Abstract
India's success in eliminating wild polioviruses (WPVs) has been acclaimed globally. Since the last case on January 13, 2011 success has been sustained for two years. By early 2014 India could be certified free of WPV transmission, if no indigenous transmission occurs, the chances of which is considered zero. Until early 1990s India was hyperendemic for polio, with an average of 500 to 1000 children getting paralysed daily. In spite of introducing trivalent oral poliovirus vaccine (tOPV) in the Expanded Programme on Immunization (EPI) in 1979, the burden of polio did not fall below that of the pre-EPI era for a decade. One of the main reasons was the low vaccine efficacy (VE) of tOPV against WPV types 1 and 3. The VE of tOPV was highest for type 2 and WPV type 2 was eliminated in 1999 itself as the average per-capita vaccine coverage reached 6. The VE against types 1 and 3 was the lowest in Uttar Pradesh and Bihar, where the force of transmission of WPVs was maximum on account of the highest infant-population density. Transmission was finally interrupted with sustained and extraordinary efforts. During the years since 2004 annual pulse polio vaccination campaigns were conducted 10 times each year, virtually every child was tracked and vaccinated - including in all transit points and transport vehicles, monovalent OPV types 1 and 3 were licensed and applied in titrated campaigns according to WPV epidemiology and bivalent OPV (bOPV, with both types 1 and 3) was developed and judiciously deployed. Elimination of WPVs with OPV is only phase 1 of polio eradication. India is poised to progress to phase 2, with introduction of inactivated poliovirus vaccine (IPV), switch from tOPV to bOPV and final elimination of all vaccine-related and vaccine-derived polioviruses. True polio eradication demands zero incidence of poliovirus infection, wild and vaccine.
Keywords: EPI, eradication, polio, vaccine, wild polioviruses
Introduction
The monumental task of interrupting wild poliovirus (WPV) transmission has been achieved and sustained in India already for two years1. Extensive and exhaustive search for WPVs among children with any disease even remotely resembling poliomyelitis has proved negative since January 13, 2011. Sewage waters in Mumbai, Delhi, Patna and Kolkata have also been diligently searched week after week; these have been negative for WPVs in 2011 and 2012. On February 25, 2012, the World Health Organization (WHO) removed India from the list of ‘polio-endemic’ countries2.
India's success has silenced critics who predicted that polio itself was non-eradicable; or that polio was not eradicable in India with its low standards of sanitation and hygiene; or that wild polioviruses (WPVs) cannot be eradicated using live oral poliovirus vaccine (OPV); or that polio was not worth eradicating as it was a low priority disease but with very high cost of eradication. Each viewpoint had an element of rationale that had been long neglected by India's policy makers, resulting in delays in interruption of WPVs, originally targeted for 2000, but achieved 11 years later. Every other country in the WHO South East Asia Region had succeeded by 2000. India had to overcome not only formidable biomedical obstacles but also serious programmatic deficiencies that contributed to the delay in achieving the elimination of WPVs. The technical obstacles were thought to be the worst in the world, hence insurmountable. India's success proves that WPVs can be eliminated elsewhere where the obstacles are technically less formidable. While India was able to overcome the programmatic deficiencies, elsewhere these remain formidable on account of socio-political reasons3.
We are not yet out of the woods, but at its edge. In 2012, three countries continue to be polio-endemic: Nigeria and Pakistan with WPV types 1 and 3 and Afghanistan with WPV type 14. One country, Chad, continued to have WPV type 1 transmission following importation4. There was also a single case of polio in Niger as a result of importation from Nigeria. As long as WPVs circulate anywhere, these may re-infect polio-free countries. During the past decade, 40 countries including 22 in the last five years, developed outbreaks of poliomyelitis due to imported WPVs5. WPV 1 of Indian origin began spreading in Tajikistan in 2009 and resulted in a large epidemic with 587 cases6. In 2011, China had an outbreak of polio due to WPV 1; the virus originated in Pakistan7. Interestingly, polio outbreaks resulting from importation have occurred only in countries exclusively using the live oral poliovirus vaccine (OPV); this observation has implications for the future of polio eradication in India as well as globally.
The ease and frequency of spread of WPVs across continents - from India to Angola and Yemen to Indonesia, the saturation of preschool children with WPV infection, the lack of upward age shift of polio and the tenacity of WPVs circulating in spite of high OPV coverage are all features characteristic of highly contagious agents that transmit person-to-person via droplets/aerosol, but not of agents transmitted exclusively via faecal-oral route. The teaching that in developing countries transmission is via faecal-oral route, but in developed countries it is via droplets/aerosol is long overdue for revision. It is very likely that both routes apply in all countries, their relative importance varying according to circumstances of crowding and sanitation. In very young infants the likelihood is more for air-borne transmission. The extremely high force of transmission (FOT) of WPVs in Uttar Pradesh and Bihar, with median age of polio always below 18 months, may well have been associated with the density of infant population, the highest in India, rather than with poor sanitation that prevails in many other States also8. For these reasons, the risk of WPV importation into India from endemic or re-infected countries is high. Most international spread seems to have been through adults acting as ‘carriers’. Pakistan and India share a very long border; there is direct air connection to Afghanistan. Our currently excellent OPV coverage is no guarantee against importation. Vaccination coverage can drop rapidly in some locations among our large annual birth cohort, and even minor fall in critical places may lead to spread of imported WPVs. Immunity gaps should not be allowed to develop now or in the near future.
True polio eradication is zero transmission of not only WPVs but also vaccine polioviruses9,10. The elimination of WPVs using OPV is the first phase, and elimination of vaccine polioviruses using inactivated poliovirus vaccine (IPV) is the second phase9,10,11,12,13. This concept originated in India and WHO has very recently endorsed it14. India will have to implement the second phase in the near future.
During the 1970s, 1980s and continuing into early1990s, polio was hyperendemic in India, with 200,000 to 400,000 cases annually15. Today we are free from WPVs. This review presents India's journey from hyperendemic to eradication of polio in five sections: the period prior to establishing nation-wide polio immunization, the first decade of Expanded Programme on Immunization (EPI), pre-eradication period, eradication phase 1, and the future prospects of phase 2 and beyond.
Polio situation prior to the adoption of EPI
Among developing countries, India was the worst affected with polio prior to its decline in the 1990s. But India was also the pioneer-leader in polio research - epidemiology, vaccine-prevention - and in the manufacture of both OPV and IPV. India's lead position was squandered in later years due to short-sighted policies and capricious decisions, a blot in our history of public health.
The Indian Council of Medical Research (ICMR) had established, with great foresight and vision, a Polio Research Unit (now Enterovirus Research Centre, EVRC) in Bombay (now Mumbai), in 1949. Data on epidemiology of urban poliomyelitis analysis were collected by pioneers16,17,18. The earliest attempt to isolate poliovirus was by CG Pandit, the first Director of ICMR (Deshpande JM, personal communication, 2012). Investigating a polio outbreak in Andamans in early 1950s he inoculated monkeys with human specimens, serially passed paralysis-causing agent six times, but lost the strain subsequently, before confirmation of viral identity. After introducing primary monkey kidney cell culture in late 1950s, EVRC isolated polioviruses easily in cell culture and confirmed by virus neutralization with antiserum.
The second polio research unit in India was the Enterovirus Laboratory, established in 1964, in the Christian Medical College (CMC), Vellore, Tamil Nadu. Studies from both centres showed that the country was hyperendemic for poliovirus infection and paralytic poliomyelitis. In longitudinal community survey the prevalence of subclinical poliovirus infection in Vellore town was 242 per 100 child-years below 5 yr19. While in the town 4 per cent of faecal samples yielded polioviruses, in a nearby rural community, the frequency of polioviruses was 2.7 per cent, suggesting lower FOT in rural children20. The incidence of paralytic polio in India was among the highest reported anywhere. In Mumbai and Vellore, 5-8 per cent of cases occurred in infants less than 6 months and the median age of polio was between 12 and 18 months, characteristic of contagious (respiratory transmitted) diseases with very high FOT21.
