Abstract
Indian childhood cirrhosis (ICC), a disease considered to have been endemic in and unique to India has now been documented in children of non-Indian origin from other countries. More recently available findings from a large multicentre study sponsored by the Indian Council of Medical Research (ICMR) have dispelled some of the generally accepted notions and have established several new facts on different aspects of the disease. All relevant reports on ICC and ICC-like diseases, till date, were reviewed to obtain a proper perspective on the current state of our understanding on this non-Wilsonian copper overload liver disease. A primary role of exogenous copper in causing the disease was earlier debated on the basis of studies in India but investigators abroad studying some sporadic cases and a series of endemic ICC-like diseases supported a hepatotoxic injury by ingested copper in genetically susceptible infants and children in ICC- like disease and in ICC. Epidemiologic and morphologic findings in the well controlled ICMR study based on 225 cases of ICC and 426 controls, all confirmed on liver biopsy, have however, convincingly refuted this concept. Additionally, this study revealed that unlike what has been believed earlier, older children more than 3 yr age can get the disease and that in its natural course the hepatic histology can transform between the characteristic one considered diagnostic and some other patterns, any one of which can be the morphologic manifestation at first presentation of the patient. Older children and cases with milder morphologic changes at presentation had longer survival. The overall inference from critical analysis of all available data is that ICC and ICC-like diseases clinically manifest in a child of any age though common in younger ones, and a clinical diagnosis must be made in any child with so-called ‘cryptogenic cirrhosis’. Exposure to exogenous copper in food, milk and water should not be a prerequisite for this consideration. A liver biopsy whenever feasible should be mandatory for confirmation with the understanding that the morphologic changes in liver can present a few other patterns besides the characteristic one currently taken to be diagnostic. The ascribed current decline in encountering ICC is likely to be due partly to missing a diagnosis and partly to a true reduction in incidence consequent on time related economic and socio-cultural changes.
Keywords: ICC, children, copper, cryptogenic cirrhosis, endemic, hypatic, Mallory hyaline
Introduction
It was one and a quarter century back that the existence of a serious form of liver disease affecting infants and children in India was announced to the medical world at a meeting in Kolkata (then called Calcutta), the seat of British India at that time. The disease became recognized worldwide as a special entity, restricted to India, under the name of Indian childhood cirrhosis (ICC). Several aspects of the disease including its cause remained elusive until the early1980s when it was found that the liver in children with ICC had heavy deposits of copper, though in all other respects it was different from Wilson's disease. Soon, studies on children with ICC and controls reported controversial views on the aetiopathogenetic role of exogenous copper ingestion in this disease. To resolve this and several other issues the Indian Council of Medical Research (ICMR) instituted a multicentre study on ICC in mid 1983 through mid 1987 that collected extensive data on several parameters from a large number of ICC cases and matched controls. For unavoidable reasons a full report on this study by ICMR was first made available only in 20061 and a small part of the findings, particularly those related to the role of copper was reported in a later publication2.
In the meantime some sporadic cases of ICC-like copper overload liver disease and a series of identical cases considered endemic in one region were reported in non-Indian origin children from outside India under a variety of names3. A report on analysis of these cases documented till 1998 drew analogy from the view expressed earlier by one group of workers on ICC and confirmed that exogenous copper ingestion resulting in copper overload in the liver had a direct hepatotoxic effect in ICC and like diseases3. On the other hand, the results of the large ICMR study on ICC not only refuted an aetiopathogenic role of exogenous copper but also highlighted several as yet unknown aspects of the disease. This review will start with an outline of the historical aspects of ICC, followed by an account of all relevant information on the ICC and ICC-like disease available till date with particular focus on the important new findings from the ICMR multicentre study.
Historical perspective
Occurrence of an unusual form of serious liver disease affecting infants and young children in India was first reported from Calcutta (known as Kolkata now), then the capital of British India as far back as the late 1880s4 when most paediatric liver diseases of toady were unknown. Initial clinicopathologic accounts on the disease then called ‘Infantile cirrhosis’ or ‘Infantile biliary cirrhosis’, were published during the early 1930s but it is only in the post-independence era that a large volume of information collected by several groups of investigators from different regions of the country revealed the high prevalence of this disease with unique clinical features and frequent fatality5,6,7,8. Soon after independence, the newly established Indian Council of Medical Research (ICMR), constituted a committee of eminent medical professionals to review all available material and study reports on this health problem of considerable national concern. The committee's report is a landmark documentation of the clinicopathologic spectrum of the disease available up to that time5. Five years later Achar and his colleagues from their large experience on this disease at Madras (now Chennai) suggested the name ‘Indian childhood cirrhosis’ for the disease, arguing that young children are in fact more often affected than infants6. This nomenclature stayed on, as in the experience of investigators around the country the age structure was acceptable and an aetiopathogenetic basis for assigning a more appropriate name was not available. It was during the two decades starting mid 1950s that a large number of valuable contributions covering various aspects of this elusive liver disease were made by paediatricians and pathologists working in several regions of the country, particularly Chennai and Vellore in the south, Delhi and Chandigarh in the north, Bombay (now Mumbai) and Gujarat in the west and Gwalior and Uttar Pradesh in central India. The first breakthrough in helping to put ICC as a specific entity was the identification of histologic changes in the liver characteristic of the established phase of disease with hepatocellular damage accompanied by significant amounts of intracellular hyaline indistinguishable from Mallory bodies of alcoholic liver injury7,8. This morphologic picture, not encountered in any other hepatic disorder including known types of cirrhosis, was considered to represent injury caused by a toxic agent and was found to relate to the fatality common in the disease. Subsequent to these reports, the WHO group which formulated the first ever aetiology based classification of cirrhosis approved ICC as a special category with specific histologic changes but no recognized cause9, and the international community of hepatologists and paediatricians became conversant with the hepatic alterations characteristic of this childhood disease10. The aetiology of the disease however, remained obscure. The suggestion made earlier by some investigators that ICC may be a manifestation of hepatitis virus infection, was found untenable on the basis of later studies using specific serological and tissue markers for these infections11,12. Unlike its morphologic identity, the aetiopathogenesis of ICC, therefore, remained an open issue till the early 1980s.
Current state of knowledge
Search for a putative causative agent started with the observation that livers from children with the disease contained excess of Orcein stain positive copper binding protein (CuBP)12,13 and histochemically as well as analytically detectable copper14,15. Interestingly, while the amount of hepatic copper in ICC was found to be far in excess of that seen in Wilson's disease, the well known inherited copper metabolic disorder of childhood14,15,16, the former disease lacked most clinicopathologic features of the latter. Notwithstanding this because of history of liver disease in siblings and close family observed in some cases of ICC8,11 and the finding of heavy hepatic copper accumulation in the usually fatal established stage of disease8,17 investigators in Delhi designed studies to explore the genealogical aspect of ICC and the role of copper if any, in initiating liver injury in this disease. Analysis of pedigree charts of 20 families with an index case of ICC and of 70 families of age matched controls showed no evidence of autosomal recessive, partial sex linkage or double recessive inheritance and it was suggested that the familial predisposition to development of ICC was most likely based on a multifactorial inheritance18. Morphologic studies using light and electron microscopic evaluation of structural changes and of stainable copper and CuBP in liver tissue from children with ICC, age-matched asymptomatic siblings of ICC cases and controls revealed mild to moderate excess of copper (and CUBP) in a majority of sibling livers which was not associated with any structural or functional alteration. This copper deposit declined after the age of 5 years17,19. The authors concluded that some inherited genetic factor inducesa temporary abnormality in copper homeostasis during infancy and early childhood that imparts susceptibility to development of ICC following toxic injury by external agent/s. It was hypothesized that excessive hepatic copper in ICC was most likely a result rather than the cause of hepatocytic injury17.
Three years later, reporting an epidemiologic study on children with ICC and age matched controls from Pune and neighbouring areas, Tanner and associates20 suggested that the hepatic copper overload and liver injury in ICC resulted from early top feeding of animal milk containing high levels of copper from storage in brass utensils. These investigators later reported that copper chelation by penicillamine therapy while not having any effect on advanced cases of ICC, reduced mortality in less advanced cases by about 50 per cent with regression of hepatic morphologic and functional abnormalities21. This was considered by them as supportive evidence for a causal role of copper in ICC. At this time the ICMR, instituted a prospective multicentre study involving six centres located in different regions of the country to keen to resolve several unanswered issues on this generally fatal paediatric health problem endemic in the country including its natural history and the debated causal role of copper.
