Abstract
Glutathione (GSH) significantly declines in the aging rat liver. Because GSH levels are partly a reflection of its synthetic capacity, we measured the levels and activity of γ-glutamylcysteine ligase (GCL), the rate-controlling enzyme in GSH synthesis. With age, both the catalytic (GCLC) and modulatory (GCLM) subunits of GCL decreased by 47% and 52%, respectively (P < 0.005). Concomitant with lower subunit levels, GCL activity also declined by 53% (P < 0.05). Because nuclear factor erythroid2-related factor 2 (Nrf2) governs basal and inducible GCLC and GCLM expression by means of the antioxidant response element (ARE), we hypothesized that aging results in dysregulation of Nrf2-mediated GCL expression. We observed an ≈50% age-related loss in total (P < 0.001) and nuclear (P < 0.0001) Nrf2 levels, which suggests attenuation in Nrf2-dependent gene transcription. By using gel-shift and supershift assays, a marked reduction in Nrf2/ARE binding in old vs. young rats was noted. To determine whether the constitutive loss of Nrf2 transcriptional activity also affects the inducible nature of Nrf2 nuclear translocation, old rats were treated with (R)-α-lipoic acid (LA; 40 mg/kg i.p. up to 48 h), a disulfide compound shown to induce Nrf2 activation in vitro and improve GSH levels in vivo. LA administration increased nuclear Nrf2 levels in old rats after 12 h. LA also induced Nrf2 binding to the ARE, and, consequently, higher GCLC levels and GCL activity were observed 24 h after LA injection. Thus, the age-related loss in GSH synthesis may be caused by dysregulation of ARE-mediated gene expression, but chemoprotective agents, like LA, can attenuate this loss.
One of the hallmarks of aging is a reduced capacity of cellular homeostatic mechanisms that protect the body against a variety of oxidative, toxicological, and pathological insults (1–3). Nowhere is this loss more pronounced than in the age-related decline in hepatic glutathione (GSH) levels. GSH is the principal low molecular weight thiol antioxidant and the cosubstrate for a variety of antioxidant and anti-xenobiotic (Phase II) enzymes (4–7). Decline in constitutive GSH levels adversely affects cellular thiol redox balance and potentially leaves the cell susceptible to a number of internal and environmental stresses. Conversely, increasing GSH steady-state levels and/or its rate of synthesis confers enhanced protection against oxidative insult (8, 9). Due to the central role of GSH in cellular protective mechanisms, the induction of enzymes required for its synthesis represents a key adaptive response to oxidative injury. In aging, however, when basal levels of oxidative stress become elevated, GSH and the enzymes from which it is synthesized do not concomitantly increase but actually decline in many tissues (4, 7, 10, 11). This lack of a cellular compensatory response to loss in GSH and the existence of a prooxidant state in aging cells suggest that the coordination of cellular antioxidant defenses may be altered with age.
The synthesis of GSH from its constituent amino acids involves the actions of two ATP-dependent enzymes, γ-glutamylcysteine ligase (GCL) and GSH synthetase. GCL, the rate-controlling enzyme in the overall pathway, is a heterodimer composed of a catalytic (GCLC; 73 kDa) and a modulatory (GCLM; 30 kDa) subunit. GCLC retains all of the catalytic activity; GCLM improves catalytic efficiency by lowering the Km for glutamate and increasing the Ki for GSH (12).
The basal and inducible expression of these GCL substituents seem to be mediated by means of the antioxidant response element (ARE) (13–15). The ARE is a cis-acting enhancer sequence that transcriptionally regulates Phase II detoxification enzymes, which are critical for maintaining cellular redox status and protecting against oxidative damage (16). A potential loss in GCL transcriptional regulation associated with aging may be indicative of a global decline in Phase II defense systems.
Recent studies show that nuclear factor erythroid2-related factor 2 (Nrf2) is the principal transcription factor that regulates ARE-mediated gene transcription (17, 18). Direct evidence for this finding is provided by the observed reduced basal expression of GCL and other Phase II detoxification enzymes in Nrf2-null mice (17, 19–21). These mice also display a marked increased susceptibility to toxicological insult (17, 19–21). We previously showed that aged animals also exhibit similar losses in steady-state GSH levels and vulnerability to toxins as shown for Nrf2-null mice (22, 23). These striking similarities between aging and Nrf2-null mice raise the question as to whether disruptions in Nrf2-mediated gene expression also occur during aging.
Thus, the aims of this study were to determine whether the age-associated decline in GSH status that we observed is due, at least in part, to the loss of Nrf2-dependent regulation of GCL expression. Moreover, previous studies show that thiol-reactive substances such as 3H-1,2-dithiole-3-thione (D3T), pyrrolidine dithiocarbamate (PDTC) (24), sulforaphane, and (R)-α-lipoic acid (LA) (7, 25, 26) act as potent chemopreventive agents that increase cellular GSH and Phase II response. Thus, we also sought to understand whether potential lesions in basal regulation of Nrf2 prevent its activation by the disulfide chemoprotectant, lipoic acid.
