Abstract
Background & objectives:
The complementary and alternative medicines (CAM) have not been systematically evaluated for the management of HIV/AIDS patients. In a prospective, single-site, open-label, non-randomized, controlled, pilot trial, we evaluated a polyherbal formulation (PHF) for its safety and efficacy in treating subjects with HIV-AIDS.
Methods:
A total of 32 and 31 subjects were enrolled under the PHF and highly active antiretroviral treatment (HAART) arms, respectively, and followed up for a period of 24 months. Plasma viral RNA, CD4 cell count and blood chemistry were monitored at 3-month intervals. Following mid-term safety evaluation, 12 subjects from the PHF arm were shifted to HAART and were followed separately as PHF-to-HAART arm, for the rest of the period.
Results:
The HAART arm was characterized by significant improvements in CD4 cell count (154.4 cells/μl/year, P<0.001) and reduction in plasma viral load within 3 to 6 months (-0.431+ 0.004 log10 IU/month, P<0.001). In contrast, the PHF arm showed a profile of CD4 cell loss at remarkably slower kinetics (14.3 cells/μl/year, P=0.021) and insignificant reduction in the viral load. The PHF and HAART arms did not differ significantly in the occurrence of AIDS-related illnesses over the study period of 24 months. In the PHF-to-HAART arm, the rates of CD4 count and reduction in viral load were significant and comparable to that of the HAART group. In the PHF arm, at 1 month, a significant increase in CD4 cell count and a concomitant decrease in viral load were seen.
Interpretation & conclusions:
The PHF appears to have provided protection by delaying the kinetics of CD4 cell reduction. Given the several study limitations, drawing assertive inferences from the data is challenging. Future studies with a stringent study design are warranted to confirm these findings.
Keywords: Chronic immune activation, complementary and alternative medicine, disease progression, HIV-AIDS, human clinical trial, immunomodulation, polyherbal formulation
Despite success in improving clinical prognosis, the application of highly active anti-retroviral therapy (HAART) for HIV/AIDS suffers from several limitations including drug resistance, toxicity, adherence, treatment duration, high costs, limited immune reconstitution and lack of viral eradication. Further, in the absence of an effective vaccine, complementary and alternative medicines (CAM) have become an alternative option to vast majorities of people in industrialized as well as resource-constrained countries regardless of the economic and cultural differences1. Only a few clinical trials have been conducted to evaluate the immunomodulatory properties of CAM and herbal medicines for HIV/AIDS. Most of these clinical trials were limited in terms of study design, allocation concealment, high rates of loss to follow up, lack of pre-defined end points, inadequate drug description, small study size and short duration, and absence of rigorous statistical evaluation, etc2,3.
India is native for several systems of CAM including Ayurveda, Siddha, Unani and Yoga in addition to the popular use of Homeopathy and Naturopathy and many others4,5. Several thousands of medicines presently in use and listed in modern pharmacopoeia are of herbal origin with many of these having anti-microbial and/or immuno-modulatory properties6. The pathogenesis of HIV-1 infection is significantly associated with the loss and/or dysfunctioning of the CD4 T-cell which is believed to be the consequence of chronic activation of the immune system7. Any therapeutic strategy that could alleviate the immune activation, therefore, must confer significant clinical benefit in HIV/AIDS.
We report here the evaluation of an Indian polyherbal formulation (PHF), a unique herbal extract and mineral combination devoid of heavy metals, in a pilot clinical trial, for its safety and efficacy for treating patients with HIV-AIDS. We also report findings on stabilization of CD4 count and viral load and clinical progression in study participants during a follow up of 24 months.
Material & Methods
Study participants: All the study participants were recruited, counseled, and monitored at the Seva Free Clinic which provides free clinical services in the city of Bangalore, Karnataka, India, under the auspices of Samraksha, a non-governmental organization (NGO). The potential volunteers were subjected to four or five rounds of counselling by trained counsellors at the Seva Free Clinic and admitted to the study after obtaining an informed consent form. The study participants were balanced at baseline for several characteristics including gender, age group, location, income, occupation, education and marital status (Table I). A total of 475 potential volunteers were screened for CD4 count and 63 of them were qualified by the selection criteria. The study participants, all hailing from the communities within and around the city of Bangalore, were regular attendees receiving medical attention at the Seva Free Clinic. The study participants have been recruited in several batches over a period of 9 months (Fig. 1).
Table I.
Baseline characteristics of the study participants
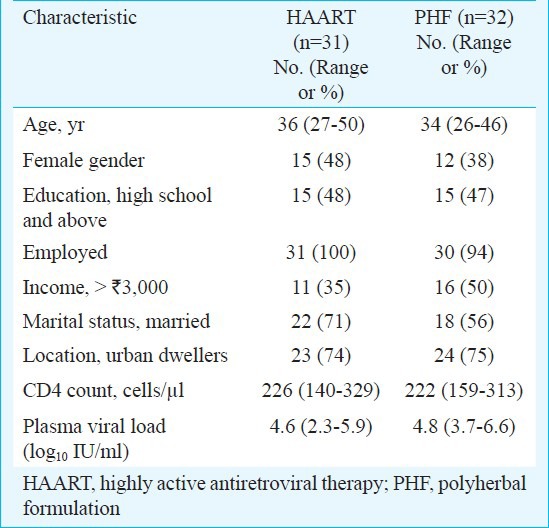
Fig. 1.
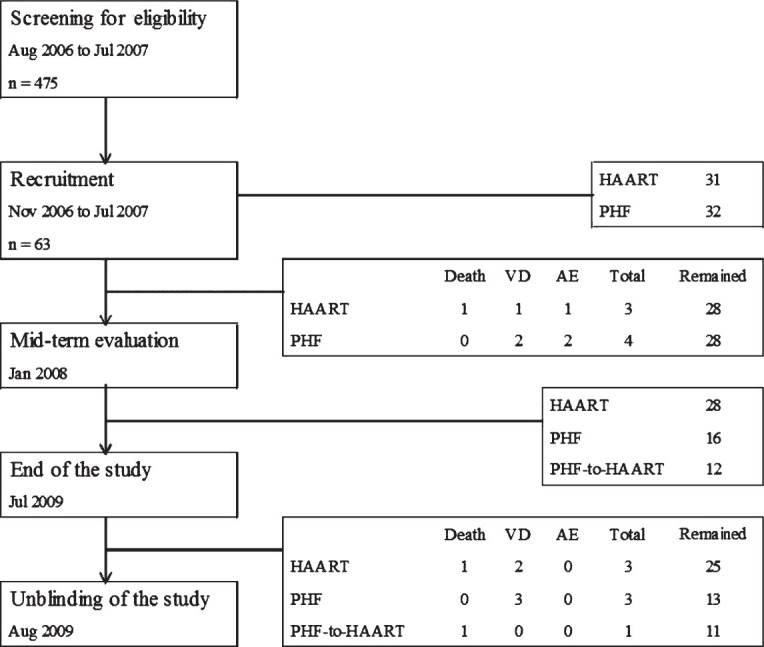
Trial profile. PHF, polyherbal formulation; VD, voluntary dropout; AE, adverse event; HAART, highly active antiretroviral treatment.
