tolerance test performed on mice cotransplanted with 5 × 106 LV-GFP–modified pseudoislets (n = 3) or LV-US2/Spi9–modified islets (n = 3) and PPI-directed CTLs (target:effector 100:1). n represents the number of transplanted mice each performed with a different donor. (a) Human insulin release values and (b) human C-peptide concentration after Intraperitoneal Glucose Tolerance Test. Three tolerance tests have been performed per mice in the first 3 weeks after transplantation (4, 11, and 19 days). Data are represented as scatter plot showing every glucose tolerance test results (a total of 9 values from bleeding at day 4, 11, and 19 for insulin and a total of 6 values from bleeding at day 11 and 19 for C-peptide). NT mice were used as negative control (n = 2). **Statistically significant; ***extremely statistically significant. CTL, cytotoxic T lymphocyte; GFP, green fluorescent protein; PPI, peptide of the preproinsulin; NT, non-transplanted.
Discussion
Islet transplantation offers a promising approach for restoring endogenous insulin secretion in patients with diabetes. However, recurrent islet autoimmunity has been shown to be a major hurdle thwarting the clinical efficacy of β-cell replacement therapy. Here, we propose an alternative with transplantation of genetically immunoprotected pseudoislets. We demonstrate that third generation lentiviral vectors can be used as efficient gene carriers for protecting primary human β cells without affecting their function, thereby confirming earlier studies in intact human islets.29 As described earlier for rodents,30 the self-aggregation phenomenon of islet cells offers an attractive opportunity to protect these endocrine “micro-organs” to a large extent before transplantation. Although we did not study the precise composition of these pseudoislets in detail, but focused on β cells only, our data show that both treatments (viz. dispersion and transduction) are not accompanied by a significant loss in insulin production rate in response to glucose. These data are in line with a recent study aiming at the evaluation of immunoisolation method and showing that pseudoislets of rat islet cells are superior to primary intact islets in term of survival and function.31
The luciferase-based survival assay presented here is an alternative to the currently used traditional tracer-release assays, which have their limitations for monitoring cell cultures (reviewed in ref. 32). This assay requires a limited number of cells (from 5,000 to 10,000 cells per sample) and the results obtained appeared to be relatively insensitive to variations in the purity of islet preparations. The use of other reporters and substrates (e.g., an amino luciferin derivative specific for caspases 3, 7, 8, or 9) are currently under investigation and may provide new insights into the molecular mechanisms involved in the killing process.33,34 The assay relies on the specificity of the promoter: the use of a ubiquitously active promoter to drive luciferase-gene expression in assays to measure β-cell survival was confounded by the increase in luciferase activity due to the expansion of other cell types (data not shown).26
Besides systemic immunosuppressive injection or immunomodulatory therapy using educated regulatory T cells,35 other strategies to protect or improve insulin secretion of the transplanted β cells, for example using encapsulated islets, are currently under investigation. However, the perfect material allowing selective permeability and reduced bioreactivity is not available yet (reviewed in ref. 36). Similarly to our approach, the direct genetic modification of islets before transplantation has also been explored in studies aiming at improving islet function by expression of heme oxygenase (HO-1), downregulation of protein tyrosine phosphatase using siRNA, or prevention of β-cell death by overexpression of viral proteins (Epstein–Barr virus vIL-10, baculovirus p35, and the adenovirus gp19 and RID α/β).37,38,39,40 However, studies illustrating the feasibility in human islets are rare but efficient thereby lentivirus-mediated expression of cFLIP in as little as 10% of the human islet cells was sufficient to protect these cells from IL-1β, TNF-α, and IFN-γ proinflammatory cytokines treatment.41
Herpesviruses have the capacity to establish a lifelong infection of their host, a phenomenon associated with the expression of proteins with potent immunosuppressive properties, eluding CD8+ and CD4+ T cells, natural killer cells and innate immunity.42,43 Among the different immunevasins, US2 is extremely effective in targeting MHC-I molecules for destruction. Shortly after their synthesis, most of the newly generated MHC class I heavy chains are degraded.24,44 US2 has been suggested to downregulate all HLA-A and -G and most HLA-B alleles, whereas several HLA-B alleles and all HLA-C and -E alleles are likely to be insensitive to US2-mediated degradation. Importantly, the fact that US2 does not downregulate HLA-E may be useful in the context of immune protection, as HLA-E serves as an inhibitor of natural killer cells.