The reported incidence of polio was very high in both urban and rural communities in Eastern Uttar Pradesh22. In a rural block outside Vellore town, the prevalence of post-polio paralysis was 6/1000 preschool children23. In Vellore town it was 8/1000 children in first grade in schools24. That could be extrapolated to polio incidence of 24 cases/100,000 population/year. Clinical poliomyelitis cases were counted in northern India and Basu calculated annual incidence of >40/100,000 population25. In Vellore town and nearby rural areas the annual incidence was measured to be 20-22/100,000 population. In both communities, more than 2 children among 1000 under-fives developed polio paralysis annually23,26. Thus, polio was a huge problem, both humanitarian and economic27. The old calculation of cost-benefit of polio control did not take into account the disability-associated productivity loss which is an important element in current cost-benefit analysis28. Due to productivity loss of large numbers of polio-affected, the nation's annual loss was colossal. However, advocacy for polio control went largely unheard29,30. Post-independence India had prioritized diseases for targeted control - such as tuberculosis, malaria, leprosy and kala azar - but not poliomyelitis, in spite of the availability, in 1955, of IPV with proven safety and efficacy. OPV became available in 1962.
Polio immunization using imported OPV was introduced in Mumbai by the city corporation in 1964 and in Vellore by CMC in 1965. Soon thereafter problems of OPV efficacy were detected and were systematically studied in Vellore31. In 1972, the first definitive study on the problem of low immunogenic efficacy of OPV with standard potency was published32,33. Low vaccine efficacy (VE) was corroborated by counting children developing poliomyelitis in spite of the recommended 3 doses of OPV34. A study in New Delhi also showed very low immunogenic efficacy35. Low vaccine efficacy was also confirmed in Mumbai36. In short, there was ample warning that India had problems with VE of OPV years before, and at the time of the launch of EPI in the country. On the other hand, IPV had showed excellent VE in clinical studies37.
India faced a choice. Salk's IPV was widely used from 1955 in USA, Canada, UK and north European countries resulting in rapid control (>95% reduction) of polio. Finland interrupted WPV transmission in 1962 using IPV in campaign mode38. Unfortunately, IPV could not be used to control polio in India as it was not licensed for use even in the private sector. One manufacturer made IPV under Maharashtra State license in 1985/86 but had to discontinue under directions of the Government of India (GOI)31,37. Ultimately IPV was licensed in 2006 when it became apparent that IPV was the vaccine of the future.
Sabin's OPV was licensed in USA in 1961as monovalent and in 1963 as trivalent vaccine. In 1966 Sabin donated his vaccine strains to the Pasteur Institute (Coonoor, Tamil Nadu) and personally trained the staff to manufacture OPV. Under the leadership of Veeraraghavan and Balasubramanian OPV was successfully manufactured; after 6 batches were released during 1968-1974, instead of expanding manufacturing capacity the OPV unit was closed down31. Ironically, WHO was preparing recommendation of OPV's wide usage in EPI that was launched globally in 1974. Since closure of OPV unit in the Pasteur Institute, no Indian manufacturer has so far been able to make OPV in India.
Polio in India during the first decade of the EPI era
WHO launched EPI in 1974 and India adopted it in 1978. Public sector units were already manufacturing DPT vaccine and BCG. Therefore, EPI began large scale inoculations with both these vaccines. Since its manufacture was discontinued in 1974, OPV had to be imported for use in EPI. So its introduction was delayed and staggered, at first only in urban populations during 1979-80 and extended to rural communities during 1981-8239. In a polio hyperendemic country, routine inoculations with DPT should not have been introduced without first vaccinating against poliomyelitis, as DPT was notorious for provoking polio meelitis40. According to distributed EPI reports, during 1978-1979, 1979-1980, 1980-1981 and 1981-1982, 27, 24, 24 and 29 million children were injected with DPT, while the numbers of children given three doses of OPV in those years were zero, 0.5 million, 1.3 million and 2.3 million, respectively41. The world's largest iatrogenic provocation poliomyelitis outbreak seems to have been caused in India during the early 1980s42,43.
Even after introduction of OPV in EPI, the number of polio cases did not fall for about 10 years, as shown in Fig. 131. In those years there was no surveillance for polio; a sentinel surveillance system was operating but the numbers of cases reported were estimated to be about 10 per cent of the total cases. However, with the use of identical reporting system over the entire period the pattern was valid; only the numbers remained underestimated. Two opposing forces operated: the vaccine-induced downward pull and injection-induced upward push. In 1981, there was nation-wide polio epidemic, on the background of already hyperendemic status. The next nation-wide epidemic was in 1987-1988. During this decade, after introduction of OPV in EPI, the estimated annual numbers of cases were 200,000 to 400,000; translated to daily averages, some 500 to 1000 children were developing polio paralysis each day. Assuming annual productivity loss of 50 per cent of per capita gross national product (amounting to 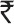 50,000), resulting in loss to national economy of
50,000), resulting in loss to national economy of 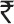 15 lakhs per paralyzed child, extended over 30 years of productivity, for 300,000 victims of polio, the total annual loss to the nation was
15 lakhs per paralyzed child, extended over 30 years of productivity, for 300,000 victims of polio, the total annual loss to the nation was 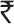 45,000 crores. This is in addition to the expenses incurred for travel and treatment, rehabilitation, calipers and wheel chair etc27. Parental loss of work added to the family's impoverishment. Polio control was necessary not only for humanitarian reasons but also for the socio-economic development of the country. High and medium income countries grasped this reality very early and rapidly controlled polio in the 1960s as soon as vaccines became available.
45,000 crores. This is in addition to the expenses incurred for travel and treatment, rehabilitation, calipers and wheel chair etc27. Parental loss of work added to the family's impoverishment. Polio control was necessary not only for humanitarian reasons but also for the socio-economic development of the country. High and medium income countries grasped this reality very early and rapidly controlled polio in the 1960s as soon as vaccines became available.
Fig. 1.

Total number of wild poliovirus cases in India from 1974 to 1994 (Source: Ref. 31).
Many cases of polio reported during the 1970s and 1980s were in children who had already taken 3 doses of OPV, on account of the low VE of OPV. In Vellore region, the proportion of such vaccine failure cases steadily increased from 10 per cent in 1979 to 30 per cent in 1986, 50 per cent in 198931. In order to improve VE several modifications were tried - as OPV did not have prime-boost effect, giving additional doses were shown to increase VE proportional to the first dose VE20. Thus, five doses given in infancy, to take advantage of 5 contacts for other EPI vaccines, was recommended as a routine, but not accepted by EPI44. Alternatively, pulsing three doses of OPV was shown to enhance VE45. In a visionary experiment, ‘pulse immunization’ using OPV was conducted in Vellore, making it the first Indian town to be polio-free; the concept and name of pulse immunization were created in Vellore46.
In 1985, ICMR commissioned a study in the North Arcot district (population 5 million), of which Vellore was the administrative headquarters. Half the population continued with OPV, but achieving highest possible coverage at the recommended ages and in the other half IPV was offered, 2 doses at 10 and 14 wk and a third at 9 months. The incidence of polio declined in both sides, with faster decline in the IPV side in spite of achieving lower 3-dose coverage31,47.
The reported number of cases of poliomyelitis under sentinel surveillance was 28,757 in 1987, not any lower that in the pre-EPI era (Fig. 1). Only by 1989, one decade after the launch of EPI in India, did the number of polio cases decline to the pre-EPI levels. This decline is attributed to the cumulative effect of increasing coverage with the vaccine - both direct vaccine efficacy and the indirect herd effect. However, the fall was still short of polio “control”, if defined as >95 per cent decline in incidence.