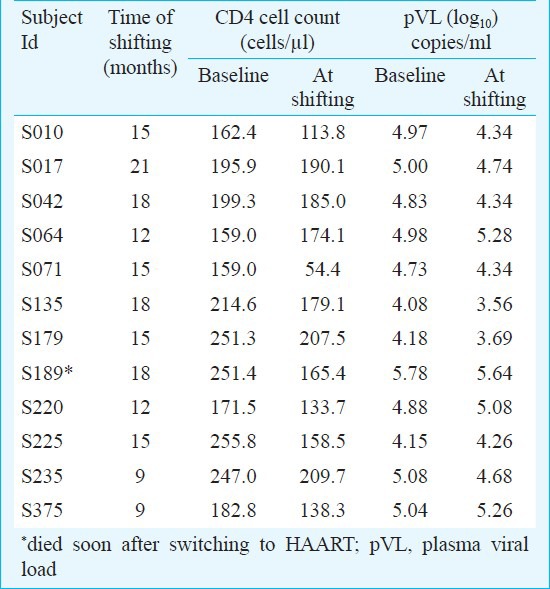
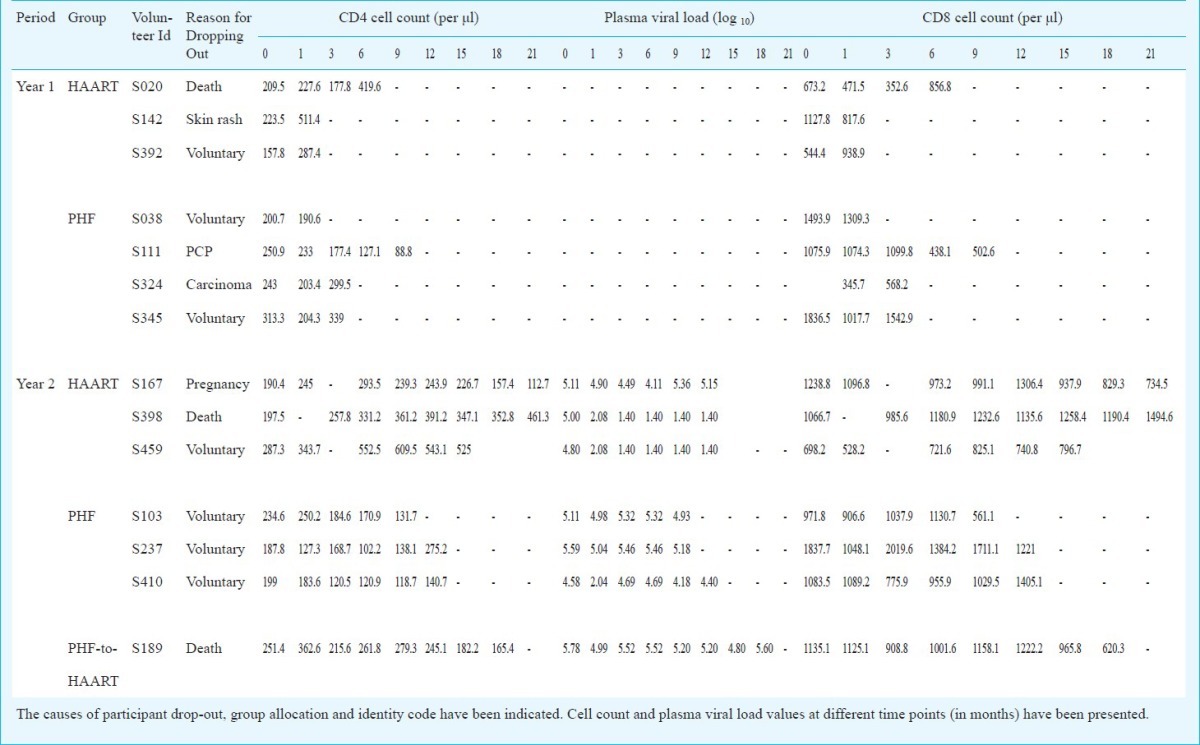
Trial characteristics: In a prospective, single-site, open-label, non-randomized, controlled, investigator-blinded trial, polyherbal formulation (PHF) was compared with highly active anti-retroviral therapy (HAART) for safety and efficacy in treating HIV/AIDS. Participants in both arms of the study, 31 and 32 under HAART and PHF, respectively, were balanced at baseline for several parameters (Table I). The major inclusion criteria were a CD4 count between 200-250 cells/μl and the subjects being drug naïve and free of presenting AIDS-related clinical symptoms. The major exclusion criteria were the presence of opportunistic infections and complications including dementia, signs of acute systemic illness, prior antiretroviral treatment, pregnancy and age below 18 years. During the first year of the study, only two arms of intervention were examined. After midterm safety evaluation, the PHF arm was further divided into two subgroups (Fig. 1) and all the three arms were followed up for the second year of the study. At mid-term safety evaluation, performed approximately 18 months after study initiation and 6 months after recruitment of the last volunteer, it was noted that in some of the participants under the PHF arm, the CD4 counts dropped below 200 cells/μl although there were no clinical manifestations in any of the study participants. Following the institutional ethics committee (IEC) directive, the study participants were informed of this development, counselled to shift to HAART and the decision was left to each participant whether to continue in the PHF arm or to be shifted to HAART. Twelve of the 28 participants of the PHF arm decided to be shifted to HAART and one of them died shortly. Clinical profile of these 12 PHF subjects who have been shifted to HAART has been summarized (Table II). Of the remaining 16 participants who continued in the PHF arm, six had CD4 cells below 200 cells (S006, S103, S170, S177, S237 and S410). Eventually, three of these six participants (S103, S237 and S410) dropped out of the study voluntarily while the other three continued. At mid-term evaluation, three of the 13 participants (S006, S170 and S177) had a CD4 count of approximately 180 cells/μl. After the interim safety analysis, co-trimoxazole prophylaxis was provided to several study participants as per the standard clinical practice.
Table II.
Clinical, immunological and virological profiles of the 12 PHF-to-HAART participants
While the study participants, clinicians and the counselors were in the knowledge of the group allocation, the research laboratory at Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR), Bangalore, responsible for measuring CD4 cell count and viral load was blinded until unblinding was performed. The protocols, donor information brochures, any amendments and patient consent forms were approved by the Institutional Ethics Committee of the NGO Samraksha which monitored the progress of the clinical trial (CTRI No. CTRI/2008/091/000021). The IEC of JNCASR also approved the study. Given that there was no other similar study earlier and that clinical benefits accrued by this formulation were not known, the number of study participants and effect size required for power calculations could not be calculated. It was decided to include 30 participants in each arm of the study hoping that withdrawal rate would be minimal given the involvement of the NGO. The identity of the participants was protected during the study and after it ended. After the clinical trial was formally concluded, all the study participants of the antiretroviral arms were transferred to the National ART programme sponsored by the Government of India.
Medicines and treatment administration: The HAART arm primarily received the administration of 30 mg of stavudine, 150 mg of lamivudine and 200 mg of nevirapine, twice a day as per the 2004 guidelines of The National AIDS Control Organization (NACO), India8. Alternative regimens, with nevirapine substituted with efavirenz/indinavir or stavudine replaced by zidovudine were used as required.
