Abstract
We investigated the production of hyaluronan (HA) and its effect on cell motility in cells expressing the v-src mutants. Transformation of 3Y1 by v-src virtually activated HA secretion, whereas G2A v-src, a nonmyristoylated form of v-src defective in cell transformation, had no effect. In cells expressing the temperature-sensitive mutant of v-Src, HA secretion was temperature dependent. In addition, HA as small as 1 nM, on the other side, activated cell motility in a tumor-specific manner. HA treatment strongly activated the motility of v-Src–transformed 3Y1, whereas it showed no effect on 3Y1- and 3Y1-expressing G2A v-src. HA-dependent cell locomotion was strongly blocked by either expression of dominant-negative Ras or treatment with a Ras farnesyltransferase inhibitor. Similarly, both the MEK1 inhibitor and the kinase inhibitor clearly inhibited HA-dependent cell locomotion. In contrast, cells transformed with an active MEK1 did not respond to the HA. Finally, an anti-CD44–neutralizing antibody could block the activation of cell motility by HA as well as the HA-dependent phosphorylation of mitogen-activated protein kinase and Akt. Taken together, these results suggest that simultaneous activation of the Ras-mitogen-activated protein kinase pathway and the phosphoinositide 3-kinase pathway by the HA-CD44 interaction is required for the activation of HA-dependent cell locomotion in v-Src–transformed cells.
INTRODUCTION
Cell transformation by Rous sarcoma virus (RSV) is mediated by the v-src gene product, v-Src, a membrane-bound tyrosine protein kinase (Jove and Hanafusa, 1987). On cell transformation, v-Src educes multifarious changes in cellular function (Hanafusa et al., 1977). Among them, the accumulation of hyaluronan (HA) was observed many years ago (Kabat, 1939; Harris et al., 1954; Erichsen et al., 1961); yet its role in cell transformation by v-src remains largely unclear.
HA, a nonsulfated high-molecular-mass glycosaminoglycan, is one of the major components of the extracellular matrix (Laurent and Fraser, 1992). With cell lines other than RSV-transformed cells, evidence that suggests the importance of HA in fundamental cell functions has accumulated. HA, with its cell-surface receptors such as CD44, appears to be involved in cell adhesion, migration, and proliferation (Lokeshwar et al., 1997). In addition, increasing evidence suggests the importance of HA in tumor progression (Knudson et al., 1989; Rooney et al., 1995; Knudson, 1996). Tumor-specific accumulation of HA was widely observed in human tumors, including colon cancer (Ropponen et al., 1998), lung cancer (Horai et al., 1981), breast cancer (Bertrand et al., 1992), and glioma (Delpech et al., 1993). In human glioma, inhibition of CD44 expression by an antisense oligonucleotide completely arrested the invasion (Merzak et al., 1994). Overexpression of HA synthase 2, Has2, in the human fibrosarcoma cell line activated anchorage-independent growth (Kosaki et al., 1999), whereas expression of HA synthase 1, Has1, in mouse mammary carcinoma cells activated metastasis (Itano et al., 1999a).
Increasing evidence suggests that HA serves not only as a component of the extracellular matrix but also as an extracellular signaling molecule (Lee and Spicer, 2000). HA binding to CD44 activated NF-kappa B through Ras and protein kinase C (Fitzgerald et al., 2000). HA-CD44 binding was found to activate mitogen-activated protein kinase (MAPK) (Slevin et al., 1998; Serbulea et al., 1999). Finally, Camenisch et al. (2000) reported that disruption of the Has2 gene abrogated cardiac morphogenesis and transformation of epithelium to mesenchyme. This defect was reversed by the expression of activated Ras, whereas expression of a dominant-negative Ras in wild-type heart explants reproduced the same defect, suggesting the critical role of Ras in HA-dependent signaling.
To search for the role of HA in cell transformation by v-src, we examined the effect of HA stimulation on cell motility. Here we show evidence that activation of HA production in cells strongly correlates with the transforming activity of v-src, and HA activates cell locomotion in a tumor-specific manner. In addition, we show that constitutive activation of both Ras-MAPK signaling and phosphoinositide 3-kinase (PI3 kinase) signaling is required for the activation of tumor-specific cell locomotion by HA.
MATERIALS AND METHODS
Cell Culture
The rat fibroblast cell line, 3Y1, was used throughout this study (Hamaguchi and Hanafusa, 1987). 3Y1 transfected with various v-src mutants, v-Src3Y1, G2A3Y1, and ts3Y1, were prepared as described previously (Machida et al., 2000). S17NRas v-Src3Y1 (Thant et al., 1997), 3Y1 transformed with MEK1EEΔN3 (Kurata et al., 2000), and v-Crk3Y1 (Liu et al., 2000) were prepared as described previously. Balb3T3 transfected with v-src, v-Src Balb3T3, was prepared as described previously (Machida et al., 2000).
Treatment of Cells with HA and Pharmacological Inhibitors
HA, purified from culture medium of Streptococcus equi, was kindly supplied by Chugai Pharmaceutical (Tokyo, Japan) and Denki Kagaku Kogyo (Tokyo, Japan). HA, 800 kDa, was boiled for 10 min and suspended in serum-free medium before use. HA fragments of various sizes, 32, 200, 800, and 1900 kDa, were kindly supplied by Denki Kagaku Kogyo. HA was added to the medium at a final concentration of 100 μg/ml. Chondroitin sulfate A and keratan sulfate were purchased from Seikagaku (Tokyo, Japan). 4-Methylumbelliferone (4MU; Sigma-Aldrich Chemical, Milwaukee, WI) was added at a concentration of 100 μM with 0.1% DMSO as described previously (Kosaki et al., 1999). 2-[4-Molpholinyl]-8-phenyl-4H-1-benzopyran-4-one (LY294002: Calbiochem, La Jolla, CA), wortmannin (Calbiochem), and PD98059 (New England BioLabs, Beverly, MA; Dudley et al., 1995) were added at final concentrations of 10, 50, and 50 μM, respectively.
Assay of HA Production
The amounts of HA in culture media were measured by sandwich binding protein assay as described previously (Itano et al., 1999b).
Immunoblotting
Analysis of Src protein, tyrosine phosphorylated proteins, Ras, and phosphorylated MAPKp42/44 by immunoblotting with specific antibodies was described previously (Hamaguchi et al., 1988, 1993, 1995).