Materials and Methods
LA was a gift of Asta Medica (Frankfurt, Mainz, Germany). Rabbit polyclonal antibodies to GCL catalytic and modulatory subunits were used (4). Histone H1 antibody from Calbiochem (EMD Biosciences, San Diego) was used as a means to verify equal loading in all lanes for immunoblot analysis. Nrf2 antibodies were obtained from Santa Cruz Biotechnology. All high performance liquid chromatography solvents were HPLC grade reagents from Fisher Scientific. All other chemicals were reagent grade or the highest quality available from Sigma.
Animals. Rats (Fischer 344, virgin male, outbred albino), both young (2–5 months: n = 25) and old (24–28 months, n = 25; National Institute of Aging animal colonies), were acclimatized in the Oregon State University animal facilities for at least 1 week before experimentation. Animals were maintained on a standard chow diet, and food and water were given ad libitum.
LA (40 mg/ml) was dissolved in 2 M NaOH containing 154 mM NaCl, and the pH was adjusted to 7.4 with concentrated HCl. LA solutions were sterile-filtered and made fresh each day of use. LA (40 mg/kg of body weight) was administered by i.p. injection. To reduce diurnal variations, animals were killed between 10:00 and 11:00 a.m. each morning.
Rats were anesthetized with diethyl ether, and a midline incision was made in the abdomen. Heparin (0.4 mg/ml) was injected by means of the iliac vein, and Hanks' balanced salt buffer (HBSS; pH 7.4) was perfused through the portal vein for 5 min to remove blood. Livers were quickly removed and washed twice in ice-cold HBSS.
GSH Analysis and Measurement of GCL Activity. Briefly, tissues were homogenized in 10% perchloric acid (wt/vol) containing 5 mM EDTA. After deproteinization, hepatic GSH and glutathione disulfide (GSSG) concentrations were determined according to the method of Faris and Reed (27). To determine the amount of GSH bound to proteins, the acid-precipitated pellets were dissolved in 0.1 M phosphate buffer, and the pH was readjusted to 7.4 with 3 M KOH. These samples were incubated in the presence of 10 mM DTT for 30 min at 37°C, and the amount of GSH released was determined by HPLC-UV analysis.
Hepatic GCL activity was detected as described (4). Briefly, tissues were homogenized in 0.25 M sucrose containing 1 mM EDTA, 20 mM Tris·HCl (pH 7.4), and 1% (vol/vol) protease inhibitor mixture P8340 (Sigma). The cytosolic protein fraction from crude homogenates was obtained by centrifugation and was subsequently filtered through microcon-10 (Millipore) tubes to remove endogenous inhibitors and substrates for GCL. GCL activity was initiated by adding protein (0.5 mg/ml) to a reaction buffer containing 20 mM l-glutamic acid, 5 mM cysteine, 5 mM DTT, 10 mM ATP, 0.1 M Tris·HCl (pH 8.2), 150 mM KCl, 20 mM MgCl2, 2 mM EDTA, and 0.04 mg/ml acivicin. The samples were incubated in a water bath at 37°C for 45 min. Reactions were stopped by mixing 150 μl of the sample with an equal volume of 10% (vol/vol) perchloric acid. The amount of GCL formed was detected by using the same HPLC protocol for monitoring GSH levels. Quantitation was obtained by integration relative to a GCL external standard.
Western Blotting Analysis. Tissues were homogenized and processed as described for the analysis of GCL activity. An aliquot of tissue homogenate (30 μg of cytosolic soluble proteins) was used for determining GCL protein content by Western blotting as described (4). GCLC and GCLM were identified according to molecular weight markers. Relative densities of the bands were digitally quantified by using nih image analysis software.
Real-Time PCR of GCLC and GCLM mRNA. A portion of each liver was excised and stored in RNALater (Ambion, Austin, TX) at 20°C and homogenized by using a Dounce homogenizer. Total RNA was isolated from both young and old rat livers (n = 2 and n = 4, respectively) by using an RNeasy Midi Kit (Qiagen, Valencia, CA). cDNA was prepared from 12.5 μg of total RNA per group by using SuperScript II (Life Technologies, Gaithersburg, MD) and oligo(dT) primers (Qiagen) in a 50-μl reaction. Semiquantitative real-time PCR with mRNA-specific primers spanning exon/exon boundaries was performed by using the DNA Engine Opticon II system (MJ Research, Waltham, MA). Specifically, 62.5 ng of each cDNA pool, 0.3 μM of each primer (GCLC-F, 5′-GTCTTCAGGTGACATTCCAAGC-3′; GCLC-R, 5′-TGTTCTTCAGGGGCTCCAGTC-3′; GCLM-F, 5′-CTGCTAAACTGTTCATTGTAGG-3′; GCLM-R, 5′-CTATTGGGTTTTACCTGTG-3′; Qiagen) (where “F” indicates forward primers corresponding to the sense strand whereas antisense reverse primers are designated with an “R”) and Finnzymes' DyNAmo Master Mix containing SYBR Green (MJ Research) was used for the PCR reaction according to the manufacturer's instructions. Samples were run concurrently with standard curves derived from PCR products, and serial dilutions were performed to obtain appropriate template concentrations. Lamin A/C (F, 5′-GGTGGATGCTGAGAACAG-3′; R, 5′-CTCCAGCTCCTTCTTATACTGCTCC-3′; Qiagen) was used as a control for RNA recovery and reverse transcription efficiency. GCLC and GCLM values were normalized to lamin A/C mRNA levels and expressed as arbitrary units. Agarose gel electrophoresis and thermal denaturation (melt curve analysis) were used to confirm specific replicon formation.
