Abstract
Background & objectives:
Various materials have been used as scaffolds to suit different demands in tissue engineering. One of the most important criteria is that the scaffold must be biocompatible. This study was carried out to investigate the potential of HA or TCP/HA scaffold seeded with osteogenic induced sheep marrow cells (SMCs) for bone tissue engineering.
Methods:
HA-SMC and TCP/HA-SMC constructs were induced in the osteogenic medium for three weeks prior to implantation in nude mice. The HA-SMC and TCP/HA-SMC constructs were implanted subcutaneously on the dorsum of nude mice on each side of the midline. These constructs were harvested after 8 wk of implantation. Constructs before and after implantation were analyzed through histological staining, scanning electron microscope (SEM) and gene expression analysis.
Results:
The HA-SMC constructs demonstrated minimal bone formation. TCP/HA-SMC construct showed bone formation eight weeks after implantation. The bone formation started on the surface of the ceramic and proceeded to the centre of the pores. H&E and Alizarin Red staining demonstrated new bone tissue. Gene expression of collagen type 1 increased significantly for both constructs, but more superior for TCP/HA-SMC. SEM results showed the formation of thick collagen fibers encapsulating TCP/HA-SMC more than HA-SMC. Cells attached to both constructs surface proliferated and secreted collagen fibers.
Interpretation & conclusions:
The findings suggest that TCP/HA-SMC constructs with better osteogenic potential compared to HA-SMC constructs can be a potential candidate for the formation of tissue engineered bone.
Keywords: Bone, ceramics phosphate, fibrin, scaffold, tissue engineering
Current therapeutic approaches in repairing critical size bone defects such as those caused by tumour resection or trauma include implantation of autografts, allografts, metal devices, porous glasses and ceramics. However, each of these techniques has their own disadvantages and limitations. Tissue engineering emerged as a solution to challenge these limitations with the primary objective to regenerate structural and functional tissue, using living cells in combination with scaffold1,2. Tissue engineering revolves around an important concept, i.e. to mimic the structure and physiological function of the tissue concern. Three key elements in recreating the living tissue are progenitor cells of the tissue concerned, biomaterial as cell carrier and tissue scaffolding, and growth factors. In bone tissue engineering, the best osteoprogenitor sources are a debatable issue. Angela et al3, have shown that periosteum provides a rich source of osteoprogenitor cells that readily proliferate and lay down bone. However, bone marrow derived mesenchymal stem cell is still the method of choice due to limited availability of periosteal tissue.
The ability of particular materials to serve as a scaffold on which bone cells can attach, proliferate, and mineralized is called osteoconduction4. It has been shown that a complete supply of calcium and phosphate source is crucial during the bone regeneration phase. Lacking in mineralization, the tissue formed will be void of mechanical strength. Hence calcium phosphate ceramics are gaining popularity as implants alone or as the biomaterial for tissue-engineered bone construct. These biomaterials are considered bioactive due to its active interaction with normal bone surfaces during bone regeneration, hence allowing osteointergration of the biomaterial and bone interface. This is important in bone tissue regeneration as the bone undergoes dynamic tissue remodelling during regeneration process3,4,5,6.
Calcium phosphate and its analogues, hydroxyapatite (HA), the crystalline form of calcium phosphate being the predominant form, constitute 60-70 per cent of bone tissues and provide mechanical strength to the tissue5. Both synthetic and naturally occurring HA and tricalcium phosphate (TCP) have been used for orthopaedic applications. Tricalcium phosphate is a tertiary calcium phosphate also known as bone ash [Ca3(PO4)2]. It serves as a rich source for calcium and phosphorus, which can be easily assimilated and absorbed. Beta-tricalcium phosphate is highly biocompatible and creates a resorbable interlocking network within the defect site to promote healing5,6.
We have developed a living three-dimensional (3D) bone construct that comprises living bone cells in active mineralization phase that forms bone at in vivo ectopic sites (e.g. subcutaneously in nude mice). Three import components in the tissue engineered bone construct are (i) living bone cells that can be harvested by a simple bone marrow aspiration from the iliac crest without any open surgery, (ii) sheep plasma-derived fibrin from the sheep blood to be used as an effective cell carrier and a rich source of growth factors to promote cell growth and differentiation, and (iii) a porous ceramic scaffold with constituents similar to the natural bone i.e. calcium and phosphate, which is osteoinductive and resorbes slowly over time as it is being replaced by newly formed bone. This study was undertaken to investigate the potential of HA or TCP/HA scaffold seeded with osteogenic induced sheep marrow cells (SMC) for bone tissue engineering.