Anti-Src monoclonal antibody, mAb327, was kindly provided by Dr. J. S. Brugge (Harvard Medical School, Boston, MA; Lipsich et al., 1983). Anti-phosphotyrosine monoclonal (PY20H) and anti-pan Ras antibodies were purchased from Transduction Lab (Lexington, KY) and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. Anti-phospho-MAPK, anti-Erk2, anti-phospho-Akt, and anti-Akt antibodies were purchased from New England BioLabs.
Inhibition of HA Binding to CD44 by Neutralizing Antibody
Anti-mouse CD44-neutralizing antibody (KM114) was purchased from Research Diagnostic (Flanders, NJ; Oliferenko et al., 2000). Cells were pretreated with the indicated doses of KM114 for 30 min, washed with phosphate-buffered saline, and subsequently treated with HA (10 μg/ml) in the presence of the indicated doses of KM114.
Cell Motility Assay
Cells were assayed for their motility by a computer-assisted modification of the phagokinetic assays with gold colloid-coated glass plates previously described (Albrecht-Buehler and Lancaster, 1976). Briefly, cells (2 × 104 cells/3.5-cm plate) were seeded on colloidal gold particle-coated glass coverslips and incubated for 18 h. After fixation with 4% paraformaldehyde, the coverslips were mounted onto the glass microscope slides, and photographs were taken by a computer assisted digital camera (model HS-300, Olympus, Tokyo, Japan) connected to a microscope. The areas of particle swept where cells moved around during incubation were measured by NIH image (version 1.62) and statistically analyzed by Stat View (version 4.51). For the assay, 12 fields per sample were randomly selected and four to five cells per field were examined for their motility.
Statistical Analysis
Data were expressed as the means of three independent experiments ± SD. The statistical analysis of the results was done with the use of Student's t test. P values <0.001 in each condition were considered statistically significant.
RESULTS
HA Production in Cells Transfected with v-src Mutants
We first assayed the levels of HA production in cells transfected with various v-src mutants. Wild-type v-src and its mutant forms were ligated into pBabe vector and transfected into a rat cell line, 3Y1. Drug-resistant clones were isolated and expression of Src protein was probed as described previously (Miyazaki et al., 1999; Machida et al., 2000). v-Src3Y1, ts3Y1, and G2A3Y1 are cell lines expressing wild-type v-Src, a temperature-sensitive mutant of v-Src, tsNY72–4, and a nonmyristoylated form of v-Src, G2ASrc, respectively (Machida et al., 2000). Cells of each cell line ( 2 × 105 in 12-well tissue culture plates) were cultured in the presence of 2% fetal calf serum (FCS), and HA accumulated in the media was assayed. As shown in Figure 1, 3Y1 transformed with v-Src had augmented secretion of HA as reported in chicken embryonic fibroblasts transformed with RSV. v-Src3Y1 secreted ∼1 μg/ml HA (5.5 ng/μg cellular proteins) within 72 h, which was approximately five times higher than 3Y1. When cells were cultured at a density of 1 × 106/plate, v-Src3Y1 secreted approximately 5 μg/ml/d of HA, which was again five times higher than 3Y1, to the medium. We found that all three isoforms of HA synthases, HAS1, HAS2, and HAS3, were activated in v-Src3Y1 (N. Itano, Y. Sohara, K. Kimata and M. Hamaguchi, unpublished data).
Figure 1.
HA production in cells transfected with various v-src mutants. Cells (2 × 105) of v-Src3Y1 (●), 3Y1 (○), ts3Y1 cultured at the nonpermissive temperature (▵) or at the permissive temperature (▴), G2A3Y1 (▪), v-Crk3Y1 (♦), and MEK1EEΔN3 (⊙) were incubated in the presence of 2% FCS for the indicated hours. To examine the effect of serum, v-Src3Y1 was also incubated with 0.5% FCS (□). Conditioned media from these cells were collected and clarified by centrifugation. HA amounts in the media were measured by the sandwich binding protein assay as described in MATERIALS AND METHODS. The results represent the mean values of three independent experiments.
G2A3Y1 expresses a mutant form of v-Src in which Gly at position 2 of v-Src was substituted for Ala by polymerase chain reaction (Machida et al., 2000). This nonmyristoylated form of v-Src is active in protein kinase but defective in membrane binding so that it lacks transforming activity (Kamps et al., 1985). In G2A3Y1, HA production was suppressed to a level smaller than that of 3Y1, although tyrosine phosphorylation was activated in this cell line. In ts3Y1, HA production was regulated in a temperature-sensitive manner. At the nonpermissive temperature (39.5°C), HA secretion was limited to a level smaller than 3Y1. In contrast, HA secretion was activated at the permissive temperature (34.5°C). Thus, the activation of HA secretion in cells transfected with mutant src genes showed good correlation with the transforming activity of v-Src.
We also examined HA secretion in v-Crk-transformed 3Y1 (v-Crk3Y1; Liu et al., 2000) and MEK1EEΔN3-transformed 3Y1 (MEK1EE3Y1; Kurata et al., 2000). v-Crk, identified as an oncogene product of the CT10 retrovirus, consists of a viral Gag sequence fused to the SH2 and the SH3 domains (Mayer et al., 1988). Although v-Crk lacks a catalytic kinase domain, it can activate the multiple signaling pathways mainly by protein-protein binding with its SH2/SH3 domains. MEK1EEΔN3 has substitutions in Ser-218 to Glu and in Ser-222 to Glu that yield constitutive activation of the kinase and lacks the nuclear export signal (amino acid deletion from position 33–41). This gene has strong transforming activity due to the enhanced activation of the MAPK (Kurata et al., 2000). We found that HA secretion in both v-Crk3Y1 and MEK1EE3Y1 was activated compared with that in 3Y1 but not so dramatically as in v-Src3Y1 (Figure 1).
In this experiment, cells were cultured in the presence of 2% FCS to minimize the effect of FCS. We found, however, that HA secretion was dramatically decreased in v-Src3Y1 cultured with 0.5% FCS to a level similar to that of 3Y1 with 2% FCS, suggesting the requirement of serum stimulation for the augmented HA secretion in transformed cells.
