Abstract
Background & objectives:
Subinhibitory concentrations (sub-MICs) of antibiotics, although not able to kill bacteria, but influence bacterial virulence significantly. Fluoroquinolones (FQs) which are used against other bacterial pathogens creates resistance in non-targeted Streptococcus pyogenes. This study was undertaken to characterize the effect of sub-MICs of FQs on S. pyogenes biofilm formation.
Methods:
Biofilm forming six M serotypes M56, st38, M89, M65, M100 and M74 of S. pyogenes clinical isolates were challenged against four FQs namely, ciprofloxacin, ofloxacin, levofloxacin and norfloxacin. The antibiofilm potential of these FQs was analysed at their subinhibitory concentrations (1/2 to 1/64 MIC) using biofilm assay, XTT reduction assay, scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM).
Results:
Among the four FQs tested, ofloxacin and levofloxacin at 1/2 MIC showed the maximum inhibition (92%) of biofilm formation against M56 and M74 serotypes. FQs effectively interfered in the microcolony formation of S. pyogenes isolates at 1/2 to 1/8 sub-MICs. Inhibition of biofilm formation was greatly reduced beyond 1/16 MICs and allowed biofilm formation. XTT reduction assay revealed the increase in metabolic activity of S. pyogenes biofilm against the decrease in FQs concentration. SEM and CLSM validated the potential of sub-MICs of FQs against the six S. pyogenes.
Interpretation & conclusions:
Our results showed that the inhibitory effect all four FQs on S. pyogenes biofilm formation was concentration dependent. FQs at proper dosage can be effective against S. pyogenes and lower concentrations may allow the bacteria to form barriers against the antibiotic in the form of biofilm.
Keywords: Biofilms, confocal laser scanning microscopy, fluoroquinolones, Streptococcus pyogenes, subinhibitory concentration
Streptococcus pyogenes is an important human pathogen responsible for a wide array of infections such as pharyngitis, scarlet fever, cellulitis, bacteremia, impetigo, acute rheumatic fever, glomerulonephritis, necrotizing fascitis and streptococcal toxic shock syndrome. S. pyogenes possess the inherent capacity to form biofilms that are associated with specific M serotypes1,2. Biofilms play an important role in more than 50 per cent of the human bacterial infections3. The biofilms of S. pyogenes have been observed in skin and root canal infections4,5.
The presence of exopolysaccharide matrix in biofilm leads to increased resistance to antimicrobial treatments and host defenses which favour the growth of microorganisms in hostile or suboptimal environments6. Generally higher concentration of the antibiotics is required to kill bacteria in the biofilm phase than their planktonic counterparts7. Significant limitations to biofilm penetration have been reported for beta-lactams and aminoglycosides class of antibiotics8. Penicillin remains the drug of choice for the treatment of S. pyogenes infections because it remains susceptible to this antibiotic despite its intensive use. The increasing use of fluoroquinolones (FQs) due to their excellent activities against some other bacterial pathogens, has led to the emergence of FQs resistant S. pyogenes strains9. Several studies showed the emergence of FQs resistance in S. pyogenes isolates though these were not used against S. pyogenes infections10,11,12. It has been implied that subinhibitory concentrations (sub-MICs) of certain antibiotics can suppress the formation of biofilms by disrupting the adhering capacity13 and higher concentrations of FQs may result in the induction of resistance14. Hence concentrations of antibiotics may influence the bacterial virulence parameters such as adherence15, motility, biofilm formation16 and sensitivity to oxidative stress17.
Therefore, studying the effect of sub-MICs of antibiotics on microorganisms is of continuing interest to microbiologists18,19. In this study, we investigated the effect of subinhibitory concentrations of FQs namely, ciprofloxacin (CIP), levofloxacin (LEV), norfloxacin (NOR) and ofloxacin (OFL) on biofilm production by S. pyogenes.
Material & Methods
Six clinical isolates of S. pyogenes with M serotypes of M56, st38, M89, M65, M100 and M74 already identified as effective biofilm formers in our previous study2 were used. These isolates were obtained from pharyngitis patients, attending Government Rajaji Hospital, Madurai, India using 5 per cent sheep blood agar plates and routinely maintained in Tryptose agar plates (Hi media Laboratories, India).
