Abstract
Background & objectives:
Intensive regular physical exercise training is associated with a physiological changes in left ventricular (LV) morphology and functions. This cardiac remodeling observed in the athletes is associated with the specific haemodynamic requirements of the exercise undertaken. The main objective of this study is to evaluate the effect of endurance training on cardiac morphology, systolic and diastolic LV functions and haemodynamic parameters both in male and female athletes.
Methods:
Seventy nine healthy athletes (age 20.0 ± 2.6 yr; 49% male) and 82 healthy sedentary adolescent (age 20.8 ± 2.2 yr, 49% male) volunteered to participate in this study. All subjects underwent transthoracic echocardiography and impedance cardiography.
Results:
Both female and male athletes had greater LV end-diastolic cavity sizes, LV mass and stroke volume (SV) values when compared with controls. Also, in male athletes, LV mass index was higher than in female athletes. While male athletes had lower resting heart rate compared to female athletes, they had higher mean arterial blood pressure. In male athletes, basal septal and mid septal strain values were higher compared to controls. There were no significant differences in strain and peak systolic strain rate values between female athletes and controls. In male athletes, there was a weak positive correlation between SV and LV mass, basal lateral and septal strain values. In female athletes, only a weak positive correlation was found between SV and basal septal strain values.
Interpretation & conclusions:
Endurance-trained male and female athletes had higher LV mass, LV cavity dimensions and SV compared to sedentary controls. Although there was no difference in diastolic cardiac functions between athletes and controls, local enhanced systolic function was found with increase of SV. Both morphologic and haemodynamic differences were more evident in male athletes.
Keywords: Athlete's heart, endurance training, impedance cardiography, strain imaging, tissue Doppler
Intensive regular physical exercise training is associated with a physiological increase in left ventricular (LV) wall thickness, cavity size and mass that have been known as “athlete's heart”1. The extent to which LV dilatation and hypertrophy are altered depends on the type of physical training, endurance or strength. This cardiac remodelling observed in the athletes is associated with the specific haemodynamic requirements of the exercise undertaken. It remains a question whether the athlete's heart is merely a physiologic phenomenon or poses cardiovascular risk.
Studies up to now about athlete's heart primarily includes echocardiographic evaluation to detect morphological and functional changes2,3,4. There were conflicting results of these studies about systolic and diastolic functional changes in athlete's heart5,6,7,8,9. Not much attention has been paid to haemodynamic changes and difference between male and female athletes. Also, cardiac morphologic and haemodynamic changes were studied in different studies. Therefore, we aimed to obtain further data on the morphological and haemodynamic characteristics of the athletic heart in males and females with use of impedance cardiography and echocardiographic examination including tissue Doppler and strain imaging.
Tissue Doppler imaging and strain rate imaging are new echocardiographic modalities based on Doppler tissue imaging and these are capable of assessing systolic and diastolic myocardial functions5,10. Impedance cardiography is a reliable and valid non invasive technique for measuring various haemodynamic indices of cardiovascular function in critical care environments and laboratory settings11. In the previous studies, impedance cardiography was used during active exercise12,13. In this study, we used impedance cardiography in athletes at rest and we aimed to evaluate chronic haemodynamic changes in athletes who are doing intensive regular physical exercise and correlations between chronic haemodynamic changes with cardiac function and morphology in both gender.
Material & Methods
The study was conducted between January 2010 and September 2010 in the departments of Cardiology & Biophysics, Mersin University, Mersin, Turkey. Seventy nine healthy athletes (age 20.0 ± 2.6 yr; range 16 to 28 yr) volunteered to participate in this study. Thirty nine athletes were male (49 %). Athletes were recruited from local sports teams and Sports Academy. Inclusion criteria for athletes were: (i) age 16-40 yr; (ii) engaged in endurance-training activities greater than 5 h/wk for the past year. Exclusion criteria for athletes included: (i) moderate or severe valvular disease; (ii) history of coronary artery disease, myocardial infarction, hypertension or diabetes mellitus; (iii) ejection fraction less than 50 per cent by echocardiogram; and (iv) history of cardiomyopathy, congenital heart defect, open heart surgery, or ongoing arrhythmia.
Five sporting disciplines predominantly made up the study group: hokey, running, football, swimming, gymnastic. The number of hours of intensive training averaged was 11.6 ± 3.1 h/wk (range 8 to 16 h). The athletic history was 7.7 ± 3.6 yr (range 2 to 15 yr). Body surface area was significantly greater in male athletes compared to female athletes (1.88±0.13 m² vs 1.67±0.11 m²).
The control group comprised 82 healthy adolescent volunteers (age 20.8±2.2 yr, 49 % male). All control subjects were free of any cardiovascular or pulmonary disease with normal electrocardiogram during rest and none of whom were engaged in sporting activities other than at the recreational level. Control subjects were recruited from local healthy volunteers who were students at the university or medical workers in the hospital. Controls were of similar age, gender and body surface area the athletes (Table I). All subjects underwent physical examination, 12-lead electrocardiogram, transthoracic echocardiography and impedance cardiography. At the time of the study, all volunteers were at rest in the supine position for at least 20 minutes. Written consent was obtained from all subjects, and institutional ethics committee approved the study protocol.