Activation of Cell Motility by HA
Because several lines of evidence suggest the involvement of HA in cell locomotion, we next assayed the effect of HA treatment on cell motility by a method with the use of glass plates coated with colloidal gold particles as described in MATERIALS AND METHODS. Cells were seeded at low density (2 × 104 cells/3.5-cm plate) onto colloidal gold particle-coated glass plates and incubated with or without serum and HA for 18 h. In the area where cells migrated during incubation, gold particles were removed by the cells so that the levels of cell motility could be visualized by the gold particle-free area (Albrecht-Buehler and Lancaster, 1976). In the serum-free medium, both 3Y1 and v-Src3Y1 displayed restricted motility without HA treatment (Figure 2A). In contrast, HA-stimulated v-Src3Y1 exhibited high motile activity approximately three- to fourfold higher than HA-untreated v-Src3Y1. 3Y1 also responded to HA, but its activation was only 1.5-fold higher than untreated 3Y1. In addition, HA-treated v-Src3Y1 showed high motile activity ∼2.5-fold higher than HA-treated 3Y1. When cell motility was assayed in the presence of 2% serum, HA-dependent cell motility was more activated, but cell motility without HA stimulation was also activated.
Figure 2.
Migration of HA-stimulated cells on colloidal gold-coated glass plates and immunoblotting of v-Src and tyrosine phosphorylated proteins. (A) Photographs show representative results of the cell motility assay with colloidal gold-coated glasses. Cells were seeded on colloidal gold glass plates and incubated with or without HA for 18 h. The colloidal gold-free areas (white areas) represent the phagokinetic activity of the cells. (a and b), 3Y1; (c and d), v-Src3Y1; (e and f), G2A3Y1. (a, c, and e), incubation without HA; (b, d, and f), incubation with HA (8 × 105 Da, 100 μg/ml). (B) After 18 h of incubation with or without HA in the presence or absence of 2% FCS, the colloidal gold glass plates were fixed and photographs were taken by a computer assisted digital camera. The gold colloid-free areas were measured by NIH image (version 1.62). The data are mean averages ± SD of gold particle-free areas. *, p < 0.0001; **, p < 0.001. (C) The same set of cells was stimulated with HA and subjected to immunoblotting with anti-phosphotyrosine antibody. (D) Immunoblotting with anti-Src.
Effect of HA on Motility of Cells Transfected with Mutant src and on Cellular Protein Phosphorylation
We next examined HA-dependent cell motility in mutant src-transfected cells. Although G2A3Y1, whose mutant Src is active in kinase but defective in cell transformation, showed slightly increased locomotion in the absence of HA stimulation (Figure 2B), it did not exhibit a clear response in cell motility to HA stimulation. We also observed HA-dependent activation of cell motility in ts3Y1 in a temperature-dependent manner. We confirmed that HA treatment did not grossly suppress or activate the expression of v-Src and tyrosine phosphorylation of cellular proteins (Figure 2, C and D). These results suggest that activation of cell locomotion by HA is closely associated with the transforming activity of v-src.
Specificity, Effective Dose, and Size of HA for the Activation of Cell Motility
We examined the specificity, effective dose, and size of HA for motility. As shown in Figure 3A, treatment of v-Src3Y1 with other glycosaminoglycans, keratan sulfate and chondroitin sulfate A, did not activate cell motility of v-Src3Y1. We observed activation of cell motility in a dose as small as 0.5 μg/ml HA but found a sharp decline of activation between 1.0 and 0.5 μg/ml. Under the conditions used here, 0.1 μg/ml HA did not show an obvious effect on cell motility. We used 800-kDa HA for this assay so that 1 μg/ml HA corresponds to 1. 25 nM. Because 2 × 105 of v-Src3Y1 cultured with 0.5% FCS secreted 67 ng of HA within 24 h of incubation, we estimate that 2 × 104 cells of v-Src3Y1 cultured without FSC for cell motility assay may secrete <10 ng of HA during the assay. This dose is far smaller than the ED for the motility assay so that it may be a negligible dose for cell motility.
Figure 3.
Specificity and ED of HA for the activation of cell motility. (A) v-Src3Y1 cells were stimulated with 100 μg/ml HA, keratan sulfate (KS), or chondroitin sulfate (Ch-S) in the absence of serum for 18 h. After incubation, cell locomotive activities were assayed as in Figure 2. (B) v-Src3Y1 was stimulated with the indicated doses of HA for 18 h in the absence of serum, and cell motile activities were assayed. N.S., not significant.
In this study, we routinely used HA boiled for 10 min to avoid possible contamination of growth factors and chemokines. In addition, we examined the effect of highly purified HA of different sizes, 32, 200, 800, and 1900 kDa. All of these highly purified HA sizes showed activation of cell motility in a tumor-specific manner (Table 1). We found, however, that 800 kDa showed the highest activity, whereas 32-kDa HA had the lowest activity compared with other sizes of HA.
Table 1.
Specificity of HA size on the activation of cell motility
| Size of HA (kDa) | 32 | 300 | 800 | 1900 | |
|---|---|---|---|---|---|
| % motilea | 35.3 ± 11.5 | 55.7 ± 24.4 | 88.1 ± 28.7 | 100 | 94.5 ± 28.4 |
| Relative activation (fold) | 1 | 1.58 | 2.49 | 3.00 | 2.83 |
Relative motility was expressed by as the percentage compared with the result of 800-kDa HA.
Effect of 4-Methylumbelliferone (4-MU) on HA Production and Cell Locomotion
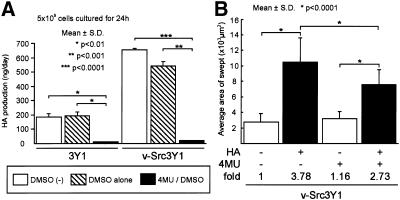
Although v-Src3Y1 may secreted a larger amount of HA than 3Y1 during the motility assay, we found that v-Src3Y1 required serum stimulation for efficient activation of HA secretion (Figure 1). To confirm our observations, we studied the effect of 4MU, a potent inhibitor of HA synthases (Nakamura et al., 1995), on the activation of cell motility by HA. As shown in Figure 4, treatment of cells with 4MU completely abolished the production of HA; yet 4MU–treated v-Src3Y1 retained its response of cell motility to HA treatment. These results suggest that the difference between 3Y1 and v-Src3Y1 in the degree of HA-dependent cell motility does not depend on the activated production of HA in v-Src3Y1 but rather reflects the change in locomotive function that is closely associated with cell transformation by v-src.
Figure 4.