The ICMR Multicentre National Collaborative Study on ICC
The study centres for this collaborative investigation conducted between 1983 and 1987 were selected on the basis of: (i) different geographic locations in India, (ii) published reports on ICC from each of these regions, and (iii) availability of investigators with expertise and experience on ICC and paediatric liver disease. The six centres were in Maharashtra, Madhya Pradesh, Gujarat, Tamil Nadu and Delhi (2 Centres). The principal objectives of the study were to ascertain the natural history of ICC, explore the clinical manifestations and hepatic structural changes in early and other phases of disease and identify the likely aetiopathogenesis with particular reference to exogenous copper toxicity. All efforts were made to obtain clinical and investigational follow up data and repeat liver biopsies in as many subjects as possible. No copper chelation therapy was given to any subject. Within the collection period an all centre total pool of 1161 children was entered in the study but only 885 of these on whom adequate initial liver biopsy was available, were considered as study subjects for further analysis.
The clinical categorization on the basis of agreed upon and pre-tested clinical criteria was as follows: Highly suggestive ICC; Suspected ICC; Early ICC; High risk ICC and Non-ICC. The histopathological categorization of liver biopsy on agreed upon and pre-tested morphologic criteria were Definitive ICC (DICC); Probable ICC (PICC); Inactive cirrhosis (CIRR); Abnormal liver unclassified (ALU) and Other identifiable disease. The biopsy was considered normal (NORMAL) when no significant change was seen. A label of DICC was given when unequivocal changes accepted as characteristic for ICC8,10,11 were present. The histlogic categorization of the 885 study cases was as follows: 227 DICC, 95 PICC, 137 CIRR (Inactive, mainly micronodular cirrhosis including some chronic hepatitis with fibrosis), and the remaining 426 non-ICC conditions that included 137 cases of other known paediatric liver diseases, 222 ALU and 67 cases categorized as NORMAL. On matching the clinical categories of the 885 subjects with their histologic categorization 11.6 per cent of clinically non-ICC subjects turned out to have a diagnosis of Definitive ICC on liver biopsy. It was, therefore, decided early in the study that all final analyses will be focussed on comparing the data on DICC cases with those on the 426 subjects with histological non-ICC conditions as controls. Follow up clinical data and serial liver biopsy findings on PICC and CIRR cases which were analyzed separately revealed that these two categories of cases were related to ICC, representing different phases of the disease.
Considerably long time was spent on compilation, processing, analysis and interpretation of the large bulk of information obtained from the study and the full account on and conclusions from the study became available in a special ICMR report in 20061 followed by a brief account of some important findings particularly highlighting the causal role of copper in 20082. This well controlled exhaustive study yielded important and new information on the disease including its natural history and protean manifestations as well as convincing evidence refuting a causal toxic role of exogenous copper.
Copper in aetiopathogenesis of ICC and ICC-like disease
Epidemiologic and clinical studies: Following the discovery of large amounts of hepatic copper deposit in ICC, Tanner and colleagues20 conducted an epidemiologic survey on children with ICC and matched controls in the Pune area and found that while most families of both groups used brass or copper vessels for storage of water and milk and for boiling milk, compared to controls a larger proportion of children with ICC were weaned and put on top feed of animal milk at an earlier age of 3 to 6 months. None of the ICC cases were exclusively breast fed. They surmised that excess copper ingested early in infancy resulted in hepatic copper overload in ICC and was important in its aetiology20. From a subsequent study on clinical trials with penicillamine therapy this group reported that while there was no beneficial effect in children with advanced ICC, some children having milder disease without jaundice or ascites showed regression in hepatic morphologic and functional damage21. They considered this as evidence favouring an aetiopathogenetic role of copper in ICC. In contrast, Sethi and colleagues22 in a study on 120 ICC cases from another area in the same Maharastra State reported that all these children were breast fed and in 46 per cent no copper yielding (Brass) utensil was used by the families for storing or boiling milk fed to the children. The liver and serum samples in all cases had increased copper. They concluded that excess dietary copper was unlikely to cause the copper overload in ICC.
While ICC has been known to be endemic in India over past so many decades, this type of childhood liver disease is rarely encountered in other countries. In fact, there have been only about 30 sporadic cases of ICC-like disease documented in 18 reports from 11 countries in 5 continents, the first few cases having been initially labelled as Wilson’ disease and retrospectively designated as ICC-like disease3,23,24,25,26. In addition, a series of 138 endemic cases of children dying of an identical disease in western Austria then called ‘Endemic Tyrolean infantile cirrhosis’ has been reported23. All these cases having the clinical and hepatic morphologic features considered characteristic of established ICC8,11 were described under a variety of names including ‘Idiopathic copper toxicity’, ‘Indian childhood cirrhosis-like disease’, ‘Copper associated childhood cirrhosis’, ‘Copper associated liver disease in childhood’, and others23,24,25,26,27,28,29,30. Reviewing the epidemiologic features of the first 23 sporadic and the 138 Tyrolean cases of this ICC-like disease Muller and associates3 while drawing analogy with the hypothesis advanced earlier by Tanner and his group20 supported the hepatotoxic role of exogenous copper in ICC and like diseases. They justified the term ‘Idiopathic copper toxicity’ adding that the exogenous copper causes disease only in genetically predisposed children with an autosomal recessive inheritance. Subsequently, four other reports of ICC-like disease in six children, one each from Australia, Germany and Japan and three from Saudi Arabia have appeared24,28,29,30. The data related to the possible role of exogenous copper ingestion in the aetiopathogenesis of the disease from all important studies reported so far are summarized in Table I.
Table I.