We confirmed that US2 expression induces downregulation of class I in surrogate β cells and that its coexpression with serine protease inhibitor 9 protects human islet cells against autoreactive T cells. The possible risk of increased susceptibility to natural killer cells associated with MHC-I downregulation could be counteracted with Serpin-9 coexpression by blocking granzyme B activity.45 The significance of Serpin-9 in protecting β cells against PPI autoreactive T cells has been recently reinforced by a study showing that cytotoxic degranulation is the predominant modus operandi of PPI-CD8+–mediated killing.46
Environmental factors including proinflammatory cytokines might influence CTL killing capacity and β-cell survival during transplantation. The clinical transplantation setting where islets are directly infused via the portal vein might complicate the problem.15 Participation of proinflammatory cytokines, instant blood-mediated immune response and activated complement or β-cell stress on autoreactive CTL killing capacity and on β-cell antigenic peptide generation remains to be investigated.
The use of NSG mice allowed us to monitor the human islet survival in mild hyperglycemic environment (basal glucose level is about 1 mmol/l higher in mouse than human) to specifically evaluate autoreactive CTL activity without interference from innate immunity and natural killer activity.47 The killing assay using human pseudoislets and a human autoreactive T-cell clone isolated from recent onset patient with type 1 diabetes, although not relevant for a long term study of the graft, provides a suitable model to determine the fate of human islet cells in the context of human CD8 autoreactivity. The results prove that these cellular aggregates remained functional and glucose-responsive, indicating that they were capable of resisting autoimmune T cells.
The lentiviral system used to deliver the protective transgenes is believed to provide a long term expression of US2/Spi9. However, cessation of transgene expression, due to promoter silencing, is still possible, and could lead to eradication of the modified cells by immune system. The cytomegalovirus promoter, used here, is very active in finally differentiated cells, like β cells but as many viral promoters its expression could be silenced.48 When translated to the clinic, other promoter, in particular cellular promoters, should be envisaged (Ubiquitin C promoter for example) and included in a specific comparative study.
To summarize, the novelty of our report is threefold. First, we engineered a novel assay to assess specific, autoimmune-mediated destruction of primary human β cells, allowing for the definition of selective β-cell loss in vitro. This approach facilitates the creation of a screening platform for identification of new compounds that inhibit the interplay between β cells and autoreactive T cells. Second, we designed a preclinical humanized mouse model to allow assessment of the fate of primary human β cells in an autoimmune environment. Finally, we showed that lentiviral vectors represent an efficient system for gene transfer into human diploid islet cells that can be subsequently reaggregated into functional pseudoislets. This offers new possibilities for genetic modification as a means of protecting human islet cells against the effect of autoreactive and possibly alloreactive T cells. By targeting two molecular pathways (viz. MHC class I synthesis and the perforin cell death pathway), we could increase the resilience of human β cells to acute, antigen-specific autoimmune attack. This study constitutes a proof of concept in mild hyperglycemic condition. The questions of the protective effect in extreme hyperglycemic environment where the increase in insulin production (and by definition the increase of antigenic leader peptide epitope) could exacerbate CTL killing, the scaling up of gene transfer techniques under clinically applicable procedures, the stability of transgene expression, the efficacy of downregulation of different HLA haplotypes and the risk of tumor development remain to be addressed before any translational research. Yet we can speculate that this type of approach could be successfully extended to protect against any auto- and alloreactivity. A combine approach using other highly potent viral immune evasion proteins could be envisaged to specifically target the HLA type or to increase the level of protection.21 The next Holy Grail will be the translation of these evasion strategies without the use of viral carrier nor prior disruption of the islet integrity.