Material & Methods
The commercial HA and TCP/HA (G. Surgiwear Limited Shahjahanpur, India) used in the study had the following characteristics: HA: derived from natural sources with average granule size of 1.8-3.0 mm. TCP/HA: synthetic with 80 per cent TCP and 20 per cent HA and an average granule size of 1.5-3.0 mm.
Isolation of sheep marrow cell: Six healthy sheep each weighing between 15 and 20 kg, were used. The study protocol was approved by the Universiti Kebangsaan Malaysia Animal Ethical Committee (UKMAEC) with the approval number ORTHO/2003/TAN/22-SEPTEMBER/122. Sheep marrow cells (SMC) were obtained by iliac crest aspiration. The animals were anaesthetized and under sterile conditions, a 16-gauge Jamshidi needle (Cardinal Health, Dublin, Ireland) was used to aspirate 12-15 ml of bone marrow. SMCs were collected into two 20 ml syringes, each containing 1.5 ml of heparinized (250 units/ml) saline solution. The syringes were detached and inverted several times to ensure mixing6. The suspension was centrifuged at 180 g for 5 min and the supernatant removed. The bone marrow was mixed with osteogenic medium and seeded in two 75 cm2 culture flasks (Orange Scientific, Belgium). The osteogenic medium contained high-glucose Dulbecco's modified Eagle's medium (high-glucose DMEM) (GIBCO Laboratories, USA) supplemented with 10 per cent foetal bovine serum (HyClone, Logan, UT), glutamine (1 mM), penicillin G (100 U/ml), streptomycin sulphate (100 μg/ml), 10-8 M dexamethasone (Sigma, USA), 10 mM sodium β-glycerol phosphate (Sigma) and L-ascorbic acid (50 μg/ml; Sigma). Three-fourths of the culture medium was changed after 5 days, and non adherent cells were removed along with the culture medium. The medium was then replaced twice weekly. When the SMC became nearly confluent, the cells were detached and serially sub-cultured3.
Autologus fibrin preparation: For preparation of autologous fibrin, 20-30 ml sheep blood was collected into a sterile tube containing citrate phosphate dextrose (CPD) solution (2.63% sodium citrate, 0.33% citric acid, 2.32% dextrose, and 0.25% sodium dihydrogen phosphate) with one-ninth volume of the collected blood as an anticoagulant. The blood was centrifuged at 800 g for 10 min, and the upper layer, including plasma and the buffy coat (mostly white blood cells and platelets) was transferred to another tube. To spin down the buffy coat, further centrifugation of the transferred layers was performed at 1500 g for 10 min and once again the upper layer was transferred to a new tube using 0.2 μm-syringe filter for sterility. This final volume of fibrin obtained was 10-15 ml. This fibrin was used directly or stored in -20°C until further usage2.
Bone construct preparation: After 21 days, passage 1 (P1) cell culture were rinsed twice with phosphate buffer saline solution, lifted with 2 ml of 0.2 per cent trypsin, concentrated by centrifugation at 180 g for 10 min, and diluted to 1 ml of SMC suspension (3 × 106 cells/ml) in sheep plasma for seeding into ceramic grafts. HA and TPA with HA (TCP/HA) scaffolds were first pre-wetted with sheep plasma; 5-6 × 106 SMCs were then mixed with 1 ml of sheep plasma and dropped onto pre-wetted scaffolds. Polymerization of fibrin in the plasma was initiated by adding 25 μl 250 mM calcium chloride (CaCl2). HA and TCP/HA scaffold mixed with SMC and fibrin are known as HA-SMC construct and TCP/HA-SMC construct3,6.
In vitro HA-SMC and TCP/HA-SMC induction: HA-SMC and TCP/HA-SMC constructs were immersed into osteogenic medium for osteogenic induction and differentiation for 3 wk before being harvested. Medium was charged every 2 and 3 days in the culture plate. Samples were evaluated by histology and gene expression analysis. Porous TCP/HA consisting of hydroxyapatite (20%) and tricalcium phosphate (80%) with 60-70 per cent porosity of 1,5-3.00 mm and HA with the same porosity and a mean pore size 1.8-3.0 mm was used3.
Bone construct implantation: HA-SMC and TCP/HA-SMC constructs were implanted subcutaneously on both sides of the dorsum of nude mice (n=6) age ranged 8-12 wk under general anaesthesia using 50 mg/ml ketamine, 20 mg/ml xylazil and 50 mg/ml zoletil; dosage given, 0.2 ml/kg. All mice were monitered weekly. All the insicion sites were applied with a bactroban antiseptic until these dried and healed completely. After eight weeks of implantation, SMC-ceramic constructs were harvested and cleaned for histology analysis and gene expression quantification3,4.