Epidemiologic data relating to possible exogenous copper ingestion inreports on ICC and ICC-like disease
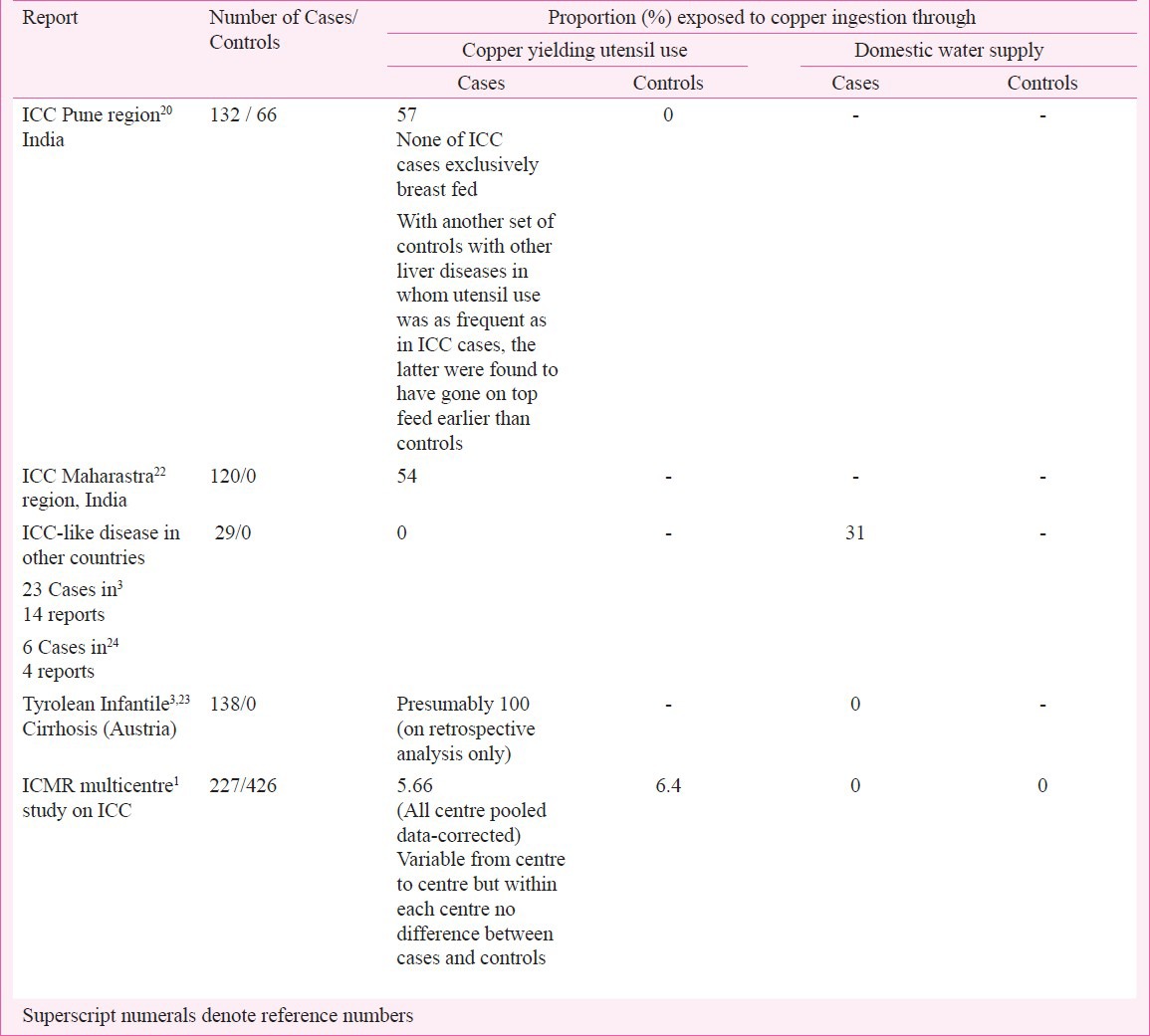
In the ICMR study copper ingestion was assessed in every subject by careful elicitation of the source of domestic water supply and of the use of ‘copper yielding utensils’ (untinned or improperly tinned brass and copper utensils) for cooking and storage of food, milk and water. The study data showed that in all urban and rural areas of India domestic water was supplied through galvanized iron pipes on taps and tube wells respectively, with no possibility of copper contamination. Domestic use of ‘copper yielding utensils’ was random and variable from region to region, mostly present in a minority (less than 10%) of the households. In fact, at two study centres, one in the west and the other in the southeast part of the country, use of such utensil was extremely rare, encountered in none of the children with definitive ICC and in only 1.7 per cent of children of the control group. At three other study centres in the north and in the west the proportion using such utensil was 15 to 20 per cent, being no different in ICC cases and controls within each centre. The highest frequency of copper yielding utensil use was observed in the centre in central part of India where these figures were 55 per cent in ICC cases and 52 per cent in controls, the difference being statistically insignificant. Thus, in the all-centre pooled data the corrected average proportion of copper yielding utensil use in the Definitive ICC cases (5.6%) was no different from that in the non-ICC controls (6.4%) (Table I). It is important to note that the liver biopsy from every case of Definitive ICC in this study showed excess copper deposit, in the majority (85%) of moderate or marked degree. In all cases a detailed history of breast and top feeding till complete weaning was obtained. In the Definitive ICC group, nine children (most of them less than 11 months old) were exclusively breast fed without any supplementation of animal milk or weaning diets2. Another 13 children with definitive ICC while being breast fed had no supplementation of animal milk or milk formula but were on additional home food using only non-copper yielding utensils1. Thus about 10 per cent cases of definitive ICC had no possibility of having had excessive ingestion of copper before being afflicted by the disease. As mentioned earlier, in the centre at Indore in central India approximately half of DICC cases and controls had copper yielding utensils used at their homes and yet DICC was relatively uncommon in Muslim families who used such utensils as often as the others. Allthese findings go against incriminating excessive copper ingestion through water, milk or food as a primary cause of either the hepatic copper overload or the liver injury in ICC. Among the 29 sporadic cases of ICC-like disease reported from outside India the copper content in the domestic water supply according to the standard laid out by WHO (2 mg/l or less) was low in 12, presumably low in eight (cases from USA, UK, Italy and Mexico quoted as ‘not done’3), and mild to moderately high in the remaining nine. In the retrospective study on 138 infants and young children of Tyrol area, Austria, then called ‘Endemic Tyrolean infantile cirrhosis’, the authors presumed that excess copper ingestion might have caused the toxic liver injury because milk prepared in untinned brass and copper vessels used during that period showed a copper concentration of 10.5 - 63.3 mg/l23. In this study, while both the diseased subjects and their asymptomatic siblings were exposed (presumably) to high copper intake, geneologic studies on families of affected children suggested a strong possibility of an autosomal recessive inheritance needed for manifestation of the disease23. Based on these observations Muller and associates3 advocated that all ICC-like disease referred to as ‘Idiopathic copper toxicity’ and by analogy ICC is caused by high copper intake in a genetically susceptible child. That such a complementary role of copper and genetic susceptibility exists in the aetiopathogenesis of ICC has been later discussed by others31,32 but without any additional study data.
In the Tyrol study23 the evidence incriminating high copper intake through milk feed for toxic injury of the liver in affected children was merely circumstantial. Most of the patients in this study were infants and very young children, some as young as 2 months succumbing to the disease at that age. For significant exogenous copper intake to initiate the fatal liver injury at such early infancy one has to assume that the milk feed from copper yielding vessels was instituted almost at birth. In none of the 29 cases of sporadic ICC-like disease reported from around the world use of brass or copper utensil was reported and in only nine the domestic water supply could have, if at all, served as the source of excess copper ingestions. These results fail as convincing evidence to incriminate ingested exogenous copper as the hepatotoxic agent in ICC-like disease. To explore the possible hepatoxic effect of high copper intake, three epidemiologic studies, one from Massachusetts, USA and two from Germany failed to reveal any liver injury in children exposed for prolonged periods to high copper concentrations in domestic water supply33,34,35.
Reporting ICC-like disease in a 20 month old Caucasian child living in rural Australia exposed to high copper intake from domestic water supply, Walker30 emphasized the very low incidence of this disorder despite a significant population at risk because of general use of copper pipes in water supply. Also, in a recent report none of the five cases of biopsy proven ICC encountered in a children's hospital at Hyderabad in south India had any source of exposure to exogenous copper ingestion36.
Morphologic studies: That the profound hepatocytic damage considered diagnostic of ICC and reason for the high mortality5 possibly resulted from a toxic injury was first suggested by investigators from Delhi when they demonstrated presence of large amounts of intracytoplasmic hyaline in the degenerated liver cells which was morphologically and histochemically identical to the Mallory hyaline typically seen in alcoholic liver disease7,37. This hypothesis was based on the known hepatotoxicity of alcohol and also the occurrence of Mallory hyaline in hepatic injury caused by other toxic agents in animals and man7,8,37. The discovery of large amounts of copper and copper associated protein in livers of children with ICC, a disease that was also known to have random familial occurrence, drew attention to a possible linkage of this condition with Wilson's disease which was by then well established as an inherited disorder of copper metabolism. However, the two diseases had several differences in clinical and pathological manifestations. For example, ICC (and ICC-like disease) affects infants and very young children while Wilson's disease almost never occurs below 4 yr age and unlike the latter disease ICC is generally and quickly fatal. Also, in Wilson's disease hepatocytic injury is neither not that severe nor has so much Mallory hyaline as in ICC which characteristically lacks the steatosis in livers of alcohol induced and Wilson's disease.
The problem of associating the heavy copper deposit with liver injury in ICC was difficult because hepatic alterations in the early stage of ICCwere not known. Taking this into consideration the Delhi group conducted a light and electron microscopic study on livers tissues obtained from ICC patients and their asymptomatic siblings as cases, compared with controls constituting age matched children and newborns dying of non-ICC liver disease or non-liver disease17,19. All but one of 27 ICC patients’ livers showed abundant copper deposits while 18 of 27 sibling livers had mild to moderate copper deposit which reduced with age and was not seen above 5 yr age. Small amount of copper was seen in liver of normal infants up to 6 months of age but above that age small amount of copper was seen only in the presence ofliver disease. While all children with ICC died, sibling livers with copper had no significant structural change, all these children remained healthy on follow up17,16. The investigators concluded that copper accumulation by itself was not hepatotoxic and that ICC would have resulted from the action of some toxin on livers with a possible inherited abnormal copper homeostatsis during infancy17.