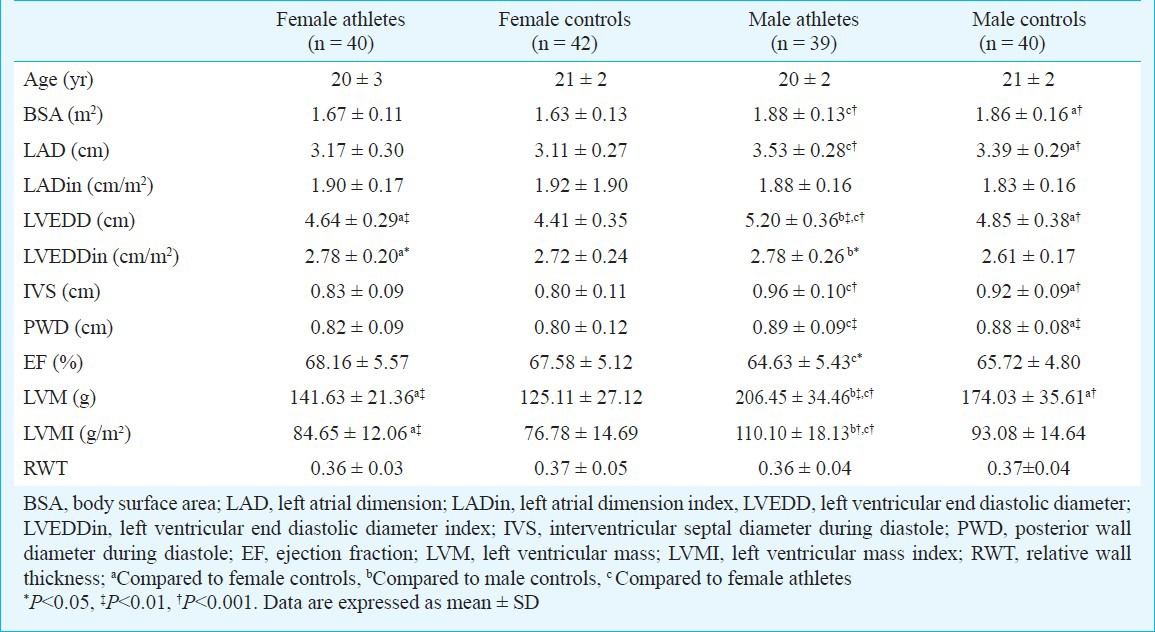
Table I.
Demographic and basal echocardiographic parameters of athletes and controls
Echocardiography: Two-dimensional echocar-diography was performed using Philips Sonograph (HD 11XE, Eindhoven, The Netherlands) with 3 MHz transducer. Images of heart were obtained in the standard parasternal long-axis, short-axis and apical four chamber planes. M-mode echocardiograms in the parasternal long axis were used for the measurement of LV end-diastolic and systolic dimensions, left atrial diameter according to American Society of Echocardiography standards14. Left ventricle wall thickness was measured from 2D parasternal views at the end-diastol with the greatest measurement within the left ventricular wall defined as the maximal wall thickness. Relative wall thickness was calculated by dividing the sum of the septal and posterior wall thickness by the LV end-diastolic diameter. Left ventricular mass was calculated from the cavity size and the wall thickness by the formula of Devereux15.
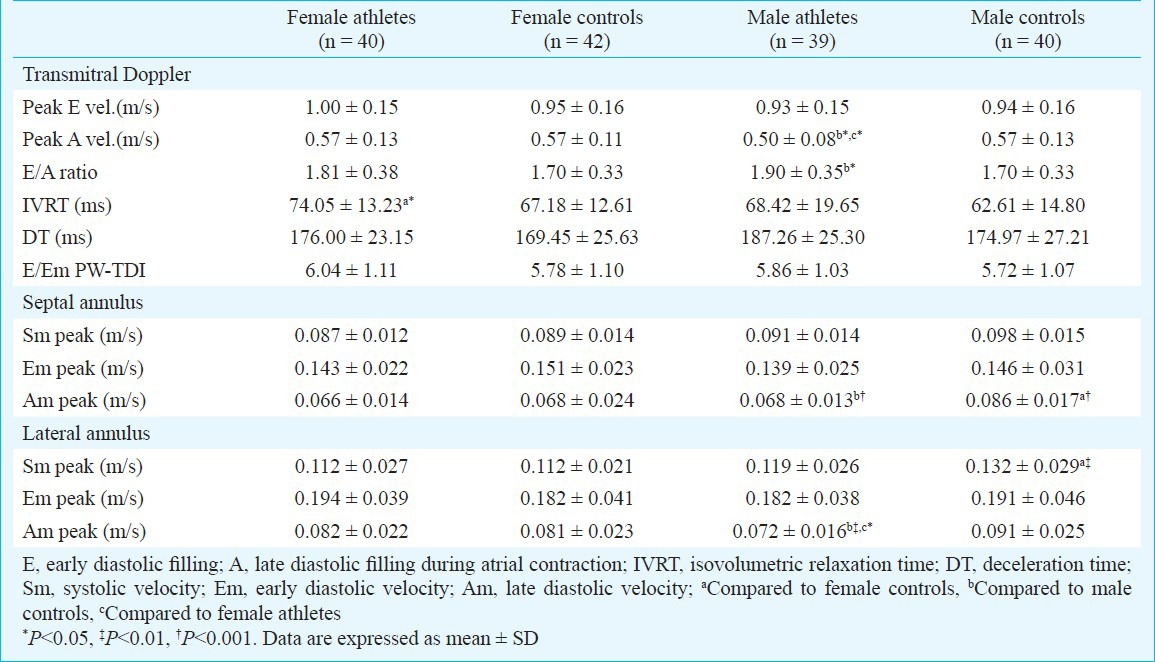
Diastolic function was previously evaluated with traditional ultrasound pulse Doppler recording at the distal margins of mitral valve leaflets. Early and late transmitral flow velocities, E velocity deceleration time and isovolumetric relaxation time were analyzed. But, this technique is dependent on preload, end-systolic volume, atrial and ventricular stiffness and left ventricular relaxation, some of which may be altered by training and it may pseudonormalize in some patients. Tissue Doppler imaging derived diastolic index, peak early diastolic filling velocity of the mitral annulus is independent of the preload and does not pseudonormalize6. Therefore, we obtained pulse wave tissue Doppler imaging (PW-TDI) indices; diastolic early (Em) and late (Am) velocities from mitral septal and lateral annulus using a sample area of 5 mm. From combination of traditional and PW-TDI indices, we obtained E/Em ratio (Em is the average of the septal and lateral mitral annulus).