Effect of 4MU, an inhibitor for HA synthases, on HA production and cell locomotion. (A) 3Y1 and v-Src3Y1 were incubated with or without 50 μM 4MU in 0.1% DMSO for 3 d, and media were replaced before the assay. Cells were subsequently incubated for 24 h in the presence or absence of 4MU, and HA secreted in the conditioned media was measured as described in MATERIALS AND METHODS. Values represent the means of three independent experiments ± SD. *, p < 0.01; ** p < 0.001; p*** < 0.0001. (B) Effect of HA treatment on cell motility in the presence or absence of 4MU was assayed by the method with colloidal gold-coated glasses. Cells were incubated for 3 d with or without 4MU and subsequently incubated with or without 100 μg/ml HA. After incubation, cells were fixed and the colloidal gold-free areas were measured. The data represents means ± SD. *, p < 0.0001.
The Role of Ras Signaling in HA-dependent Locomotion
The results that HA activated cell motility in a transformation-specific manner suggest the involvement of the signaling pathways activated by v-Src. Among the multiple signaling pathways constitutively activated by v-Src, the Ras-MAPK pathway is a major pathway that appears to be important for transformation (Smith et al., 1986; DeClue et al., 1991; Nori et al., 1991; Thant et al., 1999). In addition, HA appears to activate this pathway (Slevin et al., 1998; Serbulea et al., 1999; Camenisch et al., 2000; Fitzgerald et al., 2000; Lee and Spicer, 2000). Therefore, we studied the role of Ras signaling in HA-activated locomotion of v-Src3Y1 by use of a dominant-negative form of ras. Expression of S17N Ras, a mutant Ras with Asn substituted for Ser at position 17 of H-Ras yielding a dominant inhibitory effect on endogenous Ras, resulted in inhibition of cell growth and morphological change of v-src–transformed cells (Feig and Cooper, 1988). We established v-src-transformed cell lines in which S17N Ras was conditionally inducible under the control of mouse mammary tumor virus promoter/enhancer (Thant et al., 1997, 1999). v-Src3Y1 was transfected with S17N ras ligated into pMAM2-BSD. Blasticidin-resistant clones overexpressing Ras by dexamethasone treatment were selected by immunoblotting with anti-pan Ras (Thant et al., 1997). Figure 5 shows results with one of the representative clones. We confirmed that neither HA nor dexamethasone suppressed the expression of v-Src. By treatment with dexamethasone, S17N ras-transfected clones expressed higher levels of Ras, whereas expression of S17N Ras was not interfered with by HA treatment. We confirmed that the relative amount of GTP-bound Ras was dramatically decreased by the expression of S17N Ras (Thant et al., 1999). In this cell line, we found that HA-dependent cell motility was completely blocked by the expression of S17N Ras (Figure 6). To confirm these observations, we next examined the effect of manumycin A, a potent inhibitor of Ras farnesyltransferase (Tamanoi, 1993), on HA-dependent locomotion. v-Src3Y1 was pretreated with manumycin A and its HA-dependent cell locomotion was compared with that of untreated v-Src3Y1. As shown in Figure 6C, activation of cell locomotion by HA was completely blocked in manumycin-treated v-Src3Y1. These results strongly suggest that constitutive activation of the Ras-signaling pathway in v-Src3Y1 is required for the tumor-specific activation of cell locomotion in HA-treated cells.
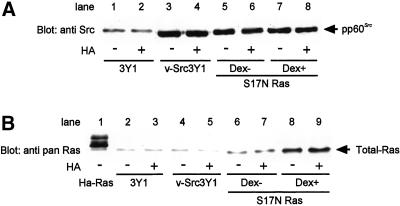
Figure 5.
Expression of S17N Ras by dexamethasone (Dex) treatment. (A) Immunoblotting of v-Src. S17N ras-transfected v-Src3Y1 cells were preincubated with 2 μM dexamethasone at interval of 12 h to 48 h. 3Y1, v-Src 3Y1, and S17N ras-transfected v-Src3Y1 were incubated in the presence (+) or absence (−) of dexamethasone and HA in serum-free medium for 18 h. Subsequently, cells were subjected to immunoblotting with anti-Src antibody. (B) The same cell lysates were probed with anti-pan Ras antibody. H-Ras-transformed 3Y1 (H-Ras) was used as a control.
Figure 6.
Effects of S17N Ras expression and manumycin A treatment on the HA-dependent cell motility of v-Src3Y1. (A) Representative photographs of the cell motility assay with colloidal gold-coated glasses. S17N ras-transfected v-Src3Y1 was incubated with (+) or without (−) 2 μM of dexamethasone (Dex) for 48 h and subsequently stimulated with HA in the presence (+) or absence (−) of dexamethasone. The photographs were taken after 18 h of incubation with or without HA and dexamethasone. (a and c), S17N ras-transfected v-Src3Y1 incubated without dexamethasone; (b and d), the same cells incubated with dexamethasone; (a and b), incubated without HA; (c and d), incubated with HA. (B) After incubation with (+) or without (−) HA and dexamethasone (Dex), cells were fixed and their motile activities were assayed. *, p < 0.0001, mean ± SD. (C) v-Src3Y1 cells were incubated with (+) or without (−) 1 μM manumycin A for 10 h. Manumycin-treated and -untreated cells were incubated with (+) or without (−) HA in serum-free medium for 18 h and assayed for their motile activity. *, p < 0.0001; N.S., not significant; results are means ± SD.
The Role of MEK1-MAPK Signaling in HA-dependent Cell Locomotion
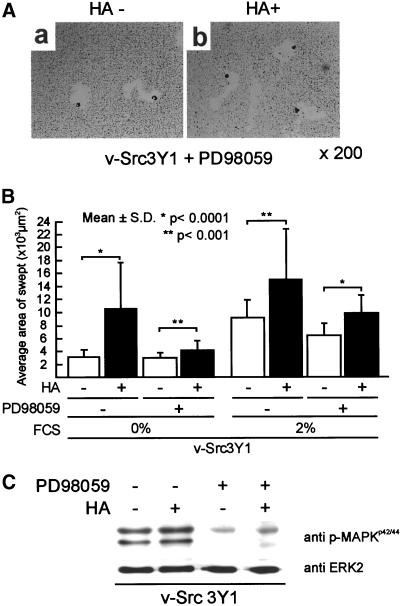
We next examined the role of MEK1-MAPK signaling, a major downstream pathway of Ras signaling, in HA-dependent cell locomotion by use of PD98059, an MEK1 inhibitor, and a constitutive active form of MEK1, MEK1EEΔN3. We found that HA treatment of v-Src3Y1 slightly activated the phosphorylation of MAPK even without serum starvation of cells (Figure 7C). v-Src3Y1 was pretreated with 50 μM of PD98059 for 6 h before HA treatment. The same amount of PD98059 was added to the medium at the interval of 6 h after HA addition and cell motility was assayed after 18 h of incubation with HA. As shown in Figure 7C, treatment of v-Src3Y1 with PD98059 strongly suppressed the phosphorylation of MAPK as we reported previously (Kurata et al., 2000), but the phosphorylation of MAPK in HA-treated cells was slightly elevated even in the presence of PD98059. Under these conditions, we found that PD98059 treatment strongly suppressed the HA-dependent cell motility of v-Src3Y1 (Figure 7, A and B). Although cell motility of the PD98059-treated v-Src3Y1 was weakly activated by HA stimulation, the level observed was similar to that of 3Y1.