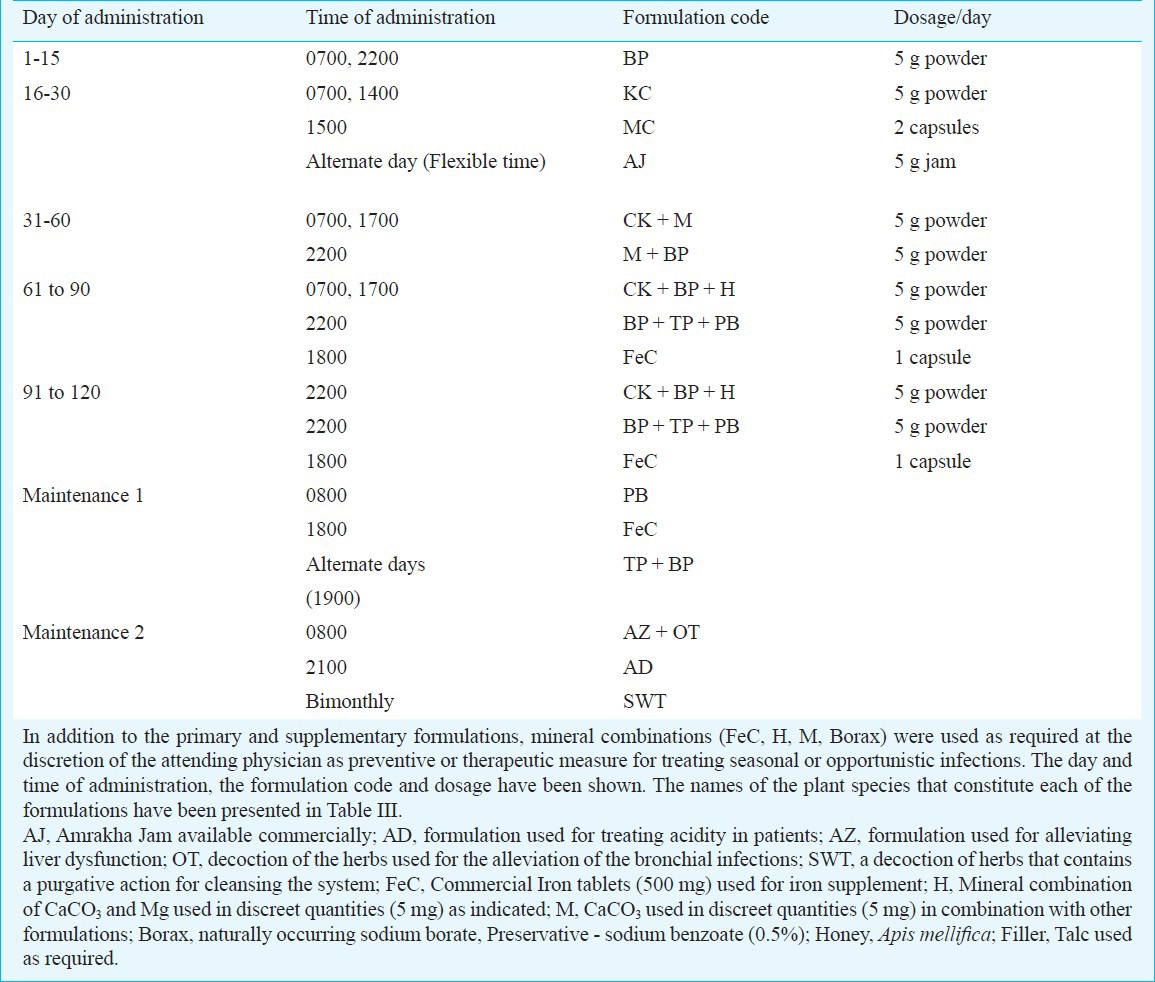
The PHF procured from Vedic Drugs Pvt. Ltd., India, consisted of ingredients from 58 different plant species (Table III) and formulated into 6 primary formulations (FBP, FTP, FKC, FMC, FCK and FPB,) and five supplementary formulations (FAJ, FAD, FAZ, FOT and FSWT). The drug administration schedule of the PHF has been depicted (Table IV). The polyherbal formulations were administered orally only for 4 months following recruitment. Study participants were counselled on the use of the PHF and provided quantities of medicines sufficient between visits to the clinic. To circumvent the generic problem of the highly variable nature of the herbal formulations, a single lot of PHF was used for the entire clinical trial, drug toxicity testing, quality analyses and in vitro virus inhibition assays.
Table III.
Plant species that constituted six primary and five supplementary polyherbal formulations
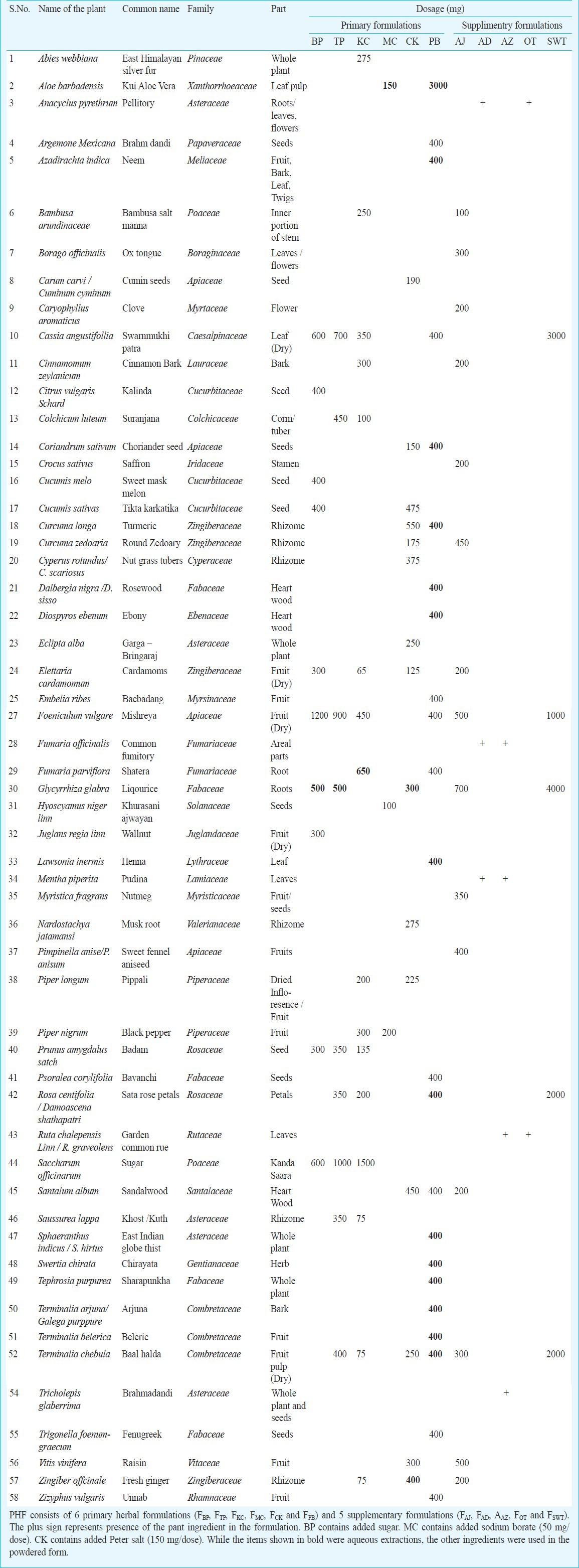
Table IV.
Drug administration schedule of the polyherbal formulation
Adherence and clinical management: Adherence to antiretroviral treatment was monitored by pill count method by checking the number of pills brought back by the participants. More than 95 per cent adherence was found among the study participants. Administration of the antiretroviral medicine was monitored by three trained Allopathy physicians throughout the study period. Side effects in the HAART arm were managed according to guidelines of NACO (National Guidelines on Second-line ART for adults and adolescents 2008)8 by one of the investigators (KSS). At least one of a team of seven physicians affiliated with the Shanmugha Arts, Science, Technology and Research Academy (SASTRA) University, Tanjavur, Tamil Nadu, India was available at the Clinic to monitor the clinical trial throughout the duration of the study. Minor complications in the PHF arm were managed by the attending CAM clinicians and serious manifestations were referred to KSS. The costs of treatment under both of the arms and other incidental costs were borne by the research project.
Unblinding of the study: The study was unblinded on August 8, 2009 in the presence of an Unblinding Committee consisting of three members not related to study. Hard copies of the data sheets containing raw CD4 counts and viral load values from the JNCASR laboratory and the allocation code from The Seva Free Clinic were presented to the Committee members. Each of the original data sheets and the clinical code were attested by all the Committee members and placed in the possession of the Committee Chairman.
Drug toxicity testing: Drug toxicity testing for the PHF was performed by Sri Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvanathapuram, Kerala, India. Three different tests - acute oral toxicity, sub-acute oral toxicity and sub and chronic oral toxicity tests - performed in rats confirmed the non-toxic nature of the polyherbal formulation (data not shown).
Study end points: The primary end points of the study were CD4 count and the plasma RNA viral load. Secondary end points were manifestation of AIDS-related clinical symptoms including opportunistic infections and/or death, as defined by Centers for Disease Control and Prevention (CDC)9.