The early phase of global polio eradication (1988 to 1995)
The World Health Assembly (WHA) resolved in 1988 to target polio for global eradication by 2000 and India was a signatory in support of the decision48. The four strategic components promoted by WHO were to reach and maintain high routine OPV coverage, to top up immunization with supplementary doses of OPV (Supplementary Immunization Activity, SIA), to establish systematic surveillance of polio with laboratory virological support, and to use local area mop up OPV campaigns to interrupt any remaining chains of WPV transmission49.
By 1990 when 80 per cent 3-dose OPV coverage was achieved, the burden of polio had begun declining in India. The achievement of 80 per cent coverage of all EPI vaccines was celebrated in the Global Child Summit of 1990 in New York31. In the absence of polio surveillance, neither the total number of cases nor the proportions accounted for by 3-dose vaccine failure was under scrutiny. The estimated number of polio cases in 1994 was 50,000; that amounted to an average of 137 children getting paralyzed every day31. Polio burden had not yet reached control status.
During the first decade of the EPI era, polio had continued to persist endemically with superimposed outbreaks at intervals of 5-7 years (Fig. 1); 1992 was an epidemic year. Thereafter, cases declined and in 1993 and 1994 the numbers dwindled to less than half of the number in the pre-EPI era. Eventually, the number of reported cases of polio declined to 3,142 in 199550. In the absence of public health surveillance and epidemiology intelligence, EPI could only deliver vaccines, not measure or monitor the disease burdens or the control trajectories of the target diseases. Disease statistics were collected through a sentinel monitoring system and the Central Bureau of Health Intelligence was putting summary data in the public domain, 2-3 years later. The reported number of cases was shown to be approximately 10 per cent of the national total numbers calculated using the actual incidence of polio15,25.
During 1960s and 1970s a few high-income countries had already eliminated polio using only IPV; others had eliminated WPVs using OPV but continued to have an occasional case of polio due to OPV, known as vaccine-associated paralytic polio (VAPP). But polio due to WPVs plagued most developing countries, and EPI was specially designed for them. However, EPI failed to control polio in most of them. Although accurate numbers are not available, WHO estimated that 150 countries had annual burden of 350,000 polio cases in 198851. This was a gross underestimate as India alone had 200,000 to 400,000 annual cases in mid-1980s52,53.
Era of polio eradication (1995 to 2012)
Although the WHA decision to eradicate polio was taken in 1988, India's efforts to implement it started on a national level only in 1995-1996. In 1995, the Global Polio Eradication Initiative (GPEI) spearheaded by WHO in partnership with UNICEF, Centers for Disease Control of USA and Rotary International designed a modus operandi for India. Thus was born the National Polio Surveillance Project (NPSP), a joint project of the WHO and GOI. India had so far not managed to bring polio under control status, but expected to eliminate its transmission within the next 4-5 years. The two factors that stood in the way of control, namely ‘failure to vaccinate’ and ‘failure of vaccine’ were not realistically addressed. While it was expedient to assign polio eradication to NPSP it was unwise since we already had the Immunization Division, the natural choice as the nodal agency for polio eradication. However, polio eradication activities were conducted in parallel with UIP, as yet another vertical national project. From inception, NPSP was headed by a WHO staff. The elimination of WPVs stands as an eloquent testimony to the effective leadership of NPSP, the close cooperation by the GOI and State Governments and the sincere work of countless health-workers, non-governmental organizations (NGOs), and local volunteers.
In 1994, a pilot polio immunization campaign was conducted in Delhi, targeting one million children up to 3 yr of age. Since then SIA was popularly called Pulse Polio Immunization (PPI) after the successful Vellore experiment46. PPI was later expanded nationally in 1995 during which a total of 88 million under-3 children were immunized. From the next year the target age group was increased to all children under the age of 5 yr. This resulted in further decline in number of polio cases to 1005 reported in 199654. In October 1997, NPSP had begun to undertake ‘active surveillance’ of acute flaccid paralysis (AFP) cases in the entire country. In 1999, the India Expert Advisory Group for polio (IEAG) was constituted by GOI to review progress and to modify tactics of SIAs for interrupting WPV transmission. The global standard of adequate surveillance was detecting annually at least one case of acute flaccid paralysis in 100,000 children below 15 yr. IEAG found that in India AFP was far more common than in South American countries and the benchmark for accepting the quality of AFP surveillance was revised to at least 2 /100,000. Unfortunately, even as >10/100,000 are reported55, the diagnoses and outcomes of such colossal numbers of children with non-polio AFP are not in the public domain, highlighting the weaknesses of India's health management system.
Till 1998-1999, PPI consisted of vaccination of children at fixed booths on two National Immunization Days (NIDs), separated by six weeks, during the winter months. After the nation-wide PPI campaigns in 1995-1996, 1996-1997, 1997-1998 and 1998-1999, WPV 2 stopped circulating by October 199956. The very last chain of transmission was in Aligarh in Uttar Pradesh (UP). So, the coverage of trivalent OPV (tOPV) had reached to a level sufficient to interrupt WPV 2; but that was insufficient to interrupt WPV 1 and 3 transmissions. The vaccine efficacy of type 2 component in tOPV was high, but not that of the types 1 and 320,32,33. Their efficacy was too low to contribute to herd effect, and the FOT of WPV 1 and 3 was too high to achieve interruption without significant herd effect.
In view of missing goal of reaching zero incidence of polio by 2000, a plan to further intensify PPI was adopted in 2000. Four nation-wide PPI rounds were conducted in October, November, December of 2000 and January 2001, followed by two sub-national rounds in 8 States (Assam, Bihar, Gujarat, Madhya Pradesh, Orissa, Rajasthan, Uttar Pradesh and West Bengal) that had continuing polio and had low EPI coverage57. In spite of reaching 94-95 per cent of target children in PPI campaigns, WPV transmission could not be interrupted in the high risk States of UP and Bihar. It was obvious that as near 100 per cent children as possible had to be vaccinated repeatedly for success. During and since 2000, therefore, another tactic was applied: in addition to booth immunization, a house-to-house search of missed children and vaccinating them on the next 2-3 days following each national and sub-national PPI58.
By 2000, the estimated number of polio cases worldwide had declined 99 per cent from 1988. Of the 150 polio-endemic nations all but six (India, Pakistan, Afghanistan, Nigeria, Niger and Egypt) had succeeded in interrupting WPV transmission57. In parallel, in 2000 and 2001 there were only 265 and 268 cases due to WPV 1 and 3, for more than 99 per cent decline in India from the 1980s. Thus polio was effectively controlled by 2000, but WPV transmission was not interrupted. Experts have debated if India should have attempted eradication before achieving control. Today, success speaks for itself; failure to target polio control in the past was not to be a reason for not targeting eradication.
In 2000, since success had eluded us and more precision was required to track all WPV transmission chains, NPSP adopted a ‘virological scheme’ of classification in place of ‘clinical’ classification of AFP cases. For this purpose, the WHO, in association with NPSP, strengthened several existing virology laboratories and networked them for virological surveillance of polio. Two consecutive-day stool samples were collected from each child with AFP and submitted to the designated laboratory under cold chain conditions. Each poliovirus isolate was analysed to distinguish vaccine virus from WPV. Only if WPV was detected was the child diagnosed with polio. By 2001, WPV circulation was limited essentially to the two northern States of UP and Bihar. GOI took polio eradication as an issue of national prestige, and declared 2005 as the target year in its National Health Policy. Yet, 2002 saw an outbreak with 1,600 cases, nearly 87 per cent of cases detected globally, mostly of type 1, and 1,363 (85%) cases in UP and Bihar alone59. In central and eastern UP 32 per cent children with non-polio AFP had received 3 or less tOPV doses, a surrogate for vaccine coverage in local children. Also, 60 per cent of WPV polio cases were from Muslim community. The failure to dislodge WPV 1 and 3 was attributed to inadequate numbers of annual PPI campaigns and also to their poor quality58. As seen in Fig. 2, epidemiologically, 2002 was an epidemic year - according to the recent 4-year periodicity of WPV 1 - 1998, 2002, and later in 2006. WPV numbers were 225, 134 and 66, respectively in 2003, 2004 and 2005 - and viruses strayed into other States, as far south as Karnataka, Kerala and Tamil Nadu58,59,60.