Susceptibility testing: Minimal inhibitory concentrations (MICs) of ciprofloxacin (CIP) (Himedia Laboratories, India), levofloxacin (LEV), norfloxacin (NOR) and ofloxacin (OFL) (Sigma, USA) were determined using modified form of broth microdilution method outlined by the Clinical and Laboratory Standards Institute20. The broth microdilution method involves exposing bacteria to decreasing concentrations of FQs in liquid media. The bacterial suspension (106 CFU/ml) was added to Todd Hewitt Broth (THB)21 supplemented with 5 per cent lysed sheep blood and the antibiotics were serially diluted two folds to give final concentrations ranging from 0.5 to 128 μg/ml and incubated at 37°C for 18 h. The lowest concentration of FQs at which there was no visible growth was taken as the MIC for that isolate.
Biofilm assay: The effects of four FQs were tested against biofilm forming M serotypes of S. pyogenes isolates in 24 well microtiter plates as described earlier20. Briefly, the FQs at sub-MICs (1/2, 1/4, 1/8, 1/16, 1/32 and 1/64) were added to THB containing the bacteria of 106 cfu/ml. Culture without adding any antibiotics was used as control and the wells containing THB alone were used as blanks. The percentage of biofilm inhibition was calculated by the formula:
Percentage of inhibition = ([Control OD570 nm- Test OD570 nm] / Control OD570 nm) × 100
XTT reduction assay: A semiquantitative measurement of metabolic activity of S. pyogenes biofilms were obtained from the 2,3-bis(2-methoxy-4-nitro-5-sulphophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium-hydroxide (XTT) reduction assay20. Biofilm formed wells were washed twice with PBS to remove planktonic as well as adhered cells. Then, 50 μl of XTT salt solution (1mg/ml in PBS) and 4 μl of menodione solution (1mM in acetone; Sigma, USA) were added to each well. Microtiter plates were incubated at 37°C in the dark for 90 min. Bacterial dehydrogenase activity reduces XTT tetrazolium salt to XTT formazan, resulting in colorimetric change (turns to orange) which was correlated with cell viability. The colorimetric changes were measured spectrophotometrically at 492 nm22.
Light microscopy: For determining the biofilm formation of S. pyogenes, small sterile glass slides (1x1cm) were placed into the wells of 24 well polystyrene plates. The four FQs at sub-MICs (1/2, 1/4, 1/8, 1/16, 1/32 and 1/64) along with THB containing the bacteria of 106 cfu/ml were added into the 24 well plates and incubated at 37°C for 24 h. After incubation the small glass slides were removed and gently washed twice to remove planktonic cells. Crystal violet staining was performed and the presence of biofilms was inspected by light microscopy (Euromex GE 3045, Holland) at magnifications of 40x.
Scanning electron microscopy (SEM): Sample preparation for SEM analysis was performed as described by Lembke et al1. Biofilms on the glass pieces were fixed for 2 h in solution containing 2.5 per cent glutaraldehyde. Further, the glass pieces were washed in 0.1 M sodium acetate buffer (pH 7.3). Samples were dehydrated through a graded series of ethanol, critical point dried, gold sputtered and examined under scanning electron microscope (Hitachi S-3000H, Japan).
Confocal laser scanning microscopy (CLSM): CLSM was used to determine the three dimensional architecture, thickness and morphology of biofilms formed by S. pyogenes isolates. Staining of biofilms and CLSM analysis were performed as described previously21. The biofilms formed on cover slips were stained with 0.2 per cent acridine orange (Hi-Media Laboratories, Mumbai) for 2 min. The stained slides were subjected to visualization under confocal laser scanning microscope (Zeiss LSM710 meta, Germany). Images were captured and processed by using Zeiss LSM Image Examiner Version 4.2.0.121.
Statistical analysis: All experiments were performed in triplicates. Comparative results for different isolates were statistically analyzed using SPSS 15.0 statistical package (SPSS Inc., Chicago, USA). All pair-wise comparisons were performed using Dunnett's test. P<0.05 was considered significant.
Results
MIC values of all six isolates against four FQs are shown in the Table. NOR demonstrated the highest MIC value of 8 μg/ml for st38 and M78 isolates. M100 showed the lowest MIC value of 0.2 μg/ml against CIP and LEV. All the four FQs at their sub-MICs did not show any antibacterial activity.
Effect of subinhibitory concentrations of FQs on S. pyogenes biofilms: FQs showed substantial inhibition in the biofilm formation of S. pyogenes isolates. It was evident that OFL and LEV showed a promising antibiofilm activity with a maximum inhibition of 92 per cent against the potent biofilm former M56 and M74 serotypes at 1/2 MIC, followed by CIP and NOR with 83 and 82 per cent inhibition against M74 and st38 serotypes respectively (Table). On an average, the FQs showed 70 and 50 per cent inhibition (P<0.05) against all the M serotypes tested at 1/4 and 1/8 MIC, respectively when compared to the control. The percentage inhibition of biofilm observed in the M65 serotype was comparatively low against all the four FQs tested, while the least was observed in M89 treated with NOR. With an increase in dilution of sub-MICs of the antibiotics, the percentage inhibition of the biofilm formation was also reduced considerably. This result demonstrates that the formation of biofilms was influenced in a concentration dependent manner.