Blood collection and processing: A peripheral blood sample of 50 ml was collected from each participant at each of the following time points, day 1, day 14, months 1, 3 and at 3 month intervals thereafter up to month 24. The blood samples were collected between 10.00 to 12.00 h and sent to the JNCASR laboratory within 4 h after collection. Although this was an open-label study, the samples were sent under a code to blind the investigators at JNCASR. A part of the blood was used directly for CD4 cell count determination while from the remaining blood, mononuclear cells (PBMC) were isolated using density gradient centrifugation using Ficol-Hypaque. PBMCs were stored in multiple aliquots in liquid nitrogen. At every sample collection, blood was analyzed for the following parameters – differential blood count, haemoglobin, erythrocyte sedimentation rate (ESR), liver enzymes (ALT, AST), random blood sugar, urea, and creatinine. Levels of sodium, potassium, and chloride ions were checked at baseline and at the end of every year. Further, thymus scans were performed at baseline and at the end of the study. Helical scan was performed starting from apices of lungs down to mid chest level by employing 5 mm sections.
CD4 cell count determination: CD4 cell counts were determined 12-24 h from the time of sample collection using MultiTest antibodies, counting beads (340499 and 349480, Becton and Dickinson, USA) and the Lyse-No-Wash protocol as described by the manufacturer. CD4 cell count of each sample was performed in duplicate. An Immunotrol control sample (660798, Beckman and Coulter) was included in each run to validate the analysis. Samples were acquired on a BD FACSCalibur and instrument calibration was performed using Calibrite beads (340486 and 340487, Becton and Dickinson, USA).
Plasma viral load quantitation: Plasma was separated from whole blood within 6 h of collection and stored frozen in a deep freezer as aliquots of 0.5 ml. Viral loads were assayed using NucliSens EasyQ v1.1.1 (bioMerieux, France). Viral loads below the detection limit of <25 IU/ml, were assigned a value of 25 IU/ml. A plasma sample of known viral load and a different sample negative for the virus were included in each run and the assay was validated only if the internal controls gave expected results. Samples were thawed only once and used immediately for estimating viral load. The CD4 cell count and viral load were determined at every time point of sample collection from every study participant.
HLA typing of the subjects using genomic DNA: HLA typing of all the study participants was performed at 2 digit resolution for MHC Class I loci A and B and the Class II loci DRB1, 3, 4 and 5. Genomic DNA extracted from frozen PBMCs using an in-house protocol was subjected to PCR using sequence-specific primers essentially as described previously10. Allele frequencies of this cohort was compared to the frequencies observed in the general southern Indian population (obtained from the dbMHC database, http://www.ncbi.nlm.nih.gov/projects/gv/mhc/main.fcgi?cmd=init). Alleles were classified as either protective or suspectible to HIV infection and disease progression11,12,13. Statistical testing was performed using chi-squared test.
In vitro virus-inhibition assay: The PHF formulation was dried and the aqueous phase was extracted in a Soxhlet extractor at 100 °C for 36 h. The crude extract was cooled and dried under reduced pressure and stored at -20 °C until use. The remaining material was dried at room temperature and extracted with ethyl acetate at 80 °C for 24 h. The extract was cooled, dried and stored at -20 °C until use. To test in vitro activity, CEM CCR5 T-cells were treated with varying amounts of the extract (aqueous extract re-dissolved in water and ethyl acetate extract in DMSO) for 72 h. Viability was tested by staining the cells with 7 amino actinomycin (7-AAD) and per cent dead cells was assessed by flow cytometry (FACSCalibur, Becton and Dickinson, USA). Camptothecin treatment was used as positive control for cell death. Cell proliferation was assessed by determining live cell counts using Trypan blue. Antiviral activity was estimated using CEM CCR5 cells infected with NL4-3 strain14. Supernatants were collected on day 5 and the levels of p24 were assayed by capture ELISA using a commercial kit (Microlisa, J. Mitra and Co, Bangalore, India).
Immunophenotying of the T-cells in the clinical samples using flow cytometry: Frozen PBMC were thawed and rested overnight in RPMI medium supplemented with 10 per cent fetal calf serum (FCS) and antibiotics. On the following day, 1x106 cells were stained with 100 μl of viability dye (L23101, Invitrogen, USA) for 30 min. Cells were washed once in 2% FCS and stained with anti- CCR7-Cy7-PE (557648, BD, USA) in a final volume of 50 μl for 20 min at 37°C in azide-free buffer. CD4 T-cells were classified as recent thymic emigrants (CD45RA+ CD31+), naïve (CD45RA+ CCR7+), central memory (CD45RA- CCR7+), effector/effector memory (CD45RA- CCR7-) and Ki67+. Cells were washed and stained with a 100 μl cocktail of antibodies to other surface antigens - CD31 PE (555446, BD, USA), CD4 PerCP (347314, BD, USA), CD45RA APC (550855, BD, USA) and CD3 APC-H7 (641397, BD, USA)- for 30 min on ice, fixed in 4 per cent PFA, permeabilized with Perm/Wash buffer (BD, USA) and fixed again in 0.5 per cent PFA. In an alternate panel, the CD31 PE antibody was replaced with intracellular Ki67 PE (556027, BD, USA) staining. Only samples with >70 per cent viable cells were included in the analysis. A minimum of 9,000 live CD3+ CD4+ events was acquired on a BD FACSCanto and analyzed using FlowJo 7.6.1 for Windows (TreeStar, USA). Compensation was performed using single-stained controls and gates were set using appropriate FMO controls. Data was analyzed using t-tests with Bonferroni correction.
Statistical analyses: Longitudinal analyses were carried out individually for each dependant variable using generalized estimating equations (GEE) with time as a repeated factor under a first-order auto-regressive covariance structure. The values of the measured clinical parameters are presented as mean + 1 SD with 95% confidence intervals (CI). Duplicate T-cell counts at each time point were averaged and viral load was log10-transformed. The analysis utilized a total of 500, 475 and 535 observations for CD4 count, CD8 count and plasma viral load, respectively. The missing values in CD4 count and plasma viral load (<10% of total observations) were not imputed. Within-group longitudinal analysis was performed as a comparison of each time point with the baseline and P values are presented with and without Bonferroni correction. Inter-group means at each time point were compared by an unpaired two-tailed t-test. Slopes of the dependant variables were obtained by linear regression. Incidence of opportunistic infections was analyzed using Kaplan-Meier survival curves and significance using logrank test. Voluntary dropouts were censored at the time of leaving the study. Statistical testing was performed with PASW18.0, Hog Kong and GraphPad Prism 5.0, USA and P values below 0.05 were considered to represent statistical significance. The data have been presented as mean, rather than median values as the number of observations has fallen to small figures in the PHF and PHF-to-HAART groups especially in year-2. The statistical analysis included all data points available at any given point even after some volunteers left the study, died or lost for follow up and this was applicable to all the three arms of the study. The missing values were ignored and not imputed for statistical evaluation. Statistical evaluations based on median values, however, provided similar inferences. Values from the month 1 time point were excluded from the linear regression as it was significantly different from the other time points and tended to alter slope values.
Results
Baseline characteristics: Between August 2006 and July 2007, a total of 475 volunteers were screened and 63 subjects were identified who were qualified for the study (Fig. 1). Following several rounds of counselling, 31 and 32 subjects voluntarily opted to join the HAART and PHF arms of the study, respectively. The study was terminated in July 2009 and the unblinding protocol was performed in August 2009. At baseline, the mean CD4 count and viral loads were comparable between the two groups (Table I). By the time the study was terminated at 24 months, 14 of the 63 participants (Fig. 1) discontinued for one of the following reasons - voluntary decision (8 subjects), adverse events (3 subjects) and death (3 subjects). The clinical profiles of the 14 subjects who dropped out of the study are summarized in Table V. Some of the participants dropped out of the study at an early point and sufficient number of data points could not be collected from them, and data from such participants were not included in the data analysis.
Table V.