Strain is a measure of tissue deformation and is defined as the change in length normalized to original length. The rate at which this change occurs is called strain rate10. For LV strain measurement, 2-D colour Doppler myocardial imaging velocity data were obtained from apical four chamber view. Analysis was independently performed on an analysis system (QLAB quantification software, Phillips HD11XE, Eindhoven, The Netherlends). For data acquisition, two complete cardiac cycles from each 2-D echo view were collected and stored in a cine-loop format. The image sector width was set as narrow as possible to allow a frame rate acquisition greater than 100 frame/s. Regional velocity analyses was performed from the basal, mid and apical segments of the interventricular septum and the lateral LV wall (Fig. 1). The location of this region of interest was manually adjusted in each systolic frame to ensure optimal tracking throughout the systole. Strain and peak systolic strain rate measurements for 2 consecutive cardiac cycles were averaged for each participant.
Fig. 1.
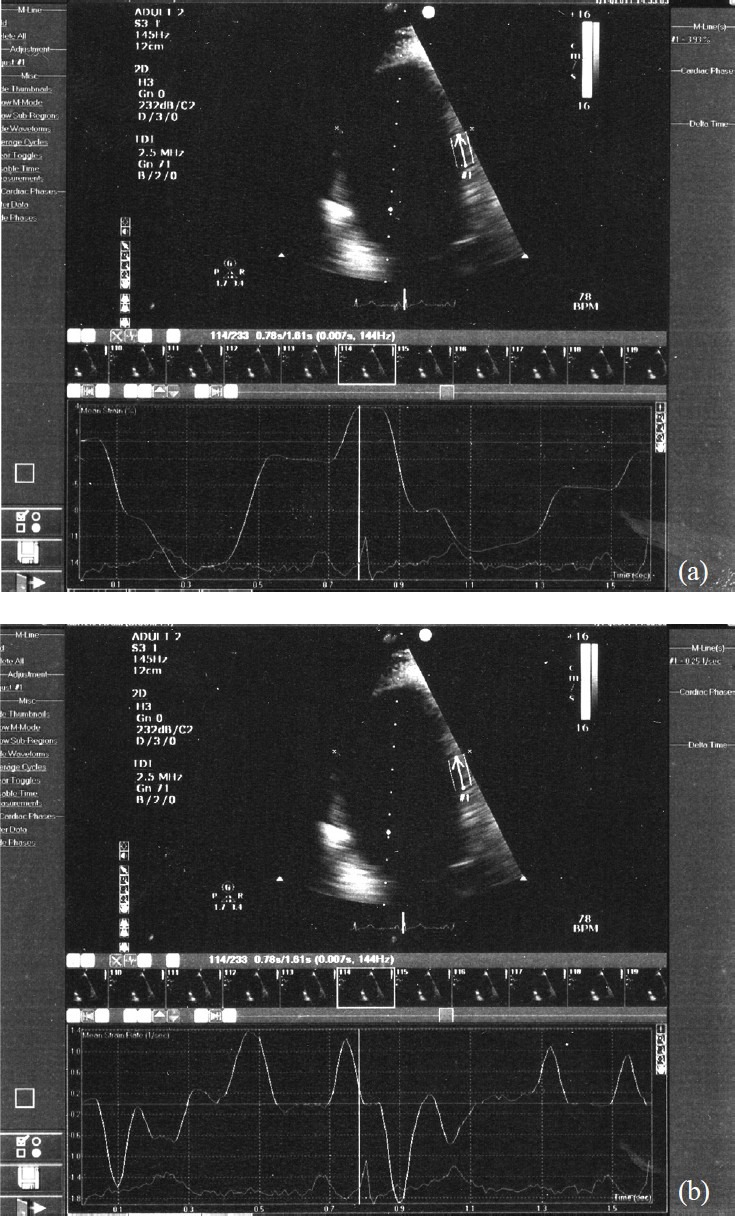
Four chamber view images with strain (a) and strain rate (b) analysis of the mid segment of interventricular septum in a control subject.
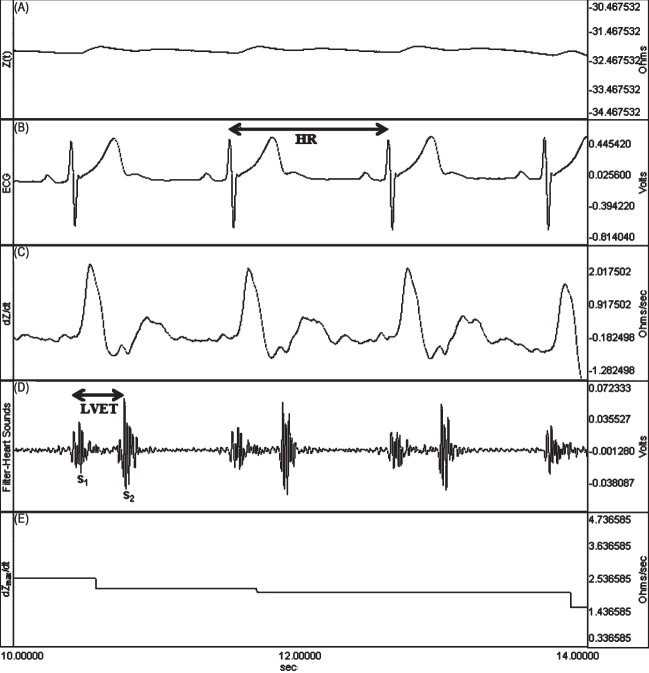
Impedance cardiography: All haemodynamic data were obtained by impedance cardiography using the EBI100C module in BIOPAC MP100 acquisition system (Santa Barbara, USA) (Fig. 2). Impedance cardiography devices use baseline and changes in thoracic bioimpedance to determine indices of blood flow (stroke index and cardiac index), resistance (total peripheral resistance and total peripheral resistance index), contractility (myocardial contractility index) and thoracic fluid content.