Figure 7.
Effect of PD98059 treatment on HA-dependent cell motility of v-Src3Y1. (A) Representative photographs of PD98059-treated v-Src3Y1 stimulated with (+) or without (−) HA. (B) v-Src3Y1 was pretreated with 50 μM of PD98059 for 6 h before HA treatment. The same dose of PD98059 was added to the culture at the interval of 6 h after HA addition. The HA-dependent cell motility was measured after 18 h of incubation with PD98059. The values were means ± SD. (C) Phosphorylation of MAPK in v-Src3Y1 incubated with (+) or without (−) PD98059 and HA. Cell lysates were proved with either anti-phospho-MAPK antibody (top) or anti-ERK2 antibody (bottom).
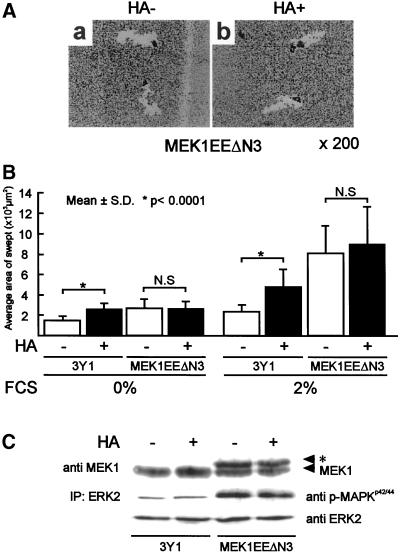
We next examined HA-dependent cell motility in cells transformed with oncogenic MEK, MEK1EEΔN3 (MEK1EE3Y1; Kurata et al., 2000). This gene has strong transforming activity due to enhanced activation of MAPK (Kurata et al., 2000). In contrast to v-Src3Y1, MEK1EE3Y1 did not respond to HA treatment (Figure 8). We found, however, serum-dependent cell locomotion of MEK1EE3Y1 was more strongly activated than that of 3Y1. These results suggest that the MEK1-MAPK pathway is required but not sufficient for the tumor-specific activation of cell locomotion by HA.
Figure 8.
HA-dependent cell motility of 3Y1 transformed with active MEK1 (MEK1EEΔN3). (A) Representative pictures of MEK1EE 3Y1 incubated with (+) or without (−) HA. (a) HA untreated; (b) HA treated. (B) 3Y1 and MEK1EE3Y1 were incubated with (+) or without (−) HA in the presence or absence of 2% FCS for 18 h. The cell motile activity was assayed as described. The data are means ± SD. *, p < 0.0001; N.S., not significant. (C) Expression of MEK1 and phosphorylation of MAPK in HA-treated and -untreated MEK1EEΔN3 were assayed by immunoblotting. *, c-Myc epitope-tagged MEK1. Top, immunoblotting with anti-MEK1; middle, immunoblotting of immunoprecipitated ERK2 with anti-phospho-MAPK; bottom, immunoblotting of immunoprecipitated ERK2 with anti-ERK2.
The Role of the PI3 Kinase Pathway in HA-dependent Cell Locomotion
Because constitutive activation of MAPK by MEK1EEΔN3 was not sufficient for the activation of cell locomotion by HA, we next examined the role of another signaling pathway, PI3 kinase, in cell locomotion. v-Src3Y1 cells were pretreated with either wortmannin or LY294002, potent inhibitors of PI3 kinase, and HA-dependent cell locomotion of these cells was compared with that of untreated v-Src3Y1. As shown in Figure 9, both of the inhibitors suppressed HA-dependent cell locomotion almost completely, whereas MAPK activity was not inhibited by treatment with LY294002. In the experiment in Figure 9B, cells were incubated with serum-free medium for 4 h and subsequently stimulated with HA for 5 min. We found that, under these conditions, clear activation of MAPK was observed in both 3Y1 and v-Src3Y1. Moreover, we observed the HA-dependent activation of MAPK in the presence of LY294002. These results suggest that, in addition to MEK1-MAPK, the PI3 kinase pathway plays an important role in the HA-dependent cell locomotion.
Figure 9.
Effect of wortmannin and LY294002 on HA-dependent cell motility of v-Src3Y1. (A) In v-Src3Y1 cells treated with wortmannin, cells were pretreated with 100 nM wortmannin for 1 h and stimulated with HA in the presence of wortmannin. Wortmannin was added to the culture at an interval of 9 h after HA stimulation. In cells treated with LY294002, cells were preincubated with 10 μM LY294002 for 1 h and stimulated with HA in the presence of LY294002. The cell motility was assayed after 18 h of incubation with HA in the absence of FCS. *, p < 0.0001, mean ± S.D. (B) Phosphorylation of MAPK in these cells was assayed by immunoblotting with anti-phospho-MAPKp42/44. Cells were incubated with serum-free medium for 4 h and subsequently treated with 10 μM LY294002 for 1 h. After incubation, cells were stimulated with 100 μg/ml HA for 5 min and subjected to immunoblotting with anti-phospho-MAPK.
Effect of the Neutralizing Antibody against CD44 on HA-dependent Activation of Cell Motility and on Signaling Cascade
HA of nanomolar order can activate cell motility (Figure 3B), suggesting the involvement of HA binding to its cellular receptor. To obtain more clues, we examined the effect of a neutralizing antibody against CD44, KM114, on HA-dependent cell motility. Because KM114 is an antibody against mouse CD44, a mouse cell line, Balb3T3, was transformed with v-Src (v-SrcBalb3T3). As shown in Figure 10, treatment of cells with 10 μg/ml HA activated the cell motility in a tumor-specific manner. However, HA-dependent activation of cell motility in v-SrcBalb3T3 was suppressed by pretreatment of cells with KM114 in a dose-dependent manner. These results suggest that activation of cell motility by HA requires, at least in part, its binding with CD44.
Figure 10.