Clinical, immunological and virological profile of the 14 participants who dropped out of the study
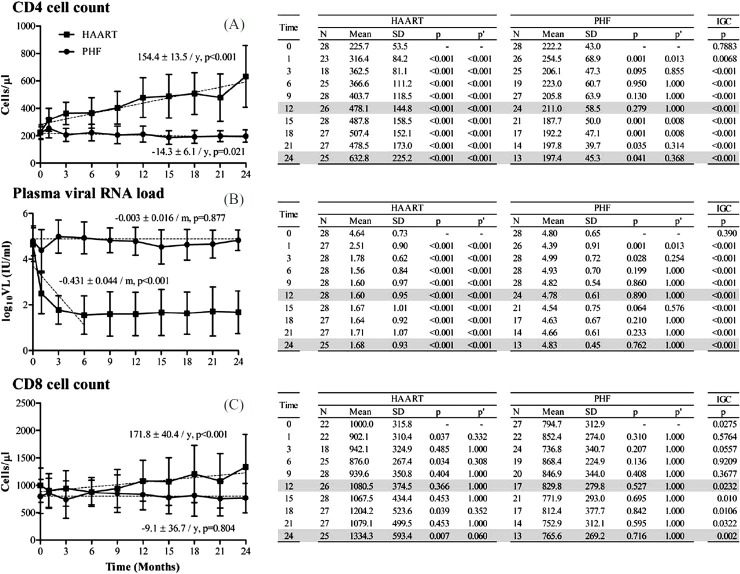
Primary end points: In the HAART arm, a progressive enhancement in the mean CD4 count was observed from the time anti-retroviral therapy was initiated. The mean CD4 count increased from 225.7 ± 53.5 cells/μl (95% CI, 205.9-245.5) at baseline to 632.8 ± 225.2 cells/μl (544.5-721.0) at 24 months (Fig. 2, top panel) translating to a recovery of 154.4 ± 13.5 cells/μl per year (R2 = 0.3419, P<0.001). Concomitantly, a steep suppression in the viral RNA load was achieved in 26 of the 28 participants within 3-6 months (Fig. 2, middle panel). The mean baseline value of 4.64 ± 0.73 log10 IU/ml (4.37-4.91) was reduced to 1.78 ± 0.62 log10 IU/ml (1.55-2.01) within 3 months (P<0.001), an approximate reduction of three orders of magnitude, and remained at the low level for the rest of the study period with a slope of -0.431 ± 0.044 log10 copies per month (R2 = 0.472, P<0.001). The CD8 count remained largely stable over this period with 1000.0 ± 315.8 (868.1-1132.0) and 1334.3 ± 593.4 cells/μl (1101.6-1566.9) at the baseline and 24 months, respectively (P= 0.007) (Fig. 2, bottom panel).
Fig. 2 (A-C).
T-cell count and plasma viral RNA load profiles in HAART and PHF arms of the study. All values calculated using generalized estimating equations model are presented as mean ± 1 SD. P values are presented without (p) or with (p’) Bonferroni correction. IGC represents P value of inter-group comparison by an unpaired t-test. The kinetics of T-cell reconstitution or viral load reduction is estimated using linear regression analysis (dotted lines). Under HAART, the regression slope for viral load analysis was based on observations only up to 6 months. The slope kinetics and P values are shown. Months 12 and 24 are highlighted only for clarification.
In the PHF arm, the mean CD4 count of 222.2 + 43.0 cells/μl (206.3-238.1) at the baseline appeared to have remained stable over the study period of 24 months. This was in spite of a perceptible loss in the mean CD4 count, to 211.0 ± 58.5 (187.6-234.4) and 197.4 ± 45.3 cells/μl (172.7-222.0) at 12 and 24 months, respectively (Fig. 2A). Using a post-hoc analysis, the observed power of the study for the difference at 24 months was 56 per cent. The slow kinetics of the CD4 cell reduction in the PHF arm, 14.3 ± 6.1 cells/μl lost per year (R2 = 0.029, P=0.021), contrasted that of the HAART and the difference between the two arms was statistically significant at all the time points (P<0.001) subsequent to the baseline. The CD4 count of the PHF participants was determined on more than one occasion, at and around baseline, at screening (typically 4 to 6 wk prior to recruitment), at recruitment (day 0) and at day 15 and found to be stable. The viral load, on the other hand, remained stable over the study period with 4.80 ± 0.65 log10 IU/ml (4.56-5.04) at the baseline and 4.78 ± 0.61 (4.54-5.03) and 4.83 ± 0.45 log10 IU/ml (4.59-5.07) at 12 and 24 months, respectively, (Fig. 2B) these changes being non-significant. Likewise, the CD8 count of 794.7 ± 312.9 cells/μl (676.7-912.7) at baseline in the PHF arm, did not change significantly at 12 or 24 months with 829.8 ± 279.8 (696.8-962.8) and 765.6 ± 269.2 cells/μl (619.3-911.9), respectively (Fig. 2C).
The secondary end points and adverse events: The occurrence of AIDS-defining illnesses was analyzed in all the three arms (Fig. 3). At the termination of the study, there was no significant difference among the three arms in either AIDS-defining or other illnesses. All the parameters of the blood chemistry determined at every visit largely remained within normal reference ranges in all the study participants (data not shown). Appearance of several adverse events has been recorded in all the three arms of the study. In the HAART arm, chronic diarrhoea, one event of lymphoma followed by death, skin rash, herpes zoster, pulmonary tuberculosis, extrapulmonary tuberculosis, hodgkins lymphoma and nevirapine-related allergy were observed. In the PHF arm, appearance of Herpes zoster, Pneumocystis carinii pneumonia, oral ulceration, persistent lymphadenopathy, skin lesion, oesophageal carcinoma, and an event of extrapulmonary tuberculosis were seen. In the PHF-to-HAART arm, thrombocytopenia, skin lesion, pulmonary tuberculosis, herpes zoster, cryptococcal meningitis and oral candidiasis were observed.
Fig. 3.
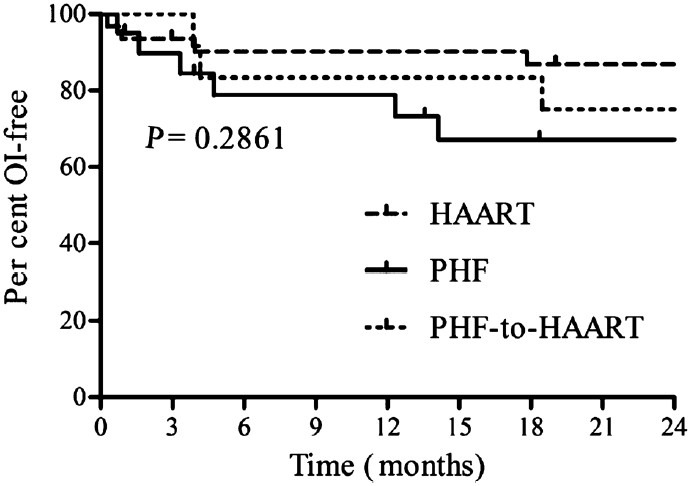
AIDS-related clinical manifestations: Kaplan-Meier plots depicting the incidence of AIDS-related illnesses including death, infectious diseases and malignancies under the HAART, PHF and PHF-to-HAART arms of the study. Opportunistic infections (OI) were classified according to the CDC system. Statistical significance was tested using logrank test.