Fig. 2.
The simultaneous recordings of thoracic electrical bioimpedance in an athlete. (A) Z0 (steady-state basal impedance). (B) HR (heart rate) (C) dZ/dt (rate of impedance change) (D) LVET (left ventricular ejection time) (E) dZmax/dt (maximum rate of impedance change).
A total of four electrodes are placed laterally on the individual's neck and thorax and a high-frequency (70 kHz), low-amplitude (2.5 mA) electrical current is applied to the outer sensors (most superior sensors on the neck and most inferior sensors on the thorax). The inner sensors measure the pulsatile changes in voltage that occur due to pulsatile changes in thoracic impedance related to the change in the size of the thoracic aorta and reflecting stroke volume (SV). Impedance within the thorax is related to the conduction properties of the tissue and volume of the path of resistance16. Using the change in voltage across the thorax to measure the change in thoracic impedance, the equations that describe the components of the equation by Bernstein17 were used to derive SV. Stroke volume (ml/beat) can be calculated as the product of four components16: volume of electrically participating tissue (VEPT in l) which is solely dependent on the distance between the inner electrodes; left ventricular ejection time (LVET in sec); maximum rate of change in impedance during ventricular ejection (dZmax/dt in Ohm/sec); and steady-state basal impedance, Z0 (Ohm). dZmax/dt is also called the ejection velocity index (EVI). Z0 is also called thoracic fluid index (TFI). The resistivity18 of all chest tissue, including skeletal muscle, cardiac muscle, lung, chest wall, subcutaneous fat, bone, and fluid (intra- and extracellular) determines Z0. Because body fluids are the most variable components of those listed, changes in Z0 primarily occur because of changes in thoracic fluid levels. Because Z0 decreases as fluid volume rises, and vice versa in an inverse relationship, it is not a convenient measurement. This reciprocal relationship was used to develop the new parameter of thoracic fluid content (TFC), defined as 1/Zo. Therefore, it parallels changes in fluid volume. To make TFC a convenient number18, both numerator and denominator are multiplied by 1000.
It was important to provide a mechanism to determine the LVET. Using the heart sounds microphone (TSD108) with the differential amplifier (DA100) and by running a selective bandpass filter, it was possible to record the aortic valve activity. Finally, to record heart rate, three ECG electrodes were placed in a lead I configuration on the thorax. The recording system recognized and eliminated the impedance changes arising from ventilation and body movements. Impedance cardiography, heart sounds and ECG signals were continuously recorded in 60 sec epochs. Heart rate, Z0, dZmax/dt, and LVET were derived from the event waveforms. Stroke volume was calculated using the Bernstein equation17. Cardiac output (CO) was determined using the equation CO (ml/min) = SV (ml/beat) × HR (beats/min). The total peripheral resistance (TPR) was calculated by the equation TPR=MAP × 80/CO, where MAP was the mean arterial pressure. Cardiac index (CI), stroke index (SI) and total peripheral resistance index (TPRI) values were calculated respectively of CO, SV and TPR dividing by body surface area. The index of myocardial contractility (IC) was derived from the thoracic impedance including the TFI and the EVI following the formula IC=EVI/TFI.
Statistical analysis: Data are expressed as mean ± SD or median (min-max) values. The sample size for each group was determined doing power analysis by using preliminary study results. The sample sizes were calculated for all statistically significant parameters and determined as 40 for minimum effect size with the power of 80 per cent and type I error of 5 per cent. Control of normal distribution of data was made with Shapiro-wilks test. The values of athletes and controls were compared using the independent t test, Mann-Whitney U test and chi-square test. Pearson's correlation coefficient was performed to test univariate relations between impedance cardiography and echocardiographic parameters. Statistical analysis was performed using SPSS for windows (Release 11.5, SPSS Inc, Chicago, Illinois, USA). P<0.05 was considered significant.
Results
The study population consisted of male and female athletes and control subjects. Athletes and control subjects were similar in regard to age, gender and body surface area. Demographic and basal echocardiographic parameters of the study population are shown in Table I. Both female and male athletes had greater LV end-diastolic cavity sizes when compared with controls (4.64 ± 0.29 cm in female athletes, 4.41 ± 0.35 cm in female controls, P<0.01; 5.20 ± 0.36 cm in male athletes, 4.85 ± 0.38 cm in male controls, P<0.001). In comparison with the male athletes, female values were much lower (P<0.001) but there was no difference in LV end-diastolic diameters when expressed relative to body surface area. In the group of female athletes, LV end-diastolic cavity dimension ranged from 3.9 to 5.3 cm and absolute values were within normal limits (LV end-diastolic diameter <5.4 cm). In the group of male athletes, 15 per cent had LV cavity size enlarged (>5.5 cm).