Fig. 2.
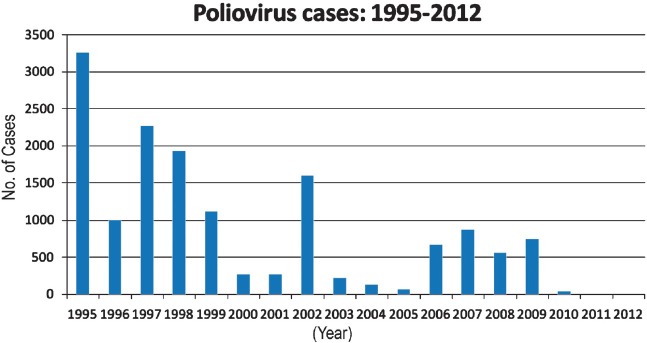
Total number of wild poliovirus cases in India from 1995 to 2012.
In 2003, the ‘under-served strategy’ was introduced as part of better communication efforts in Uttar Pradesh to reach out to and get support of marginalized sections of the society especially those living in poor Muslim communities, lacking access to basic sanitary and healthcare services, and were often missed in tOPV rounds, and thus were more likely to receive fewer doses61. The strategy was aimed at engaging universities, religious leaders and groups, local associations and individuals from underserved Muslim communities to broaden ownership and accountability for polio eradication. An improvement in poliovirus surveillance quality was seen in 2004. The programme was now able to rapidly detect poliovirus transmission anywhere in the country. It became obvious that huge numbers of people migrate for employment, mostly seasonal, and their children by and large missed receiving tOPV doses in EPI and in PPI. So the ‘transit vaccination’ strategy was launched, with teams stationed at bus stands, railway stations, highways, markets and at congregation sites and provided polio vaccine to eligible children. Beginning in 2005, NPSP and GOI and partners intensified eradication efforts with careful monitoring and implementation of immunization and surveillance activities, with particular attention paid to detailed local level micro-planning and by expanding the number of AFP reporting units throughout the country.
The extremely poor efficacy of OPV - ‘failure of vaccine’ - permitted WPV transmission in western U P and in Bihar in spite of high tOPV coverage. Thus, >95 per cent of children with polio had earlier received at least four tOPV doses. Persistent transmission was attributed to failure of vaccine and very high FOT of WPV due to the very high density of infant population60,62,63. Many children got polio in spite of seven or 10 or even 15 doses of tOPV. In 2005, monovalent OPV type 1 (mOPV-1) and type 3 (mOPV-3) were licensed based on an early Indian study showing 2.5 to 3 times higher VE of mOPV-1 and mOPV-3 than that of tOPV64. In 2005, these data were confirmed in a new research study65. SIAs added mOPV1 in April 2005, and it was used in most SIA rounds conducted during April-November in Bihar, UP, Mumbai (Maharashtra State), and polio-free States that had documented cases of WPV1 importation66. In December 2005, mOPV3 was first used in eradication activities in western UP, after detection of WPV3 in Moradabad district66.
The second but less formidable problem was inadequate coverage of under-five children with OPV doses, both under routine EPI schedule and in pulse campaigns - ‘failure to vaccinate’. Routine vaccination coverage with 3 doses of OPV continued to be low in the polio-endemic States (Bihar, 27%; western UP, 38%; and eastern UP, 45%) (UNICEF, unpublished data, 2005). To counter both factors, the number of PPI campaigns was increased to 10 each year from 2005 and the ‘under-served’ and ‘transit vaccination’ strategies were sustained. Yet, UP and Bihar remained the sites of ongoing WPV transmission in India and the source of exportation of WPV to other countries, including the polio-free countries of Angola (with spread to the Democratic Republic of Congo and Namibia), Bangladesh, and Nepal67,68.
There was a polio outbreak in 2006, with 648 cases of type 1 and 28 of type 3, again most cases occurring in UP and Bihar. The population immunity gap was found primarily in infants and very young children (<2 yr) due to very low UIP coverage with tOPV and insufficient opportunities to receive mOPV1 in pulse campaigns69. To compensate for low routine coverage, the PPI campaigns had been increased to 10 per year, and neonates and very young infants were specially targeted.
From mid-2000s the need for research to answer several questions was appreciated. The GOI and NPSP guided research confirmed the high efficacy of mOPV-1. NPSP continued with multiple campaigns using mOPV-1. Subsequently, WPV type 1 came under control, but immunity gaps remained for type 3, since routine immunization was not reaching a majority of infants and vaccination campaigns used mOPV-170. In 2006, the IPV was licensed in India. In late 2006, the IEAG for the first time requested a pilot study of supplemental dose of IPV in a few blocks of western UP in adition to giving mOPV1 birth dose.
Based on recommendations of the Global Advisory Committee on Polio Eradication and IEAG, India prioritized elimination of WPV1 from 2006/2007 because it was the most frequent cause of paralytic disease, was responsible for >90 per cent of polio cases in the country during the previous five years, and had been the agent of re-infection of a few polio-free countries69. Moreover, the next anticipated WPV1 outbreak year was 2010 and IEAG wanted to ensure that such an outbreak will not occur in 2010. In spite of these arguments, the tactic of preferentially targeting WPV-1 became controversial.
Was it wise to target WPV-1 while immunity gaps against WPV-3 would remain? Nevertheless, it seemed to have worked and ultimately areas that previously had the highest incidence of WPV1 recorded lowest numbers in subsequent years and finally its transmission ceased in January 2011. However, this tactic could not address the type 3 outbreaks that occurred in 2007-2008 in Bihar and in 2008-2009 in Uttar Pradesh, adding up to totals of 874 cases in 2007, 559 in 2008 and 741 in 2009. Most of the WPV3 cases in 2007 occurred in certain districts of western Uttar Pradesh that had never conducted a mOPV3 SIA until July 200771. These outbreaks were in part due to the very low EPI coverage with tOPV in Bihar and UP - insufficient even to prevent outbreaks. The WPV-3 outbreaks during 2007-2009 were seen as failure by some experts while the concurrent elimination of WPV-1 in UP and Bihar was lauded as success by others.
The interruption of WPV1 transmission in UP during 2007-2008 indicated that frequent mOPV1 rounds of consistently high coverage with meticulous micro-planning, enhanced technical and communication support could be successful even in areas with the most persistent transmission. However, during 2008-2009, UP was re-infected with WPV 1 that was introduced from Bihar71. The number of reported WPV3 cases in India declined steadily since the peak of the 2007 outbreak. Most WPV3 cases in 2008 occurred in districts in UP and Bihar in which less than three SIA rounds of mOPV3 had been administered during 2007. The mOPV3 rounds conducted at the end of 2007 and during 2008 appeared to have substantially reduced WPV3 transmission71.