Table.
Per cent inhibition of biofilm formation by S. pyogenes isolates on six concentrations of four fluoroquinolones
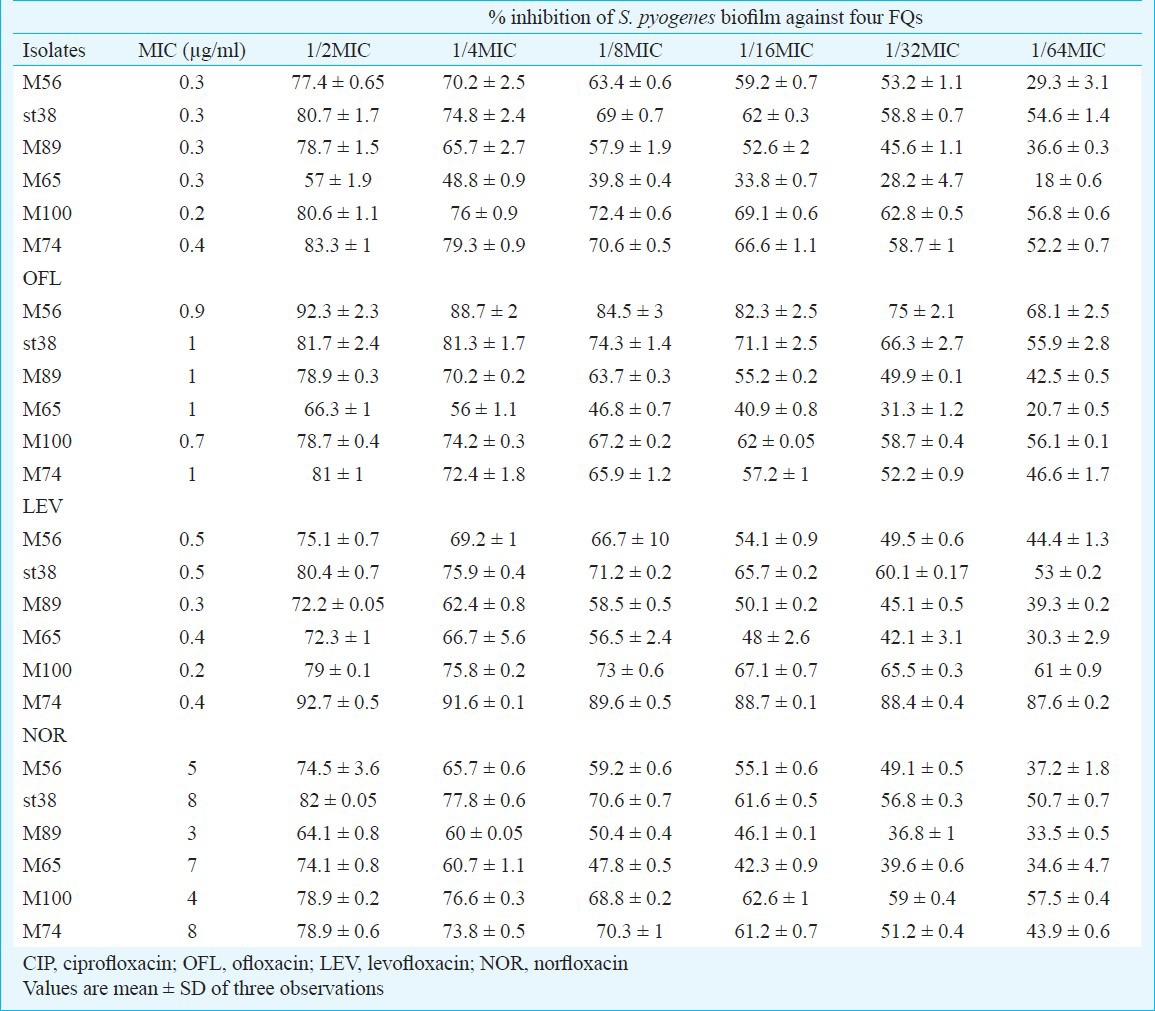
Assessment of metabolic activity on preformed biofilms: XTT assay revealed the metabolic activity of S. pyogenes biofilms against the FQs after 24 h of incubation at 37°C. Biofilms at 1/2 and 1/4 MICs showed less metabolic activity. It was evident that the metabolic activity of S. pyogenes against FQs was concentration dependent and metabolically active biofilm were more in lower sub-MICs (Fig. 1).