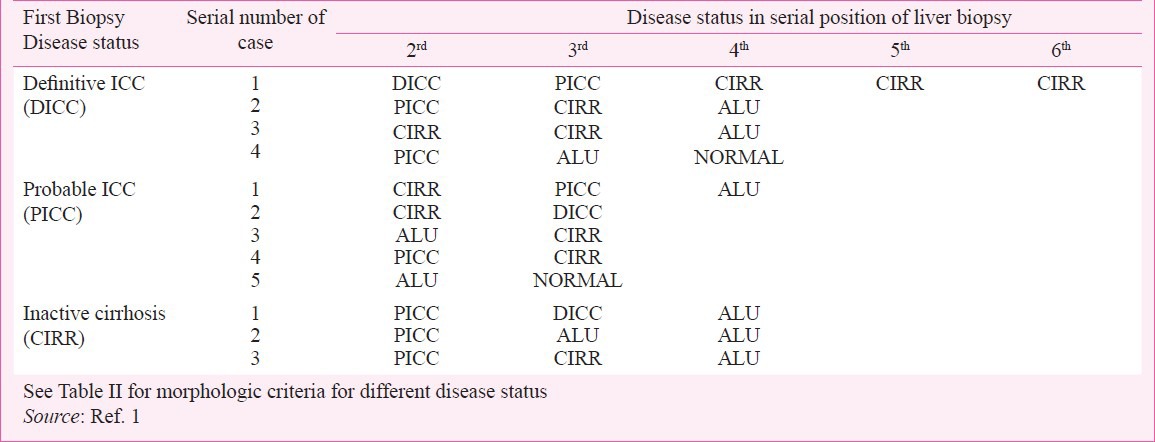
An important objective of the ICMR study1 was to identify the clinicopathologic features of early stage of ICC and explore the natural history of the disease. These were achieved by critically analyzing follow up clinical data and histologic features in livers of all study subjects with one or more serial biopsies. No copper chelation therapy was given to any child including those with histologically confirmed diagnosis of ICC. Table II shows the essential histologic features on the basis of which each liver biopsy in this study was assignedone of the following histologic categories: DICC, PICC, CIRR including some advanced chronic hepatitis, ALU and NORMAL. Almost all cases of DICC (92%) had mostly moderate to marked copper deposits, while less than 25 per cent cases of PICC had mild amounts of copper and less than 10 per cent of CIRR showed only small copper deposit. ALU and NORMAL livers had no copper deposits. Among cases with one to four follow up serial biopsies the first biopsy diagnosis was DICC in 24, PICC in 29 and CIRR in 30 (Table II). After detailed evaluation of morphologic features in serial biopsies and follow up clinical and mortality data obtained from these 83 cases it was inferred that the categories designated as PICC, DICC and CIRR represented early phase with mild injury, established phase with florid injury and quiescent phase with partly compensated injury respectively. In five children in whom the first biopsy had features of Probable ICC with only small amounts of copper deposit the second biopsy taken three to ten months later showed progression to Definitive ICC with much enhanced copper deposit. In another child this transition occurred through an intermediate phase of cryptogenic cirrhosis in the second biopsy (Tables II & III), the first two biopsies showing no copper deposit while the third biopsy with Definitive ICC had only small amount of copper. Similarly 2 of 30 cases having a status of CIRR in the first biopsy with very little or no copper progressed to DICC in the 3rd biopsy at 12 months follow up (Table III). Initial scanty copper deposit or complete absence of it in the early and quiescent phase of ICC and excess of it subsequently in the advanced phase of disease indicate that copper deposit itself does not trigger the hepaocytic injury in ICC but instead is associated with established damage that manifests later. This corroborates our earlier observations from studies on asymptomatic siblings of children with ICC17,19. The ICMR study failed to support incriminating hepatic copper deposit as the cause of liver injury1,2.
Table II.
ICMR study on ICC: Natural course of disease from follow up data on mortality and morphologic disease status in serial liver biopsies
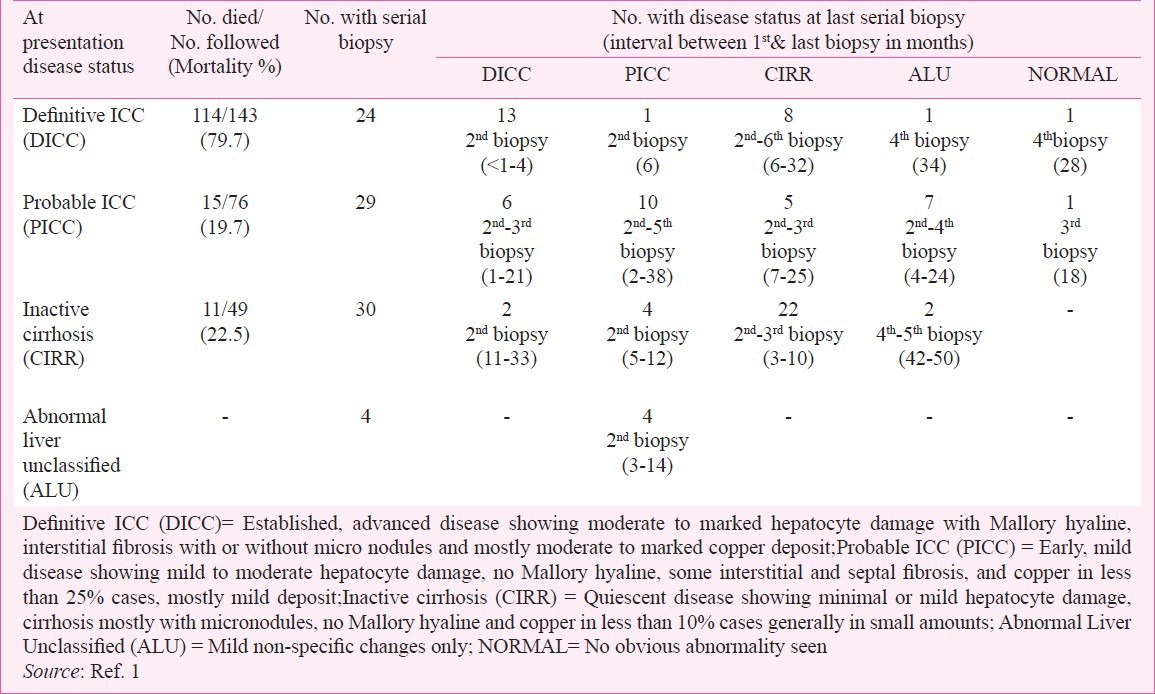
Table III.
Cases with more than one change in morphologic disease status in serial liver biopsies
The beneficial effect of chelation and removal of hepatic copper by D-penicillamine therapy in some cases of ICC reported by investigators from western India21,38 and in two cases of ICC-like disease outside India39 has been cited as one evidence favouring the causal role of copper in initiating the liver injury in this disorder3,21,31,38. This argument is however, untenable in view of some inherent limitations in the copper chelation studies21,38 and of findings on the natural evolution of ICC in the ICMR study1,2. The D-penicillamine therapy studies lacked adequate controls to asses the natural course of the disease in them, and compared the response and survival in cases with milder form of ICC with those in cases with advanced disease which mostly end fatally. Evaluation of serial biopsies and follow up information in the ICMR study where no copper chelation therapy was given has for the first time provided knowledge on the natural course of ICC. Cases diagnosed as DICC with morphologic features of advanced disease had a high mortality (79.7%) in contrast to the much lower figures for cases of PICC (19.7%) and of inactive cirrhosis (7.7%) with morphologic features of mild, early disease and quiescent disease respectively (Table II). Interestingly, within six to 32 months of detection a little less than half of the DICC cases down graded, mostly to quiescent CIRR or to the mildform of disease, PICC, and in some cases even to histologically normal or minimal, non-specific lesion. Similarly, about 45 per cent of PICC at initial encounter down graded mostly to the quiescent or non-specific form and even to normal within four to 25 months. Conversely, in variable proportions of initially PICC and CIRR the disease worsened, progressing to DICC. Thus the so-called improvement seen in ‘pre-icteric cases’ of ICC after D-penicillamine therapy21,38 very likely represents a natural course of the disease unrelated to the effect of copper chelation. In a report on hepatic pathomorphologic features in 12 German children with ICC-like disease Muller-Hocker and colleagues27 described good correlation of the severity of lesions with clinical outcome but none with the degree of hepatic copper overload. Cases with hepatic lesions of florid ICC succumbed to the disease while in all survivors the liver morphology was that of inactive micronodular cirrhosis. A recent review on the spectrum of hepatic morphology in Wilson's disease, the only established copper toxicity liver disorder, concluded steatosis and glycogenated nuclei to be the most important and consistent feature40, both of which are conspicuously absent in ICC.