Materials and Methods
Cells. HEK 293T cells were maintained in Dulbecco's modified Eagle's medium (DMEM) + 10% fetal calf serum supplemented with penicillin (100 units/ml), streptomycin (100 µg/ml); K562-A2 cells were maintained in Iscove's modified Dulbecco's media (IMDM) + 10% fetal calf serum supplemented with 0.7 mg/ml geneticin (Gibco BRL, Gaithersburg, MD), penicillin (100 units/ml), and streptomycin (100 µg/ml). PPI-specific CD8 T cells were cloned previously from a HLA-A201 patient with type 1 diabetes as described previously.28 For maintenance and expansion, PPI-specific and pp65CMV-directed CTLs were cultured for 2 weeks with irradiated allogeneic PBMCs, irradiated PPI15–24, or CMVpp65 peptide-pulsed HLA-A2–expressing EBV-LCL in IMDM supplemented with 10% human serum, 0.5% LeucoA, 0.1 ng/ml recombinant human (rh)-IL-12, 10 ng/ml rh-IL-7, 25 U/ml rh-IL-2 and 5 ng/ml rh-IL-15. Cells were frozen in a solution of 20% human pooled serum and 10% DMSO and kept in liquid nitrogen until use. Upon thawing, cells were allowed to rest in IMDM supplemented with 10% human serum, IL-2 (50 U/ml) and IL-15 (0.1 ng/ml).
DNA constructs. pLV-CMV-US2-bc-Spi9 has been generated from two intermediate cloning vectors pLV-CMV-US2-bc-GFP and pLV-CMV-Spi9-bc-GFP. pLV-CMV-US2-bc-GFP has been generated by cloning the US2-bc-GFP cassette from the LZRS-US2-bc-GFP described previously49 into the pLV-CMV vector. A pJET-Spi9 vector has been generated by cloning a Spi9 PCR fragment into pJET2.1/blunt vector (Fermentas, St Leon-Rot, Germany) using the following primers Spi9 Fw5′-TACATAAGGTTACACTAT-3′ and Spi9 Rv 5′-AACCCTTGTGTTAAGTAA-3′ on human placental cDNA (Agilent Technologies, Santa Clara, CA). pLV-CMV-Spi9 has been generated by introducing a NotI/XbaI Spi9 containing fragment from pJET-Spi9 into pLV-CMV-bc-GFP. Next, the GFP fragment from pLV-CMV-US2-bc-GFP was removed after NsiI/MscI digestion and blunting reaction, and was replaced by a SmaI/SmaI Spi9 containing fragment from pLV-CMV-Spi9-bc-GFP to generate the final pLV-CMV-US2-bc-Spi9 vector.
The HIP-derived constructs have been generated by cloning the HIP −326/+30 by PCR from human genomic DNA using the following primers FW: 5′-GCGCTCGAGTCTCCTGGTCTAATGTGGAA-3′ and Rv: 5′-GCGAAGCTTCTCTTCTGATGCAGCCTGTC-3′. The PCR product containing the XhoI/HindIII linker was then used to replace the CMV promoter in pLV-CMV-GFP to create pLV-HIP-GFP. pLV-HIP-Luc2CP was generated by cloning the HIP-Luc2CP containing fragment from pGL4-HIP-Luc2CP into lentivirus backbone.
Lentiviruses and cell transduction. All vectors are derived from pRRL-cPPT-CMV-IRES-GFP-PRE. Third generation self-inactivating lentivirus vectors were produced as described previously.50 Lentivirus vectors are quantified by antigen capture ELISA measuring HIV p24 levels (ZeptoMetrix, Buffalo, NY). The infectious titer is derived from the p24 concentration by the conversion that 1 ng p24 corresponds to 2,500 infectious particles. Whole islets or freshly dispersed islets were seeded in ultra-low attachment six-well plates and maintained in DMEM + 10% fetal calf serum + Pen/Strep. Viral supernatants were added to fresh medium supplemented with 8 µg/ml polybrene (Sigma-Aldrich, St Louis, MO), and the cells were incubated overnight. Transduction efficiency was determined by flow cytometry analysis after washing in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA) and analyzed on a FACS LSRII (BD Pharmingen, San Diego, CA).
Luciferase killing assay. Transduced dispersed islet cells were seeded in U-shape 96-well plates and incubated for 48 hours with HLA-A2–specific CTLs (directed against pp65CMV or PPI) in IMDM + 10% human serum supplemented with IL-2 (50 U/ml) and IL-15 (0.1 ng/ml). After 2 days of coculture, cells were lysed in Luciferase Lysis buffer (25 mmol/l Tris–HCl, pH 7.8, 2 mmol/l CDTA, 2 mmol/l DTT, 10% glycerol, 1% Triton X-100). Luciferase activity was determined by luminometry using the Promega Luciferase Assay Reagent (Promega, Madison, WI). Experiments were performed in triplicate for every target:effector ratio used. Results shown are represented as residual luciferase activity with the luciferase activity of transduced islets incubated without CTL set at 100%.