Fig. 1.
Metabolic activity of biofilms formed by S. pyogenes isolates at their subinhibitory concentrations (a) 1/2MIC, (b) 1/4MIC, (c) 1/8MIC, (d) 1/16MIC, (e) 1/32MIC, (f) 1/64MIC and (g) Control as quantified by XTT assay and measuring A492nm. Mean value of triplicate independent experiments and SDs are shown. Dunnett's test demonstrated significant difference between the tests and the control (P< 0.05).
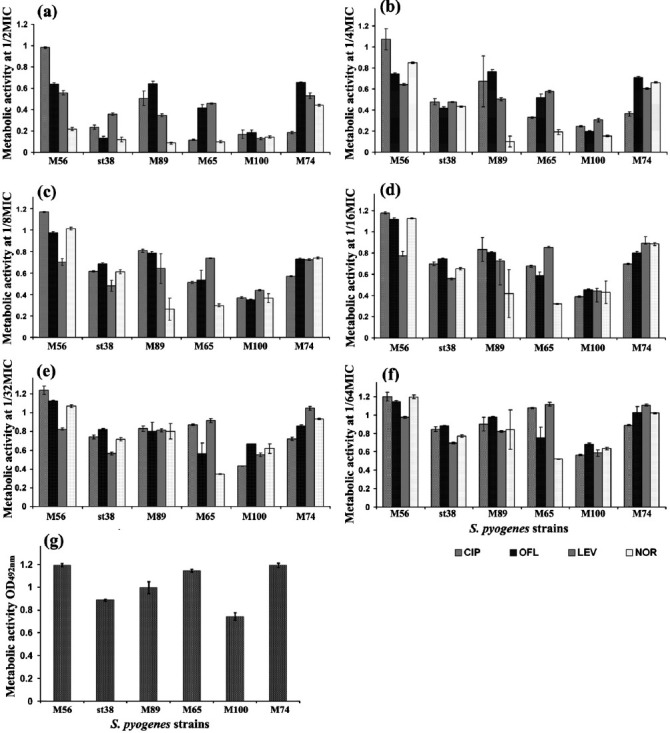
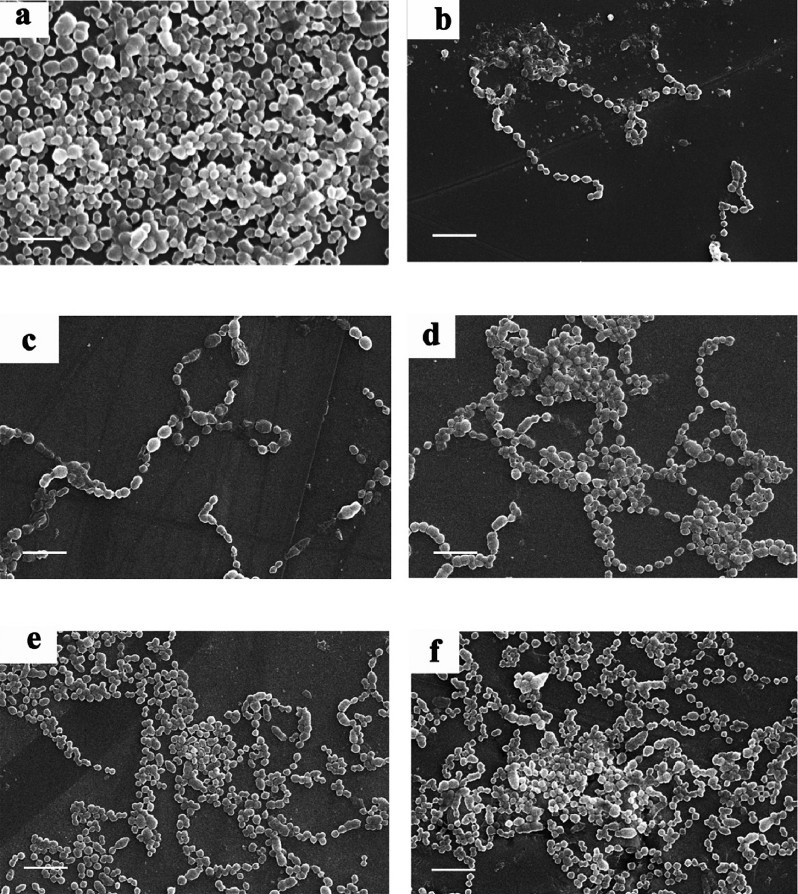
Surface topography and architecture of biofilms: The structural morphology of the biofilm formation viewed under light microscopy and was further confirmed by SEM analysis. SEM analysis revealed the potential effect of FQs against biofilm forming M serotypes of S. pyogenes. LEV at sub-MICs (1/2-1/8 MIC) restricted the attachment of bacterial cells greatly when compared to the control. Figure 2 (representative SEM images) depicts the antibiofilm activity of the FQs against the serotype M56. At sub-MICs (1/16-1/64 MIC), incompetence of FQs on biofilm formation was observed and at these lower concentrations FQs lost their antibiofilm activity and allowed the bacteria to form biofilm. Increase in biofilm formation upon exposure to lower concentrations of FQs (1/8-1/64 MIC) was observed from SEM analysis (Fig. 2c-f). Magnified SEM image showed a visible slimy matrix around S. pyogenes cell (Fig. 3) comparably more slimy outer cover than that of the control.