Total and Nuclear Nrf2 Levels. Liver tissue was homogenized (1:10) in RIPA Buffer [150 mM PBS containing 1% (vol/vol) Igepal CA630, 0.5% (wt/vol) sodium deoxycholate, 0.1% (wt/vol) SDS, and 5 μg/μl protease inhibitor mixture], pH 7.4, and 50 μg of protein was used for Western analysis of total hepatic Nrf2 levels (as described below). In other studies, nuclear extracts were prepared from liver tissue by the method of Dignam et al. (28). Protein (40 μg) was loaded in each well of a precast 12% Tris·HCl polyacrylamide gel (Bio-Rad). Separated polypeptides were transferred to nitrocellulose membranes (Amersham Pharmacia) and probed with anti-Nrf2 antibodies at a 1:2,000 titer. Chemiluminescent detection was done by an ECL Western Blotting Detection kit from Amersham Pharmacia.
Electrophoretic Mobility-Shift Assay (EMSA). Transcription factor binding to the ARE was determined by using an EMSA. Nuclear extracts were prepared as described earlier. All gel-shift assays were performed for three sample replicates in each group. A synthetic double-strand oligonucleotide probe for the ARE (5′-TGG GGA ACC TGT GCT GAG TCA CTG GAG-3′) (Santa Cruz Biotechnology) was end-labeled by using [γ-32P] (Amersham Pharmacia) and T4 polynucleotide kinase (Promega). Binding reactions containing equal amounts of protein (9 μg) and labeled oligonucleotide probes were performed for 20 min at room temperature in binding buffer (4% glycerol/1 mM MgCl2/0.5 mM EDTA/0.5 mM DTT/50 mM NaCl/10 mM Tris, pH 8.0). Specific binding was confirmed by using 100-fold excess unlabeled ARE oligonucleotide as a specific competitor. Protein–DNA complexes were separated by gel electrophoresis by using 6% nondenaturing polyacrylamide gels followed by auto-radiography for 18 h to detect the degree of retardation produced by binding to the probe.
Supershift Assay. Binding of Nrf2 to the ARE was determined by supershift assays where anti-Nrf2 antibodies were incubated with the nuclear extracts at 4°C overnight before carrying out the EMSA reaction.
Statistical Analysis. The statistical significance between means of two independent groups was determined by Student's t test, assuming equal variances. For the comparison of treatment effects of LA in old rats, a one-way analysis of variance with Bonferroni's post hoc test was used. All of the results were considered significant if the P value was <0.05. Statistical analysis was performed by using prism 4.0 software (GraphPad, San Diego).
Results
Age-Associated Changes in GSH Levels and GSH Biosynthetic Capacity. Hepatic GSH levels were measured to establish the extent of age-related changes in this key antioxidant. Compared with young rats, old animals exhibited a significant 35% decline (P < 0.05) in overall GSH concentrations, regardless of redox state (Fig. 1A). To ascertain whether this apparent loss of total GSH was due to formation of protein-GSH mixed disulfides, glutathiolation of proteins was measured. With age, the concentration of GSH bound to proteins increased by 40% from 11.6 ± 1.5 to 19.8 ± 5.2 pmol/mg of tissue (P < 0.05). Despite this increase, protein-bound GSH levels could not account for the age-dependent loss of total GSH.
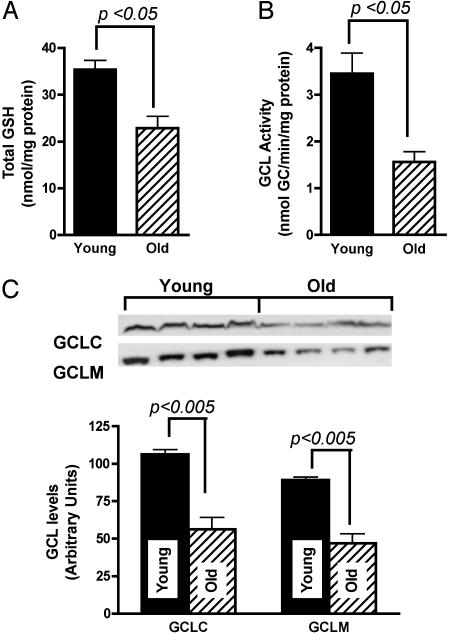
Fig. 1.