Western blot. Cells were treated with Luciferase Lysis buffer supplemented with a cocktail of protease inhibitors (Roche, Basel, Switzerland). Samples for western-blot analyses were prepared by boiling protein extracts with Sample buffer (10% glycerol, 2% SDS, 60 mmol/l Tris–HCl (pH 6.7), 2.5% mercaptoethanol, and 2.5% bromophenol blue from a 1:20 diluted saturated solution) for 5 minutes at 100 °C, and analyzed on SDS-PAGE. Proteins were transferred to Immobilon-P (Immobilon-P transfer membrane (polyvinylidene difluoride); Millipore, Etten-Leur, The Netherlands) and visualized by standard protocols with anti-insulin (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA; sc14664), and anti-actin (1:1,000 clone C4; ICN Biomedicals, Zoetermeer, The Netherlands).
Reverse-transcription PCR and real-time PCR. Total cellular RNA was extracted from K562 cells using Trizol. RNA of 500 ng was reverse transcribed using Superscript RT II kit (Invitrogen, Karlsruhe, Germany). Expression of the genes of interest was detected using the following primers: Insulin Fw 5′-GCAGCCTTTGTGAACCAACA-3′, Insulin Rv 5′-CGGGTCTTGGGTGTGTAGAAG-3′ GAPDH Fw 5′-ACAGTCAGCCGCATCTTCTT-3′, GAPDH Rv 5′-AATGAAGGGGTCATTGATGG-3′. Real-time PCR was performed in triplicate using the SybrGreen master mix kit (Applied Biosystems, Foster City, CA) and an Applied Biosystems Step One Plus machine. GAPDH mRNA was used as reference.
Human islet isolation. Human pancreata were harvested from brain-dead organ donors after informed consent was obtained in writing from family members. Leiden University Medical Centre had permission to isolate islets and to use them for scientific research if they are insufficient for clinical islet transplantation, in accordance with national laws and institutional ethical requirements. Human islet isolations were performed in the GMP-facility of the Leiden University Medical Center. Purity of the final islet preparation was assessed by 1 mmol/l dithizone (Sigma-Aldrich) staining and ranged from 75 and 95%. The purified islets were cultured in CMRL-1066 supplemented with 10% human serum, ciprofloxacin 20 µg/ml, gentamycine 50 µg/ml, l-glutamine 2 nmol/l, fungizone 250 ng/ml, HEPES 10 mmol/l, and nicotinamide 10 mmol/l, and cultured at 37 °C in a humidified atmosphere of 5% CO2 for 1–7 days before use.
Pseudoislets formation. Dissociation of primary human islets was performed by 5–10 minutes 0.05% trypsin treatment at 37 °C. After washing step in DMEM + 10% fetal calf serum (Invitrogen, Karlsruhe, Germany) containing media, dissociated cells were passed though a 40 µm filter and seeded in ultra-low attachment six-well plates at ~200.000 cells/ml (Corning, NY 14831). Spontaneous reaggregation of islet cells started to be observed 24 hours after dissociation. Pseudoislets were maintained 6 days in culture prior reimplantation and medium was refreshed every 2 days.
Mice. Male NOD SCID IL-2R−/− mice (NSG) of 6–12 weeks old were obtained from the Jackson Laboratory (Bar Harbor, ME), and bred in the animal facilities of the Leiden University Medical Center. Animals were housed under specific pathogen-free conditions. All studies were approved by the local Animal Care Committee.
Human islets/pseudoislets transplantation. Islet equivalents of 3,000 and 5 × 106 dispersed and reaggregated islet cells, either non-transduced or GFP transduced, were transplanted under the left kidney capsule, using a polyethylene catheter and precision syringe (Hamilton, Reno, NV). Grafts were removed 19 days after transplantation. Formalin-fixed paraffin-embedded sections were stained for insulin and DNA content. Insulin and DNA staining, and GFP epifluorescence were assessed by confocal microscopy. Evaluation of PPI-specific CTL killing in vivo was performed by cotransplantation of 5 × 106 reaggregated cells, either GFP or US2/Spi9 transduced, with HLA-A2 PPI-directed cytotoxic T cells at a 100:1 target:effector ratio. Islets isolated from three different donors have been genetically modified by GFP or US2/Spi9 lentiviral vectors and transplanted under the kidney capsule of three different NSG mice. Islet functionality was evaluated after Intraperitoneal Glucose Tolerance Test by human insulin and C-peptide measurement.