Manual palpation: At the time of harvest, both consturcts were palpated manually. Each motion construct was graded as solid or not solid if any motion was present. Two examiners in a blinded fashion evaluated the manual palpation assessment7.
Histological analysis: About 1.0 cm3 of the HA-SMC and TCP/HA-SMC constructs were cut for histological examination. All samples were fixed in 10 per cent phosphate buffer formalin. Samples were decalcified by immersion in 10 per cent ethylenediamine tetra acetic acid (EDTA) solution, dehydrated with a graded series of alcohol, soaked serially in xylol (Hayman England, UK), and embedded in paraffin. Sections of approximately 5 μm were then stained with hematoxylin and eosin (H&E) (Merk Darmstadt, Germany) and Alizarin (BDH, England, UK) Red (AR) (Sigma, St. Louis, MO, USA) and viewed under light microscope35.
Scanning electron microscope analysis (SEM): HA-SMC and TCP/HA-SMCs constructs were harvested after 3 wk in osteogenic induction medium and 8 wk post-implantation. Specimens were cut into the sizes of 1 cm3 slices, which were immediately transferred into small vials, immersed in primary fixation, 2.5 per cent glutaraldehyde for at least 24 h at 4°C. This was followed by three changes of 0.1M sodium cacodylate solution for 10 min each. The samples were then post-fixed with 1 per cent osmium tetraoxide for 2 h at 4°C. Next, the samples were dehydrated into a series of gradual increasing gradient of acetone (35, 50, 75, 95% for 10 min each and 3 changes in 100 per cent for 15 min). Once dehydrated, samples were transferred into specimen basket immersed in 100 per cent acetone, ready to be dried in critical point dryer (Baltec 030 CPD, Liechtenstein, Switzerland) for approximately 30 min. Samples were then mounted onto aluminum stub and sputter-coated with Au/Pd and viewed under scanning electron microscope (LEO 1455 Variable Pressure SEM, Carl Zeiss, Germany) in high pressure mode of 15 kV accelerating voltage. Samples were mounted into a stub using double-sided carbon tape, aided with stereomicroscope before sputter-coated (Polaron E5100 sputter coater, Milan, Italy) with gold-palladium. Samples were examined under scanning electron microscope (LEO 1455 Variable Pressure SEM, Carl Zeiss, Germany) in secondary electron image mode at acceleration voltage of 15kV in high-pressure mode3.
Quantitative gene expression analysis by real-time PCR: Type I collagen and osteopontin were quantitatively analyzed with real-time PCR technique3,5. The expression level of each targeted gene was normalized to glycereldehyde-3-phosphate dehydrogenase (GAPDH). Primers for sheep GAPDH (forward 5’CTGGTGCTGAGTACGTGGTG3’, reverse 5’ CGTCAGCAGAAGGTGCAGAG3’ [AF030943]), type I Collagen (forward 5’CGGCTCCTGCTCCTCTTAG3’, reverse 5’CTGTACGCAGGTGACTGGTG3’ [AF129287]) and osteopontin (5’GTCCAGATGCCACAGAGGAG3, reverse 5’GGCCTTTGGCGTGAGTTC3’ [AF152416]) were designed with Primer 3 software (http://frodo:wi.mit.edu/cgi-bin/primer3/primer3-www.cgi) and blasted with GeneBank database sequences (www.ncbi.nlm.nih.gov) to obtain primers with high specificity. The efficiency and specificity of each primer set was confirmed with standard curve (Ct value versus serial dilution of total RNA) and melting profile evaluation. Real-time PCR reaction was performed with 100 ng of total RNA, 400 nM of each primer and iScript One-Step RT-PCR kit with SYBR Green (Bio-Rad, USA) according to the manufacturer's instruction. Reactions were run using Bio-Rad iCycler with reaction profile of cDNA synthesis for 30 min at 50°C; pre-denaturation for 2 min at 94°C; PCR amplification for 38 cycles with 30 sec at 94°C, 30 sec at 60°C and 30 sec at 72°C. This series of cycles was followed by a melt curve analysis to check the reaction specificity. Expression level of each targeted gene was normalized to GAPDH and was then calculated for statistical analysis.
Statistical analysis: The data were presented as mean (±) standard error of mean (SEM). Student's t test was used to compare data between groups. Differences at 5 per cent level were considered significant.
Results
Gross examination & manual palpation: TCP/HA-SMCs were transformed into a form of bone structure with a smooth surface and hard and resistant to compression (Fig. 1A). On the other hand, there was no common structure observed in the HA-SMCs (Fig. 1B) and these were not decomposed at the end. Palpation test was applied manually on both constructs after 8 wk of implantation into nude mice. TCP/HA-SMC construct exhibited good quality of bone formation based on the hardness compared to HA-SMC construct. All six TCP/HA-SMC constructs revealed high resistant to palpation while it was missing in HA-SMC constructs.