Fig. 2.
Scanning electron micrographs of S. pyogenes serotype M56 biofilms and their treatment with LEV at sub-MICs (a) Control, (b) 1/4MIC, (c) 1/8MIC, (d) 1/16MIC, (e) 1/32MIC and (f) 1/64MIC (Scale bar = 10 μm).
Fig. 3.
Scanning electron micrographing image of (a) Control and (b) st38 cell at 1/32MIC of LEV treatment after 24h of incubation (Scale bar = 1 μm).
CLSM was used to analyze the surface topography and three dimensional architecture of the biofilm formed by S. pyogenes isolates. The thickness of S. pyogenes biofilm ranged from 60-120 μm. All FQs acted well on S. pyogenes biofilms up to 1/8 MIC whereas at 1/16-1/64 MIC there was a proportional increase in the thickness of biofilms. LEV reduced the formation of biofilms at 1/2 to 1/8 MIC in M56 serotype (Fig. 4a-d as a representative for all FQs tested) whereas antibiotics beyond 1/16 MIC showed a gradual increase in the establishment of biofilms (Fig. 4e-g).
Fig. 4.
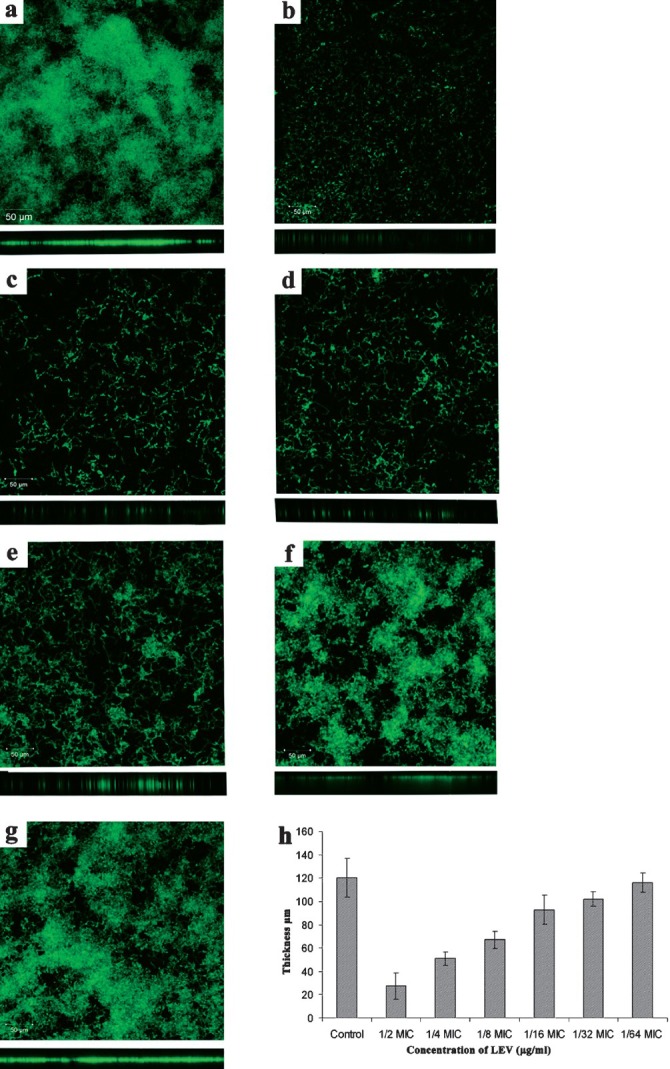
Confocal Laser Scanning Microscopic image showing gradual increase in biofilm formation by S. pyogenes serotype M56 (a) Control, (b) 1/2MIC, (c) 1/4MIC, (d) 1/8MIC, (e) 1/16MIC, (f) 1/32MIC, (g) 1/64MIC and (h) Thicknesses of biofilms were determined from merging all the z-stack images using CLSM-assosiated software. Magnification: 20 x and Scale bar = 50 μm.