The inheritance factor in ICC and ICC-like disease
An inherited predisposition to ICC has long been suspected because of random occurrence of the disease among siblings and of death due to liver disease in second line family members. Designing proper studies to verify the nature of familial susceptibility, however, had to wait for availability of morphologic criteria for unequivocal diagnosis of index cases of ICC in the early 1970s8,11. In a study from northern India, analysis of pedigree charts of 20 families with an index case of ICC and of 70 families of age matched controls Kalra and colleagues18 found no evidence of autosomal recessive, partial sex linkage or double recessive inheritance and suggested that the familial predisposition to development of ICC is most likely based on a multifactorial inheritance. Morphologic studies on liver biopsies from asymptomatic siblings of children with ICC also revealed some abnormality in handling of copper by hepatocytes resulting in excess deposit of the metal as compared to controls. This was a temporary event that was not accompanied by any associated cellular injury and corrected itself in time and was considered to be the genetic predisposition on which some other agent acts to initiate the disease17,19. For ICC-like disease in non-Indian origin children from different countries, till 1996 identical disease in siblings has been documented in 4 of 14 reports and additional family member involvement in two of these four reports3. In the four reports from then till date sibling involvement has been recorded in one but other family member involvement in none (Table IV). Parental consanguinity was recorded only in one of total 18 reports. The main support for a genetic susceptibility to this disease has come from the study of the endemic Tyrolean infantile cirrhosis in which pedigree studies on families of 38 affected children and segregation analysis was strongly suggestive of an autosomal recessive inheritance3,23 (Table IV).
Table IV.
Summary of findings related to investigation on inheritance factor in studieson ICC and ICC-like disease
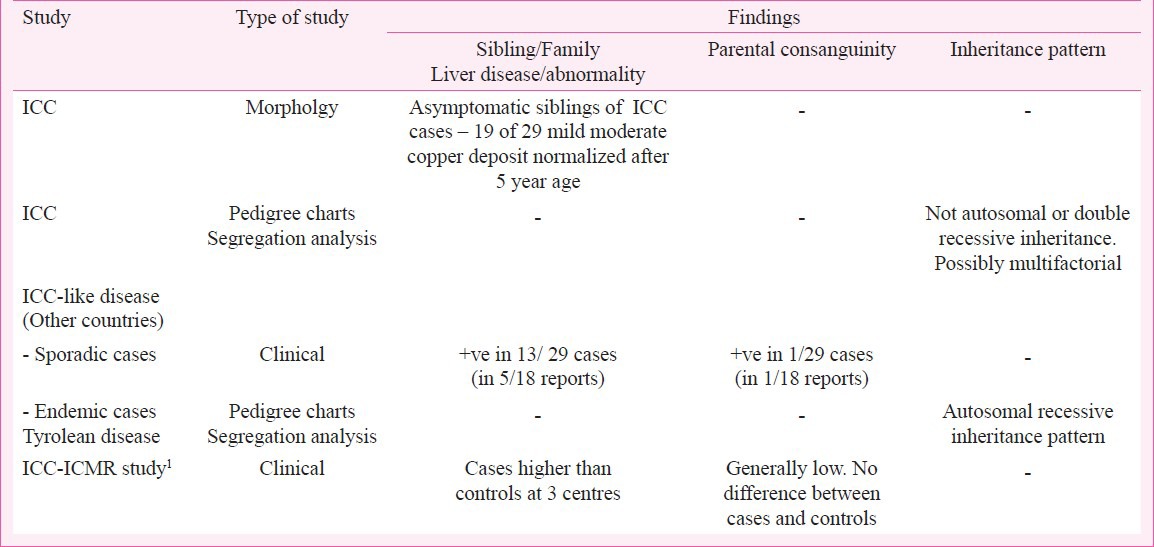
In the ICMR study1, at three of the six centres affliction of siblings and family members was significantly higher in ICC cases than in controls though parental consanguinity was no different between the two groups at any centre (Table IV). Regarding parental consanguinity increasing the occurrence of genetically inherited diseases and disease susceptibility, the ICMR study documented an interesting observation. In conformity with previous notion, this study recorded significantly less Muslim childrenin the DICC group as compared to in the control group (pooled data - 2.6 vs.7.5%, P<0.01) even though it is well known that parental consanguinity is high among Muslims who are genetically otherwise identical with Hindus1. This difference in occurrence of ICC was particularly prominent at the study centre at Indore in Central India, which has a large Muslim population (1.8 vs. 17.7%). ICC in India and ICC-like disease reported from other countries have identical clinicopathologic features which are in several respects different from those of Wilson's disease, the well recognized genetically inherited copper overload liver disease of childhood. Because of familial occurrence and hepatic copper overload in the former two diseases, a genetic aberration similar to what is seen in Wilson's disease has been considered to be the underlying cause in them. However, initial association and haplotype sharing studies on parents of children dying of the ICC-like Tyrolean cirrhosis showed that this disease was not an allelic variant of Wilson's disease41. More recent sequencing studies on the human copper metabolism gene on chromosome 2p 13-p 16 orthologous to the MURR1 gene of Bedlington terriers, the animal model of Wilson's disease, did not reveal any gene mutation in patients of ICC and ICC-like disease42. Thus, any mutation of the gene associated with copper metabolism has not as yet been identified in the ICC and ICC-like non-Wilsonian copper overload liver disease of childhood. A special breed of a rare species Ronaldsay lamb that develops hepatic injury with copper overload when given excessive copper in diet, has been suggested to be a likely model for ICC and ICC-like disease43. There has, however, been no confirmation of this possibility by gene analysis studies. In summary, some undefined inheritance manifesting as familial and sibling susceptibility very likely predisposes infants and children to occurrence of both ICC and ICC like disease. The nature of the precipitating cause continues to be elusive but exogenous copper is almost certainly not a candidate for it.
New insights into clinical and pathologic presentations
The ICMR study, by virtue of obtaining data on a large number of homogenous cases collected on the basis of standardized criteria that successfully avoided most of personal investigator biases, have been able to yield several findings on different aspects of ICC that were largely unknown and unrecognized. The important ones among these are highlighted below.
Age of occurrence: It has been generally believed that ICC and identical conditions clinically manifest and present exclusively during infancy and early childhood. In fact, because of its morbidity and mortality predominantly in infancy the disease was variously named as ‘Infantile biliary cirrhosis’, ‘Infantile cirrhosis’ and ‘Endemic Tyrolean infantile cirrhosis’. As years passed, it was realized that the disease is also shared by young children up to about 3 yr age and the name ‘Childhood disease’ got universally accepted. Most physicians, paediatricians and pathologists came to believe that ICC was restricted to infants and young children. Even though this was largely borne out in the ICMR study where 88.6 per cent of 227 DICC cases were of 6 months to 3 years age, older children were also affected and at the Madras (Chennia) centre 12 of the 14 children with DICC were above the age of 4 yr, the oldest being 11 years. This regional variation in the age incidence of ICC was striking particularly when one considers the fact that in a series of 445 cases reported from the same area decades earlier all except one child were 1 to 3 yr old44. This interesting phenomenon of a shift in the upper age limit in ICC has been confirmed in a recent report from a neighbouring area in south India where five biopsy proven cases encountered during the last decade ranged in age between 1 ½ and 12 years, three below 2 yr, and two aged 6 and 12 yr36. Anearlier report from Vellore in the same area which had been endemic for ICC documented cases of what the authors named as ‘Atypical copper cirrhosis’ in nine older children 4 to 15 yr age45 which actually represent cases of true ICC1,36. In report on ICC-like cases from other countries seven of 28 were older children of 6 to 10 yr age3. Another interesting feature was the longer survival in DICC ensuing at older age, 10 of the 12 children at the Madras centre having survived the disease at the end of the 4 year ICMR study period1.
