Abstract
Background & objectives:
Kidney transplantation is the best option for patients with end-stage renal disease (ESRD) failure. Prolonged use of immunosuppressive drugs often causes opportunistic infections and malignancies of skin and mucosae, but due to lack of a careful dermatological screening in several transplantation centers the diagnosis and the treatment of dermatological lesions in kidney transplant patients are underestimated. In addition after the introduction of interleukin (IL)-2 -receptor antagonists (basiliximab/daclizumab), mTOR inhibitors and mycophenolate mofetil (MMF)/mycophenolic acid (MPA) in new immunosuppressive protocols only a few studies have analyzed the skin and mucosal lesions in kidney transplant patients. This study was undertaken to evaluate the cutaneous and mucosal diseases after kidney transplantation, and to investigate the association between these and different immunosuppressive protocols and/or demographic features.
Methods:
A retrospective analysis was done using medical records of kidney transplantation between 2000 and 2009 at the Transplant Unit of Fondazione IRCCS Policlinico San Matteo, Pavia, Italy. The study included 183 patients (M 57.3%, F 42.7%) aged 51.5±11.8 yr) with transplant age 52.3±34.9 months. Induction therapy was basiliximab and steroids based; maintenance therapy included combination-regimes from cyclosporine, tacrolimus, steroids, mycophenolate mofetil (MM), mycophenolic acid (MPA), rapamycin, everolimus. Anti-rejection therapy was steroid and/or thymoglobulines based. Diagnosis of cutaneous disease was made through examination of skin, mucous membranes, nails and hair evaluation. Skin biopsies, specific cultures and serological tests were done when required.
Results:
Skin and mucosal diseases were reported in 173 (95.7%) of patients; 88 (50.81%) showed viral lesions; 92 (53.01%) immunosuppression-related lesions; 28 (16.39%) benign tumours; 26 (15.3%) precancers /neoplastic lesions; 24 (14.21%) mycosis; 16 (9.29%) cutaneous xerosis, 15 (8.74%) dermatitis, while absence of cutaneous disease was evident only in 8 (4.37%) cases. An association between drug side effects and anti-rejection treatment (P≤0.01) and/or calcineurin-inhibitors (CNI) exposure (P≤0.01) was found. Longer exposure to immunosuppressive drugs (>60 months) was associated with pre-malignancy and malignancy lesions.
Interpretation & conclusions:
Cutaneous diseases are frequent in kidney transplanted patients. Continuous skin monitoring is necessary to make an early diagnosis and to start appropriate treatment.
Keywords: Anti-rejection treatment, benign tumour, end-stage renal disease, immunosuppression, kidney transplant, skin lesions
Kidney transplantation is the standard form of treatment for patients with end-stage renal failure disease (ESRD). Immunosuppressive treatment, however, elicits the risk of complications, as cutaneous and mucosal diseases1. The diagnosis and treatment of dermatological lesions may be underestimated, because of the absence of dermatologists working in touch with several Transplant Units and/or the lack of a careful dermatological screening in several Transplantation Centers. The frequency of skin and mucosal lesions in kidney transplant patients has been evaluated by a limited number of studies. In addition, a few studies have been carried out after the introduction of basiliximab in induction therapy and after the use of mTOR (mammation target of rapamycin) inhibitors and mycophenolate mofetil (MMF)/mycophenolic acid (MPA) in new immunosuppressive protocols2,3,4.
Skin cancer has been a cause of concern after kidney transplantation, despite a wide spectrum of non tumoural skin and mucosal diseases affecting these patients5,6,7,8. The aim of this study was to evaluate all mucosal and cutaneous diseases found in kidney transplant patients treated with anti-interleukin-2 (IL-2) receptor monoclonal antibody basiliximab, mTOR inhibitors, calcineurin inhibitors (CNI), antimetabolites such as MMF/MPA, and steroids, and to investigate whether the mucosal and cutaneous diseases were associated with specific immunosuppressive drugs and/or demographic features.
Material & Methods
All kidney transplant patients who underwent regular clinical follow up visits between January 1, 2000 and January 1, 2010 at Transplantation Center of Policlinico San Matteo, Pavia, Italy, were included in the study. Written informed consent was obtained from each patient and the study protocol was approved by the local ethical committee.
A total of 183 patients were enrolled. They were seen regularly every 3 months at the renal transplant outpatient clinic and every 6 months a dermatologic examination, including skin, mucous membranes, nails and hair analysis was done. Dermoscopic evaluation of pigmented lesions and, when necessary, serologic, cultural and histological evaluation were done.