Age-related decline in total hepatic GSH is due to loss in GCL activity and expression. Hepatic GSH levels in young (3 mo; n = 4) and old (24 mo; n = 4) F344 rats are shown in A. Results show a 35% decline in total GSH [GSH + 2 glutathione disulfide (GSSG)] in old relative to young rats. (B) Measurement of GCL activity reveals a significant 54.8% decline with age. Western analysis of GCL subunits shows that decreased GCL activity was, in part, due to the lower levels of GCLC and GCLM in old relative to young rats (C). Results are expressed as the mean ± SEM.
To discern whether the age-related decline in hepatic GSH was due, in part, to diminished synthetic capacity, protein levels and activities of GCL were examined. Results show a 53 ± 6% loss in hepatic GCL activity in old compared with young rats (P < 0.05; Fig. 1B). Because GCL is composed of both a catalytic and modulatory subunit, loss in activity could be due to a decline in either GCLM and/or GCLC content. To assess how aging affects the steady-state levels of these subunits, Western analysis was performed. Results show that GCLC levels in old rats were on average 47 ± 8% lower than that observed in young rats (P < 0.005; Fig. 1C); GCLM levels also exhibited a similar loss with age (52 ± 14%; P < 0.005; Fig. 1C). These results indicate that the attenuated hepatic GSH content in old rats is largely accounted for by lower levels and activities of GCL.
GCL Subunit mRNA Levels. Loss of GCL subunits could be due to an age-associated attenuation in GCLC and GCLM message levels as previously observed (4). To discern whether the loss in GCL subunits in this study were due to lower expression, semiquantitative real-time PCR was performed to monitor age-related changes in GCLC and GCLM message abundance. When normalized to lamin A/C, hepatic GCLC levels in old rats were 0.33 ± 0.06 a.u., which was ≈41.1% lower than in young rats (056 ± 0.1 a.u). Similarly, steady-state levels of hepatic GCLM mRNA in old rats (1.1 ± 0.1 a.u.) were 30.0% lower than average values seen in young rats (1.5 ± 0.2 a.u.). These data suggest that the observed lower GCL activity and protein content may be due to diminished gene expression.
Age-Related Decline in Nuclear Nrf2 and Antioxidant Response Element Binding. Due to the central role of Nrf2 in regulating GCLC and GCLM gene transcription, we next examined whether cellular and nuclear Nrf2 levels are adversely affected during aging. Western blot assays showed that hepatic Nrf2 levels declined by 56 ± 2% (n = 3 young; n = 4 old; P < 0.001) in old relative to young rats. Cell fractionation and analysis of nuclear extracts also revealed that basal nuclear Nrf2 levels decreased concomitantly (51 ± 7%) with this overall loss in hepatic Nrf2 (P < 0.0001; Fig. 2A). These results suggest that normal steady-state Nrf2 levels decline in the aging rat liver and affect basal Nrf2-mediated gene transcription.
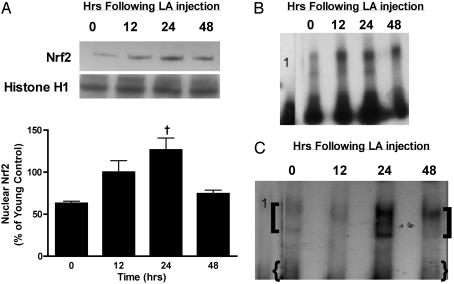
Fig. 2.
Aged rats display a significant loss in nuclear Nrf2 content and ARE-binding activity. Western analysis of Nrf2 present in nuclear extracts prepared from young and old rats are shown in A. Results are also graphically presented relative to Histone H1 loading controls and show that basal nuclear Nrf2 levels are 51% lower on an age basis. (B) EMSA analysis using nuclear extracts from young and old rats shows an overall age-related loss of transcription factor binding to the ARE consensus sequence. Lane 1 is the migration of free probe in absence of nuclear extract. A negative control using excess unlabeled cold probe is shown in lane 2. (C) Supershift analysis reveals that Nrf2 binding to the consensus ARE sequence declines with age. The two distinct supershifted bands are denoted by brackets whereas the lower band as indicated by braces denotes band shifts. Lane 1 is a competitive control where cold unlabeled probe was added in 100-fold excess. Results shown are representative of three independent experiments.
Because nuclear Nrf2 levels, in particular, are a direct reflection of ARE-mediated transcriptional activity, constitutive GCL gene expression by Nrf2 and other transcription factors binding to the ARE may decline with age. To examine whether this loss in constitutive nuclear Nrf2 levels translated into lower ARE binding, gel mobility shift assays were performed. As shown in Fig. 2B, we observed lower transcription factor binding to the consensus ARE sequence in nuclear extracts taken from old vs. young rats. Semiquantitative analysis of these EMSA experiments revealed a 40% loss in ARE-binding activity. These results are consistent with the 50% decline in nuclear Nrf2 levels as well as the observed loss in GCL subunit message levels with age.