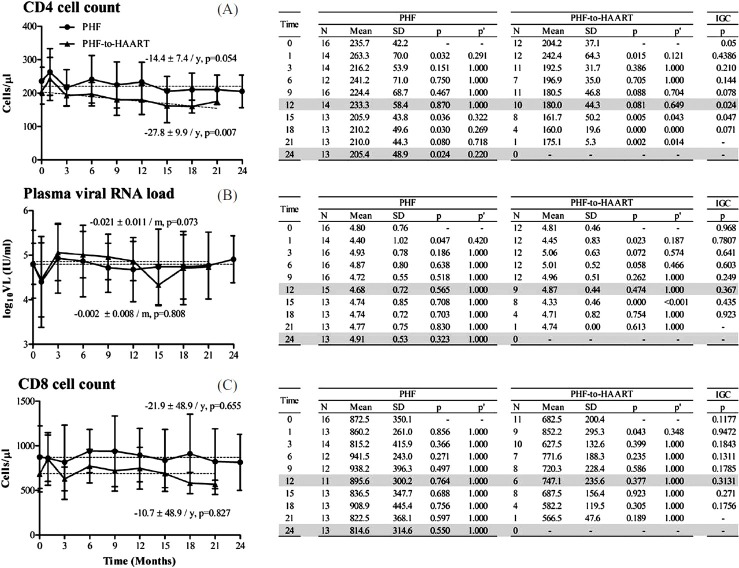
Immunological and virological responses in the PHF versus PHF-to-HAART arms: After the PHF arm was divided, a retrospective comparison performed between the 16 remaining PHF subjects and the 12 PHF-to-HAART participants led to several interesting observations (Fig. 4). First, at baseline, these two groups differed significantly in the mean CD4 count (P=0.05). Second, the CD4 count remained relatively stable in the PHF group over 24 months. In contrast, in the PHF-to-HAART group, the reduction in the CD4 count became significant at 15 months (P=0.043) and thereafter, although fewer data points were available for this analysis. Lastly, the rate of CD4 cell loss in PHF-to-HAART group (27.8 ± 9.9 cells/year) was nearly twice that of the PHF group (14.4 ± 7·4 cells/year).
Fig. 4.
T-cell count and plasma viral RNA load profiles in the PHF and PHF-to-HAART arms of the study. Data presentation as described in Fig. 2. The original PHF arm was stratified into two subgroups after the mid-term safety evaluation. All data from the PHF-to-HAART arm have been collected prior to the initiation of the antiretroviral therapy.
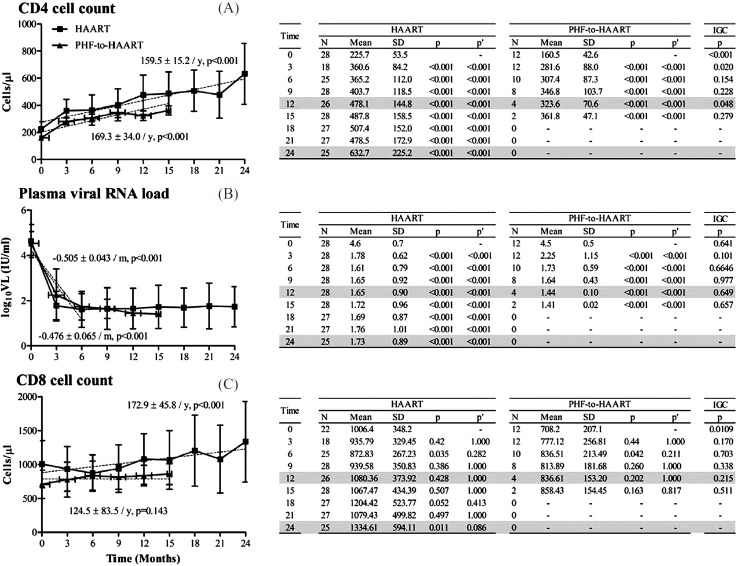
Immunological and virological responses in the HAART versus PHF-to-HAART arms: Through a similar comparative analysis between the HAART and PHF-to-HAART subgroups, a statistically significant improvement was observed in the CD4 count in both the groups at a rate comparable between HAART and PHF-to-HAART arms with 159.5 ± 15.2 and 169.3 ± 34.0 cells reconstituted per year, respectively (Fig. 5A), suggesting efficient immune reconstitution even in the PHF-to-HAART group regardless of low CD4 cell count at the inception. While the profiles of viral load reduction were indistinguishable between these arms (Fig. 5, middle panel), the CD8 count remained stable in both the arms (Fig. 5C).
Fig. 5 (A-C).
T-cell and plasma viral RNA profiles in the HAART and PHF-to-HAART arms of the study. Data presentation as described in Fig. 2. Month 1 observations from the HAART group have been omitted from the data analysis since the PHF-to-HAART arm did not contain a corresponding time point. The mean ± 1 SD values presented here under HAART therefore differ marginally from those of Fig. 2. The ‘zero’ time point under PHF-to-HAART corresponds to the first time evaluation after the subject has been started on antiretroviral therapy.
Presence of specific HLA alleles in the arms of the study: Specific HLA alleles are known to be strongly associated with protection or susceptibility in HIV-1 infection. To understand if the HLA allele distribution between the HAART and PHF arms of the study differed from each other, we determined the HLA profile of each of the study participants. Additionally, allele frequencies of the two arms were compared to the frequencies observed in the general population of southern India (retrieved from the dbMHC database, http://www.ncbi.nlm.nih.gov/projects/gv/mhc/main.fcgi?cmd=init). This analysis failed to identify a significant association between the study arms with any of the alleles known to promote disease progression or resistance in the viral infection (Fig. 6). This observation ruled out the possibility that the HLA profile differences between the study arms could have influenced the clinical outcome.
Fig. 6.
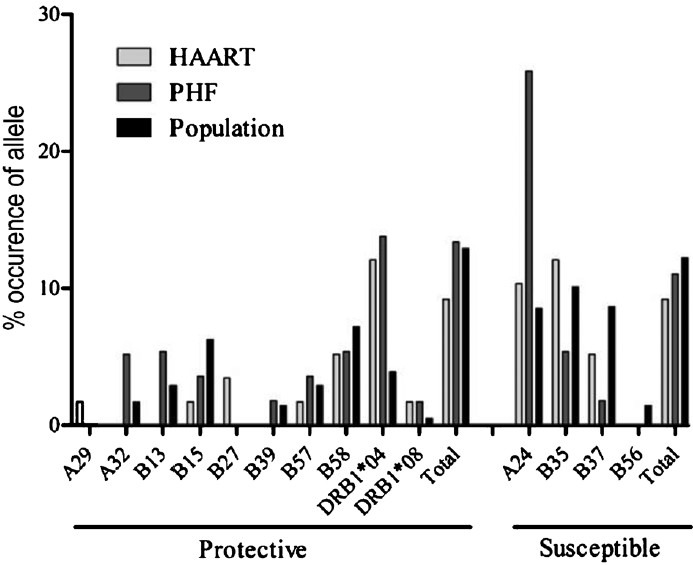
HLA alleles in the HAART and PHF groups in comparison to the general populations of India. The PHF and PHF-to-HAART arms were pooled due to insufficient numbers. Allele frequencies were calculated using the HLA comparison software available at HIV Molecular Immunology Database (http://www.hiv.lanl.gov/content/immunology/hla/hla_compare.html). HLA allele frequencies of the southern India population were obtained from dbMHC database. Statistical significance was tested using chi-squared test.
Transient enhancement in CD4 cell count and immunemodulation: Following one month of PHF administration, a significant but transient increase was observed in mean CD4 count in 17 of the 26 PHF participants. The mean CD4 count of 222.2 ± 43.0 cells/μl at baseline increased to 254.5 ± 68.9 cells/μl from (228.0-281.0, P=0.001) with a corresponding reduction in the viral load from 4.80 ± 0.65 to 4.39 ± 0.91 log10 IU (4.04 to 4.74, P=0.001) (Fig. 2A, B). A polychromatic flow cytometry analysis of the CD4 cells was done, drawn from six representative subjects - at baseline and months 1 and 3. CD4 T cells were classified as naïve (CD45RA+ CCR7+), central memory (CD45RA- CCR7+), effector (CCR7-), recent thymic emigrant (CD45RA+ CD31+) and proliferating (Ki67+) cells. There was a significant increase in the number of total CCR7+ cells (P=0.010) at month 1. When the CCR7+ cells were further divided into central memory and naïve, the increase was observed in central memory (P<0.001) but not in naïve cells. There were no notable differences in the effectors and proliferating cells. Similar transient changes in CD8 cell count was also observed although not reaching statistical significance but correlating with changes in CD4 count (data not shown). A consistent enhancement in the CD4 cells in a majority of the study participants was suggestive of the immunomodulatory property of the PHF.