Detailed clinical records were available for all patients. For each patient the following information was recorded: age, sex, transplant date, transplant age (i.e. time after transplantation), type of immunosuppressive therapy, rejection episodes, anti-rejection treatment, aetiology of end stage renal disease (ESRD), a detailed history of skin lesions and physical examination findings. Muco-cutaneous lesions were classified in eight groups: (i) viral lesions, (ii) mycotic lesions, (iii) drug side effects (DSE), (iv) xerosis, (v) dermatitis, (vi), precancer / neoplastic lesions (PN/N), (vii) benign lesions, and (viii) pigmentary disorders.
All patients were treated with the following immunosuppressive regimen:
(i) induction therapy: IL- 2 receptor antagonist (Simulect) (Novartis; Basel, CH) or anti-thymocyte immunoglobulins (Genzyme, Cambridge, MA, USA), methylprednisolone.
(ii) long-term maintenance therapy: combination of MMF 1.5-2 g per day or MPA (0.720-1.440 g per day), cyclosporine (3-9 mg/kg per day), tacrolimus (0.15-0.30 mg/kg per day), sirolimus (trough level 10-15 ng/ml per day) or everolimus (trough level 5-8 ng/ml per day).
Acute rejection was usually treated with pulse therapy with methylprednisolone (0.5-1 g per day for 3 days) and corticosteroid resistant acute rejection or vascular rejection was treated with anti-thymocyte immunoglobulins.
Statistical analysis: All data were expressed as mean and standard deviation for normally distributed variables and as median and percentiles (25 and 75) for not normally distributed variables. Frequencies were expressed as percentage. To assess the relationship between data Fisher's exact test was used. For variables with skewed distribution, non-parametric test was used (Mann-Whitney U-test). Statistical analysis was performed using stata version software (StataCorp LP, College Station, Texas, USA).
Results & Discussion
A total of 183 consecutive renal transplant patients were studied. Mean age was 51.5±11.8 yr. There was a male predominance (57.38%). Median transplant age was 49 months (range 3-230 months). A majority (42.08%) of patients had transplant age between 25 and 60 months. The main cause of renal failure was chronic glomerulonephritis (23.5%), followed by polycystic kidney (21.86%), reflux nephropathy (9.29%), nephroangiosclerosis (8.2%), genetic nephropathies (5.46%), and systemic lupus erythematous (1.64%). In our population, 20.22 per cent had chronic renal failure of uncertain origin. Diabetes, vasculitis, amyloidosis, drug nephrotoxicity, thrombotic microangiopathy and kidney stones were included in the miscellaneous group.
Among 183 patients studied, 132 (76.7%) never had acute rejection episodes, 31 (18%) had at least one rejection episode and nine (5.23%) had more than one. 11 (6.3%) had a subclinical rejection. The acute rejection in 24 patients (13.11%) was treated with thymoglobulines and in 37 patients (20.2%) with steroid pulses.
Spectrum of dermatology-mucous membranes diseases: Our retrospective analysis revealed that only six patients (4.3%) had no dermatological involvement, neither inflammatory nor neoplastic. The reported muco-cutaneous lesions were: (i) viral lesions: warts, herpes simplex 1 and 2, herpes zooster and genital warts; (ii) mycotic lesions: dermatophytosis and onychomycosis; (iii) drug side effects: telangectases, acne, sebaceous hyperplasia, gingival hyperplasia, hypertrichosis, aphthae, ecchymosis and folliculitis; (iv) dermatitides: allergic dermatitis, eczema, seborrhoeic dermatitis, psoriasis; (v) xerosis; (vi) precancer/neoplasia: actinic keratoses, dysplastic naevi, basal cell carcinomas, melanoma; and (vii) benign lesions: seborrhoeic keratosis and onycodystrophy.
Ninety nine patients (54.1%) presented with more than one kind of cutaneous lesions; two lesions were observed in 40 patients (i.e. folliculitis and xerosis), three in 29 cases, four in 17 patients and more than four in 13 cases.
The most common lesion was drug side effects and was present in 92 (DSE, 53.01%), patients; followed by viral lesions 88 (50.81%), benign tumours 28 (16.39%), pre-malignant or malignant lesions 26 (15.3%), mycosis 24 (14.21%), xerosis 16 (9.29%) and dermatitis 15 (8.74%). Among DSE, folliculitis was the most frequent disease, being 30.91% (30 cases), followed by gingival hyperplasia reported in 29 (30.00%) patients; oral aphtae in 12 (12.33%) cases; telangectases in 9 patients (9.28%); acne in 8 cases (8.24%) and hypertrichosis in four patients (4.13%). Only three patients had ecchymosis and two had sebaceous hyperplasia.