Fig. 1.
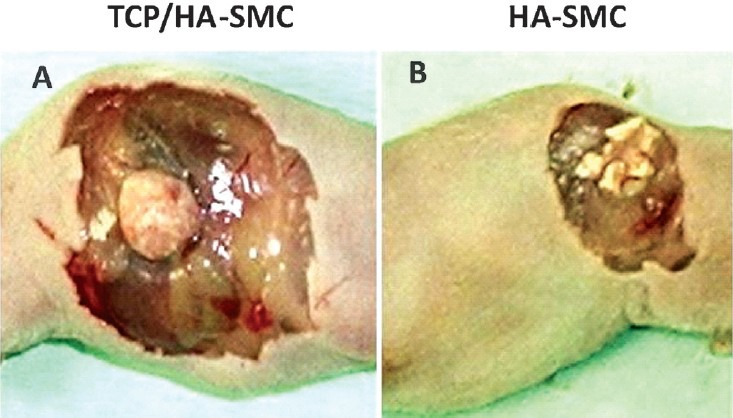
The diagram shows the formation of bone in the in vivo system (athymic mice). (A). TCP/HA-SMC showed a significant change in shape compared with the HA-SMC construct. A smooth outer surface and the hard structure was felt with the fingers. (B). HA-SMC constructs showed no change and still maintain its form.
Histological analysis: Histological evaluation using H&E revealed the formation of new bone tissue when cultured for 3 wk in induction medium and 8 wk post-implantation in mice model (Fig. 2). The interconnected pores within the seeded scaffolds were observed with cells and appeared more frequent and compact in the case of in vivo system as compared to in vitro system. After 3 wk in the induction medium, distinct nodules were visible in both seeded constructs (Fig. 2A). New bone layer with a lamellar-like feature appeared in close interaction and bonding with the biomaterial as these could be seen surrounding the periphery surface of TCP/HA-SMC (Fig. 2B). Alizarin Red staining of ceramics scaffolds seeded with SMC grown in the two different systems revealed deposition of calcium phosphate nodules within the constructs. The formation of nodules confirmed the differentiation of SMC into osteogenic lineage within the 3-D HA-SMC and TCP/HA-SMC constructs in mice model suggested higher matrix mineralization.
Fig. 2.
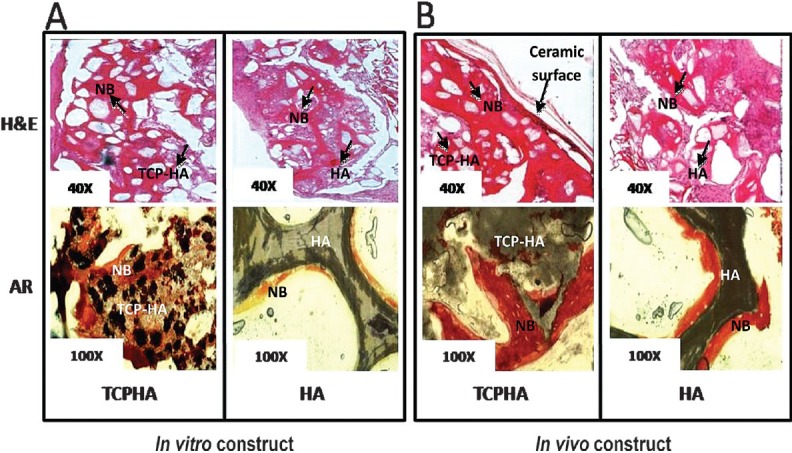
Light microscopic images of HA-SMC and TCP/HA-SMC three dimensional construct implanted in mice. (A) TCP/HA-SMC construct revealed new bone (NB) formation with some ceramics and fibrous tissue still seen. More bone area detected as compared to HA-SMC in both staining, H&E and Alizarin Red. (B) formation of new bone with a high surface area, and cell-shaped void embedded in the bone for TCP/HA-SMC construct. In HA-SMC construct there were lots of fibrous intrusion. AR staining confirmed more mineral activity in in vivo level both HA-SMC and TCP/HA-SMC Constructs.
mRNA quantification: Transcripts levels of osteogenic differentiation markers were quantified using real-time RT-PCR. Fig. 3 shows results of quantitative RT-PCR at the level of in vitro and in vivo. The rate of gene expression of collagen type 1 for in vivo TCP/HA-SMCs was significantly higher (8.43 ± 0.99, P<0.05) compared to in vitro TCP/HA-SMCs. No significant difference was seen for osteopontin (OPN) in in vivo constructs compared to in vitro constructs. Same pattern occurred in HA-SMCs in vivo constructs for collagen type 1 with (3.67 ± 0.22, P<0.05) when compared with the HA-SMC in in vitro constructs with no statistical significance.