Discussion
The main finding of the present study was the likely stabilization of the clinical profile, especially the CD4 count, in the PHF arms. However, the significance of changes in the CD4 count in the PHF arm depended partly on the statistical model used for evaluation. The mean rate of CD4 cell decrease in the PHF arm was 14.3 ± 6.1 cells/μl per year when regression analysis was performed. A more rigorous analysis performed using the Generalized Estimating Equations showed a non-significant mean CD4 cell reduction from baseline 222.2 cells/μl to 211.0 cells/μl at 12 months. At 24 months, this number dropped further which was significant compared to the baseline value but not if Bonferroni correction was applied. There is no consensus among the statisticians under what circumstances this correction must be used15. Application of Linear Mixed Model to evaluate the significance of CD4 cell changes demonstrated results similar to the GEE model (data not shown).
At the time the study was terminated, the three arms of study did not differ from one another significantly in AIDS-related clinical manifestations. Only two studies from India examined the survival rate in AIDS in subjects with <200 CD4 cells/μl. Kumarasamy et al16 observed that the median duration of survival in 71 drug naive subjects was 33 months, and Hira et al17 reported a median survival period of only 19.2 months after developing AIDS. We did not find AIDS-related mortality in the participants in the PHF arms with the exception of a single death. A few studies previously reported the existence of seropositive individuals whose CD4 counts dropped below 200 cells/μl but who remained symptom-free for extended periods18,19.
The present study had several limitations. First, the study was an open-label trial. Given the nature of the medicines, the identity of the medicines could not be concealed from the participants, therefore, the placebo effect could not be eliminated from the study outcome. The investigators at the JNCASR laboratory were however, blinded to the identity of the group allocation until the time of unblinding. Second, participant recruitment could not be randomized given that the PHF formulation was not a conformed medicine. In an open-label study, non-randomization is permitted especially for ethical reason19. Third, lack of a placebo group to compare the study outcome imposed limitations on the study interpretation. As the issue of the placebo inclusion is highly contentious21, the HAART arm was used for the purpose of comparison. Fourth, the quality control of the herbal medicines remains a universal problem with variations in the formulations, ingredients and administration. We prepared a single lot of the PHF that was used for the entire clinical trial, toxicity studies and laboratory assays thus partially addressing this question. Fifth, given the recruitment criteria, a ‘selection bias’ may have crept into the study design. Although the study subjects cannot be technically qualified as long-term non-progressors or elite controllers, some of them being intrinsically resistant to disease progression cannot be ruled out. Lastly, co-trimoxazole prophylaxis was offered to all the HAART and several PHF participants especially during year 2. Cotrimoxazole prophylaxis can have significant impact on the incidence of opportunistic infections and prognosis. However, the magnitude of benefits conferred by the prophylaxis is quite variable ranging from 0 to 50 per cent22. In spite of these limitations, inferences drawn from this prospective study were based on a large number of repeat observations from each participant. Monitoring the AIDS-related illnesses added strength to the present study.
The absence of apparent disease progression in the PHF participants could be partly explained if the CDC classification of AIDS-related clinical stages8 is not applicable for India. The validity of the CDC classification system for non-caucasoid ethnic groups of many countries has been challenged23,24,25. A recent study commissioned by The National AIDS Control Organization (NACO) of India conducted simultaneously at eight different centres using the two-color immunophenotyping technique and uniform protocols and quality control measures and involving a total of 1,200 subjects representing diverse populations of India, identified that the mean CD4 count in these populations was 918.85 ± 311.39 cells/μl26 similar to that of the Caucasoid populations. Thus, absence of AIDS-related clinical symptoms in the PHF groups at or below 200 CD4 cell/μl may not be attributed to ethnic differences in average CD4 count.
We asked if the kinetics of CD4 cell reduction, rather than the absolute CD4 count and/or cell percentage, could serve as a more reliable prognostic marker27,28 to explain the slower CD4 cell loss observed in the PHF arm. Only two studies from India examined CD4 cell loss kinetics to date, and neither of them could be representative for technical reasons29,30. Several investigators mainly from the pre-HAART era, monitored the fate of the CD4 cell kinetics in drug-naïve subjects, however, only a few considered cell loss at or below 200 cells/μl. In a study of 111 haemophiliacs in London, followed up for more than 7 years27, it was identified that the median slope for CD4 cell decline was 80 CD4 cells/year and this value was steeper for those who developed AIDS. This study proposed a uniform cell decline through all the stages of the viral infection including below 200 cells/μl. Another study involving 579 seropositive homosexual men in Baltimore, USA, with a follow up of 4 years demonstrated a CD4 cell loss of 189 cells/year in those who progressed to AIDS in less than one year18. Urassa et al31 in a prospective study with 171 seropositive subjects in Tanzania showed that the median CD4 cell loss was 52 cells/year in 35 subjects who progressed to AIDS and 22 cells in 156 participants that did not. In a prospective study among 187 homosexual men in Amsterdam and with a follow up of 5 years, the authors proposed a bimodal decline in the CD4 count among those participants who progressed to AIDS32. The CD4 cells declined at a slower rate of 40 cells/year until the level of 400 cells/μl reached and fell at a faster rate of 188 cells/year and the rate of cell reduction continued at the same pace even after 200 cells. The CD4 cell loss observed in the PHF and PHF-to-HAART arms in the present study, both in the absence of antiretroviral therapy was significantly smaller than that reported previously.
Given the evidently stabilized immune profile of the PHF arm, we tried to find if the immune function of the T-cell significantly improved in these subjects. It was reasoned that if the polyherbal formulation improved the quality of life of the study participants, such an improvement is likely reflected in the quality of the T-lymphocyte function itself. Extensive immunophenotyping of the CD4 T-cells, in a cross-sectional analysis, suggested reduced levels of immune activation of the T-cell with respect to four different biomarkers (Mangaiarkarasi et al personal communication). In this backdrop, the reduced serum levels of the liver enzymes in the PHF arm may be of significance. This is suggestive of hepatotoxicity which is manifested in several infections including in HIV-AIDS.
Considering the inherent limitations of the present study, it is not possible to ascertain the clinical benefits of the PHF. Another study, therefore, is urgently warranted with a stringent study design incorporating double-blinding, randomization and larger number of participants. To eliminate selection bias, participants with higher CD4 count need to be included, preferably with >350 cells/μl in light of the revised guidelines of the NACO8. Use of the placebo control will be the most critical component of the study design since it will permit meaningful interpretation of the data. It would also be critical to examine if the PHF formulations could complement the standard antiretroviral therapies.
Acknowledgment
Authors thank the study participants, the staff at The Seva Free Clinic and the NGO, Samraksha for their active participation, and acknowledge Dr Barbara Shacklett for help with flow cytometry protocols, Dr S. Vijaya for making FACS Canto available for polychromatic flow cytometry, Drs K.R. Srinivas and Umesh Waghmare for help with some statistical models and Aruna Waghmare for making the database for data management, and Ms. Shveta Jaishankar for help in preparing some of the data tables. Technical assistance by Mr. Rasheed Ahmed G Maniyar is acknowledged. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: pNL4-3 from Dr Malcolm Martin and bicyclam JM-2987 (hydrobromide salt of AMD-3100) from DAID. The first author (MA) was a recipient of the Council of Science and Industrial Research fellowship from the Government of India. This work was supported by grants to the last author (UR) from the Department of Science and Technology, Government of India (Grant Nos. VI-D&P/11/2003-04/TT and VI-D&P/327/09-10/TDT). Authors pay special tribute to the memory of Ms Shashikala, a counsellor who initiated this study in the clinic.