Viral lesions due to Herpes Simplex 1 and 2 were the most frequent and were found in 47 patients (51% viral lesions); Herpes Zoster lesions in 27 (29%) patients (Fig.); warts in 16 patients (17%); genital and perianal warts in three cases (3%).
Fig.
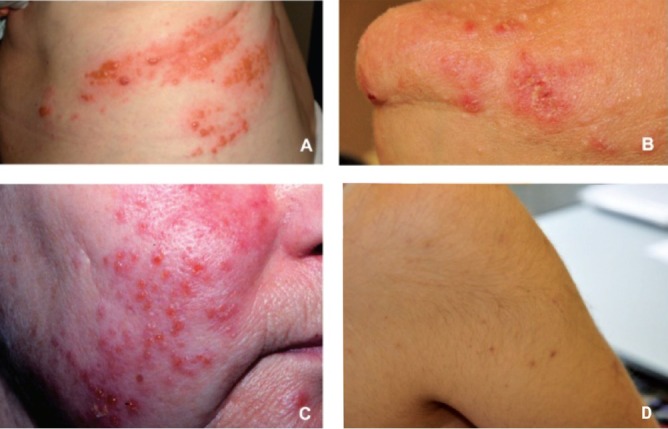
Some of most frequent skin lesions seen in kidney transplant patients: (A) Herpes Zoster; (B) folliculitis; (C) Herpes Simplex; (D) hyperthricosis.
Seborrhoeic keratosis was the most common benign lesion observed (24 cases), while onycodystrophy was reported in six patients.
Precancer and neoplastic lesions were reported in 15.3 per cent of patients: dysplastic naevi in 15 cases, non melanoma skin cancer in 15 and one case of melanoma. No case of squamous cell carcinoma was diagnosed. Diagnosis of cutaneous mycosis was reported in 25 patients, while there was only one case of onycomycosis. Skin xerosis was reported in 17 patients. Seborrhoeic dermatitis was the most frequent lesion reported in the group of dermatitides with seven cases, followed by eczema in six cases, psoriasis in five and in one case allergic dermatitis.
Association between muco-cutaneous diseases and immunosuppressive treatments: An association between DSE and anti-rejection treatment (P≤0.01) and/or calcineurin-inhibitors (CNI) exposure (P≤0.01) was found. Longer exposure to immunosuppressive drugs (> 60 months) was associated with pre-cancerous and cancerous lesions (P≤0.003). However, no association was found between thymoglobulin treatment and/or pulse steroid treatment and precancer and malignant diseases. The Table summarizes the significant associations found between single muco-cutaneous lesions and the immunosuppressive drugs or demographic features.
Table.
Significant associations between micro-cutaneous lesions and immunosuppressive treatment
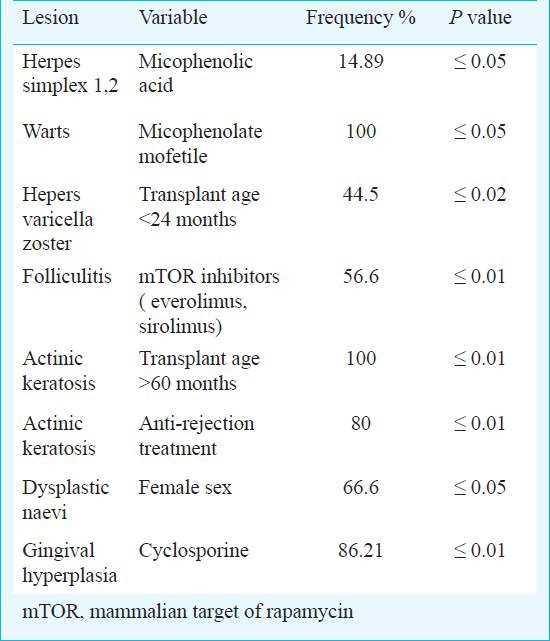
Only 8 (4.3%) patients had a normal skin, confirming the importance of a dermatological examination in renal transplant patient which should be part of the post-transplant programme for a prompt and correct diagnosis. The Fig. shows some of the most frequent skin diseases: vesicles of herpes zoster, papules and pustules of folliculitis, Herpes Simplex papules and vesicles and hyperthricosis.