Absolute LV wall thickness in female athletes ranged from 6 to 10 mm, in male athletes ranged from 8 to 12 mm. Of the 79 athletes, only two (2.5%) had 12 mm LV wall thickness and two of them were male. No athletes had LV wall thickness >12 mm consistent with hypertrophic cardiomyopathy. All female athletes had a LV wall thickness of <11 mm. Both female and male athletes had a significantly greater LV mass when compared with controls (141.63±21.36 g in female athletes, 125.11±27.12 g in female controls, P=0.004; 206.45±34.46 g in male athletes, 174.03±35.61 g in male controls, P<0.01). Left ventricular mass index was 84.65±12.06 g/m² in female athletes and 110.10±18.13 g/m² in male athletes and also significantly greater when compared with controls (P<0.001 and P<0.01). Also, in male athletes, LV mass indices were higher than female athletes (P<0.001).
Compared with sedentary control subjects, male and female athletes showed mildly enlarged left atrial dimension. Although, female athletes had smaller left atrial chamber than male athletes (3.17±0.30 and 3.53±0.28 cm respectively, P<0.001), there was no significant difference after correction for the body surface area (1.90 ± 0.17 and 1.88 ± 0.16 cm/m² respectively). Despite these morphological differences, athletes did not show alterations in ejection fraction compared with controls.
Table II lists the haemodynamic parameters by impedance cardiography of all subjects. Resting heart rate was lower in both female and male athletes compared to control subjects (71±10 beat/min in female athletes, 78 ± 9 beat/min in female controls, P<0.001; 65±9 beat/min in male athletes, 76±10 beat/min in male controls, P<0.001). While male athletes had lower resting heart rate compared to female athletes (P<0.01), they had higher mean arterial blood pressure (86.90±8.64 vs 82.04±8.18 mmHg, P<0.05).
Table II.
Haemodynamic parameters by impedance cardiography of athletes and controls
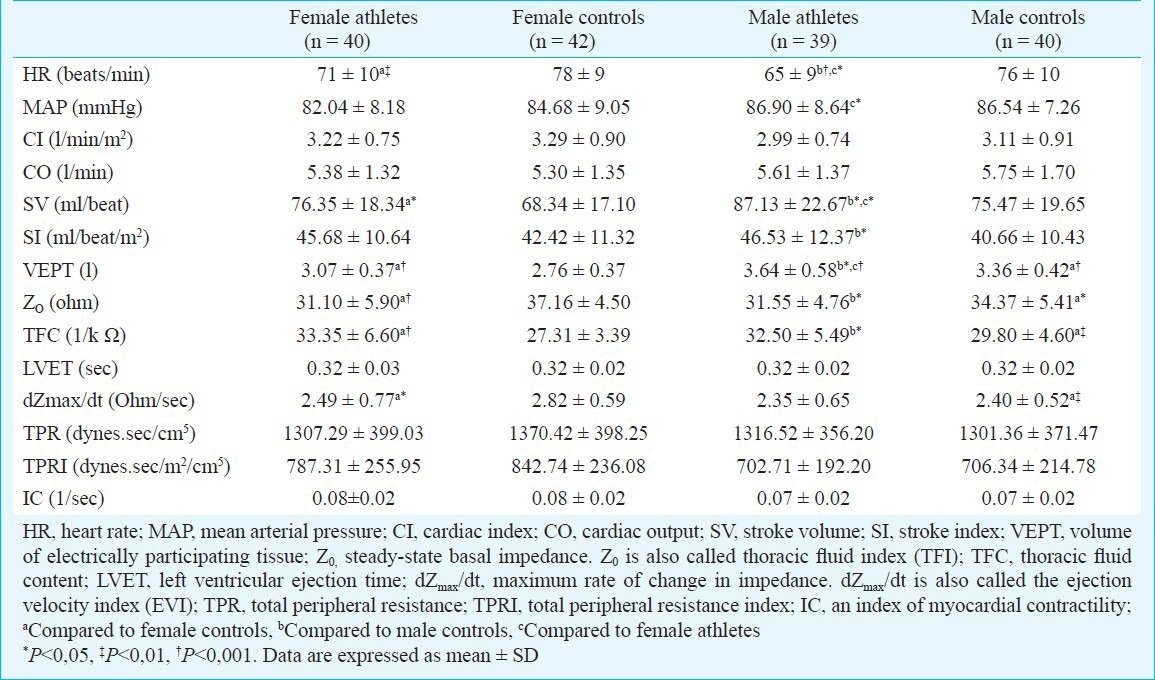
Male and female athletes had higher VEPT, TFC and SV compared to control subjects (Table II). Although resting SV values were significantly lower in female athletes compared to male athletes (76.35 ± 18.34 vs 87.13 ± 22.67 ml/beat, P<0.05), these gender difference were no longer significant when expressed relative to body surface area as SI (45.68±10.64 vs 46.53 ± 12.37 ml/beat/m2). There was no change in the CO, TPR, TPRI and IC between different gender of athletes and controls (Table II).
In both groups, results of diastolic function by traditional PW Doppler showed an apparent normal diastolic pattern. While late transmitral flow velocity was lower in male athletes than in control subjects (P<0.05), there was no differences in female athletes. Consequently male athletes showed increased ratio of early to late transmitral flow velocities (P<0.05). Male athletes had also lower lateral and septal annulus Am peak velocities (Table III). There was no significant difference in tissue Doppler parameters in female athletes compared to controls. Also, there was no difference in E/Em parameters between athletes and control subjects.
Table III.