Effect of anti-CD44 on HA-dependent cell motility and on phosphorylation of MAPK and Akt. (A) v-SrcBalb3T3 cells were preincubated with the indicated doses of KM114, a neutralizing antibody against CD44, for 30 min. Cells were further incubated in the presence (+) or absence (−) of 10 μg/ml HA. The cell motile activities after HA-stimulation in the presence (+) or absence (−) of KM114 were measured. (B) Representative photographs of the cell motility assay with colloidal gold-coated glasses. v-SrcBalb3T3 was incubated with (+) or without (−) 10 μg/ml KM114 for 30 min and subsequently stimulated with HA in the presence (+) or absence (−) of KM114. The photographs were taken after 18 h of incubation with or without HA and KM114. (C) v-SrcBalb3T3 was incubated with (+) or without (−) 10 μg/ml KM114 for 30 min and subsequently stimulated with HA for 5 min in the presence (+) or absence (−) of KM114. After stimulation, cells were subjected to immunoblotting with anti-phospho-MAPK, anti-ERK2, or anti-Akt. Cell lysates from the same set of cells were immunoprecipitated with anti-Akt and Akt in the precipitates and were proved with anti-phospho-Akt.
We next examined the effect of KM114 pretreatment on HA-dependent signaling cascade (Figure 10C). We found again that treatment of cells with 10 μg/ml HA augmented the phosphorylation of MAPK in v-SrcBalb3T3. In contrast, pretreatment of v-SrcBalb3T3 with 10 μg/ml KM114 strongly blocked HA-dependent activation of MAPK. These results suggest that the HA-dependent activation of MAPK requires HA-CD44 interaction.
We next examined HA-dependent activation of the PI3 kinase-Akt pathway by use of anti-phospho-Akt. Cells incubated with serum-free medium for 4 h were stimulated with 10 μg/ml HA for 5 min and cell lysates were prepared as described in MATERIALS AND METHODS. Akt in the lysates was immunoprecipitated with anti-Akt and probed with anti-phopho-Akt (Figure 10C). We found that HA treatment of v-SrcBalb3T3 augmented the phosphorylation of Akt, a downstream effector for the PI3 kinase, whereas KM114 treatment of cells strongly inhibited the HA-dependent phosphorylation of Akt. These results suggest that activation of the PI3 kinase-Akt pathway by HA requires HA-CD44 interaction.
DISCUSSION
In this report we showed for the first time that augmented secretion of HA in v-Src3Y1 strongly correlated with the transforming activity of v-Src, and HA stimulated cell locomotion in a tumor-specific manner. While other glycosaminoglycans, keratan sulfate and chondroitin sulfate A, did not show clear activation of cell motility, HA in a nanomolar dose could stimulate the motility. Moreover, a neutralizing antibody against CD44 could block HA-dependent activation of cell motility in a dose-dependent manner. These results suggest that HA may regulate cell motility of v-Src-transformed cells in an autocrine-like manner under the condition used here. In addition, we showed that tumor-specific activation of cell locomotion by HA required two parallel pathways, the Ras-MAPK pathway and the PI3K-Akt pathway. Inhibition of either 1 of these pathways by specific inhibitors strongly suppressed cell locomotion, whereas constitutive activation of the MAPK pathway by oncogenic MEK was not sufficient for the activation of HA-specific locomotion. Moreover, we found that pretreatment of cells with a neutralizing antibody against CD44 strongly blocked the activation of MAPK and Akt as well as cell locomotion. These results suggest that both the Ras-MAPK pathway and the PI3 kinase-Akt pathway were required, but activation of MAPK alone was not sufficient for HA-specific locomotion of transformed cells. Our results also suggest that HA may activate the dual pathways by its interaction with CD44, at least in part. In contrast, cells transformed with oncogenic MEK did not respond to HA but had a strong response to serum stimulation (Figure 8). These results suggest that HA-dependent cell locomotion may differ, in part, from serum-dependent cell locomotion in their critical signaling pathways.
The Ras-signaling pathway has drawn attention because of its role in cell transformation by v-src. Suppression of the Ras signaling either by a neutralizing antibody against Ras (Smith et al., 1986), by the dominant-negative Ras (S17N Ras; Feig and Cooper, 1988), or by p120GAP (DeClue et al., 1991; Nori et al., 1991) strongly inhibited transformation of NIH-3T3 cells by v-Src. However, in experiments with chicken embryonic fibroblasts, expression of dominant-negative Ras could not completely suppress the transformation of cells by v-src (Aftab et al., 1997), suggesting that Ras signaling alone is not sufficient for cell transformation. Consistently, another independent signaling, activation of signal transducers and activators of transcription 3, was found to be critical in cell transformation by v-src (Bromberg et al., 1998; Turkson et al., 1998). In addition to these observations, v-Src appears to activate other signaling pathways such as PI3K (Fukui and Hanafusa, 1989) and protein kinase C (Zang et al., 1995). The oncogene of the avian sarcoma virus 16 isolated by P. Vogt encodes the catalytic subunit of the PI3 kinase (Chang et al., 1997), suggesting that PI3 kinase signaling is critical for cell transformation. Recently, Penuel and Martin (1999) reported that dual pathways, Ras-MAPK and PI3 kinase-mTOR, were required for the transformation of chicken embryonic fibroblasts by v-Src. Thus, several independent signaling pathways are involved in complete cell transformation by v-src. All these studies, however, focused only on oncogenic growth of cells, and the regulation of the tumor-specific cell locomotion remains largely unclear. Our results are consistent with the report by Penuel and Martin (1999) and strongly suggest that Ras signaling together with PI3 kinase signaling plays a pivotal role in tumor-specific cell locomotion in addition to the oncogenic growth of the cells. Further studies including the identification of signaling molecules between CD44 and Ras/PI3 kinase are required for the complete comprehension of cell transformation by v-Src.
ACKNOWLEDGMENTS
We are greatly indebted to Hidesaburo Hanafusa for his continuous encouragement, support, and helpful discussion. We thank Mary Dutta for correction of English and Fumiko Yamauchi for her excellent technical assistance. This work was supported by a Grant-in-Aid for Center of Excellence Research from the Ministry of Education, Science and Culture of Japan.