Discussion
In bacteria, the formation of biofilms is the major barrier that protects the pathogen from many environmental stresses like antibiotics and human immune system. The biofilm formed by S. pyogenes restricts the diffusion of antimicrobials agents and thereby develops tolerance to these substances, which is of major clinical importance23. The effectiveness of various antibiotics at their sub-inhibitory concentrations against the adherence property and virulence factors production by the pathogens has been studied13,24. The effect of sub-MICs of various antibiotics and FQs has been studied on P. aeruginosa13, S. typhimurium19, Staphylococcus spp.25 and Streptococcus spp.26 and FQs showed a strong bactericidal activity against the biofilm forming cells than other antibiotics27. Tanaka et al27 reported that the levels of some exoproteins were increased in S. pyogenes after treatment with macrolides28, whereas gatifloxacin showed no effect on exoprotein production. The LEV has been shown to have a substantial therapeutic efficacy against biofilm producing P. aeruginosa7. Odenholt-Tornqvist et al29 reported that only a small amount of FQs is necessary to prevent normal cell growth once the bacteria have been damaged by a suprainhibitory concentration of the antibiotic. Extensive use of one particular antibiotic can develop resistance against that antibiotic in non-targeted bacteria.
In this study, the four FQs at the sub-MICs (1/2, 1/4 and 1/8) reduced the biofilm formation by 40-90 per cent in S. pyogenes. These FQs at the sub-MICs may reduce the development of biofilms by interfering with the adherence property of the bacterium30. Of the four FQs used, OFL worked efficiently in disintegrating the microcolony formation of S. pyogenes biofilms. In the present study, all four FQs showed antibiofilm activity up to 1/8 MICs whereas an earlier study27 reported P. aeroginosa showing different sub-MICs with respect to different FQs for the complete eradication of their biofilms. FQs rapidly diffuse deep into the biofilms of Gram-negative bacteria, in the similar way it might have gained entry and disrupted the biofilms of S. pyogenes, a Gram-positive bacterium31. According to Schmitz et al9 at lower concentrations, FQs act in a bacteriostatic way since these block the DNA replication process, while at higher concentrations these are bactericidal9. Similarly, the cell density of the S. pyogenes isolates at the MICs was bactericidal whereas at sub-MICs the cell density values were similar to the control, eventhough the FQs were bacteriostatic and the metabolic activity of S. pyogenes biofilms determined by XTT assay was associated with the cell density values (data not shown).
In the present study, FQs at lower sub-MICs (1/16-1/64 MIC) lost their antibiofilm effect and allowed biofilm formation in the isolates. There are several reports regarding the induction of biofilms while treatment with antibiotics. For example, Linares et al32 also reported the induction of biofilms at sub-MICs of ciprofloxacin, tobramycin and tetracycline. Another important fact is that microbes exhibit inherent antibiotic resistant mechanisms to overcome the hostile environments8,23 likewise they may induce biofilm formation as a protective mechanism. In situ, FQs used for the treatment of other bacterial pathogens may enhance biofilm formation in non-targeted S. pyogenes. Several factors may also be involved in the induction of S. pyogenes biofilm at the lower concentrations of FQs. Reduced concentrations of the antibiotics lead to an adverse condition, which in turn may activate the quorum of signaling molecules in S. pyogenes by inducing the virulence trait such as biofilm formation18.
In conclusion, our results document that all four FQs used in this study efficiently inhibited the biofilm formation at their sub-MICs (1/2-1/8 MIC) and at lower sub-MICs (1/16 and 1/64 MIC). These lower concentrations of FQs may provide a chance to protect the pathogen by forming biofilm. SEM and CLSM analyses portrayed the surface topography and architecture of biofilms formed by the six M serotypes of S. pyogenes strains. Considerable reduction in thickness of the biofilms at the (1/2-1/8 MIC) and increasing thickness at lower sub-MICs by CLSM analysis demonstrated FQs ability to interference with S. pyogenes biofilms. Further expression analysis of S. pyogenes isolates challenged with FQs at subinhibitory concentration may unravel the exact mechanism involved in the concentration dependent biofilm inhibition. Hence, the outcome of this study suggests that appropriate FQs should be used at proper dosage for other bacterial infections else their effects over non-targeted pathogens like S. pyogenes could either worsen or attenuate the disease. FQs usage based on optimal concentrations of the antibiotics and target specificity is crucial to protect the mankind from life threatening infections.