In our study the prevalent lesions resulted in DSE which were significantly related with transplant age. CNI and anti-rejection treatment were associated with a higher risk of developing DSE. A longer duration of immunosuppressive treatment and higher doses of anti-rejection drugs increase the risk of developing DSE. The DSE included common cosmetic lesions such as acneiform eruptions, hypertrichosis, gingival hyperplasia, and folliculitis. Moloney et al1 demonstrated that cosmetic skin problems had most impact on quality of life than a history of skin cancer. This might contribute to the poor compliance with immunosuppressant regimens which is a major cause of graft failure9. Acne and folliculitis, very frequently observed in our study (39.1%), were related to sirolimus-based immunosuppressive regimen. In literature, the frequency of acne related to sirolimus, has been reported between 15 and 25 per cent10. A French study described acne in 45 per cent of renal transplant recipients, erupting soon, mainly in men. These authors proposed that the role of sirolimus in the pathogenesis of acne might be due to direct toxic effect on follicles or to a toxic modification of sebum.
Our results agree with the studies performed in Caucasic race2, but not with Indian and Latin-American which show a high prevalence of skin infections, but probably this difference is justified by different climatic and health-social conditions3,4,11,14. Viral lesions, the second most frequent, were manifested more often in the first two years. We suppose that the immunosuppressive regimen, including basiliximab and MMF/MPA15, and our choice to not use a cytomegalovirus (CMV) prophylactic treatment, may justify this high prevalence, because it is known that CMV can reactivate Herper Simplex or Zoster (HSV or HZV) infection16. According to this hypothesis, a higher incidence of HZV infection was found, within the first four years after transplantation17,18,19, because the patients were treated with antiviral treatment which postponed the acquisition of immunologic memory. A low occurrence of non-melanoma skin cancer was found in our patient population, may be because of periodical dermatologic screening and education about sun exposure. Moreover our patients live at latitude with a lower risk of skin cancer compared to other populations6,20,21,22,23,24,25. Finally, the follow up of our study (median 5 years) was short to see neoplastic degeneration.
In conclusion, our study demonstrated the importance of a careful dermatological screening and follow up, associated with an appropriate education in kidney transplant recipients in reducing muco-cutaneous complications.
References
- 1.Moloney FJ, Keane S, O’Kelly P, Conlon PJ, Murphy GM. The impact of skin disease following renal transplantation on quality of life. Br J Dermatol. 2005;153:574–8. doi: 10.1111/j.1365-2133.2005.06699.x. [DOI] [PubMed] [Google Scholar]
- 2.Formicone F, Fargnoli MC, Pisani F, Rascente M, Famulari A, Peris K. Cutaneous manifestations in Italian kidney transplant recipients. Transplant Proc. 2005;37:2527–8. doi: 10.1016/j.transproceed.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 3.Prakash J, Singh S, Prashant GK, Kar B, Tripathi K, Singh PB. Mucocutaneous lesions in transplant recipient in tropical country. Transplant Proc. 2004;36:2162–4. doi: 10.1016/j.transproceed.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Sandoval M, Ortiz M, Diaz C, Majerson D, Molgò M. Cutaneous manifestation in renal transplant recipients of Santiago, Chile. Transplant Proc. 2009;41:3752–4. doi: 10.1016/j.transproceed.2009.05.041. [DOI] [PubMed] [Google Scholar]
- 5.Barba A, Tessari G, Boschiero L, Chieregato GC. Renal transplantation and skin disease: review of the literature and results of a 5-years follow-up of 285 patients. Nephron. 1996;73:131–6. doi: 10.1159/000189029. [DOI] [PubMed] [Google Scholar]
- 6.Strumia R, Perini L, Tarroni G, Fiocchi O, Gilli P. Skin lesions in kidney transplant recipients. Nephron. 1992;62:137–41. doi: 10.1159/000187021. [DOI] [PubMed] [Google Scholar]
- 7.Rascente M, Pisani F, Barletta A, D’Angelo M, Giammaria A, Parzanese I, et al. Malignancies after kidney transplantation. Transplant Proc. 2005;37:2529–31. doi: 10.1016/j.transproceed.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 8.Ramsay HM, Fryer AA, Hawley CM, Smith AG, Harden PN. Non-melonoma skin cancer risk in the Queensland renal transplant population. Br J Dermatol. 2002;147:950–6. doi: 10.1046/j.1365-2133.2002.04976.x. [DOI] [PubMed] [Google Scholar]