Fig. 3.
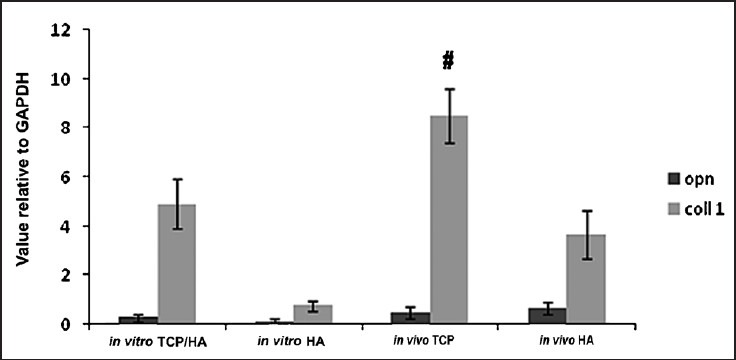
Transcript level related to osteogenic gene expression of osteopontin (OPN) and type 1 collagen (col 1) quantified by real time RT-PCR. The rate of gene expression of collagen type 1 for in vivo TCP/HA-SMC was significantly higher (8.43 ± 0.99, P<0.05) when compared to in vitro TCP/HA-SMC. But no significant difference was shown for OPN in in vivo constructs compared to in vitro constructs. B) Same pattern has occurred in HA-SMC in vivo constructs for collage type 1 with (3.67 ± 0.22, P<0.05) when compared with the HA-SMC in vitro constructs, but no significant value provided by OPN when compared between the two constructs.
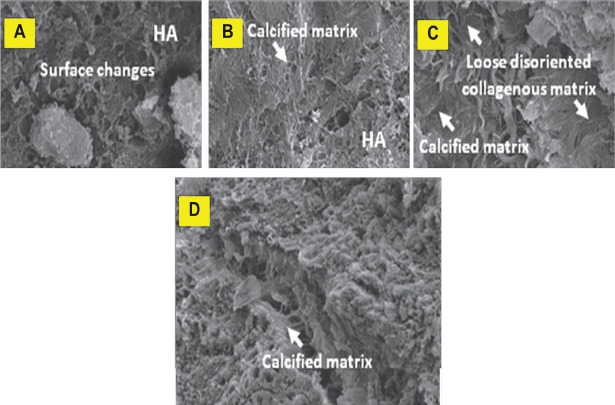
Scanning electron microscopy: HA-SMC and TCP/HA-SMCs constructs were prepared for in vitro cultivation (Fig. 4). After 3 wk most pores were filled with new tissue as observed by SEM especially in TCP/HA-SMC constructs. Flattened cells attached and proliferated and at the same time vesicles matrix were produced. The calcification process occurred and it resulted from the thick collagen bundle in the constructs (Fig. 4A). Less calcification was seen in HA-SMC constructs. In in vivo result, SEM showed the formation of thick collagen fibers encapsulated TCP/HA-SMC constructs more than HA-SMC constructs. Cells were attached to the both constructs surface horizontally to proliferate and secrete collagen fibers. The commencement of the calcification resulted in the formation of thick collagen fibers (Fig. 5A), but this phenomenon was not seen in HA-SMC constructs as shown (Fig. 6C).
Fig. 4.
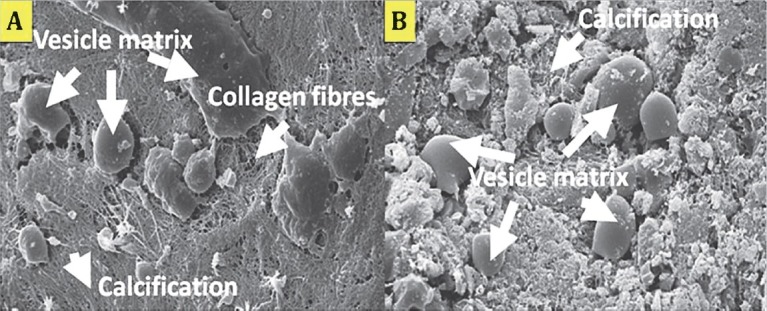
Scanning electron micrograph of HA-SMC and TCP/HA-SMC constructs in osteogenic medium for three weeks. Flattened cells were detected attached to the constructs and started to proliferated and secreting matrix. (A) In TCP/HA-SMC constructs, a lots of calcification process and vesicles matrix were detected. (B) In HA-SMC constructs, less vesicles matrix and collagen fibres can be seen.