To identify whether Nrf2 was, in part, responsible for the age-related attenuation in transcription factor binding to the ARE, supershift analysis was performed by using specific antibodies to Nrf2 (Fig. 2C). Results showed two distinct bands that were retarded in nuclear extracts from both young and old rats. Both of these bands were specific for Nrf2 as demonstrated by competition controls where the unlabeled ARE probe was added in 100-fold excess. These bands may reflect differences in heterodimer partners with Nrf2. Both of these shifted bands markedly declined with age, therefore demonstrating a distinct loss in Nrf2-dependent ARE binding activity.
Pharmacological Activation of Nrf2-Dependent ARE-Gene Transcription. Lower constitutive nuclear Nrf2 levels also suggest that normal signals to induce Nrf2 nuclear translocation may be altered with age. If true, disulfide chemopreventive agents, which readily induce Phase II enzyme response with respect to Nrf2, may not be beneficial to old rats. To test the degree of Nrf2 activation in old rats, we treated animals with LA, a disulfide compound known to activate Nrf2 (25, 26).
As shown in Fig. 3, hepatic nuclear Nrf2 levels in old rats treated with LA showed a marked increase over that seen in vehicle-treated controls. Age-related deficits in nuclear Nrf2 were largely abated within 12 h after LA treatment. This elevated level of nuclear Nrf2 was still evident 24 h after LA administration but declined sharply after 48 h. Thus, responsiveness of Nrf2 nuclear translocation when exposed to chemopreventive agents remains intact in the aging rat liver.
Fig. 3.
LA induces nuclear Nrf2 levels and increases its ARE binding activity. The time-dependent changes in nuclear Nrf2 levels after LA injection (40 mg/kg of body weight) in old rats were determined by Western blot analysis (A). The histone H1 normalized values are graphically represented and show an increase in nuclear Nrf2, with maximum induction seen within 24 h after LA injection (A). (B) EMSA analysis of nuclear extracts shows increased transcription factor binding to the ARE consensus sequence within 12 h, which was maintained for 48 h. Lane 1 is a competition with cold unlabeled probe. (C) Results from supershift assays, which indicate that LA increases Nrf2 binding to the ARE in a time-dependent manner and show maximal binding at 24 h after LA injection. Lane 1 shows a negative control using an antibody against the P65 subunit of NF-κB. Results are representative of three independent experiments. †, The group that is significantly (P < 0.05) different from old control rats.
To further examine whether LA treatment caused increased transcription factor binding to the ARE, EMSA experiments were performed. As expected by the increase in nuclear levels of Nrf2, ARE transcription factor binding activity increased in response to LA as early as 12 h and remained elevated for up to 48 h (Fig. 3B).
Supershift analysis showed that LA increased Nrf2 binding to the ARE vs. the vehicle control. A time course revealed that Nrf2 binding was maximal 24 h after LA treatment and declined after 48 h. Both of the bands associated with Nrf2 were similarly affected with LA (Fig. 3C). These results demonstrate that Nrf2 is responsive to exogenous activators in old rats.
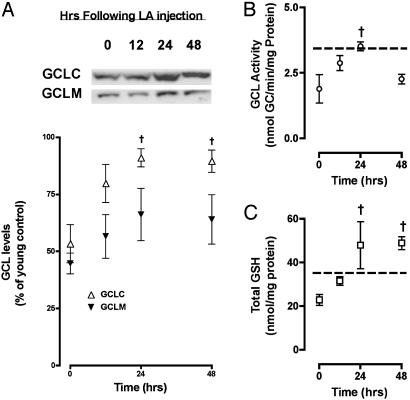
To understand whether the LA-mediated increase in Nrf2 binding translated into elevated GCL levels, we measured GCLC and GCLM protein content in rats treated with LA or saline. LA treatment reversed the age-related decline in GCLC levels within 24 h (1.7-fold increase; P < 0.05; Fig. 4A) after treatment. GCLM levels also increased; however, the heightened levels of this subunit did not reach statistical significance relative to vehicle controls (Fig. 4A). As predicted by higher GCL protein content, hepatic GCL activity and GSH levels also significantly increased after LA treatment. Heightened GCL activity was observed over the 48-h time course and directly correlated with the LA-induced increase in GCLC and GCLM protein (Fig. 4B). Concomitant with heightened GCL levels and activity, hepatic GSH levels also increased and were actually higher than that seen in young untreated animals 24 h after LA administration (Fig. 4C). These results demonstrate that, just as in young animals, the nuclear translocation of Nrf2 efficiently elevates GSH synthesis in old rats.
Fig. 4.