References
- 1.Astin JA. Why patients use alternative medicine: results of a national study. JAMA. 1998;279:1548–53. doi: 10.1001/jama.279.19.1548. [DOI] [PubMed] [Google Scholar]
- 2.Ozsoy M, Ernst E. How effective are complementary therapies for HIV and AIDs?--A systematic review. Int J STD AIDS. 1999;10:629–35. doi: 10.1258/0956462991913088. [DOI] [PubMed] [Google Scholar]
- 3.Mills E, Wu P, Ernst E. Complementary therapies for the treatment of HIV: in search of the evidence. Int J STD AIDS. 2005;16:395–403. doi: 10.1258/0956462054093962. [DOI] [PubMed] [Google Scholar]
- 4.Ravishankar B, Shukla VJ. Indian systems of medicine: a brief profile. Afr J Tradit Complement Altern Med. 2007;4:319–37. doi: 10.4314/ajtcam.v4i3.31226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaidya AD, Devasagayam TP. Current Status of Herbal Drugs in India: An Overview. J Clin Biochem Nutr. 2007;41:1–11. doi: 10.3164/jcbn.2007001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li JW, Vederas JC. Drug discovery and natural products: end of an era or an endless frontier? Science. 2009;325:161–5. doi: 10.1126/science.1168243. [DOI] [PubMed] [Google Scholar]
- 7.Boasso A, Shearer GM. Chronic innate immune activation as a cause of HIV-1 immunopathogenesis. Clin Immunol. 2008;126:235–42. doi: 10.1016/j.clim.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Available from: http://nacoonline.org/NACO/QuickLinks/Publication/Treatment-Care_Support/Operational_Technicalguidelines_and_policies/Operational_guidelines_for_ART_/services/
- 9.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1993;41:1–19. [PubMed] [Google Scholar]
- 10.Smith L, Fidler S. HLA typing technologies. In: Mehra NK, Kaur G, McCluskey J, Christiansen FT, Claas FH, editors. The HLA complex in biology and medicine: A resource book. New Delhi: JP Medical Publishers; 2010. pp. 175–87. [Google Scholar]
- 11.Carrington M, O’Brien SJ. The influence of HLA genotype on AIDS. Annu Rev Med. 2003;54:535–51. doi: 10.1146/annurev.med.54.101601.152346. [DOI] [PubMed] [Google Scholar]
- 12.Kaur G, Mehra NK. Genetic determinants of HIV-1 infection and progression to AIDS: immune response genes. Tissue Antigens. 2009;74:373–85. doi: 10.1111/j.1399-0039.2009.01337.x. [DOI] [PubMed] [Google Scholar]
- 13.Papasteriades CH, Economidou J, Pappas H, Kapsimali V, Psarra K, Katsarou O, et al. HLA antigens as predictors of disease progression in HIV-infected haemophilia patients (a 22 years’ follow up) Haemophilia. 2005;11:371–5. doi: 10.1111/j.1365-2516.2005.01113.x. [DOI] [PubMed] [Google Scholar]
- 14.Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, et al. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–91. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998;316:1236–8. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumarasamy N, Solomon S, Flanigan TP, Hemalatha R, Thyagarajan SP, Mayer KH. Natural history of human immunodeficiency virus disease in southern India. Clin Infect Dis. 2003;36:79–85. doi: 10.1086/344756. [DOI] [PubMed] [Google Scholar]
- 17.Hira SK, Shroff HJ, Lanjewar DN, Dholkia YN, Bhatia VP, Dupont HL. The natural history of human immunodeficiency virus infection among adults in Mumbai. Natl Med J India. 2003;16:126–31. [PubMed] [Google Scholar]
- 18.Hoover DR, Rinaldo C, He Y, Phair J, Fahey J, Graham NM. Long-term survival without clinical AIDS after CD4+ cell counts fall below 200 x 10(6)/l. AIDS. 1995;9:145–52. [PubMed] [Google Scholar]
- 19.Sabin CA, Mocroft A, Bofill M, Janossy G, Johnson M, Lee CA, et al. Survival after a very low (< 5 x 10(6)/l) CD4+ T-cell count in individuals infected with HIV. AIDS. 1997;11:1123–7. doi: 10.1097/00002030-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Karlberg JPE, Speers MA. Features of Clinical Trials. In: Karlberg JPE, Speers MA, editors. Reviewing clinical trials: A guide for the ethics committee. Hong Kong: Karlberg, Johan Petter Einar; 2010. pp. 30–58. [Google Scholar]
- 21.Lau JT, Mao J, Woo J. Ethical issues related to the use of placebo in clinical trials. Hong Kong Med J. 2003;9:192–8. [PubMed] [Google Scholar]
- 22.Walker A, Ford D, Gilks C, Munderi P, Ssali F, Reid A, et al. Daily co-trimoxazole prophylaxis in severely immunosuppressed HIV-infected adults in Africa started on combination antiretroviral therapy: an observational analysis of the DART cohort. Lancet. 2010;375:1278–86. doi: 10.1016/S0140-6736(10)60057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anglaret X, Diagbouga S, Mortier E, Meda N, Verge-Valette V, Sylla-Koko F, et al. CD4+ T-lymphocyte counts in HIV infection: are European standards applicable to African patients? J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:361–7. doi: 10.1097/00042560-199704010-00009. [DOI] [PubMed] [Google Scholar]
- 24.Ramalingam S, Kannangai R, Zachariah A, Mathai D, Abraham C. CD4 counts of normal and HIV-infected south Indian adults: do we need a new staging system? Natl Med J India. 2001;14:335–9. [PubMed] [Google Scholar]
- 25.Murugavel KG, Balakrishnan P, Mohanakrishnan J, Solomon SS, Shankar EM, Muthu Sundaram SP, et al. Establishment of T-lymphocyte subset reference intervals in a healthy adult population in Chennai, India. Indian J Med Res. 2009;129:59–63. [PubMed] [Google Scholar]
- 26.Thakar MR, Abraham PR, Arora S, Balakrishnan P, Bandyopadhyay B, Joshi AA, et al. Establishment of reference CD4+ T cell values for adult Indian population. AIDS Res Ther. 2011;8:35. doi: 10.1186/1742-6405-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips AN, Lee CA, Elford J, Janossy G, Timms A, Bofill M, et al. Serial CD4 lymphocyte counts and development of AIDS. Lancet. 1991;337:389–92. doi: 10.1016/0140-6736(91)91166-r. [DOI] [PubMed] [Google Scholar]
- 28.Cozzi LA, Sabin CA, Phillips AN, Lee CA, Pezzotti P, Rezza G. The rate of CD4 decline as a determinant of progression to AIDS independent of the most recent CD4 count. The Italian Seroconversion Study. Epidemiol Infect. 1998;121:369–76. doi: 10.1017/s095026889800140x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehendale SM, Bollinger RC, Kulkarni SS, Stallings RY, Brookmeyer RS, Kulkarni SV, et al. Rapid disease progression in human immunodeficiency virus type 1- infected seroconverters in India. AIDS Res Hum Retroviruses. 2002;18:1175–9. doi: 10.1089/08892220260387913. [DOI] [PubMed] [Google Scholar]
- 30.Ding M, Tarwater P, Rodriguez M, Chatterjee R, Ratner D, Yamamura Y, et al. Estimation of the predictive role of plasma viral load on CD4 decline in HIV-1 subtype C-infected subjects in India. J Acquir Immune Defic Syndr. 2009;50:119–25. doi: 10.1097/QAI.0b013e3181911991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urassa W, Bakari M, Sandstrom E, Swai A, Pallangyo K, Mbena E, et al. Rate of decline of absolute number and percentage of CD4 T lymphocytes among HIV-1-infected adults in Dar-es-Salaam, Tanzania. AIDS. 2004;18:433–8. doi: 10.1097/00002030-200402200-00009. [DOI] [PubMed] [Google Scholar]
- 32.Schellekens PT, Tersmette M, Roos MT, Keet RP, de Wolf F, Coutinho RA, et al. Biphasic rate of CD4+ cell count decline during progression to AIDS correlates with HIV-1 phenotype. AIDS. 1992;6:665–9. doi: 10.1097/00002030-199207000-00008. [DOI] [PubMed] [Google Scholar]