Fig. 5.
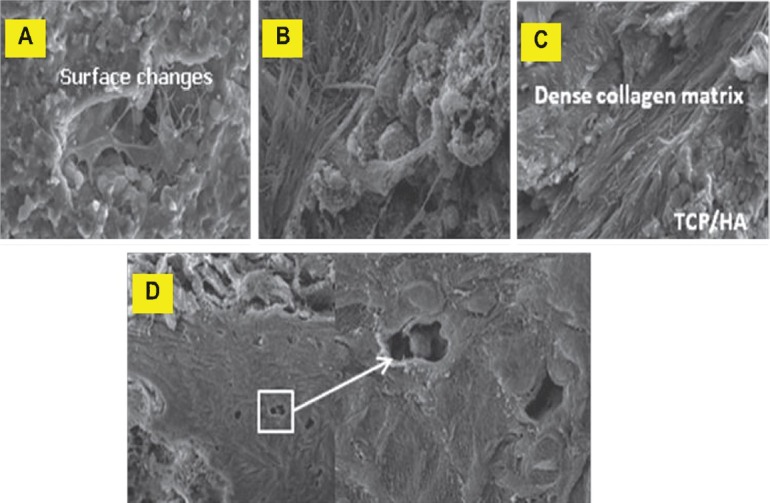
Scanning electron micrographs of TCP/HA-SMC scaffolds. Development of osteoblasts was seen to progress along with the development of extracellular matrix (ECM) in threee phases; cell proliferation with ECM secretion (A and B), ECM maturation (C) and finally, ECM mineralization (D) (arrow).
Fig. 6.
Scanning electron micrographs of HA-SMC scaffolds. Stages of matrix maturation in in vivo HA-SMC post-implantation (oppositional bone growth). (A and B) Cells transforming into mineralized. (C and D) sheets which then broke off to fuse with other nodules, then produced the overall bone-like matrix.
Stages of matrix maturation in in vivo TCP/HA-SMC post-implantation: Development of osteoblasts was in progress along with the development of extracellular matrix (ECM) in the three phases; cell proliferation with ECM secretion (Fig. 5A & B), ECM maturation (5C) and finally, ECM mineralization (5D).
Stages of matrix maturation in in vivo HA-SMCs post-implantation (oppositional bone growth) are shown in Fig. 6A & 6B. Cells were transformed into mineralized (Fig. 6C and 6D) sheets, which then broke off to fuse with other nodules and produced the overall bone-like matrix.
Discussion
The interconnected pores within the ceramics scaffolds can be observed together with the cells attached onto it. Some investigators reported that distinct nodules were visible when scaffolds were seeded with cells8. They found that the presence of osteogenic factors showed deposition of calcium phosphate nodules within the scaffold matrix. Von Kossa staining was used to detect the calcium phosphate nodules9. Kim et al8 found abundant calcification area and collagen fibres on the scaffolds surface and the interconnecting pores. Immunohistochemistry of type 1 collagen was positive. In the present study, we achieved similar results by detecting the mineralization area via Alizarin Red. Intense Alizarin Red staining in seeded scaffolds suggested higher matrix mineralization. The observed calcified nodules were formed within the pores of the scaffolds, revealing cell-cell and cell-matrix interaction during differentiation. Moreover, H&E and Alizarin Red staining showed large scale of new bone formation in our seeded scaffolds, TCP/HA-SMC construct compared to the HA-SMC construct. However, both ceramic-SMC constructs still exhibited ceramics residue due to short incubation period. The TCP/HA-SMC construct showed greater new bone formation in both the in vitro and in vivo systems compared to HA-SMCs. Less bone formation showed in HA-SMCs constructs with some granule residues can be seen on the scaffolds.