REFERENCES
- Aftab DT, Kwan J, Martin GS. Ras-independent transformation by v-Src. Proc Natl Acad Sci USA. 1997;94:3028–3033. doi: 10.1073/pnas.94.7.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht-Buehler G, Lancaster RM. A quantitative description of the extension and retraction of surface protrusions in spreading 3T3 mouse fibroblasts. J Cell Biol. 1976;71:370–382. doi: 10.1083/jcb.71.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand P, Girard N, Delpech B, Duval C, d'Anjou J, Dauce JP. Hyaluronan (hyaluronic acid) and hyaluronectin in the extracellular matrix of human breast carcinomas: comparison between invasive and non-invasive areas. Int J Cancer. 1992;52:1–6. doi: 10.1002/ijc.2910520102. [DOI] [PubMed] [Google Scholar]
- Bromberg JF, Horvath CM, Besser D, Lathem WW, Darnell JE., Jr Stat3 activation is required for cellular transformation by v-src. Mol Cell Biol. 1998;18:2553–2558. doi: 10.1128/mcb.18.5.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, Kubalak S, Klewer SE, Mcdonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HW, Aoki M, Fruman D, Auger KR, Bellacosa A, Tsichlis PN, Cantley LC, Roberts TM, Vogt PK. Transformation of chicken cells by the gene encoding the catalytic subunit of PI3-kinase. Science. 1997;276:1848–1850. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- DeClue JE, Zhang K, Redford P, Vass WC, Lowy DR. Suppression of src transformation by overexpression of full-length GTPase-activating protein (GAP) or of the GAP C-terminus. Mol Cell Biol. 1991;11:2819–2825. doi: 10.1128/mcb.11.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpech B, Maingonnat C, Girard N, Chauzy C, Maunoury R, Olivier A, Tayot J, Creissard P. Hyaluronan and hyaluronectin in the extracellular matrix of human brain tumor stroma. Eur J Cancer. 1993;29A:1012–1017. doi: 10.1016/s0959-8049(05)80214-x. [DOI] [PubMed] [Google Scholar]
- Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erichsen S, Eng J, Morgan HR. Infection of chicken iris epithelium with the Rous sarcoma virus, tumor cells and chick embryo cells transformed in vitro by virus. 1. Production of mucopolysaccharides, J. Exp Med. 1961;114:435–440. doi: 10.1084/jem.114.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig LA, Cooper GM. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol Cell Biol. 1988;8:3235–3243. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KA, Bowie AG, Skeffington BS, O'Neill LAJ. Tas, protein kinase Cξ, and IκB kinases 1, and 2 are downstream effectors of CD44 during the activation of NF-κB by hyaluronic acid fragments in T-24 carcinoma cells. J Immunol. 2000;164:2053–2063. doi: 10.4049/jimmunol.164.4.2053. [DOI] [PubMed] [Google Scholar]
- Fukui Y, Hanafusa H. Phosphatidylinositol kinase activity associates with viral p60src protein. Mol Cell Biol. 1989;9:1651–1658. doi: 10.1128/mcb.9.4.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi M, Grandori C, Hanafusa H. Phosphorylation of cellular proteins in Rous sarcoma virus-infected cells: analysis by use of anti-phosphotyrosine antibodies. Mol Cell Biol. 1988;8:3035–3042. doi: 10.1128/mcb.8.8.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi M, Hanafusa H. Association of p60src with Triton X-100-resistant cellular structure correlates with morpho1ogical transformation. Proc Natl Acad Sci USA. 1987;84:2312–2316. doi: 10.1073/pnas.84.8.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi M, Matsuyoshi N, Ohnishi Y, Gotoh B, Takeichi M, Nagai Y. p60v-src causes tyrosine phosphorylation and inactivation of the N-cadherin-catenin cell adhesion system. EMBO J. 1993;12:307–314. doi: 10.1002/j.1460-2075.1993.tb05658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi M, Yamagata S, Thant AA, Xiao H, Iwata H, Mazaki T, Hanafusa H. Augmentation of metalloproteinase (gelatinase) activity secreted from Rous sarcoma virus-infected cells correlates with transforming activity of src. Oncogene. 1995;10:1037–1043. [PubMed] [Google Scholar]
- Hanafusa H, Halpern CC, Buchhagen DL, Kawai S. Recovery of avian sarcoma virus from tumors induced by transformation-defective mutants. J Exp Med. 1977;146:1735–1747. doi: 10.1084/jem.146.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RJ, Malmgreen H, Sylven B. The polysaccharides of Rous sarcoma. Br J Cancer. 1954;8:141–146. doi: 10.1038/bjc.1954.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horai T, Nakamura N, Tateishi R, Hattori S. Glycosaminoglycans in human lung cancer. Cancer. 1981;48:2016–2021. doi: 10.1002/1097-0142(19811101)48:9<2016::aid-cncr2820480918>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Itano N, Sawai T, Miyaishi O, Kimata K. Relationship between hyaluronan production and metastatic potential of mouse mammary carcinoma cells. Cancer Res. 1999a;59:2499–2504. [PubMed] [Google Scholar]
- Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y, Miyauchi S, Spicer AP, McDonald JA, Kimata K. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem. 1999b;274:25085–25092. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- Jove R, Hanafusa H. Cell transformation by the viral src oncogene. Annu Rev Cell Biol. 1987;3:31–56. doi: 10.1146/annurev.cb.03.110187.000335. [DOI] [PubMed] [Google Scholar]
- Kabat EA. A polysaccharide in tumors due to a virus of leucosis and sarcoma of fowls. J Biol Chem. 1939;130:143–147. [Google Scholar]
- Kamps MP, Buss JE, Sefton BM. Mutation of NH2-terminal glycine of p60src prevents both myristoylation and morphological transformation. Proc Natl Acad Sci USA. 1985;82:4625–4628. doi: 10.1073/pnas.82.14.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson W. Tumor-associated hyaluronan: providing an extracellular matrix that facilitates invasion. Am J Pathol. 1996;148:1721–1726. [PMC free article] [PubMed] [Google Scholar]