In 2008, Kosi River bank communities in Bihar were identified as a key reservoir of WPVs71 Plan was drawn up to intensify and focus efforts in Kosi River areas. High-risk blocks were mapped, and additional stay points built for enhanced supervision and efforts in the hardest-to-reach areas where children were being missed. In November 2009, the IEAG declared that 107 blocks in western UP and central Bihar were holding the key to eradication in India72. In late 2009, India had planned to conduct additional mOPV3 SIA rounds as needed to prevent further WPV3 outbreaks while continuing to use mOPV1 for most SIAs72. Based on preliminary data from a clinical trial conducted in 2009, the IEAG recommended the use of bivalent OPV containing types 1 and 3 (bOPV) instead of mOPV-3 to address both WPV-1 and 373.
Towards the end of 2009, while WPV1 had virtually disappeared, there was tension and disappointment that WPV3 was still causing outbreaks in spite of intensive efforts over many years, repetitive and massive OPV campaigns, improved tactics and large expenditure71. From a layman's viewpoint it did not matter if WPV 1 or WPV 3 was causing polio outbreak - but IEAG was clear in its objective of sequential elimination of WPV1 first and WPV3 later. The fear of a resurgence of WPV 1 in 2010 was the one factor that encouraged IEAG to stay the course with targeting WPV1 and the result was success. GOI proposed a reduction in the tempo of eradication efforts and to accept ‘control’ of WPVs as the realistic goal that could be achieved74. Fortunately, IEAG guided the battle against polio with continued vigour. Only 42 WPV cases were detected in 2010. This emboldened the GOI to recommend responding to each case of polio as a public health emergency75. Finally, there was only one case in 2011 and the responsive mop-up immunization was exemplary.
The introduction of bOPV in SIAs beginning in January 2010 contributed substantially to the sustainment of simultaneous reduction in WPV1 and WPV3 cases. A clinical trial earlier had demonstrated the superiority of bOPV compared with tOPV and non-inferiority compared with mOPV1 and mOPV373. Seroprevalence (of polio antibodies) studies among infants aged 6-7 months in highrisk areas of UP and Bihar indicated that after bOPV introduction, seroprevalence against WPV3 had increased and high levels of sero-prevalence against WPV1 were maintained (Enterovirus Research Center, Mumbai, India, unpublished data, 2010). In India, the last confirmed WPV3 case had occurred on October 22, 2010 in Jharkhand, not UP or Bihar1,75. Similarly, the last WPV1 case occurred on January 13, 2011, in Howrah, West Bengal and not UP or Bihar1,75. Subsequently, India was removed from the list of polio endemic countries after completing a year without reporting any more WPV isolate from case or environmental samples.
As no WPV was identified throughout the high-transmission season in 2012, India is regarded as free of WPV polio. This places the WHO SEA Region, of which India is a member, on track to be certified polio-free as early as 2014. Certification of polio eradication occurs at three levels. The National Certification Committee will collect all relevant documentation not only to confirm the absence of WPV circulation but also to ensure that no laboratory is keeping any clinical specimen likely to contain poliovirus or any past laboratory virus isolate or virus stock for research or diagnostic studies. India has a mechanism to ensure such laboratory containment under the ICMR. WHO does not certify individual countries but a Region. The SEA Regional Certification Committee will review all information and will consider certification after three consecutive years have passed from the very last WPV isolate in the region (January 13, 2011). Therefore, we may expect Regional Certification some time after January 2014. Once that happens SEA Region joins the other already certified Regions, (Pan American, Western Pacific and European). Eastern Mediterranean Region (with Afghanistan and Pakistan) and African region (with Nigeria) will remain to achieve WPV elimination and certification, hopefully in 2015 or 2016. Only after all Regions are so certified will the Global Certification Committee recommend to WHO to declare the world free of WPVs.
The second phase of polio eradication and beyond
All countries that used OPV had to face the rare adverse event of VAPP from the very beginning76. Countries that eliminated polio exclusively using IPV did not get burdened with VAPP76. Therefore, it was obvious that continued use of OPV after the eradication of WPVs would be ethically unacceptable (Table). During the early years of global efforts to eradicate polio, the general expectation was that OPV could be discontinued after achieving WPV eradication. WHO had clearly recommended that all countries using OPV must monitor VAPP76. Unfortunately India did not comply and the problem of VAPP was ignored until pointed out by researchers from CDC and NPSP77. From the time polio case classification based on virus isolation was introduced, only WPV detection was taken as the criterion to classify a case as polio; consequently all VAPP cases from whom vaccine-like viruses were isolated were classified as ‘non-polio’. In 1999, there were 181 VAPP cases77. On reanalysis it was found that India has globally the highest incidence of VAPP - 1 case for 143,000 birth cohorts in comparison with 1/750,000 in the USA and 1/400,000 in Norway78.
Table.
Different phases leading to final eradication of polio
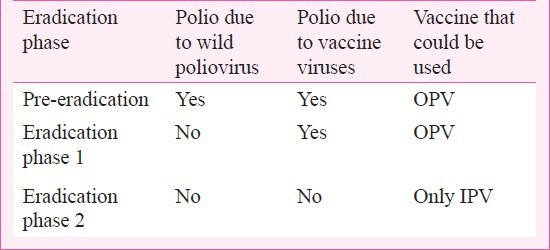
OPV was developed in the era prior to the development of molecular virology, but Sabin viruses were known to cause secondary spread and also to regain phenotypic property of neurovirulence from the very early days of its use. Once the genetic basis of attenuation was discovered, its easy genetic reversibility was also described. Based on this information, it was pointed out that true polio eradication must mean zero transmission of poliovirus, wild and vaccine9,11,12,20. Phase 2 is inevitable to complete and conclude polio eradication.
Vaccine viruses are not only genetically prone to reversal to neurovirulence causing VAPP, but also epidemiologically prone to transmission to unvaccinated children, causing polio outbreaks. The longer vaccine viruses replicate in human intestines, the greater the mutations that bring it closer to the genetic sequences of WPV79. Such back-mutated revertant virus is called “vaccine-derived poliovirus” (VDPV) to distinguish it from vaccine-like poliovirus strains frequently found in the stools of children in any community using OPV79. If one genotype of VDPV is detected in two or more children with polio, that strain is called “circulating VDPV” (cVDPV)79. To complicate matters, cVDPV may silently circulate for many months, even 1-2 years, before showing up with polio cases79. Under these circumstances, the day OPV is discontinued, silent transmission of VDPV may already be happening in the community. Therefore the emergence of cVDPV must be pre-empted using IPV under cover of which OPV withdrawn (Table). After discontinuation of all OPV, any cVDPV detected must be interrupted using only IPV. India achieved WPV elimination using OPV but will have to move to phase 2 during which OPV will have to be withdrawn under immunity cover of IPV8,9,11.
Since today VAPP overwhelmingly outnumbers polio due to WPVs, OPV has to be discontinued as early as feasible, for ethical reasons. When OPV is withdrawn, there will be a time overlap when children shedding vaccine viruses may transmit infection to immunity-naive infants and children, seeding the emergence of VDPV uninhibited by immunity. Such early lineages of VDPV will remain hidden in silent circulation until conditions are right for them to cause polio outbreaks10. By then, their containment will be difficult. Thus, allowing the emergence and circulation of cVDPV is unwise and irresponsible. The emergence of VDPVs should be pre-empted using IPV10. The elimination of VDPVs using IPV has been called phase 2 of polio eradication10. For countries using OPV to eradicate WPVs, the need for a second phase is essential for the eradication of vaccine polioviruses (Table).
The known risk factors of the emergence of VDPV are gaps in population immunity and continued use of OPV. India detected VDPV polio cases in 2009 and since then every year one or more cases have been detected - 21 in 2009, 5 in 2010 and 7 in 201180. In 2012, there was one case of VDPV polio - this does not negate the status of the absence of WPV in human infection over two years as of January 2013.