Immunostaining and confocal microscopy. Intact islets or pseudoislets were fixed in PBS containing 4% paraformaldehyde. Permeabilization was performed using PBS/1% Triton. Blocking was done with PBS/BSA 3%, and first and secondary antibodies were diluted in PBS/BSA 3%. Anti-Insulin (H86; Santa Cruz Biotechnology) was used at 1:200 and secondary antibody coupled to Alexa 568 was used at a dilution of 1:500. Nuclei were stained with 4′-6-diamidino-2-phenylindole (DAPI). Samples were subjected to optical sectioning using a laser scanning confocal microscope (Zeiss, Thornwood, NY).
Glucose-induced insulin-secretion test. Intact islets or pseudoislets were washed in PBS and transferred to a 8 µm pore size transwell plate (Corning HTS Transwell-96 well, Sigma-Aldrich) and preincubated in a modified Krebs-Ringer Bicarbonate buffer (KRBH) containing 115 mmol/l NaCl, 5 mmol/l KCl, 24 mmol/l NaHCO3, 2.2 mmol/l CaCl2, 1 mmol/l MgCl2, 20 mmol/l HEPES, 2 g/l human serum albumin (Cealb, Sanquin, The Netherlands), pH 7.4 for 2 hours. The transwell plate was then successively transferred for 1 hour to KRBH with 2 mmol/l and 20 mmol/l glucose at 37 °C. Insulin concentrations were determined in the supernatants by ELISA (Mercodia, Uppsala, Sweden).
Intraperitoneal glucose-tolerance test. For the intraperitoneal glucose-tolerance tests, mice were fasted overnight and injected with 2 g/kg glucose 1 mol/l i.p. blood was drawn from the tail vein at t = 0, t = 30 and t = 45 minutes. Intraperitoneal Glucose Tolerance Test was validated by determination of blood glucose concentrations measured using a glucose meter (Accu Chek, Roche). Human insulin content and human C-peptide concentrations were determined by ELISA according to the manufacturer's instructions. (Human insulin ELISA 10-113-10 and human C-peptide ELISA 10-1136-01; Mercodia, Uppsala, Sweden).
Statistical analysis. All data were presented as mean ± SD. For human insulin and human C-peptide values presented in Figure 6, data were subjected to nonparametric statistical analysis using two-way analysis of variance test with Bonferroni correction using GraphPad Prism software (GraphPad, San Diego, CA). A value of P < 0.05 was considered statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. CTLs killing capacity. Figure S2. Effect of US2/Spi9 on islets functionality. Data. CTL killing assay.
Acknowledgments
The authors thank Steve J Cramer, Martijn JWE Rabelink, and Annemieke M Tons (Leiden University Medical Center, The Netherlands) for expert technical assistance. This work has been supported by the Dutch Diabetes Research Funds (grants DFN 2005.00.0212, DFN 2007.00.0015, and Expert Center grant 2008.40.001). M.P. and S.A. receive support from the UK Department of Health via the National Institute for Health Research Biomedical Research Centre Award to Guy's and St Thomas' National Health Service Foundation Trust in partnership with King's College London. The authors declare no conflict of interest.