LA improves GSH synthetic capacity and hepatic GSH levels. Western analysis of GCL subunits was performed after LA injection at times indicated in A. Results show that GCLC levels in old rats were maximally increased in response to LA within 24 h (A). GCLM also exhibited a similar time-dependent increase, but the difference observed was not statistically significant (A). Paralleling the increase in GCL levels, enzyme activity increased by two-fold within 24 h after LA injection (B). The changes in GCL levels and enzyme activity resulted in an overall increase in hepatic GSH 24 h post LA injection (C). The dashed lines in B and C indicate mean experimental values seen in young animals. Results are expressed as the mean ± SEM. †, Groups that are significantly (P < 0.05) different from old controls.
Discussion
The present study demonstrates that one potential mechanism underlying the loss of GSH synthesis in old animals is a decline in Nrf2-mediated transcription of GCL proteins. More than 200 antioxidant and detoxication enzymes are regulated by the Keap1-Nrf2 pathway (29); thus, alterations in basal hepatic and nuclear Nrf2 levels and ARE binding activity are likely to have broad effects on cellular antioxidant and xenobiotic responses. In support of this notion, we also have preliminary evidence that NQO1 activity, a quintessential Phase II enzyme regulated by the ARE, declines with age (S.V.S., unpublished results). This finding raises the intriguing possibility of a global repression of Phase II response during aging.
Several lines of evidence [e.g., Nrf2-null mice, Nrf2 overexpression systems, and Nrf2-dominant negative mutants (17, 19–21, 30–32)] implicate this transcription factor as a central transcriptional regulator of ARE-containing Phase II detoxication genes. For example, Nrf2-null mice share remarkable similarity to old animals, including loss of constitutive GCL levels and activity and a concomitant decline in GSH (22). The loss of stress tolerance capacity exhibited by Nrf2-null mice against toxicants, such as acetaminophen, mirrors a similar loss in aging rats (23). Thus, age-associated changes in GCL activity and in GSH levels may be a useful surrogate marker in understanding the mechanisms underlying transcriptional dysregulation of ARE-containing genes.
Like other Phase II genes, the enhancer regions of both GCLM and GCLC contain the ARE, which is most critical for basal and inducible expression of GCL (8, 22, 33, 34). Although the ARE seems to be the principal regulator of GCL expression, it is also noteworthy that its 5′ promoter region also contains multiple binding sites for other transcription factors, such as NF-κB, SP-1, activator protein-1 and -2 (AP-1 and -2), and metal response (MRE) and antioxidant response elements (33, 35, 36). Interestingly, a study using rats reported that basal and inducible GCL transcription depends on AP-1 rather than the ARE, as reported in humans and mice (33). Although the reasons for this discrepancy are not clear, one possibility is that the ARE sequence that is functionally important for GCL regulation resides in a region not examined in that study (33). Although this finding raises the possibility of AP-1 involvement in regulating rat GCL, the results from the current study, as well as those from a previous report, support at least a role for Nrf2 in regulating GCL expression, even in rats (37). Further studies are needed to delineate how the ARE and other responsive elements interact to govern GCL transcription and the role of other transcription factors in the age-related loss of GSH biosynthesis.
The control of Nrf2-dependent transcription can be achieved at multiple levels: (i) by Nrf2 interactions with Kelch-associated protein 1 (Keap1), a cytosolic repressor protein, and/or (ii) by Nrf2 interactions with other bZip transcription factors. Nrf2 bound to Keap1 has a short half-life (<20 min) and is rapidly degraded by ubiquitin-26S proteosomes (38). Keap1 [inhibitor of Nrf2 (INrf2) in rats] has abundant free cysteine residues (25 cysteines), which makes it an ideal redox-sensing partner (39). A recent study reported that the specific modification of Cys-151 leads to the dissociation of Nrf2 from Keap1 (39), which prevents Nrf2 degradation and allows its nuclear translocation. The paradoxical decline in nuclear Nrf2 levels despite an increased age-associated pro-oxidant cellular milieu, suggests that there may be potential alterations in the redox-sensing capacity of Keap1. To partially address this issue, we treated old rats with LA, a disulfide compound known to activate Phase II gene transcription (25, 26). We found that LA potently increases hepatic nuclear Nrf2 levels in a time-dependent manner. These results demonstrate that the pathways leading to Nrf2 activation remain intact in old animals.
Aside from changes in Nrf2 and Keap1 interactions, other factors may be responsible for the observed decline in Nrf2 levels. Nrf2 gene expression is itself governed by the ARE (40); therefore, the intriguing possibility exists that the dysregulation that alters ARE-mediated gene transcription also detrimentally represses Nrf2 transcription. Corroborating this hypothesis, we also observed an age-dependent loss of total cellular Nrf2 levels. It will be important to examine the exact nature and mechanism(s) underlying the age-related decline of Nrf2.