Acknowledgment
The authors acknowledge the financial assistance rendered by University Grants Commission (UGC), New Delhi (F. No. 34-263/2008(SR)) and the computational and bioinformatics facility provided by the Alagappa University Bioinformatics Infrastructure Facility, Karaikudi (funded by Department of Biotechnology, Government of India; Grant No. BT/BI/25/001/2006).
References
- 1.Lembke C, Podbielski A, Hidalgo-Grass C, Jonas L, Hanski E, Kreikemeyer B. Characterization of biofilm formation by clinically relevant serotypes of group A Streptococci. Appl Environ Microbiol. 2006;72:2864–75. doi: 10.1128/AEM.72.4.2864-2875.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thenmozhi R, Nithyanand P, Rathna J, Karutha Pandian S. Antibiofilm activity of coral associated bacteria against different clinical M serotypes of Streptococcus pyogenes. FEMS Immunol Med Microbiol. 2009;57:284–94. doi: 10.1111/j.1574-695X.2009.00613.x. [DOI] [PubMed] [Google Scholar]
- 3.Spoering AL, Lewis K. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J Bacteriol. 2001;183:6746–51. doi: 10.1128/JB.183.23.6746-6751.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akiyama H, Morizane S, Yamasaki O, Oono T, Iwatsuki K. Assessment of Streptococcus pyogenes microcolony formation in infected skin by Confocal Laser Scanning Microscopy. J Dermatol Sci. 2003;32:193–9. doi: 10.1016/s0923-1811(03)00096-3. [DOI] [PubMed] [Google Scholar]
- 5.Takemura N, Noiri Y, Ehara A, Kawahara T, Noguchi N, Ebisu S. Single species biofilm forming ability of root canal isolates on gutta percha points. Eur J Oral Sci. 2004;112:523–9. doi: 10.1111/j.1600-0722.2004.00165.x. [DOI] [PubMed] [Google Scholar]
- 6.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 7.Ishida H, Ishida Y, Kurosaka Y, Otani T, Sato K, Kobayashi H. In vitro and in vivo activities of levofloxacin against biofilm-producing Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42:1641–5. doi: 10.1128/aac.42.7.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart PS. Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol. 2002;292:107–13. doi: 10.1078/1438-4221-00196. [DOI] [PubMed] [Google Scholar]
- 9.Schmitz FJ, Higgins P, Mayer S, Fluit A, Dalhoff A. Activity of quinolones against Gram-positive cocci: mechanisms of drug action and bacterial resistance. Eur J Clin Microbiol Infect Dis. 2002;21:647–59. doi: 10.1007/s10096-002-0788-z. [DOI] [PubMed] [Google Scholar]
- 10.Rivera A, Rebollo M, Sanchez F, Navarro F, Miro E, Mirelis B, et al. Characterisation of fluoroquinolone resistant clinical isolates of Streptococcus pyogenes in Barcelona, Spain. Clin Microbiol Infect. 2005;11:759–61. doi: 10.1111/j.1469-0691.2005.01216.x. [DOI] [PubMed] [Google Scholar]
- 11.Alberti S, Cortés G, Garcia-Rey C, Rubio C, Baquero F, Garcia-Rodriguez JA, et al. Streptococcus pyogenes pharyngeal isolates with reduced susceptibility to ciprofloxacin in Spain: mechanisms of resistance and clonal diversity. Antimicrob Agents Chemother. 2005;49:418–20. doi: 10.1128/AAC.49.1.418-420.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smeesters PR, Vergison A, Junior DC, Van Melderen L. Emerging fluoroquinolone-non-susceptible Group A Streptococci in two different paediatric populations. Int J Antimicrob Agents. 2009;34:44–9. doi: 10.1016/j.ijantimicag.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Fonseca AP, Extremina C, Fonseca AF, Sousa JC. Effect of subinhibitory concentration of piperacillin/tazobactam on Pseudomonas aeruginosa. J Med Microbiol. 2004;53:903–10. doi: 10.1099/jmm.0.45637-0. [DOI] [PubMed] [Google Scholar]
- 14.Pletz MWR, McGee L, Van Beneden CA, Petit S, Bardsley M, Barlow M, et al. Fluoroquinolone resistance in invasive Streptococcus pyogenes isolates due to spontaneous mutation and horizontal gene transfer. Antimicrob Agents Chemother 2005. 2006;50:943–8. doi: 10.1128/AAC.50.3.943-948.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolter JM, McCormack JG. The effect of subinhibitory concentrations of antibiotics on adherence of Pseudomonas aeruginosa to cystic fibrosis (CF) and non-CF-affected tracheal epithelial cells. J Infect. 1998;37:217–23. doi: 10.1016/s0163-4453(98)91859-x. [DOI] [PubMed] [Google Scholar]