- Knudson W, Biswas C, Li XQ, Nemec RE, Toole BP. The role and regulation of tumor-associated hyaluronan. Ciba Found Symp. 1989;143:150–159. doi: 10.1002/9780470513774.ch10. , discussion 159–169, 281–285. [DOI] [PubMed] [Google Scholar]
- Kosaki R, Watanabe K, Yamaguchi Y. Overproduction of hyaluronan by expression of the hyaluronan synthase HAS2 enhances anchorage-independent growth and tumorigenicity. Cancer Res. 1999;59:1141–1145. [PubMed] [Google Scholar]
- Kurata H, Thant AA, Matsuo S, Senga T, Okazaki K, Hotta N, Hamaguchi M. Constitutive activation of MAP kinase kinase (MEK1) is critical and sufficient for the activation of MMP-2. Exp Cell Res. 2000;254:180–188. doi: 10.1006/excr.1999.4738. [DOI] [PubMed] [Google Scholar]
- Laurent TC, Fraser JRE. Hyaluronan. FASEB J. 1992;6:2397–2404. [PubMed] [Google Scholar]
- Lee JY, Spicer AP. Hyaluronan: a multifunctional, megaDalton, stealth molecule. Curr Opin Cell Biol. 2000;12:581–586. doi: 10.1016/s0955-0674(00)00135-6. [DOI] [PubMed] [Google Scholar]
- Lipsich LA, Lewis AJ, Brugge JS. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983;48:352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu E, Thant AA, Kikkawa F, Kurata H, Tanaka S, Nawa A, Mizutani S, Matsuda S, Hanafusa H, Hamaguchi M. The Ras-MEK1 pathway is critical for the activation of MMP secretion and the invasiveness in v-crk-transformed 3Y1. Cancer Res. 2000;60:2361–2364. [PubMed] [Google Scholar]
- Lokeshwar VB, Oebek C, Soloway MS, Block NL. Tumor-associated hyaluronic acid a new sensitive and specific urine marker for bladder cancer. Cancer Res. 1997;57:773–777. [PubMed] [Google Scholar]
- Machida K, Matsuda S, Yamaki K, Senga T, Thant AA, Kurata H, Miyazaki K, Hayashi K, Okuda T, Kitamura T, Hayakawa T, Hamaguchi M. v-Src suppresses SHPS-1 expression via the Ras-MAP kinase pathway to promote the oncogenic growth of cells. Oncogene. 2000;19:1710–1718. doi: 10.1038/sj.onc.1203497. [DOI] [PubMed] [Google Scholar]
- Mayer BJ, Hamaguchi M, Hanafusa H. A novel viral oncogene with structural similarity to phospholipase C. Nature. 1988;332:272–275. doi: 10.1038/332272a0. [DOI] [PubMed] [Google Scholar]
- Merzak A, Koocheckpour S, Pilkington GJ. CD44 mediates human glioma cell adhesion and invasion in vitro. Cancer Res. 1994;54:3988–3992. [PubMed] [Google Scholar]
- Miyazaki K, Senga T, Matsuda S, Tanaka M, Machida K, Takenouchi Y, Nimura U, Hamaguchi M. Critical amino acid substitutions in the Src SH3 domain that convert c-Src to be oncogenic. Biochem Biophys Res Commun. 1999;263:759–764. doi: 10.1006/bbrc.1999.1464. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Takagaki K, Shibata S, Tanaka K, Higuchi T, Endo M. Hyaluronic-acid-deficient extracellular matrix induced by addition of 4-methyl-umbelliferone to the medium of cultured human skin fibroblasts. Biochem Biophys Res Commun. 1995;208:470–475. doi: 10.1006/bbrc.1995.1362. [DOI] [PubMed] [Google Scholar]
- Nori M, Vogel US, Gibbs JB, Weber MJ. Inhibition of v-src-induced transformation by a GTPase-activating protein. Mol Cell Biol. 1991;11:2812–2818. doi: 10.1128/mcb.11.5.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliferenko S, Kaverina I, Small JV, Huber LA. Hyaluronic acid (HA) binding to CD44 activates Rac1, and induces lamellipodia outgrowth. J Cell Biol. 2000;148:1159–1164. doi: 10.1083/jcb.148.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penuel E, Martin GS. Transformation by v-Src: Ras-MAPK and PI3K-mTOR mediate parallel pathways. Mol Biol Cell. 1999;10:1693–1703. doi: 10.1091/mbc.10.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney P, Kumar S, Ponting J, Wang M. The role of hyaluronan in tumor neovascularization. Int J Cancer. 1995;60:632–636. doi: 10.1002/ijc.2910600511. [DOI] [PubMed] [Google Scholar]
- Ropponen K, Tammi M, Parkkinen J, Eskelinen M, Tammi R, Lipponen P, Agren U, Alhava E, Kosma VM. Tumor cell-associated hyaluronan as an unfavorable prognostic factor in colorectal cancer. Cancer Res. 1998;58:342–347. [PubMed] [Google Scholar]
- Serbulea M, Kakumu S, Thant AA, Miyazaki K, Machida K, Senga T, Ohta S, Yoshioka K, Hotta N, Hamaguchi M. Hyaluronan activates mitogen-activated protein kinase via Ras-signaling pathway. Int J Oncol. 1999;14:733–738. doi: 10.3892/ijo.14.4.733. [DOI] [PubMed] [Google Scholar]
- Slevin M, Krupinski J, Kumar S, Gaffney J. Angiogenic oligosaccharides of hyaluronan induce protein tyrosine kinase activity in endothelial cells and activate a cytoplasmic signal transduction pathway resulting in proliferation. Lab Invest. 1998;78:987–1003. [PubMed] [Google Scholar]
- Smith MR, DeGudicibus SJ, Stacey DW. Requirement for c-ras proteins during viral oncogene transformation. Nature. 1986;320:540–543. doi: 10.1038/320540a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamanoi F. Inhibitors of Ras farnesyltransferases. Trends Biochem Sci. 1993;18:349–353. doi: 10.1016/0968-0004(93)90072-u. [DOI] [PubMed] [Google Scholar]
- Thant AA, Sein TT, Liu E, Machida K, Kikkawa F, Koike T, Seiki M, Matsuda S, Hamaguchi M. Ras pathway is required for the activation of MMP-2 secretion and for the invasion of src-transformed 3Y1. Oncogene. 1999;18:6555–6563. doi: 10.1038/sj.onc.1203049. [DOI] [PubMed] [Google Scholar]
- Thant AA, Serbulea M, Kikkawa F, Liu E, Tomotaka Y, Hamaguchi M. c-Ras is required for the activation of the matrix metalloproteinases by concanavalin A in 3Y1 ce11s. FEBS Lett. 1997;406:28–30. doi: 10.1016/s0014-5793(97)00230-5. [DOI] [PubMed] [Google Scholar]
- Turkson J, Bowman T, Garcia R, Caldenhoven E, De Groot RP, Jove R. Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol Cell Biol. 1998;18:2545–2552. doi: 10.1128/mcb.18.5.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Q, Frankel P, Foster DA. Selective activation of protein kinase C isoforms by v-Src. Cell Growth Differ. 1995;6:1367–1373. [PubMed] [Google Scholar]