A further complicating factor in characterizing the age-related loss of ARE-dependent gene transcription is the influence of different transcription partners to Nrf2. Nrf2 forms heterodimers with other bZip proteins, including the Jun/Fos family, Fra, small Maf, and ATF4 proteins (8, 16–18). Depending on the partnering factor, Nrf2-dependent gene expression can be modulated, either positively or negatively, to a significant degree. It is not known whether relative proportions of these partner proteins are altered in the aging rat liver. It will be important to analyze potential age-related changes in these factors, particularly, the Jun/Fos family of proteins and their effect on ARE-mediated gene transcription.
In summary, the evidence presented in this paper shows a reduced Nrf2-mediated gene expression in the aging rat liver. Remarkably, activation of Nrf2 can be achieved by treating old rats with LA. In addition to contributing to our understanding of the aging process, these findings suggest new strategies to improve stress resistance in the elderly. Related chemopreventive strategies have garnered much interest in cancer research but, to our knowledge, have heretofore not been advocated as a possible means to improve the morbidity and mortality associated with aging. The results presented herein strongly suggest that further research, in this regard, is warranted (41–43).
Acknowledgments
We gratefully acknowledge the expert technical assistance of Ms. Shi-Hua Du Heath, and we thank Stephen Lawson and John Nides for their comments and careful review of the manuscript. This work was supported by National Institutes of Health Grants RO1 AG17141A and P01 AT002034-01 (to T.M.H.) and RO1 ES11831-01 (to R.L.U.), and by the Environmental Health Sciences Center, Oregon State University (ES00210).
Abbreviations: GSH, glutathione; GCL, γ-glutamylcysteine ligase; GCLC, GCL catalytic subunit; GCLM, GCL modulatory subunit; Nrf2, nuclear factor erythroid2-related factor 2; ARE, antioxidant response element; LA, (R)-α-lipoic acid; Keap1, Kelch-associated protein 1; EMSA, electrophoretic mobility-shift assay.
References
- 1.Harman, D. (1992) Mutat. Res. 275, 257-266. [DOI] [PubMed] [Google Scholar]
- 2.Shigenaga, M. K., Hagen, T. M. & Ames, B. N. (1994) Proc. Natl. Acad. Sci. USA 91, 10771-10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckman, K. B. & Ames, B. N. (1998) Physiol. Rev. 78, 547-581. [DOI] [PubMed] [Google Scholar]
- 4.Liu, R. & Choi, J. (2000) Free Radical Biol. Med. 28, 566-574. [DOI] [PubMed] [Google Scholar]
- 5.Uejima, Y., Fukuchi, Y., Teramoto, S., Tabata, R. & Orimo, H. (1993) Mech. Ageing Dev. 67, 129-139. [DOI] [PubMed] [Google Scholar]
- 6.Nakata, K., Kawase, M., Ogino, S., Kinoshita, C., Murata, H., Sakaue, T., Ogata, K. & Ohmori, S. (1996) Mech. Ageing Dev. 90, 195-207. [DOI] [PubMed] [Google Scholar]
- 7.Hagen, T. M., Vinarsky, V., Wehr, C. M. & Ames, B. N. (2000) Antioxid. Redox. Signal 2, 473-483. [DOI] [PubMed] [Google Scholar]
- 8.Wild, A. C. & Mulcahy, R. T. (2000) Free Radical Res. 32, 281-301. [DOI] [PubMed] [Google Scholar]
- 9.Moinova, H. R. & Mulcahy, R. T. (1998) J. Biol. Chem. 273, 14683-14689. [DOI] [PubMed] [Google Scholar]
- 10.Liu, R. M. (2002) J. Neurosci. Res. 68, 344-351. [DOI] [PubMed] [Google Scholar]
- 11.Rebrin, I., Kamzalov, S. & Sohal, R. S. (2003) Free Radical Biol. Med. 35, 626-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu, S. C. (1999) FASEB J. 13, 1169-1183. [PubMed] [Google Scholar]
- 13.Wild, A. C., Gipp, J. J. & Mulcahy, T. (1998) Biochem. J. 332, 373-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moinova, H. R. & Mulcahy, R. T. (1999) Biochem. Biophys. Res. Commun. 261, 661-668. [DOI] [PubMed] [Google Scholar]
- 15.Wild, A. C., Moinova, H. R. & Mulcahy, R. T. (1999) J. Biol. Chem. 274, 33627-33636. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen, T., Sherratt, P. J. & Pickett, C. B. (2003) Annu. Rev. Pharmacol. Toxicol. 43, 233-260. [DOI] [PubMed] [Google Scholar]