Hepatic morphology for identification of disease: The unique histologic hallmark of ICC that makes this condition stand out from the rest of paediatric or even most adult liver diseases8,11 have stood the test of time. These also characterize all cases of ICC-like disease reported from other countries, irrespective of the variety of names given to it. The ICMR study1 explored not only the natural history of disease to and from this morphologic stage but also delineated the clinical and pathologic features of early stage disease and determined if ICC at presentation can be identified on features of hepatic morphology different from the one already known. Serial follow up biopsies have provided valuable information in clarifying these issues. The important impact of these observations is that natural up and down changes in hepatic morphology occurs in ICC and that this can result in catching ICC at first encounter with histologic picture different from the conventional one, be it of the milder type or the inactive cirrhosis type.
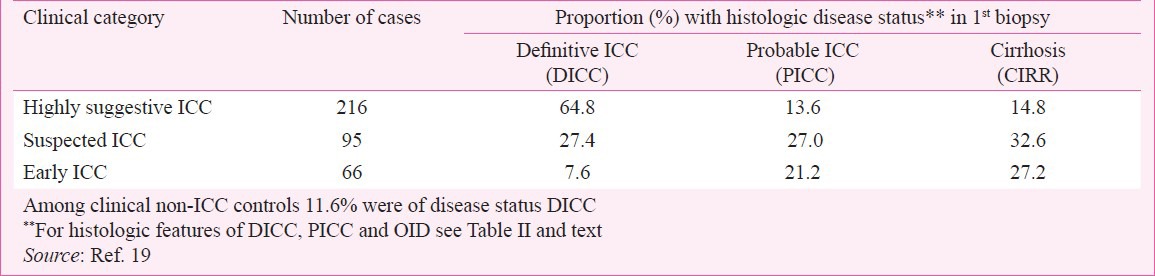
Variations in clinical features at first presentation: For homogeneity in primary subject input data the paediatricians in the ICMR multicentre study, all of whom had long experience on ICC had to decide on criteria for different categories of cases and controls to be strictly followed at each centre. The agreed upon criteria for the 3 categories of ICC cases were as follows:(i) Highly suspected ICC - Hard liver along with any three from among clinical manifestation of hepatocellular dysfunction andportal hypertension, and sibling/family member death due to liver disease; (ii) Suspected ICC - Firm liver with palpable spleen, distension of abdomen, ascites and/or edema, sibling history present or not; (iii) Early ICC – Firm liver with sibling/family history of liver disease and symptoms of poor appetite, sticky stools, and bowel movement abnormality46,47. The adequacy of criteria for each category in making correct clinical identification of ICC was determined by matching it with the histologic status in the first biopsy taking DICC as the gold standard of ICC and also PICC and CIRR which were found to represent other phases of the disease. These comparisons obtained in the ICMR study are shown in Table V. DICC was detected inabout two-thirds, one fourth and less than one tenth of clinical category ‘Highly suggestive ICC’, ‘Suspected ICC’ and ‘Early ICC’, respectively. On the other hand, more of PICC and CIRR were encountered in the latter two than in the former clinical category (Table V). Even among non-ICC controls, 11 per cent biopsies were labelled as DICC. These findings indicate that firstly, while we seem to have no foolproof set of clinical presenting features that will identify most cases of ICC with the diagnostic histology of established disease, a majority of them and a good share of probable ones in other phases of ICC can be detected on the clinical criteria used in the ICMR study1. Secondly, cases of true ICC can present with clinical features as yet not considered typical of the disease and that supplementation by a liver biopsy only can confirm the diagnosis of established or other phase of ICC.
Table V.
ICMR-ICC study - At presentation histologic disease status in different clinical categories of cases and non-ICC controls*
Future prospects and approaches
The foregoing account of all information on ICC occurring in India and ICC-like disease occurring in other countries from reports available till date and their critical analysis provide reasonable clarification of several generally held notions and add new facts on many aspects of this childhood disease. The notion that excessive copper intake in infancy through food or water contaminated by the metal from copper yielding utensilsor copper tubing seems ill founded. The evidence on which this concept was based was inadequate and largely circumstantial. Epdemiologic, clinical and morphological data from large, well controlled studies have failed to incriminate exogenous copper in causing a toxic injury or the copper overloading of liver in ICC. The argument that the disease has been prevented or eradicated on stopping domestic use of brass or copper vessels23,48 is untenable as this association happens to be coincidental and not cause-effect related. Deposition of copper in the liver, far in excess of what is seen in other hepatic disorders including those with cholestasis, particularly in the established phase of ICC and like diseases is undoubtedly a consistent and characteristic finding. The explanation of this is currently uncertain though it is probable that the increased accumulation of the compound results from advancing injury of hepatocytes that have a pre-existing defect in handling copper metabolism. With exogenous copper excluded as a primary cause, the uncertainty of aetiopathogensis of the disease looms large2. Occurrence of disease in siblings and other family members has often been recorded both in ICC and ICC-like diseases which is very likely related to some inherited susceptibility rather than to a common environmental agent. The exact pattern of inheritance however, remains undefined because limited family tree studies have expressed divergent views18,23. The possibility of some exogenous hepatotoxic agent initiating and propagating the disease in individuals with a temporary defect in copper homeostasis during infancy and early childhood was suggested earlier on the basis of morphologic studies in patients and their asymptomatic siblings17,19. The fact that in ICC with established and active diseasethe injured liver cells consistently show Mallory hyaline, identical to what is seen in hepatoxic injury by alcohol and other agents was considered as evidence of a probable toxic injury of the liver in ICC7,8,17. In all cases of ICC-like disease reported from outside India also this morphogic abnormality has been observed49. A review on occurrence of Mallory hyaline in liver disease reported the highest incidence in ICC, more than in alcoholic hepatitis and alcoholic cirrhosis and about three times that in Wilson's disease50. Also, immunohistochemical analysis of Mallory body in livers of ICC and ICC-like disease has shown that like in other toxic injuries these structures are products of toxic oxidative injury to liver cells49. Thus, while we are presently equipped with knowledge to suspect and recognize ICC and ICC-like disease in the different forms it presents, there is almost no light at the end of the tunnel on its aetiopathogenesis.
There has been a sharp decline in reports and research on ICC in the last three decades apparently because of a significant drop in encountering cases in clinical practice and pathologic material45,51. This can be ascribed to either one or more likely to both of two issues namely, a true decrease in incidence of disease and to our inability in recognizing the changing patterns and differing faces of disease presentation we are not acquainted with. It would be prudent to discuss the second issue first since it is supported by some tangible evidence. It has been a general understanding among paediatricians and pathologists that ICC is a disease of infants and very young children only, and that a diagnosis of the disease must rest on finding the ‘classical’ liver histology of established disease. This notion led Ramakrishna and colleagues to exclude considering ICC for the copper overload liver disease in older children45. A report from Andhra Pradesh36 shows how a clinical diagnosis of ICC was not considered in five children mainly because there was no source of exposure to excess dietary copper and in addition two of them were 6 and 11 year old, a diagnosis of ICC having been made in each case on liver biopsy only. This report also exemplifies how detection of ICC can be missed even in specialist health centres if a clinical suspicion is not aroused and a liver biopsy is not resorted to. The other reason for the decline in encountering cases of ICC is an actual reduction in its occurrence, an issue that is important but on which, except for personal views of concerned professionals around the country, no measurable evidence is available. It may be mentioned here that when setting up centres for the ICMR study was being planned, Calcutta (now Kolkata) from where the first report of large numbers of ICC originated was not chosen because for years no reports on the disease had come from there1. Even in Madras (now Chennai) ICC cases (then called Infantile cirrhosis) reported in multiples of hundred in the 1930s through 1950s6,44, the numbers had declined in subsequent decades. Decline in cases has also been observed in other parts of the country. However, an upward shift in the age of ICC afflicted children observed with advancing time, as emphasized earlier, may have partly camouflaged an undetermined proportion of these cases from being detected. Be that as it may, the probability of some decline in the incidence of ICC cannot be ignored. As for ICC-like disease occurring outside India its occurrence has been so infrequent that time trend changes are not possible. Only the disease in Tyrol area of Austria which had been endemic there seems to have disappeared since 1974 allegedly due to replacement of copper utensils by modern industrial ones23. In this context, it can be argued that examples of diseases endemic to some areas undergoing natural decline, extinction or eradication with time do exist. Banti's disease once endemic in Italy and Southern Europeand its counterpart non-cirrhotic portal fibrosis (NCPF) once endemic in India and Idiopathic portal hypertension endemic in Japan are less frequently encountered now52. Even though the aetiopathogenesis of these diseases have never been established, a toxic injury of the portal venous system has been suggested53. Some infectious and other environmental toxin induced diseases have also shown decline with time. Along with economic development and socio-cultural changes, reduced amounts or elimination of available environmental toxins and infectious agents will result in lowering of incidence in diseases caused by them. A similar explanation may well be applicable to ICC in which an as yet unidentified environmental toxin, other than copper, has been suggested to play a prime role in aetiopathogenesis.