Bone formation is a dynamic process and requires an understanding of how bone cells interact with its surroundings. This study demonstrated the behaviour of bone cells in the process of adhesion, growth, collagen synthesis and calcification characteristics. There are two types of proteins in bone extracellular matrix: the collagens, mostly type I collagen, which account for 90 per cent of the bone matrix proteins10 and the noncollagenous proteins, including osteocalcin and osteopontin11,12,13. Osteocalcin, the most abundant noncollagenous protein of the mineralized bone matrix, binds calcium and plays an important role in matrix mineralization, and represents the later state of osteoblastic differentiation14. Osteopontin is a highly sulphated, phosphorylated, and glycosylated protein that mediates cell attachment through a RGD motif (RGD is the single letter code for arginine-glycine-aspartate) to extracellular matrices15. Due to its highly negatively charged characteristics, osteopontin can sequester calcium ions while conserving polyglutamate regions, which have hydroxyapatite crystal nucleation potential16. In the absence of osteocalcin, osteopontin could contribute to an overall metabolic shift toward new bone formation17,18,19. Induction of osteoblastic differentiation by osteogenic medium also leads to the expression of both noncollagenous proteins, (osteocalcin and osteopontin) and collagen type I. These noncollagenous protein and collagen type I are major components for ECM deposition. Our findings showed evidence of bone formation as indicated by expression of collagen type I and osteopontin in these osteogenic-inducing cells in this study. These genes are related to bone extracellular matrix formation. Initially it was thought that the SMC in a ceramics construct form gradually lost its phenotypes during the process of in vitro incubation. However, it was found that the SMC retained its phenotypes and it was highly expressed in the SMC-TCP/HA construct compared to the HA-SMC construct.
In SEM analysis, it was observed that mineralization had taken place in the two different types of constructs. The mechanism of bone formation in TCP/HA-SMC bone constructs underwent a physiological process known as collagen calcification in disoriented manner. Development of osteoblast along osteogenic pathway was reported to progress along the development of extracellular matrix in three phases; cell proliferation with ECM secretion, maturation and finally mineralization20. The mechanism of calcification in HA-SMC constructs differed in a few ways. Flattened cells could be seen gradually transforming into mineralized sheet formation. Similar results have been earlier3,20. Various calcified accretion were formed from these mineralized sheets which broke off to fuse with other nodules to produce the overall bone-like matrix. However, this phenomenon occurred in a small region of the HA-SMC constructs.
The sheep fibrin prepared in our laboratory was found to be a suitable cell carrier and acted as a temporary scaffold for the formation of stable constructs in bone tissue engineering. It fulfilled the criteria of being biocompatible, osteoinductive and resorbable3,5. Due to lack of mechanical strength, its usage may be limited to non-load bearing or minor bone defects. Torigoe et al21 investigated the effect of autologous fibrin and bone marrow on β-tricalcium phosphate on a monkey ectopic bone formation model. They found that autologous plasma efficiently promotes osteogenesis. Thus, in our laboratory we developed the engineered bone tissue with addition of autologous plasma incorporated with calcium phosphate granule due for implantation. We also created a system that eliminated the addition of bovine thrombin as used in other studies22,23. This reduces the risk of immune rejection after transplantation and the risk of contamination.
In conclusion, TCP-HA appears to be a scaffold of choice for bone tissue engineering based on the ideal surface for attachment and synthesis of matrix.
Acknowledgment
This study was supported by the IRPA grants 06-02-02-0059-EA292 and 06-02-02-003 BTK/ER/022 from Ministry of Science, Technology and Innovation (MOSTI) Malaysia, Faculty of Medicine, National University of Malaysia, National University of Malaysia Hospital and Faculty of Veterinary Medicine, University Putra Malaysia.
References
- 1.Aminuddin BS, Ruszymah BHI. Tissue engineering research in developing countries, the significant and differences as compared to the developed countries. Med J Malaysia. 2008;63(Suppl A):47–8. [PubMed] [Google Scholar]
- 2.Ruszymah BHI, Chua KH, Mazlyzam AL, Fuzina NH, Aminuddin BS. Human serum verses animal serum in human cartilage tissue engineering. Int Med J. 2003;2:6–7. [Google Scholar]