Standard transmitral Doppler echocardiograhic data and assessment of mitral annulus spectral pulse Doppler velocities
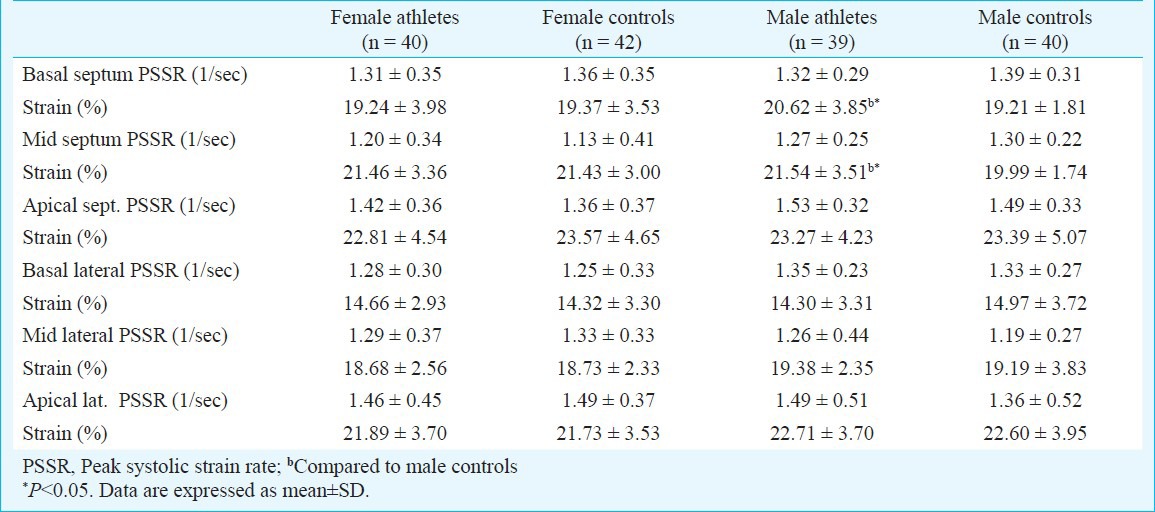
In male athletes, basal septal and mid septal strain values were higher compared to controls. There were no significant differences in strain and peak systolic strain rate values between female athletes and controls (Table IV). Both in male and female athletes, SV was correlated with TPR (in male r=-0.76, P<0.001; in female r=-0.81, P<0.001), and SI was correlated with TPRI (in male r=-0.65, P<0.001; in female r=-0.72, P<0.001). In male athletes, there was a weak correlation between SV and LV mass (r=0.28, P=0.29) and SV and basal lateral and basal septal strain values (r=0.57, P<0.001; r=0.52, P=0.001). In female athletes, only a weak correlation was found between SV and basal septal strain values (r=0.31, P=0.04).
Table IV.
Peak systolic strain rate and peak strain values in LV of athletes and controls
Discussion
In this study, both male and female athletes had greater LV end-diastolic cavity sizes, LV mass and LV mass index when compared with controls. Left ventricular mass of female athletes was lower than that of male athletes. These results were also correlated with previous studies4,19,20,21.
Gender difference in LV mass was not completely explained by the different body size of the female athletes, because LV mass index was also significantly different between them. One mechanism implicated for gender-related difference is the lower increase in absolute blood pressure with peak exercise in women22,23. Zemva et al23 found that lower LV mass index in female dancers was related to lower peak exercise systolic blood pressure. Although we measured the resting blood pressure, female athletes had lower mean arterial blood pressure values compared with male athletes. Androgenic hormone levels and genetic factors may affect the gender related difference. Testosteron and oestrogen sensitive receptors are described in the cardiac muscle, the former increasing cardiac muscle mass, the latter protecting against cardiac hypertrophy24. The role of hormonal effects was confirmed in the study of Pavlik et al24. They could not find any difference in relative LV muscle mass in female athletes older than 45 yr, but LV mass was lower in female athletes aged below 45 yr. All athletes in our study were below 40 yr of age. The level of training can also contribute to the difference in LV mass. However, it is not easy to form two groups of different gender who are at the same level of training even if they perform the same sport. In this study, we tried to select male and female athletes with similar intensity of training and competition.
The upper limits of left ventricular wall thickness did not exceed 11 mm in female athletes and only two male athletes had 12 mm LV wall thickness. No athletes had LV wall thickness >12 mm. Sharma et al2 also found that no female athlete had a LV wall thickness >11 mm in elite female athletes and only 0.4 per cent of male athletes had LV wall thickness >12 mm. In another study3 performed in elite male and female British athletes, none of female athletes showed LV wall thickness >11 mm. So, if a female athlete has a LV wall thickness greater than 12 mm, instead of physiologic result of chronic exercise training, an expression of primary cardiac hypertrophy must be considered.
Although LV mass was higher in male athletes, there was no difference in LV passive filling (transmitral E velocity and tissue Doppler Em velocities) and in LV stiffness (deceleration time). Also, we could not find any difference in E/Em parameters between male and female athletes and control subjects. In spite of increased LV mass in male and female athletes, left ventricular relaxation was not affected. While an improved diastolic function was reported in athletes compared with controls in some studies6,7, most of studies showed no significant differences regarding the diastolic myocardial velocities5,8,9,20.