Indian childhood cirrhosis, thus christened for being a disease endemic in and unique to India with specific clinical and pathologic features has later been documented in children of non-Indian origin in other countries. Now that many new facets of the disease have been unveiled and several notions, some supported by studies on ICC-like disease from outside India, have been dispelled, it is time to consider approaches to further exploration on the still elusive aspects of the disease. To identify the disease in clinical practice it is necessary to leave the narrow alley of our earlier notions restricted by limited age range, sibling/family history of disease, prior exposure to exogenous copper, the ‘classical’ hepatic morphology and the like, and widen the area of scanning cases of paediatric liver disease. Fortunately, unlike in adults, most hepatopathies in children manifest in infancy and very early childhood while older children are generally spared. With the availability of specific serologic, biochemical and imaging techniques the aetiology of a majority of liver diseases can now be identified. Thus in all so-called ‘cryptogenic’ liver disease in a child of any age a diagnosis of ICC must be considered and a liver biopsy done to evaluate the histologic abnormalities. In the latter, the entire spectrum of changes manifesting at different phases of ICC should be looked for including those with minimal non-specific changes. Such overall scrutiny is likely toreveal cases of ICC as yet unrecognized in our population and a proper record and follow up on these cases will provide information on incidence and prevalence of the disease as well help studies on determining its currently uncertain aetiopathogenesis.
References
- 1.Sriramachari S, editor. New Delhi: Indian Council of Medical Research; 2006. Indian Childhood Cirrhosis (ICC) - A Multicentre National Collaborative Study. [Google Scholar]
- 2.Sriramachari S, Nayak NC. Indian childhood cirrhosis: several dilemmas resolved. Indian J Med Res. 2008;128:93–6. [PubMed] [Google Scholar]
- 3.Muller T, Muller W, Feichtinger H. Idiopathic copper toxicosis. Am J Clin Nutr. 1998;67(Suppl 5):1082S–6S. doi: 10.1093/ajcn/67.5.1082S. [DOI] [PubMed] [Google Scholar]
- 4.Sen B. Infantile cirrhosis. Indian Med Gaz. 1887;22:338–42. [Google Scholar]
- 5.Infantile cirrhosis of the liver in India (Synonym-Infantile biliary cirrhosis) Indian J Med Res. 1955;43:723–47. No authors listed. [PubMed] [Google Scholar]
- 6.Achar ST, Raju VB, Sriramachari S. Indian childhood cirrhosis. J Pediatr. 1960;57:744–58. doi: 10.1016/s0022-3476(60)80170-9. [DOI] [PubMed] [Google Scholar]
- 7.Nayak NC, Sagreiya K, Ramalingaswami V. Indian childhood cirrhosis. The nature and significance of cytoplasmic hyaline of hepatocytes. Arch Pathol. 1969;88:631–7. [PubMed] [Google Scholar]
- 8.Nayak NC, Visalakshi S, Singh M, Chawla V, Chandra RK, Ramalingaswami V. Indian childhood cirrhosis-a re-evaluation of its pathomorphologic features and their significance in the light of clinical data and natural history of the disease. Indian J Med Res. 1972;60:246–59. [PubMed] [Google Scholar]
- 9.Anthony PP, Ishak KG, Nayak NC, Poulsen HE, Scheuer PJ, Sobin LH. The morphology of cirrhosis. Recommendations on definition, nomenclature, and classification by a working group sponsored by the World Health Organization. J Clin Pathol. 1978;31:395–414. doi: 10.1136/jcp.31.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nayak NC. Indian childhood cirrhosis. In: MacSween, RNM Anthony PP, Scheuer PJ., editors. Pathology of the liver. London: Churchill Livingstone; 1979. pp. 268–9. [Google Scholar]
- 11.Nayak NC, Ramalingaswami V. Indian childhood cirrhosis. Clin Gastroenterol. 1975;4:333–49. [PubMed] [Google Scholar]
- 12.Nayak NC, Ramalingaswami V, Roy S, Sachdeva R. Hepatitis-B virus and Indian childhood cirrhosis. Lancet. 1975;2:109–11. doi: 10.1016/s0140-6736(75)90008-2. [DOI] [PubMed] [Google Scholar]
- 13.Portmann B, Tanner MS, Mowat AP, Williams R. Orcein-positive liver deposits in Indian childhood cirrhosis. Lancet. 1978;1:1338–40. doi: 10.1016/s0140-6736(78)92407-8. [DOI] [PubMed] [Google Scholar]
- 14.Popper H, Goldfischer S, Sternlieb I, Nayak NC, Madhavan TV. Cytoplasmic copper and its toxic effects. Studies in Indian childhood cirrhosis. Lancet. 1979;1:1205–8. doi: 10.1016/s0140-6736(79)91894-4. [DOI] [PubMed] [Google Scholar]
- 15.Tanner MS, Portmann B, Mowat AP, William R, Pandit AN, Mills CF, et al. Increased hepatic copper concentration in Indian childhood cirrhosis. Lancet. 1979;1:1203–5. doi: 10.1016/s0140-6736(79)91893-2. [DOI] [PubMed] [Google Scholar]
- 16.Datta DV, Narang AP, Arya P, Sahni MM, Banerjee CK, Walia, et al. Indian chilhood cirrhosis. Lancet. 1979;II:641. [PubMed] [Google Scholar]
- 17.Marwaha N, Nayak NC, Roy S, Kalra V, Ghai OP. The role of excess hepatic copper in the evolution of Indian childhood cirrhosis. Indian J Med Res. 1981;73:395–403. [PubMed] [Google Scholar]
- 18.Kalra V, Roy S, Ghai OP, Jain JP. Indian childhood cirrhosis - a heritable disease. Hum Hered. 1982;32:170–5. doi: 10.1159/000153285. [DOI] [PubMed] [Google Scholar]
- 19.Nayak NC, Marwaha N, Kalra V, Roy S, Ghai OP. The liver in siblings of patients with Indian childhood cirrhosis: a light and electron microscopic study. Gut. 1981;22:295–300. doi: 10.1136/gut.22.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanner MS, Kantarjian AH, Bhave SA, Pandit AN. Early introduction of copper-contaminated animal milk feeds as a possible cause of Indian childhood cirrhosis. Lancet. 1983;2:992–5. doi: 10.1016/s0140-6736(83)90980-7. [DOI] [PubMed] [Google Scholar]
- 21.Tanner MS, Bhave SA, Pradhan AM, Pandit AN. Clinical trials of penicillamine in Indian childhood cirrhosis. Arch Dis Child. 1987;62:1118–24. doi: 10.1136/adc.62.11.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sethi S, Grover S, Khodaskar MB. Role of copper in Indian childhood cirrhosis. Ann Trop Paediatr. 1993;13:3–5. doi: 10.1080/02724936.1993.11747618. [DOI] [PubMed] [Google Scholar]
- 23.Muller T, Feichtinger H, Berger H, Muller W. Endemic Tyrolean infantile cirrhosis: an ecogenetic disorder. Lancet. 1996;347:877–80. doi: 10.1016/s0140-6736(96)91351-3. [DOI] [PubMed] [Google Scholar]
- 24.Abiodun PO, Albarki AA, Dewan M, Annobil SH. Indian childhood-like cirrhosis in three Saudi Arabian siblings. Ann Trop Paediatr. 2000;20:61–6. doi: 10.1080/02724930092093. [DOI] [PubMed] [Google Scholar]