The roadmap for phase 2 has been clearly defined in a series of papers from India: introduce IPV in UIP and achieve very high coverage and thereafter withdraw OPV nationally synchronously9,10,11,12,13. The tempo of clinical (AFP) surveillance and virological investigation of every child with AFP will have to be continued until a minimum of three consecutive years after the total withdrawal of OPV and after the last poliovirus isolation, whichever is later. The second phase will require a more expensive vaccine, IPV, but it only needs to be given in 2 +1 dose schedule provided the first dose is given after 8 wk of age and the second dose is given with a minimum of 8 wk20. The third dose ought to be given at least four months after the second dose. IPV third dose could be given along with measles vaccine. This schedule was developed in the demonstration project of IPV use in North Arcot District, 1985 to 1992 (unpublished data). The design of a district level disease surveillance, a model for UIP, shows a graph depicting the dramatic fall in cases of polio47.
In preparing the nation for using IPV, a public sector company to manufacture IPV was established by GOI but it was closed down in 1992, primarily due to the lack of license to market it in India. The North Arcot IPV schedule was at variance with the basic UIP schedule of contacts with infants. If UIP schedule is to be adhered to, then the IPV schedule should be 3+1 with the third dose given in the second year of life - each dose coinciding with the scheduled DPT. The problems and prospects of completing polio eradication with transitioning from OPV to IPV were covered in detail in two national round table discussions in New Delhi in 2003 and 201081,82.
In 2012, the WHO has announced the steps to complete and conclude polio eradication, as the “polio eradication end game strategy”83. The first step will be to universally introduce IPV and to remove vaccine virus type 2 from trivalent OPV - tOPV to bOPV switch84. Since 1999 last quarter all type 2 polio cases have been VAPP and caused by VDPV. This is ethically untenable and Sabin 2 virus has to be withdrawn. During the last 10 years 85 per cent of cVDPV cases and in the last three years about 95 per cent cases, have been due to Sabin type 285. Therefore, there is great urgency for tOPV to bOPV switch. However, it will be unwise to create an immunity vacuum for poliovirus type 2, a hitherto unprecedented situation with unforeseen risks, particularly of unchecked cVDPV outbreaks. Therefore, the introduction of IPV has been now approved by the GPEI Partnership and presented to both the Strategic Advisory Group of Experts (SAGE) on Immunization and the WHA. Both have approved the switch plan and the need for universal introduction of IPV in countries now using OPV exclusively83.
Since WPVs are getting eradicated globally, the use of WPVs to manufacture IPV will pose some risk of inadvertent leak - WHO will not apparently allow India to re-introduce IPV manufacture using WPVs. Therefore, WPV-based IPV will have to be imported. The 2+1 schedule is to reduce the overall cost of vaccination. Another method to reduce cost is to give IPV intradermally in fractional doses86,87,88. National level upscaling of routine intradermal IPV inoculation will certainly pose immense problems. The simpler solution of giving IPV in UIP schedule will cost more, but will be immunologically highly effective89,90,91.
In summary, India has achieved polio free status in January 2011, and is maintaining it till date, as of writing this piece. The special need of the hour is to maintain vigilance and not to lower guard against any future resurgence of polio, indigenous or imported, wild or vaccine, so that the gains made during the last two decades of intensive efforts are not allowed to be lost. Although our journey has been extremely difficult, it was also exhilarating and has taught us several lessons that will stand us in good stead in future disease control/elimination efforts. Perhaps we could have made the programme less expensive and succeeded in shorter time with different tactics being designed and deployed. However, the fact remains that we have eliminated wild poliovirus transmission altogether. Now it remains for us to eliminate all risks of polio due to vaccine viruses, including vaccine-derived viruses.
References
- 1.Centers for Disease Control and Prevention (CDC). Progress toward interruption of wild poliovirus transmission - worldwide, January 2011-March 2012. MMWR Morb Mortal Wkly Rep. 2012;61:353–7. [PubMed] [Google Scholar]
- 2.India Officially Removed from the List of Polio-endemic Countries. [accessed on July 25, 2012]. Available from: http://new.paho.org/hq/index.php?option=com_content&task=view&id=6484&Itemid=2244&lang=en .
- 3.Kew O. Reaching the last one per cent: progress and challenges in global polio eradication. Curr Opin Virol. 2012;2:188–98. doi: 10.1016/j.coviro.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Global Polio Eradication Initiative. Polio this week - As of 18 July 2012. [accessed on July 25, 2012]. Available from: http://www.polioeradication.org/Dataandmonitoring/Poliothisweek.aspx .
- 5.Cases of wild poliovirus by country and by year 2000–2011. [accessed on July 25, 2012]. Available from: http://www.polioeradication.org .
- 6.World Health Organization. Poliomyelitis in Tajikistan: first importation since Europe certified polio-free. Wkly Epidemiol Rec. 2010;85:57–4. [PubMed] [Google Scholar]
- 7.Global Alert and Response (GAR). Confirmed international spread of wild poliovirus from Pakistan. [accessed on July 25, 2012]. Available from: http://www.who.int/csr/don/2011_09_20/en/index.html .
- 8.John TJ. Will India need inactivated poliovirus vaccine (IPV) to complete polio eradication? Indian J Med Res. 2005;122:365–7. [PubMed] [Google Scholar]
- 9.John TJ. Common strategy and flexible tactics in our war on polioviruses. Public Health Rev. 1993;94(21):151–2. [PubMed] [Google Scholar]
- 10.John TJ, Vashishtha VM. Eradication of vaccine viruses: why, when and how? Indian J Med Res. 2009;130:491–4. [PubMed] [Google Scholar]
- 11.John TJ. Can we eradicate poliomyelitis? In: Sachdev HPS, Choudhury P, editors. Frontiers in pediatrics. New Delhi: Jaypee Bros; 1996. pp. 76–90. [Google Scholar]
- 12.John TJ. Polio eradication in India: What is the future? Indian Pediatr. 2003;40:455–62. [PubMed] [Google Scholar]
- 13.John TJ. The final stages of the global eradication of polio. N Engl J Med. 2000;343:806–7. doi: 10.1056/NEJM200009143431111. [DOI] [PubMed] [Google Scholar]
- 14.Ending polio, one type at a time. Bull World Health Organ. 2012;90:482–3. doi: 10.2471/BLT.12.020712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.John TJ. Poliomyelitis in India: prospects and problems of control. Rev Infect Dis. 1984;6(Suppl):S438–41. doi: 10.1093/clinids/6.supplement_2.s438. [DOI] [PubMed] [Google Scholar]
- 16.Gharpure PV, Bhatt PR. Unusual manifestations of human polio. Indian J Med Sci. 1952;6:576–8. [Google Scholar]
- 17.Iyer CGS, Swaminathan CS. The isolation and study of poliomyelitis virus in Bombay. Indian J Mes Sci. 1952;6:764–74. [Google Scholar]
- 18.Gharpure PV, Swaminathan CS, Dave KH. Five years of polio research in Bombay. Child Health. 1953;5:125–9. [Google Scholar]
- 19.John TJ, Kamath KR, Feldman RA, Christopher S. Infection and disease in a group of South Indian Families. IX. Poliovirus infection among pre-school children. Indian J Med Res. 1970;58:551–5. [PubMed] [Google Scholar]
- 20.John TJ. Immunization against polioviruses in developing countries. Rev Med Virol. 1993;3:149–60. [Google Scholar]