Supplementary Material
References
- In't Veld P, Lievens D, De Grijse J, Ling Z, Van der Auwera B, Pipeleers-Marichal M, et al. Screening for insulitis in adult autoantibody-positive organ donors. Diabetes. 2007;56:2400–2404. doi: 10.2337/db07-0416. [DOI] [PubMed] [Google Scholar]
- Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54 Suppl 2:S97–107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- Coppieters KT, Dotta F, Amirian N, Campbell PD, Kay TW, Atkinson MA, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med. 2012;209:51–60. doi: 10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J, Benoist C, Mathis D. Major histocompatibility complex class I molecules are required for the development of insulitis in non-obese diabetic mice. Eur J Immunol. 1993;23:3358–3360. doi: 10.1002/eji.1830231244. [DOI] [PubMed] [Google Scholar]
- Serreze DV, Leiter EH, Christianson GJ, Greiner D, Roopenian DC. Major histocompatibility complex class I-deficient NOD-B2mnull mice are diabetes and insulitis resistant. Diabetes. 1994;43:505–509. doi: 10.2337/diab.43.3.505. [DOI] [PubMed] [Google Scholar]
- Wicker LS, Leiter EH, Todd JA, Renjilian RJ, Peterson E, Fischer PA, et al. Beta 2-microglobulin-deficient NOD mice do not develop insulitis or diabetes. Diabetes. 1994;43:500–504. doi: 10.2337/diab.43.3.500. [DOI] [PubMed] [Google Scholar]
- Hamilton-Williams EE, Palmer SE, Charlton B, Slattery RM. Beta cell MHC class I is a late requirement for diabetes. Proc Natl Acad Sci USA. 2003;100:6688–6693. doi: 10.1073/pnas.1131954100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay TW, Parker JL, Stephens LA, Thomas HE, Allison J. RIP-beta 2-microglobulin transgene expression restores insulitis, but not diabetes, in beta 2-microglobulin null nonobese diabetic mice. J Immunol. 1996;157:3688–3693. [PubMed] [Google Scholar]
- Chervonsky AV, Wang Y, Wong FS, Visintin I, Flavell RA, Janeway CA., Jret al. (1997The role of Fas in autoimmune diabetes. Cell 8917–24. [DOI] [PubMed] [Google Scholar]
- Apostolou I, Hao Z, Rajewsky K, von Boehmer H. Effective destruction of Fas-deficient insulin-producing beta cells in type 1 diabetes. J Exp Med. 2003;198:1103–1106. doi: 10.1084/jem.20030698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kägi D, Odermatt B, Ohashi PS, Zinkernagel RM, Hengartner H. Development of insulitis without diabetes in transgenic mice lacking perforin-dependent cytotoxicity. J Exp Med. 1996;183:2143–2152. doi: 10.1084/jem.183.5.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas HE, Trapani JA, Kay TW. The role of perforin and granzymes in diabetes. Cell Death Differ. 2010;17:577–585. doi: 10.1038/cdd.2009.165. [DOI] [PubMed] [Google Scholar]
- Roep BO, Atkinson M. Animal models have little to teach us about type 1 diabetes: 1. In support of this proposal. Diabetologia. 2004;47:1650–1656. doi: 10.1007/s00125-004-1517-1. [DOI] [PubMed] [Google Scholar]
- Roep BO, Atkinson M, von Herrath M. Satisfaction (not) guaranteed: re-evaluating the use of animal models of type 1 diabetes. Nat Rev Immunol. 2004;4:989–997. doi: 10.1038/nri1502. [DOI] [PubMed] [Google Scholar]
- Harlan DM, Kenyon NS, Korsgren O, Roep BO, Immunology of Diabetes Society Current advances and travails in islet transplantation. Diabetes. 2009;58:2175–2184. doi: 10.2337/db09-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkse GG, Tysma OH, Bergen CA, Kester MG, Ossendorp F, van Veelen PA, et al. Autoreactive CD8 T cells associated with beta cell destruction in type 1 diabetes. Proc Natl Acad Sci USA. 2005;102:18425–18430. doi: 10.1073/pnas.0508621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huurman VA, Hilbrands R, Pinkse GG, Gillard P, Duinkerken G, van de Linde P, et al. Cellular islet autoimmunity associates with clinical outcome of islet cell transplantation. PLoS ONE. 2008;3:e2435. doi: 10.1371/journal.pone.0002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huurman VA, Velthuis JH, Hilbrands R, Tree TI, Gillard P, van der Meer-Prins PM, et al. Allograft-specific cytokine profiles associate with clinical outcome after islet cell transplantation. Am J Transplant. 2009;9:382–388. doi: 10.1111/j.1600-6143.2008.02479.x. [DOI] [PubMed] [Google Scholar]