- 25.Baker A, Gormally S, Saxena R, Baldwin D, Drunam B, Bonham J, et al. Copper-associated liver disease in childhood. J Hepatol. 1995;23:538–43. doi: 10.1016/0168-8278(95)80059-x. [DOI] [PubMed] [Google Scholar]
- 26.Dieter HH, Schimmelpfennig W, Meyer E, Tabert M. Early childhood cirrhoses (ECC) in Germany between 1982 and 1994 with special consideration of copper etiology. Eur J Med Res. 1999;4:233–42. [PubMed] [Google Scholar]
- 27.Muller-Hocker J, Summer KH, Schramel P, Rodeck B. Different pathomorphologic patterns in exogenic infantile copper intoxication of the liver. Pathol Res Pract. 1998;194 doi: 10.1016/s0344-0338(98)80027-7. HAHN MD. [DOI] [PubMed] [Google Scholar]
- 28.Nagasaka H, Kobayashi K, Yorifuji T, Kage M, Kimura A, Takayangi M, et al. Indian childhood cirrhosis-like disease in a Japanese boy undergoing liver transplantation. J Pediatr Gastroenterol Nutr. 1999;29:598–600. doi: 10.1097/00005176-199911000-00024. [DOI] [PubMed] [Google Scholar]
- 29.Trollmann R, Neureiter D, Lang T, Dorr HG, Behrens R. Late manifestation of Indian childhood cirrhosis in a 3-year-old German girl. Eur J Pediatr. 1999;158:375–8. doi: 10.1007/s004310051095. [DOI] [PubMed] [Google Scholar]
- 30.Walker NI. Copper toxicosis in an Australian child. Eur J Med Res. 1999;4:249–51. [PubMed] [Google Scholar]
- 31.Pankit AN, Bhave SA. Copper metabolic defects and liver disease: environmental aspects. J Gastroenterol Hepatol. 2002;17(Suppl 3):S403–7. doi: 10.1046/j.1440-1746.17.s3.35.x. [DOI] [PubMed] [Google Scholar]
- 32.Tanner MS. Role of copper in Indian childhood cirrhosis. Am J ClinNutr. 1998;67(Suppl 5):1074S–81S. doi: 10.1093/ajcn/67.5.1074S. [DOI] [PubMed] [Google Scholar]
- 33.Dassel de Vergara J, Zietz B, Schneider HB, Dunkelberg H. Determination of the extent of excessive copper concentrations in the tap-water of households with copper pipes and an assessment of possible health hazards for infants. Eur J Med Res. 1999;4:475–82. [PubMed] [Google Scholar]
- 34.Scheinberg IH, Sternlieb I. Is non-Indian childhood cirrhosis caused by excess dietary copper? Lancet. 1994;344:1002–4. doi: 10.1016/s0140-6736(94)91649-7. [DOI] [PubMed] [Google Scholar]
- 35.Zietz BP, de Vergara JD, Dunkelberg H. Copper concentrations in tap water and possible effects on infant's health-results of a study in Lower Saxony, Germany. Environ Res. 2003;92:129–38. doi: 10.1016/s0013-9351(03)00037-9. [DOI] [PubMed] [Google Scholar]
- 36.Patra S, Vij M, Kancherala R, Samal SC. Is Indian childhood cirrhosis is an extinct disease now? - An observational study. Indian J Pediatr. 2012 doi: 10.1007/s12098-012-0935-1. In press. [DOI] [PubMed] [Google Scholar]
- 37.Nayak NC, Roy S. Morphological types of hepatocellular hyalin in Indian childhood cirrhosis: an ultrastructural study. Gut. 1976;17:791–6. doi: 10.1136/gut.17.10.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bavdekar AR, Bhave SA, Pradhan AM, Pandit AN, Tanner MS. Long term survival in Indian childhood cirrhosis treated with D-penicillamine. Arch Dis Child. 1996;74:32–5. doi: 10.1136/adc.74.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horslen SP, Tanner MS, Lyon TD, Fell GS, Lowry MF. Copper associated childhood cirrhosis. Gut. 1994;35:1497–500. doi: 10.1136/gut.35.10.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johncilla M, Mitchell KA. Pathology of the liver in copper overload. Semin Liver Dis. 2011;31:239–44. doi: 10.1055/s-0031-1286055. [DOI] [PubMed] [Google Scholar]
- 41.Wijmenga C, Muller T, Murli IS, Brunt T, Feichtinger H, Schonitzer D, et al. Endemic Tyrolean infantile cirrhosis is not an allelic variant of Wilson's disease. Eur J Hum Genet. 1998;6:624–8. doi: 10.1038/sj.ejhg.5200235. [DOI] [PubMed] [Google Scholar]
- 42.Muller T, van de Sluis B, Zhernakova A, van Binbergen E, Janecke AR, Bavdekar A, et al. The canine copper toxicosis gene MURR1 does not cause non-Wilsonian hepatic copper toxicosis. J Hepatol. 2003;38:164–8. doi: 10.1016/s0168-8278(02)00356-2. [DOI] [PubMed] [Google Scholar]
- 43.Haywood S, Muller T, Mackenzie AM, Muller W, Tanner MS, Heinz-Erian P, et al. Copper-induced hepatotoxicosis with hepatic stellate cell activation and severe fibrosis in North Ronaldsay lambs: a model for non-Wilsonian hepatic copper toxicosis of infants. J Comp Pathol. 2004;130:266–77. doi: 10.1016/j.jcpa.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Narayanamurthi K, Tirumurti TS. The study of the epidemiology of infantile cirrhosis of the liver. Indian J Pediatr. 1939;6:85–92. [Google Scholar]
- 45.Ramakrishna B, Date A, Kirubakaran C, Raghupathy P. Atypical copper cirrhosis in Indian children. Ann Trop Paediatr. 1995;15:237–42. doi: 10.1080/02724936.1995.11747778. [DOI] [PubMed] [Google Scholar]
- 46.Parekh SR, Patel BD. Epidemiologic survey of Indian childhood cirrhosis. Indian Pediatr. 1972;9:431–9. [PubMed] [Google Scholar]
- 47.Patel BD, Parekh SR, Chitale AR. Histopathological evolution of Indian childhood cirrhosis with emphasis on criteria of early diagnosis. Indian Pediatr. 1974;11:19–28. [PubMed] [Google Scholar]
- 48.Bhave SA, Pandit AN, Singh S, Walia BN, Tanner MS. The prevention of Indian childhood cirrhosis. Ann Trop Paediatr. 1992;12:23–30. doi: 10.1080/02724936.1992.11747542. [DOI] [PubMed] [Google Scholar]
- 49.Muller T, Langner C, Fuchsbichler A, Heinz-Erian P, Ellemunter H, Schlenck B, et al. Immunohistochemical analysis of Mallory bodies in Wilsonian and non-Wilsonian hepatic copper toxicosis. Hepatology. 2004;39:963–9. doi: 10.1002/hep.20108. [DOI] [PubMed] [Google Scholar]
- 50.Jensen K, Gluud C. The Mallory body: morphological, clinical and experimental studies (Part 1 of a literature survey) Hepatology. 1994;20:1061–77. doi: 10.1002/hep.1840200440. [DOI] [PubMed] [Google Scholar]
- 51.Pediatric Liver Study Group of India. Metabolic liver diseases in childhood: Indian scenario. Indian J Pediatr. 1999;66(Suppl 1):S97–103. [PubMed] [Google Scholar]
- 52.Sarin SK, Kumar A, Chawla YK, Baijal SS, Dhiman RK, Jafri W, et al. Members of the APASL Working Party on Portal Hypertension. Noncirrhotic portal fibrosis/idiopathic portal hypertension: APASL recommendations for diagnosis and treatment. HepatolInt. 2007;1:398–413. doi: 10.1007/s12072-007-9010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nayak N. Phlebothrombotic nature of non-cirrhotic portal fibrosis. In: Okuda K, editor. International symposium on Idiopathic portal hypertension; 1983. Tokyo: University of Tokyo Press; 1983. pp. 292–302. [Google Scholar]