- 21.Athavale VB. Acute anterior poliomyelitis. Indian J Pediatr. 1960;27:325–7. doi: 10.1007/BF02819842. [DOI] [PubMed] [Google Scholar]
- 22.Chaturvedi UC, Mathur A, Singh UK, Khushwaha MRS, Mehrotra RML, Kapoor AK, et al. The problem of paralytic poliomyelitis in the urban and rural population around Lucknow, India. J Hyg Camb. 1978;81:179–87. doi: 10.1017/s0022172400025006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pabhakar N, Srilatha V, Mukarji D, John A, Rajarathnam A, John TJ. The epidemiology and prevention of poliomyelitis in a rural community in South India. Indian Pediatr. 1981;18:527–32. [PubMed] [Google Scholar]
- 24.John TK, John TJ. Is poliomyelitis a serious problem in developing countries? The Vellore experience. J Trop Pediatr. 1982;28:11–3. doi: 10.1093/tropej/28.1.11. [DOI] [PubMed] [Google Scholar]
- 25.Basu RN. Magnitude of problem of poliomyelitis in India. Indian Pediatr. 1981;18:507–11. [PubMed] [Google Scholar]
- 26.Srilatha V, Mukarji D, John TJ. The prevalence of poliomyelitis in rural school children in South India. J Trop Pediatr. 1984;30:68–9. doi: 10.1093/tropej/30.2.68. [DOI] [PubMed] [Google Scholar]
- 27.John TJ. The costs and benefits of [polio] immunization in India. Indian Pediatr. 1981;18:513–6. [PubMed] [Google Scholar]
- 28.Deogaonkar R, Hutubessy R, Putten I, Evers S, Jit M. Systematic review of studies evaluating the broader economic impact of vaccinations in low and middle income countries. BMC Public Health. 2012;16(12):1. doi: 10.1186/1471-2458-12-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.John TJ. Towards a National Policy on poliomyelitis. Indian Pediatr. 1981;18:503–5. [Google Scholar]
- 30.John TJ. How shall we control poliomyelitis in India? Indian J Pediatr. 1981;48:565–8. doi: 10.1007/BF02821573. [DOI] [PubMed] [Google Scholar]
- 31.John TJ. Understanding the scientific basis of preventing polio by immunization. Pioneering contributions from India. Proc Indian Natl Sci Acad. 2003;B69:393–422. [Google Scholar]
- 32.John TJ. Problems with oral polio vaccine in India. Indian Pediatr. 1972;9:252–6. [PubMed] [Google Scholar]
- 33.John TJ, Jayabal P. Oral polio vaccination of children in the tropics. 1. The poor seroconversion rates and the absence of viral interference. Am J Epidemiol. 1972;96:263–9. doi: 10.1093/oxfordjournals.aje.a121457. [DOI] [PubMed] [Google Scholar]
- 34.Ratnaswamy L, John TJ, Jadhav M. Paralytic poliomyelitis: clinical and virological studies. Indian Pediatr. 1973;10:443–7. [PubMed] [Google Scholar]
- 35.Ghosh S, Kumari S, Balaya S, Bhargava SK. Antibody response to oral polio vaccine in infancy. Indian Pediatr. 1970;7:78–80. [PubMed] [Google Scholar]
- 36.Pangi NS, Master JM, Dave KH. Efficacy of oral polio vaccine in infancy. Indian Pediatr. 1977;14:523–8. [PubMed] [Google Scholar]
- 37.John TJ. The golden jubilee of vaccination against polio. Indian J Med Res. 2004;119:1–17. [PubMed] [Google Scholar]
- 38.Lapinleimu K. Elimination of poliomyelitis in Finland. Rev Infect Dis. 1984;6(Suppl 2):S457–60. doi: 10.1093/clinids/6.supplement_2.s457. [DOI] [PubMed] [Google Scholar]
- 39.Basu RN. Expanded Programme on Immunisation in India. Indian J Paediatr. 1980;47:362–8. doi: 10.1007/BF02759826. [DOI] [PubMed] [Google Scholar]
- 40.John TJ. DPT and poliomyelitis in developing countries. Curr Sci. 1998;74:185–7. [Google Scholar]
- 41.Basu RN, Sokhey J. New Delhi: EPI Section, Directorate General of Health Services; 1982. The Expanded Programme on Immunisation. A Review. [Google Scholar]
- 42.Wyatt HV. Provocation paralysis. Lancet. 1993;341:61–2. doi: 10.1016/0140-6736(93)92544-4. [DOI] [PubMed] [Google Scholar]
- 43.John TJ. Did India have the world's largest outbreak of poliomyelitis associated with injections of adjuvanted DPT? Indian Pediatr. 1998;35:73–5. [PubMed] [Google Scholar]
- 44.John TJ. Antibody response of infants in tropics to five doses of oral polio vaccine. BMJ. 1976;1:812. doi: 10.1136/bmj.1.6013.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.John TJ, Joseph A, Vijayarathnam P. A better system for polio vaccination in developing countries? BMJ. 1980;281:542. doi: 10.1136/bmj.281.6239.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.John TJ, Pandian R, Gadomski A, Steinhoff MC, John M, Ray M. Control of poliomyelitis by pulse immunisation in Vellore, India. BMedJ. 1983;286:31–2. doi: 10.1136/bmj.286.6358.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.John TJ, Samuel R, Balraj V, John R. Disease surveillance at district level: a model for developing countries. Lancet. 1998;352:58–61. doi: 10.1016/s0140-6736(97)10494-9. [DOI] [PubMed] [Google Scholar]
- 48.Geneva: Forty-first World Health Assembly; 1988. May 2-13, World Health Assembly. Global eradication of poliomyelitis by the year 2000. Resolution 41.28. [Google Scholar]
- 49.World Health Organization. Global eradication of poliomyelitis Report of the fifth meeting of the Global Technical Consultative Group for Poliomyelitis Eradication, Geneva, May 8-10, 2000. [accessed on December 25, 2012]. Available from: http://www.who.int/vaccines-documents/DocsPDF00/www553.pdf .
- 50.MMWR. Progress toward Global Eradication of Poliomyelitis, 1995. [accessed on December 25, 2012]. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/00042830.htm .
- 51.Global Polio Eradication Initiative; History of polio. [accessed on December 25, 2012]. Available from: http://www.polioeradication.org/Polioandprevention/Historyofpolio.aspx .
- 52.MMWR. Progress Toward Poliomyelitis Eradication - India, December 1995 and January 1996. [accessed on December 25, 2012]. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/00041414.htm . [PubMed]
- 53.Andrus JK, Banerjee K, Hull BP, Smith JC, Mochny I. Polio eradication in the World Health Organization South-East Asia Region by the year 2000: midway assessment of progress and future challenges. J Infect Dis. 1997;175(Suppl 1):S89–96. doi: 10.1093/infdis/175.supplement_1.s89. [DOI] [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention (CDC). Update: Progress toward Poliomyelitis Eradication - South East Asia Region, 1995-1997. [accessed on April 20, 2013]. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/00047733.htm . [PubMed]
- 55.National Polio surveillance Project. Non-polio AFP rate. [accessed on April 20, 2013]. Available from: http://www.npspindia.org/nonpolio2006.asp .
- 56.Centers for Disease Control and Prevention. Apparent global interruption of wild poliovirus type 2 transmission. MMWR Morb Mort Wkly Rep. 2001;50:222–4. [PubMed] [Google Scholar]
- 57.Progress toward poliomyelitis eradication - South-East Asia, January 2000-June 2001. [accessed on July 31, 2012]. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5034a3.htm . [PubMed]
- 58.Centers for Disease Control and Prevention (CDC). Progress toward poliomyelitis eradication - India, 2003. MMWR Morb Mortal Wkly Rep. 2004;53:238–41. [PubMed] [Google Scholar]
- 59.Centers for Disease Control and Prevention (CDC). Progress Toward Global Eradication of Poliomyelitis, 2002. [accessed on April 20, 2013]. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5216a4.htm .
- 60.Centers for Disease Control and Prevention (CDC). Progress toward interruption of wild poliovirus transmission--worldwide, January 2004-March 2005. MMWR Morb Mortal Wkly Rep. 2005;54:408–12. [PubMed] [Google Scholar]
- 61.UNICEF. From 200,000 to Zero - The Journey to Polio Free India. [accessed on April 20, 2013]. Available from: http://www.unicef.org/india/Polio_Booklet-final_(22-02-2012)V3.pdf .