- 17.Itoh, K., Chiba, T., Takahashi, S., Ishii, T., Igarashi, K., Katoh, Y., Oyake, T., Hayashi, N., Satoh, K., Hatayama, I., et al. (1997) Biochem. Biophys. Res. Commun. 236, 313-322. [DOI] [PubMed] [Google Scholar]
- 18.Venugopal, R. & Jaiswal, A. K. (1996) Proc. Natl. Acad. Sci. USA 93, 14960-14965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMahon, M., Itoh, K., Yamamoto, M., Chanas, S. A., Henderson, C. J., McLellan, L. I., Wolf, C. R., Cavin, C. & Hayes, J. D. (2001) Cancer Res. 61, 3299-3307. [PubMed] [Google Scholar]
- 20.Hayes, J. D., Chanas, S. A., Henderson, C. J., McMahon, M., Sun, C., Moffat, G. J., Wolf, C. R. & Yamamoto, M. (2000) Biochem. Soc. Trans. 28, 33-41. [DOI] [PubMed] [Google Scholar]
- 21.Chan, K., Han, X. D. & Kan, Y. W. (2001) Proc. Natl. Acad. Sci. USA 98, 4611-4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan, J. Y. & Kwong, M. (2000) Biochim. Biophys. Acta 1517, 19-26. [DOI] [PubMed] [Google Scholar]
- 23.Enomoto, A., Itoh, K., Nagayoshi, E., Haruta, J., Kimura, T., O'Connor, T., Harada, T. & Yamamoto, M. (2001) Toxicol. Sci. 59, 169-177. [DOI] [PubMed] [Google Scholar]
- 24.Wild, A. C. & Mulcahy, R. T. (1999) Biochem. J. 338, 659-665. [PMC free article] [PubMed] [Google Scholar]
- 25.Flier, J., Van Muiswinkel, F. L., Jongenelen, C. A. & Drukarch, B. (2002) Free Radical Res. 36, 695-699. [DOI] [PubMed] [Google Scholar]
- 26.Cao, Z., Tsang, M., Zhao, H. & Li, Y. (2003) Biochem. Biophys. Res. Commun. 310, 979-985. [DOI] [PubMed] [Google Scholar]
- 27.Fariss, M. W. & Reed, D. J. (1987) Methods Enzymol. 143, 101-109. [DOI] [PubMed] [Google Scholar]
- 28.Dignam, J. D., Lebovitz, R. M. & Roeder, R. G. (1983) Nucleic Acids Res. 11, 1475-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwak, M. K., Wakabayashi, N., Itoh, K., Motohashi, H., Yamamoto, M. & Kensler, T. W. (2003) J. Biol. Chem. 278, 8135-8145. [DOI] [PubMed] [Google Scholar]
- 30.Chanas, S. A., Jiang, Q., McMahon, M., McWalter, G. K., McLellan, L. I., Elcombe, C. R., Henderson, C. J., Wolf, C. R., Moffat, G. J., Itoh, K., et al. (2002) Biochem. J. 365, 405-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan, K. & Kan, Y. W. (1999) Proc. Natl. Acad. Sci. USA 96, 12731-12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishii, T., Itoh, K., Takahashi, S., Sato, H., Yanagawa, T., Katoh, Y., Bannai, S. & Yamamoto, M. (2000) J. Biol. Chem. 275, 16023-16029. [DOI] [PubMed] [Google Scholar]
- 33.Mulcahy, R. T., Wartman, M. A., Bailey, H. H. & Gipp, J. J. (1997) J. Biol. Chem. 272, 7445-7454. [DOI] [PubMed] [Google Scholar]
- 34.Zipper, L. M. & Mulcahy, R. T. (2003) Toxicol Sci. 73, 124-124. [DOI] [PubMed] [Google Scholar]
- 35.Gipp, J. J. & Mulcahy, R. T. (2000) Cytogenet. Cell Genet. 88, 130-132. [DOI] [PubMed] [Google Scholar]
- 36.Gipp, J. J., Bailey, H. H. & Mulcahy, R. T. (1995) Biochem. Biophys. Res. Commun. 206, 584-589. [DOI] [PubMed] [Google Scholar]
- 37.Sekhar, K. R. & Freeman, M. L. (1999) J. Enzyme Inhib. 14, 323-330. [DOI] [PubMed] [Google Scholar]
- 38.Itoh, K., Wakabayashi, N., Katoh, Y., Ishii, T., O'Connor, T. & Yamamoto, M. (2003) Genes Cells 8, 379-391. [DOI] [PubMed] [Google Scholar]
- 39.Dinkova-Kostova, A. T., Holtzclaw, W. D., Cole, R. N., Itoh, K., Wakabayashi, N., Katoh, Y., Yamamoto, M. & Talalay, P. (2002) Proc. Natl. Acad. Sci. USA 99, 11908-11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwak, M. K., Itoh, K., Yamamoto, M. & Kensler, T. W. (2002) Mol. Cell. Biol. 22, 2883-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagen, T. M., Moreau, R., Suh, J. H. & Visioli, F. (2002) Ann. N.Y. Acad. Sci. 959, 491-507. [DOI] [PubMed] [Google Scholar]
- 42.Liu, J., Head, E., Gharib, A. M., Yuan, W., Ingersoll, R. T., Hagen, T. M., Cotman, C. W. & Ames, B. N. (2002) Proc. Natl. Acad. Sci. USA 99, 2356-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu, J., Killilea, D. W. & Ames, B. N. (2002) Proc. Natl. Acad. Sci. USA 99, 1876-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]