In the present study, to define the effect of endurance training on regional LV systolic function, we also used LV longitudinal strain and strain rate imaging because, peak systolic strain rate measures local contractile function and it is relatively volume independent. Also, strain and strain rate are less susceptible to translational motion and tethering artifacts and thus may be superior to tissue velocity in depicting regional and global myocardial function10. While ejection fraction and IC were similar between athletes and controls, basal septal and mid septal strain values were higher in male athletes compared to controls. There were no significant differences in strain and peak systolic strain rate values between female athletes and controls. There were conflicting results about strain imaging studies in the literature. While longitudinal strain was similar between endurance athletes and normal subjects in studies5,8,9,25,26, enhanced systolic functions in athletes were detected in others6,7. For instance, Tumuklu et al6 found higher mid-septum and mid-lateral peak systolic rate values in professional football players. Poulsen et al8 found that systolic longitudinal strain rate was similar in endurance athletes and controls. The reason of this diversity may be the presence of significant variability among athletes. In most of the studies, both male and female athletes were evaluated together or only male athletes were taken. Therefore, especially gender, ethnicity, age, genetic profile, sport and training type may contribute to this variability.
Both male and female athletes had higher SV compared to control subjects. In trained athletes, physiologic cardiac adaptations occur to the haemodynamic load with chronic exercise training. LV volume overload might induce greater LV end-diastolic diameter and earlier and better early diastolic stretching of myocardial fibers, which in turn could be able to induce an enhanced SV through a better use of the Frank-Starling mechanism27. Endurance trained female athletes had lower SV and SI than males but latter difference was no longer significant. Because of the smaller body mass, female athletes have smaller heart volume which may reduce LV end-diastolic volume and SV2,4,21. Wiebe et al28 found that compared to male athletes, female athletes had lower SV values but when expressed relative to body mass, there was no gender difference.
In a previous study, an independent correlation of global longitudinal strain with the sum of LV wall thicknesses was shown29. In this study, there was a weak positive correlation between SV and LV mass and also basal lateral and basal septal strain values, especially in male athletes. Left ventricular hypertrophy, mostly developed in response to pressure overload, seems to induce an enhancement of myocardial systolic function, able to better sustain the rapid increases of systolic blood pressure typical of static isometric exercise.
In this study, our interest defines the effect of chronic endurance training on myocardial functions in resting state. So, all exams were performed with the subject at rest. We did not investigate on haemodynamics during exercise. The findings in our study apply to the young endurance athlete, since it is unknown how morphological changes are related to haemodynamic functions in the older athletes (>40 yr).
In conclusion, the combined use of echocardiography and impedance cardiography may better clarify the effects of endurance training on LV morphology, functions and haemodynamic parameters. Endurance-trained male and female athletes had higher LV mass, LV cavity dimensions and SV compared to sedentary controls. Although there was no difference in diastolic cardiac functions between athletes and controls, local enhanced systolic function was found with increase of SV. Both morphologic and haemodynamic differences were more evident in male athletes compared to female athletes.
Acknowledgment
The authors thank Dr Bahar Tasdelen, for statistical analyses.
References
- 1.Maron B. Structural features of the athlete heart as defined by echocardiography. J Am Coll Cardiol. 1986;7:190–203. doi: 10.1016/s0735-1097(86)80282-0. [DOI] [PubMed] [Google Scholar]
- 2.Sharma S, Maron BJ, Whyte G, Firoozi S, Elliott PM, McKenna WJ. Physiologic limits of left ventricular hypertrophy in elite junior athletes: relevance to differential diagnosis of athlete's heart and hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40:1431–6. doi: 10.1016/s0735-1097(02)02270-2. [DOI] [PubMed] [Google Scholar]
- 3.Whyte GP, George K, Sharma S, Firoozi S, Stephens N, Senior R, et al. The upper limit of physiological cardiac hypertrophy in elite male and female athletes: the British experience. Eur J Appl Physiol. 2004;92:592–7. doi: 10.1007/s00421-004-1052-2. [DOI] [PubMed] [Google Scholar]
- 4.Pelliccia A, Maron BJ, Spataro A, Proschan MA, Spirito P. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. N Engl J Med. 1991;324:295–301. doi: 10.1056/NEJM199101313240504. [DOI] [PubMed] [Google Scholar]
- 5.Saghir M, Areces M, Makan M. Strain rate imaging differentiates hypertensive cardiac hypertrophy from physiologic cardiac hypertrophy (athlete's heart) J Am Soc Echocardiogr. 2007;20:151–7. doi: 10.1016/j.echo.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Tumuklu MM, Etikan I, Cinar CS. Left ventricular function in professional football players evaluated by tissue Doppler imaging and strain imaging. Int J Cardiovasc Imaging. 2008;24:25–35. doi: 10.1007/s10554-007-9218-8. [DOI] [PubMed] [Google Scholar]
- 7.Baggish AL, Yared K, Weiner RB, Wang F, Demes R, Picard MH, et al. Differences in cardiac parameters among elite rowers and subelite rowers. Med Sci Sports Exerc. 2010;42:1215–20. doi: 10.1249/MSS.0b013e3181c81604. [DOI] [PubMed] [Google Scholar]