- Hilbrands R, Huurman VA, Gillard P, Velthuis JH, De Waele M, Mathieu C, et al. Differences in baseline lymphocyte counts and autoreactivity are associated with differences in outcome of islet cell transplantation in type 1 diabetic patients. Diabetes. 2009;58:2267–2276. doi: 10.2337/db09-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondet JJ, Carlson AM, Kobayashi T, Jie T, Bellin M, Hering BJ, et al. The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Surg Clin North Am. 2007;87:1477–501, x. doi: 10.1016/j.suc.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Griffin BD, Verweij MC, Wiertz EJ. Herpesviruses and immunity: the art of evasion. Vet Microbiol. 2010;143:89–100. doi: 10.1016/j.vetmic.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Horst D, Verweij MC, Davison AJ, Ressing ME, Wiertz EJ. Viral evasion of T cell immunity: ancient mechanisms offering new applications. Curr Opin Immunol. 2011;23:96–103. doi: 10.1016/j.coi.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Zaldumbide A, Hoeben RC. How not to be seen: immune-evasion strategies in gene therapy. Gene Ther. 2008;15:239–246. doi: 10.1038/sj.gt.3303082. [DOI] [PubMed] [Google Scholar]
- Wiertz EJ, Tortorella D, Bogyo M, Yu J, Mothes W, Jones TR, et al. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 1996;384:432–438. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- Medema JP, de Jong J, Peltenburg LT, Verdegaal EM, Gorter A, Bres SA, et al. Blockade of the granzyme B/perforin pathway through overexpression of the serine protease inhibitor PI-9/SPI-6 constitutes a mechanism for immune escape by tumors. Proc Natl Acad Sci USA. 2001;98:11515–11520. doi: 10.1073/pnas.201398198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlotti F, Zaldumbide A, Loomans CJ, van Rossenberg E, Engelse M, de Koning EJ, et al. Isolated human islets contain a distinct population of mesenchymal stem cells. Islets. 2010;2:164–173. doi: 10.4161/isl.2.3.11449. [DOI] [PubMed] [Google Scholar]
- Bishop AE, Polak JM. The Anatomy, Organisation and Ultrastructure of the Islets of Langerhans. Blackwell Science: Oxford; 1997. [Google Scholar]
- Skowera A, Ellis RJ, Varela-Calviño R, Arif S, Huang GC, Van-Krinks C, et al. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J Clin Invest. 2008;118:3390–3402. doi: 10.1172/JCI35449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobinger GP, Deng S, Louboutin JP, Vatamaniuk M, Matschinsky F, Markmann JF, et al. Transduction of human islets with pseudotyped lentiviral vectors. Hum Gene Ther. 2004;15:211–219. doi: 10.1089/104303404772680010. [DOI] [PubMed] [Google Scholar]
- Callewaert H, Gysemans C, Cardozo AK, Elsner M, Tiedge M, Eizirik DL, et al. Cell loss during pseudoislet formation hampers profound improvements in islet lentiviral transduction efficacy for transplantation purposes. Cell Transplant. 2007;16:527–537. doi: 10.3727/000000007783464948. [DOI] [PubMed] [Google Scholar]
- O'Sullivan ES, Johnson AS, Omer A, Hollister-Lock J, Bonner-Weir S, Colton CK, et al. Rat islet cell aggregates are superior to islets for transplantation in microcapsules. Diabetologia. 2010;53:937–945. doi: 10.1007/s00125-009-1653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerome KR, Sloan DD, Aubert M. Measurement of CTL-induced cytotoxicity: the caspase 3 assay. Apoptosis. 2003;8:563–571. doi: 10.1023/A:1026123223387. [DOI] [PubMed] [Google Scholar]
- O'Brien MA, Daily WJ, Hesselberth PE, Moravec RA, Scurria MA, Klaubert DH, et al. Homogeneous, bioluminescent protease assays: caspase-3 as a model. J Biomol Screen. 2005;10:137–148. doi: 10.1177/1087057104271865. [DOI] [PubMed] [Google Scholar]
- Kanno A, Umezawa Y, Ozawa T. Detection of apoptosis using cyclic luciferase in living mammals. Methods Mol Biol. 2009;574:105–114. doi: 10.1007/978-1-60327-321-3_9. [DOI] [PubMed] [Google Scholar]