- 62.Centers for Disease Control and Prevention (CDC). Progress toward interruption of wild poliovirus transmission - worldwide, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:308–12. [PubMed] [Google Scholar]
- 63.Grassly NC, Fraser C, Wenger J, Deshpande JM, Sutter RW, Heymann DL, et al. New strategies for the elimination of polio from India. Science. 2006;314:1150–3. doi: 10.1126/science.1130388. [DOI] [PubMed] [Google Scholar]
- 64.John TJ, Devarajan LV, Balasubramanian A. Immunisation in India with trivalent and monovalent oral polovirus vaccines of enhanced potency. Bull World Health Organ. 1976;54:115–7. [PMC free article] [PubMed] [Google Scholar]
- 65.Grassly NC, Wenger J, Durrani S, Bahl S, Deshpande JM, Sutter RW, et al. Protective efficacy of a monovalent oral type 1 poliovirus vaccine: a case-control study. Lancet. 2007;369:1356–62. doi: 10.1016/S0140-6736(07)60531-5. [DOI] [PubMed] [Google Scholar]
- 66.Centers for Disease Control and Prevention (CDC). Progress toward interruption of wild poliovirus transmission---worldwide, January 2005 - March 2006. MMWR Morb Mortal Wkly Rep. 2006;55:458–62. [PubMed] [Google Scholar]
- 67.Centers for Disease Control and Prevention (CDC). Resurgence of wild poliovirus type 1 transmission and consequences of importation - 21 countries, 2002-2005. MMWR Morb Mortal Wkly Rep. 2006;55:145–50. [PubMed] [Google Scholar]
- 68.Roberts L. Polio experts strive to understand a puzzling outbreak. Science. 2006;312:1581. doi: 10.1126/science.312.5780.1581. [DOI] [PubMed] [Google Scholar]
- 69.Centers for Disease Control and Prevention (CDC). Progress toward poliomyelitis eradication - India, January 2006-September 2007. MMWR Morb Mortal Wkly Rep. 2007;56:1187–91. [PubMed] [Google Scholar]
- 70.Progress towards poliomyelitis eradication in India, January 2005 to June 2006. Wkly Epidemiol Rec. 2006;81:285–92. [PubMed] [Google Scholar]
- 71.Centers for Disease Control and Prevention (CDC). Progress toward poliomyelitis eradication - India, January 2007-May 2009. MMWR Morb Mortal Wkly Rep. 2009;58:719–23. [PubMed] [Google Scholar]
- 72.Centers for Disease Control and Prevention (CDC). Progress toward poliomyelitis eradication - India, January 2009-October 2010. MMWR Morb Mortal Wkly Rep. 2010;59:1581–5. [PubMed] [Google Scholar]
- 73.Sutter RW, John TJ, Jain H, Agarkhedkar S, Ramanan PV, Verma H, et al. Immunogenicity of bivalent types 1 and 3 oral poliovirus vaccine: a randomized, double-blind, controlled trial. Lancet. 2010;376:1624–5. doi: 10.1016/S0140-6736(10)61230-5. [DOI] [PubMed] [Google Scholar]
- 74.John TJ. Lessons from the polio eradication campaign. [accessed on July 24, 2012];Seminar. 2012 631:16–20. Available from: http://www.indiaseminar.com/semframe.html . [Google Scholar]
- 75.Centers for Disease Control and Prevention (CDC). Progress toward poliomyelitis eradication - India, January 2010-September 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1482–6. [PubMed] [Google Scholar]
- 76.WHO Consultative Group. The relation between acute persisting spinal paralysis and poliomyelitis vaccine. Bull World Health Organ. 1982;60:231–42. [PMC free article] [PubMed] [Google Scholar]
- 77.Kohler KA, Banerjee K, Hlady WG, Andrus JK, Sutter RW. Vaccine-associated paralytic poliomyelitis in India during 1999: decreased risk despite massive use of oral polio vaccine. Bull World Health Organ. 2002;80:210–6. [PMC free article] [PubMed] [Google Scholar]
- 78.John TJ. Vaccine-associated paralytic poliomyelitis in India. Bull World Health Organ. 2002;80:917. [PMC free article] [PubMed] [Google Scholar]
- 79.Kew O, Morris-Glasgow V, Landaverde M, Burns C, Shah J, Garib Z. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science. 2002;296:356–9. doi: 10.1126/science.1068284. [DOI] [PubMed] [Google Scholar]
- 80.Vaccine-Derived Polioviruses. [accessed on January 7, 2013]. Available from: www.npspindia.org/hjrev.doc .
- 81.Sood OP, Rattan A, editors. Gurgaon: Ranbaxy Science Foundation; 2003. India and the global eradication of polio. [Google Scholar]
- 82.Sood OP, John TJ, editors. Polio eradication: challenges and opportunities. Gurgaon: Ranbaxy Science Foundation; 2010. [Google Scholar]
- 83.World Health Organization. Meeting of the Strategic Advisory Group of Experts on Immunization, November 2012 - Conclusions and Recommendations. Wkly Epidemiol Rec. 2013;88:1–16. [PubMed] [Google Scholar]
- 84.John TJ. Two good reasons to drop type 2 virus from oral polio vaccine. Lancet. 2004;364:1666. doi: 10.1016/S0140-6736(04)17351-0. [DOI] [PubMed] [Google Scholar]
- 85.Circulating vaccine-derived poliovirus (cVDPV) 2000-2013. [accessed on April 20, 2013]. Available from: http://www.polioeradication.org/DataandMonitoring/PolioThisWeek/CirculatingVaccineDerivedPoliovirus.aspx .
- 86.Samuel BU, Cherian T, Sridharan G, Mukundan P, John TJ. Immune response to intradermally injected inactivated poliovirus vaccine. Lancet. 1991;338:343–4. doi: 10.1016/0140-6736(91)90480-d. [DOI] [PubMed] [Google Scholar]
- 87.Resik S, Tejeda A, Lago PM, Diaz M, Carmenates A, Sarmiento L, et al. Randomized controlled clinical trial of fractional doses of inactivated poliovirus vaccine administered intradermally by needle-free device in Cuba. J Infect Dis. 2010;201:1344–52. doi: 10.1086/651611. [DOI] [PubMed] [Google Scholar]
- 88.Estívariz CF, Jafari H, Sutter RW, John TJ, Jain V, Agarwal A, et al. Immunogenicity of supplemental doses of poliovirus vaccine for children aged 6-9 months in Moradabad, India: a community-based, randomised controlled trial. Lancet Infect Dis. 2012;12:128–35. doi: 10.1016/S1473-3099(11)70190-6. [DOI] [PubMed] [Google Scholar]
- 89.Dutta AK, Varghese VP, Pemde HK, Mathew LG, Ortiz E. Immunogenicity and safety of a pentavalent diphtheria, tetanus, acellular pertussis, inactivated poliovirus, Haemophilus influenzae type b conjugate combination vaccine (Pentaxim) with hepatitis B vaccine. Indian Pediatr. 2009;46:975–82. [PubMed] [Google Scholar]
- 90.Dutta AK, Varghese VP, Pemde HK, Mathew LG, Ortiz E. Immunogenicity and safety of a DTaP-IPV/PRP-T vaccine booster dose during the second year of life in Indian children primed with the same vaccine. Indian Pediatr. 2012;49:793–8. doi: 10.1007/s13312-012-0191-5. [DOI] [PubMed] [Google Scholar]
- 91.John TJ. Inactivated poliovirus vaccine: The fog of uncertainty is lifting. Indian Pediatr. 2012;49:787. doi: 10.1007/s13312-012-0179-1. [DOI] [PubMed] [Google Scholar]