- 8.Poulsen SH, Hjortshøj S, Korup E, Poenitz V, Espersen G, Søgaard P, et al. Strain rate and tissue tracking imaging in quantitation of left ventricular systolic function in endurance and strength athletes. Scand J Med Sci Sports. 2007;17:148–55. doi: 10.1111/j.1600-0838.2006.00538.x. [DOI] [PubMed] [Google Scholar]
- 9.Teske AJ, Prakken NH, De Boeck BW, Velthuis BK, Doevendans PA, Cramer MJ. Echocardiographic deformation imaging reveals preserved regional systolic function in endurance athletes with left ventricular hypertrophy. Br J Sports Med. 2010;44:872–8. doi: 10.1136/bjsm.2008.054346. [DOI] [PubMed] [Google Scholar]
- 10.Abraham TP, Dimaano VL, Liang HY. Role of tissue Doppler and strain echocardiography in current clinical practice. Circulation. 2007;116:2597–609. doi: 10.1161/CIRCULATIONAHA.106.647172. [DOI] [PubMed] [Google Scholar]
- 11.Van De Water JM, Miller TW, Vogel RL, Mount BE, Dalton ML. Impedance cardiography: The next vital sign technology? Chest. 2003;123:2028–33. doi: 10.1378/chest.123.6.2028. [DOI] [PubMed] [Google Scholar]
- 12.Boutcher SH, McLaren PF, Cotton Y, Boutcher Y. Stroke volume response to incremental submaximal exercise in aerobically trained, active, and sedentary men. Can J Appl Physiol. 2003;28:12–26. doi: 10.1139/h03-002. [DOI] [PubMed] [Google Scholar]
- 13.Miles DS, Cox MH, Verde TJ, Gotshall RW. Application of impedance cardiography during exercise. Biol Psychol. 1993;36:119–29. doi: 10.1016/0301-0511(93)90085-m. [DOI] [PubMed] [Google Scholar]
- 14.Sahn DJ, DeMaria A, Krisslo J, Weyman A. Recommendations regarding quantification of M-mode echocardiographic results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–85. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 15.Devereux RB. Detection of left ventricular hypertrophy by M-mode echocardiography; anatomic validation, standardization and comparison to other methods. Hypertension. 1987;9(Suppl II):II19–26. doi: 10.1161/01.hyp.9.2_pt_2.ii19. [DOI] [PubMed] [Google Scholar]
- 16.De Pasquale MJ, Fossa AA. Use of bioimpedance for measuring cardiac output in the conscious dog. Drug Dev Res. 1996;38:105–13. [Google Scholar]
- 17.Bernstein DP. A new stroke volume equation for thoracic electrical bioimpedance: theory and rational. Crit Care Med. 1986;14:904–9. doi: 10.1097/00003246-198610000-00017. [DOI] [PubMed] [Google Scholar]
- 18.van de Water JM, Mount BE, Chandra KM, Mitchell BP, Woodruff TA, Dalton ML. TFC (thoracic fluid content): A new parameter for assessment of changes in chest fluid volume. Am Surg. 2005;71:81–6. [PubMed] [Google Scholar]
- 19.Wernstedt P, Sjöstedt C, Ekman I, Du H, Thuomas KA, Areskog NH, et al. Adaptation of cardiac morphology and function to endurance and strength training. A comparative study using MR imaging and echocardiography in males and females. Scand J Med Sci Sports. 2002;12:17–25. doi: 10.1034/j.1600-0838.2002.120104.x. [DOI] [PubMed] [Google Scholar]
- 20.Wilhelm M, Roten L, Taner H, Wilhelm I, Schmid J-P, Saner H. Gender differences of atrial and ventricular remodeling and autonomic tone in nonelite athletes. Am J Cardiol. 2011;108:1489–95. doi: 10.1016/j.amjcard.2011.06.073. [DOI] [PubMed] [Google Scholar]
- 21.Pelliccia A, Maron BJ, Culasso F, Spataro A, Caselli G. Athlete's heart in women echocardiographic characterization of highly trained elite female athletes. JAMA. 1996;276:211–5. doi: 10.1001/jama.276.3.211. [DOI] [PubMed] [Google Scholar]
- 22.Karjalainen J, Mäntysaari M, Viitasalo M, Kujala U. Left ventricular mass, geometry, and filling in endurance athletes: association with exercise blood pressure. J Appl Physiol. 1997;82:531–7. doi: 10.1152/jappl.1997.82.2.531. [DOI] [PubMed] [Google Scholar]
- 23.Zemva A, Rogel P. Gender differences in athlete's heart: association with 24-h blood pressure. A study of pairs in sport dancing. Int J Cardiol. 2001;77:49–54. doi: 10.1016/s0167-5273(00)00417-4. [DOI] [PubMed] [Google Scholar]
- 24.Pavlik G, Olexo Z, Banhegyi A, Sido Z, Frenki R. Gender difference in the echocardiographic characteristics of the athletic heart. Acta Physiol Hung. 1999;86:272–8. [PubMed] [Google Scholar]
- 25.Stefani L, Pedrizzetti G, De Luca A, Mercuri R, Innocenti G, Galanti G. Real-time evaluation of longitudinal peak systolic strain (speckle tracking measurement) in left and right ventricles of athletes. Cardiovasc Ultrasound. 2009;7:17. doi: 10.1186/1476-7120-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galanti G, Toncelli L, Del Furia F, Stefani L, Cappelli B, De Luca A, et al. Tissue doppler imaging can be useful to distinguish pathological from physiological left ventricular hypertrophy: a study in master athletes and mild hypertensive subjects. Cardiovasc Ultrasound. 2009;7:48. doi: 10.1186/1476-7120-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warburton DE, Haykowsky MJ, Quinney HA, Blackmore D, Teo KK, Humen DP. Myocardial response to incremental exercise in endurance-trained athletes: influence of heart rate, contractility and the Frank-Starling effect. Exp Physiol. 2002;87:613–22. doi: 10.1113/eph8702372. [DOI] [PubMed] [Google Scholar]
- 28.Wiebe CG, Gledhill N, Warburton DER, Jamnik VK, Ferguson S. Exercise cardiac function in endurance-trained males versus females. Clin J Sport Med. 1998;8:272–9. doi: 10.1097/00042752-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 29.D’Andrea A, Cocchia R, Riegler L, Scarafile R, Salerno G, Gravino R, et al. Left ventricular myocardial velocities and deformation indexes in top-level athletes. J Am Soc Echocardiogr. 2010;23:1281–8. doi: 10.1016/j.echo.2010.09.020. [DOI] [PubMed] [Google Scholar]