- Kleijwegt FS, Laban S, Duinkerken G, Joosten AM, Koeleman BP, Nikolic T, et al. Transfer of regulatory properties from tolerogenic to proinflammatory dendritic cells via induced autoreactive regulatory T cells. J Immunol. 2011;187:6357–6364. doi: 10.4049/jimmunol.1101638. [DOI] [PubMed] [Google Scholar]
- O'Sullivan ES, Vegas A, Anderson DG, Weir GC. Islets transplanted in immunoisolation devices: a review of the progress and the challenges that remain. Endocr Rev. 2011;32:827–844. doi: 10.1210/er.2010-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JD, Ellett JD, Chen M, Smith KM, Fialkow LB, McDuffie MJ, et al. Viral IL-10-mediated immune regulation in pancreatic islet transplantation. Mol Ther. 2005;12:360–368. doi: 10.1016/j.ymthe.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Hollander K, Bar-Chen M, Efrat S. Baculovirus p35 increases pancreatic beta-cell resistance to apoptosis. Biochem Biophys Res Commun. 2005;332:550–556. doi: 10.1016/j.bbrc.2005.04.156. [DOI] [PubMed] [Google Scholar]
- Kojaoghlanian T, Joseph A, Follenzi A, Zheng JH, Leiser M, Fleischer N, et al. Lentivectors encoding immunosuppressive proteins genetically engineer pancreatic beta-cells to correct diabetes in allogeneic mice. Gene Ther. 2009;16:340–348. doi: 10.1038/gt.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Wu H, Gu P, Du H, Shao J, Wang J, et al. Improved glucose-stimulated insulin secretion by intra-islet inhibition of protein-tyrosine phosphatase 1B expression in rats fed a high-fat diet. J Endocrinol Invest. 2012;35:63–70. doi: 10.3275/7766. [DOI] [PubMed] [Google Scholar]
- Fenjves ES, Ochoa MS, Cechin S, Gay-Rabinstein C, Pérez-Alvarez I, Ichii H, et al. Protection of human pancreatic islets using a lentiviral vector expressing two genes: cFLIP and GFP. Cell Transplant. 2008;17:793–802. doi: 10.3727/096368908786516828. [DOI] [PubMed] [Google Scholar]
- Wiertz E, Hill A, Tortorella D, Ploegh H. Cytomegaloviruses use multiple mechanisms to elude the host immune response. Immunol Lett. 1997;57:213–216. doi: 10.1016/s0165-2478(97)00073-4. [DOI] [PubMed] [Google Scholar]
- Wiertz EJ, Mukherjee S, Ploegh HL. Viruses use stealth technology to escape from the host immune system. Mol Med Today. 1997;3:116–123. doi: 10.1016/S1357-4310(96)10059-9. [DOI] [PubMed] [Google Scholar]
- Barel MT, Pizzato N, Le Bouteiller P, Wiertz EJ, Lenfant F. Subtle sequence variation among MHC class I locus products greatly influences sensitivity to HCMV US2- and US11-mediated degradation. Int Immunol. 2006;18:173–182. doi: 10.1093/intimm/dxh362. [DOI] [PubMed] [Google Scholar]
- Falk CS, Mach M, Schendel DJ, Weiss EH, Hilgert I, Hahn G. NK cell activity during human cytomegalovirus infection is dominated by US2-11-mediated HLA class I down-regulation. J Immunol. 2002;169:3257–3266. doi: 10.4049/jimmunol.169.6.3257. [DOI] [PubMed] [Google Scholar]
- Knight RR, Kronenberg D, Zhao M, Huang GC, Eichmann M, Bulek A, et al. Human ß-cell killing by autoreactive preproinsulin-specific CD8 T cells is predominantly granule-mediated with the potency dependent upon T-cell receptor avidity. Diabetes. 2013;62:205–213. doi: 10.2337/db12-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- Brooks AR, Harkins RN, Wang P, Qian HS, Liu P, Rubanyi GM. Transcriptional silencing is associated with extensive methylation of the CMV promoter following adenoviral gene delivery to muscle. J Gene Med. 2004;6:395–404. doi: 10.1002/jgm.516. [DOI] [PubMed] [Google Scholar]
- de la Garza-Rodea AS, Verweij MC, Boersma H, van der Velde-van Dijke I, de Vries AA, Hoeben RC, et al. Exploitation of herpesvirus immune evasion strategies to modify the immunogenicity of human mesenchymal stem cell transplants. PLoS One. 2011;6:e14493. doi: 10.1371/journal.pone.0014493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlotti F, Bazuine M, Kekarainen T, Seppen J, Pognonec P, Maassen JA, et al. Lentiviral vectors efficiently transduce quiescent mature 3T3-L1 adipocytes. Mol Ther. 2004;9:209–217. doi: 10.1016/j.ymthe.2003.11.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.