- 9.Didlake RH, Dreyfus K, Kerman RH, Van Buren CT, Kahan BD. Patient noncompliance: a major cause of late graft failure in cyclosporine-treated renal transplants. Transplant Proc. 1988;20:63–9. [PubMed] [Google Scholar]
- 10.Kahan BD. Efficacy of sirolimus compared with azathioprin for reduction of acute renal allograft rejection: a randomized multicenter study. The Rapamune US study group. Lancet. 2000;356:194–202. doi: 10.1016/s0140-6736(00)02480-6. [DOI] [PubMed] [Google Scholar]
- 11.Mahè E, Morelon E, Lechaton S, Drappier JC, de Prost Y, Kreis H, et al. Acne in recipients of renal transplantation treated with sirolimus: clinical, microbiological, histologic, therapeutic and pathogenica aspects. J Am Acad Dermatol. 2006;55:139–42. doi: 10.1016/j.jaad.2005.11.1072. [DOI] [PubMed] [Google Scholar]
- 12.George L, John GT, Jacob CK, Eapen P, Pulimood S, George R. Skin lesions in renal transplant recipients: a single center analysis. Indian J Dermatol Venereol Leprol. 2009;75:255–61. doi: 10.4103/0378-6323.51241. [DOI] [PubMed] [Google Scholar]
- 13.Grimaldi A, Barletta A, Rascente M, Pisani F, Iaria G, Maccarone D, et al. Infectious complications in the renal transplant recipient. Transplant Proc. 2005;37:2502–3. doi: 10.1016/j.transproceed.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Chugh KS, Sharma SC, Singh V, Sakhuja V, Jha V, Gupta KL. Spectrum of dermatological lesions in renal allograft recipients in a tropical environment. Dermatology. 1994;188:108–12. doi: 10.1159/000247112. [DOI] [PubMed] [Google Scholar]
- 15.Song A, Abdala E, Bonazzi P, Bacchella T, Machado M. Does mycophenolate mofetil increase the risk of cytomegalovirus infection in solid organ transplant recipients? A mini-review. Braz J Infect Dis. 2006;10:132–8. doi: 10.1590/s1413-86702006000200011. [DOI] [PubMed] [Google Scholar]
- 16.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601–14. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 17.Arness T, Pedersen R, Dierkhising R, Kremers W, Patel R. Varicella zoster virus-associated disease in adult kidney transplant recipients: incidence and risk-factor analysis. Transpl Infect Dis. 2008;10:260–8. doi: 10.1111/j.1399-3062.2007.00289.x. [DOI] [PubMed] [Google Scholar]
- 18.Ritter ML, Pirofski L. Mycophenolate mofetil: effects on cellular immune subsets, infectious complications, and antimicrobial activity. Transpl Infect Dis. 2009;4:290–7. doi: 10.1111/j.1399-3062.2009.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lilleri D, Zelini P, Fornara C, Comolli G, Revello MG, Gerna G. Human cytomegalovirus-specific CD4+ and CD8+ T cell responses in primary infection of the immunocompetent and the immunocompromised host. Clin Immunol. 2009;131:395–403. doi: 10.1016/j.clim.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Gallagher MP, Kelly PJ, Jardine M, Perkovic V, Cass A, Craig JC, et al. Long-term cancer risk of immunosuppressive regimens after kidney transplantation. J Am Soc Nephrol. 2010;21:852–8. doi: 10.1681/ASN.2009101043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moyal DD, Fourtanier AM. Broad-spectrum sunscreens provide better protection from solar ultraviolet-simulated radiation and natural sunlight-induced immunosuppression in human being. J Am Acad Dermatol. 2008;58:149–54. doi: 10.1016/j.jaad.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 22.Urwin HR, Jones PW, Hander PN, Ramsay HM, Hawley CM, Nicol DL, et al. Predicting risk of Nonmelanoma skin cancer and premalignant skin lesions in renal transplant recipients. Transplantation. 2009;87:1667–71. doi: 10.1097/TP.0b013e3181a5ce2e. [DOI] [PubMed] [Google Scholar]
- 23.Tessari G, Naldi L, Boschiero L, Nacchia F, Fior F, Forni A, et al. Incidence and clinical predictors of a subsequent nonmelanoma skin cancer in solid organ transplant recipients with a first nonmelanoma skin cancer. Arch Dermatol. 2010;146:294–9. doi: 10.1001/archdermatol.2009.377. [DOI] [PubMed] [Google Scholar]
- 24.Bordea C, Wojnarowska F, Millard PR, Doll H, Welsh K, Morris PJ. Skin cancers in renal-transplant recipients occur more frequently than previously recognized in a temperate climate. Transplantation. 2004;77:574–9. doi: 10.1097/01.tp.0000108491.62935.df. [DOI] [PubMed] [Google Scholar]
- 25.Naldi L, Belloni Fortina A, Lovati S, Barba A, Gotti E, et al. Risk of non-melanoma skin cancer in italian organo transplant recipients. A registry-based study. Transplantation. 2000;70:1479–84. doi: 10.1097/00007890-200011270-00015. [DOI] [PubMed] [Google Scholar]