- 16.Drago L, De Vecchi E, Mombelli B, Nicola L, Valli M, Gismondo MR. Activity of levofloxacin and ciprofloxacin against urinary pathogens. J Antimicrob Chemother. 2001;48:37–45. doi: 10.1093/jac/48.1.37. [DOI] [PubMed] [Google Scholar]
- 17.Hassett DJ, Elkins JG, Ma JF, McDermott TR. Pseudomonas aeruginosa biofilm sensitivity to biocides: Use of hydrogen peroxide as model antimicrobial agent for examining resistance mechanisms. Methods Enzymol. 1999;310:599–608. doi: 10.1016/s0076-6879(99)10046-6. [DOI] [PubMed] [Google Scholar]
- 18.Rachid S, Ohlsen K, Witte W, Hacker J, Ziebuhr W. Effect of subinhibitory antibiotic concentrations on polysaccharide intercellular adhesin expression in biofilm-forming Staphylococcus epidermidis. Antimicrob Agents Chemother. 2000;44:3357–63. doi: 10.1128/aac.44.12.3357-3363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haddadin RNS, Saleh S, Al Adham ISI, Buultjens TEJ, Collier PJ. The effect of subminimal inhibitory concentrations of antibiotics on virulence factors expressed by Staphylococcus aureus biofilms. J Appl Microbiol. 2009;108:1281–91. doi: 10.1111/j.1365-2672.2009.04529.x. [DOI] [PubMed] [Google Scholar]
- 20.Wayne, PA: CLSI; 2006. Clinical and Laboratory Standards Institute (CLSI). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, Approved Standard Document M7-A7. [Google Scholar]
- 21.Thenmozhi R, Balaji K, Kumar R, Rao TS, Pandian SK. Characterization of biofilms in different clinical M serotypes of Streptococcus pyogenes. J Basic Microbiol. 2011;51:196–204. doi: 10.1002/jobm.201000006. [DOI] [PubMed] [Google Scholar]
- 22.Martinez LR, Casadevall A. Cryptococcus neoformans biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light. Appl Environ Microbiol. 2007;73:4592–601. doi: 10.1128/AEM.02506-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 24.Herbert S, Barry P, Novick RP. Subinhibitory clindamycin differentially inhibits transcription of exoprotein genes in Staphylococcus aureus. Infect Immun. 2001;69:2996–3003. doi: 10.1128/IAI.69.5.2996-3003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majtán J, Majtánová, Xu M, Majtán V. In vitro effect of subinhibitory concentrations of antibiotics on biofilm formation by clinical strains of Salmonella enterica serovar Typhimurium isolated in Slovakia. J Appl Microbiol. 2008;104:1294–301. doi: 10.1111/j.1365-2672.2007.03653.x. [DOI] [PubMed] [Google Scholar]
- 26.Opavski N, Dukic S, Mijac V, Ranin L. Influence of decreased penicillin susceptibility on growth rate of beta haemolytic streptococci. Indian J Med Res. 2004;119:237–41. [PubMed] [Google Scholar]
- 27.Abdi-Ali A, Mohammadi-Mehr M, Agha Alaei Y. Bactericidal activity of various antibiotics against biofilm-producing Pseudomonas aeruginosa. Int J Antimicrob Agents. 2006;27:196–200. doi: 10.1016/j.ijantimicag.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka M, Hasegawa T, Okamoto A, Torii K, Ohta M. Effect of antibiotics on Group A Streptococcus exoprotein production analyzed by two-dimensional gel electrophoresis. Antimicrob Agents Chemother. 2005;49:88–91. doi: 10.1128/AAC.49.1.88-96.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Odenholt-Tornqvist I, Lowdin E, Cars O. Pharmacodynamic effects of subinhibitory concentrations of beta-lactam antibiotics in vitro. Antimicrob Agents Chemother. 1991;35:1834–9. doi: 10.1128/aac.35.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reid G, Sharma S, Advikolanu K, Tieszer C, Martin RA, Bruce AW. Effects of ciprofloxacin, norfloxacin, and ofloxacin on in vitro adhesion and survival of Pseudomonas aeruginosa AK1 on urinary catheters. Antimicrob Agents Chemother. 1994;38:1490–5. doi: 10.1128/aac.38.7.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vrany JD, Stewart PS, Suci PA. Comparison of recalcitrance to ciprofloxacin and levofloxacin exhibited by Pseudomonas aeruginosa bofilms displaying rapid-transport characteristics. Antimicrob Agents Chemother. 1997;41:1352–8. doi: 10.1128/aac.41.6.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]