- 3.Angela Ng MH, Tan KK, Phang MY, Aziyati O, Tan GH, Isa MR, et al. Differential osteogenic activity of osteoprogenitor cells on HA and TCP/HA scaffold engineered bone. Biomed M Res. 2008;85A:301–2. doi: 10.1002/jbm.a.31324. [DOI] [PubMed] [Google Scholar]
- 4.Wongwitwichot P, Kaewsrichan J, Chua KH, Ruszymah BHI. Comparison of TCP and TCP/HA hybrid scaffolds for osteoinductive activity. Biomed Eng J. 2010;4:279–85. doi: 10.2174/1874120701004010279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angela Ng MH, Aminuddin BS, Tan KK, Tan GH, Isa M Ros, Fauziah O, et al. Comparison of bioengineered human bone construct from four sources of osteogenic cells. J Ortho Sci. 2005;10:192–9. doi: 10.1007/s00776-004-0884-2. [DOI] [PubMed] [Google Scholar]
- 6.Tan KK, Shamsul BS, Aminuddin BS, Tan GH, Chua KH, Angela Ng MH, et al. Bone graft substitute hydroxyapatite scaffold seeded with tissue engineered autologous osteoprogenitor cells in spinal fusion: early result in sheep as a model. Med J Malaysia. 2005;60:53–8. [PubMed] [Google Scholar]
- 7.Boden SD, Schimandle JH, Hutton WC, Damien CJ, Benedict JJ, Baranowski C, et al. In vivo evaluation of a resorbable osteoinductive composite as a graft substitute for lumbar spinal fusion. J Spinal. 1997;10:1–11. [PubMed] [Google Scholar]
- 8.Kim HJ, Kim UJ, Kim HS, Li C, Wada M, Leisk GG, et al. Bone Tissue Engineering with Premineralized silk scaffolds. Bone. 2008;42:1226–34. doi: 10.1016/j.bone.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandal BB, Kundu CC. Non-mulberry silk gland fibroin 3-D scaffold for enhanced differentiation of human mesenchymal stem cell into osteocytes. Acta Biomater. 2009;5:2579–90. doi: 10.1016/j.actbio.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 10.Gehron-Robey P. Bone matrix proteoglycans and glycoproteins. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of bone biology. San Diego, CA, USA: Academic Press; 1996. pp. 155–16. [Google Scholar]
- 11.Denhardt DT, Guo X. Osteopontin: A protein with diverse functions. FASEB J. 1993;7:1475–82. [PubMed] [Google Scholar]
- 12.Bianco P, Fisher LW, Young MF, Termine JD, Robey PG. Expression of bone sialo protein (BSP) in developing human tissues. Calcif Tissue Int. 1991;49:421–6. doi: 10.1007/BF02555854. [DOI] [PubMed] [Google Scholar]
- 13.Hauschka PJ, Lian Cole D, Gundberg C. Osteocalcin and matrix Gla protein: Vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Ni M, Zhang M, Ratner B. Calcium Phosphate - chitosan composite scaffolds for bone tissue engineering. Tissue Eng. 2003;9:337–45. doi: 10.1089/107632703764664800. [DOI] [PubMed] [Google Scholar]
- 15.Ganss B, Kim RH, Sodek J. Bone sialoprotein. Crit Rev Oral Biol Med. 1999;10:79–98. doi: 10.1177/10454411990100010401. [DOI] [PubMed] [Google Scholar]
- 16.Hunter GK, Goldberg HA. Nucleation of hydroxyapatite by bone Sialoprotein. Proc Natl Acad Sci USA. 1993;90:8562–5. doi: 10.1073/pnas.90.18.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roach HI. Why does bone matrix contain noncollagenous proteins? The possible roles of osteocalcin, osteonectin, osteopontin and bone sialoprotein in bone mineralisation and resorption. Cell Biol Int. 1994;18:617–28. doi: 10.1006/cbir.1994.1088. [DOI] [PubMed] [Google Scholar]
- 18.Stein GS, Lian JB, Owen TA. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J. 1990;4:3111–23. doi: 10.1096/fasebj.4.13.2210157. [DOI] [PubMed] [Google Scholar]
- 19.Kondo H, Ohyama T, Ohya K, Kasugai S. Temporal changes of mRNA expression of matrix proteins and parathyroid hormone and parathyroid hormone-related protein (PTH/PTHrP) receptor in bone development. J Bone Miner Res. 1997;12:2089–97. doi: 10.1359/jbmr.1997.12.12.2089. [DOI] [PubMed] [Google Scholar]
- 20.Phang MY, Angela NMH, Tan KK, Aminuddin BS, Ruszymah BHI, et al. Evaluation of suitable biodegradable scaffolds for engineered bone tissue. Medical J Malaysia. 2004;59(Suppl B):198–9. [PubMed] [Google Scholar]
- 21.Torigoe I, Sotome S, Tsuchiya A, Yoshii T, Maehara H, Sugata Y, et al. Bone regeneration with autologous plasma, bone marrow stromal cells, and porous b-tricalcium phosphate in nonhuman primates. Tissue Eng Part A. 2009;15:1489–99. doi: 10.1089/ten.tea.2008.0317. [DOI] [PubMed] [Google Scholar]
- 22.Niehoff P, Springer IN, Açil Y, Lange A, Marget M, Roldán JC, et al. HDR brachytherapy irradiation of the jaw - as a new experimental model of radiogenic bone damage. J Craniomaxillofac Surg. 2008;36:203–9. doi: 10.1016/j.jcms.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Smucker JD, Aggarwal D, Atkinson BL, Bobst JA, Nepola JV, Fredericks DC. B2a Peptide on ceramic granules enhances posterolateral spinal fusion in rabbits compared with autograft. Spine. 2008;33:1324–9. doi: 10.1097/BRS.0b013e3181732a74. [DOI] [PubMed] [Google Scholar]