Abstract
We hypothesized that targeting key points in the ischemic cascade with combined neuroglobin (Ngb) overexpression and c-jun N-terminal kinase (JNK) inhibition (SP600125) would offer greater neuroprotection than single treatment after in vitro hypoxia/reoxygenation and in a randomized, blinded in vivo experimental stroke study using a clinically relevant rat strain. Male spontaneously hypertensive stroke-prone rats underwent transient middle cerebral artery occlusion (tMCAO) and were divided into the following groups: tMCAO; tMCAO+control GFP-expressing canine adenovirus-2, CAVGFP; tMCAO+Ngb-expressing CAV-2, CAVNgb; tMCAO+SP600125; tMCAO+CAVNgb+SP600125; or sham procedure. Rats were assessed till day 14 for neurologic outcome before infarct determination. In vitro, combined lentivirus-mediated Ngb overexpression+SP600125 significantly reduced oxidative stress and apoptosis compared with single treatment(s) after hypoxia/reoxygenation in B50 cells. In vivo, infarct volume was significantly reduced by CAVNgb, SP600125, and further by CAVNgb+SP600125. The number of Ngb-positive cells in the peri-infarct cortex and striatum was significantly increased 14 days after tMCAO in animals receiving CAVNgb. Neurologic outcome, measured using a 32-point neurologic score, significantly improved with CAVNgb+SP600125 compared with single treatments at 14 days after tMCAO. Combined Ngb overexpression with JNK inhibition reduced hypoxia/reoxygenation-induced oxidative stress and apoptosis in cultured neurons and reduced infarct and improved neurologic outcome more than single therapy after in vivo experimental stroke in hypertensive rats.
Keywords: antiapoptotic, antioxidant, combined therapy, hypertensive rat, transient focal ischemia
Introduction
Currently, reperfusion therapy with recombinant tissue plasminogen activator (rt-PA) is the cornerstone of treatment for acute ischemic stroke, yet therapy can only be delivered to a fraction of patients because of a narrow therapeutic window and risk of cerebral hemorrhage.1 Extensive research has identified excitotoxicity, oxidative stress, inflammation, and cell death (necrosis and apoptosis) as key contributory pathways underlying lesion progression.2 These diverse injury mechanisms may explain why pharmacotherapy clinical trials geared to manipulate single pathways have failed. A polytherapy approach targeting distinct pathways may be more effective and has shown promise in preclinical models,3, 4 with the majority assessing the potential to extend the therapeutic window of rt-PA. Of these, 97% used young, healthy animals.3 The revised STAIR5 and other guidelines covering preclinical stroke studies6 have highlighted the need to include comorbidities in preclinical stroke models to better model the clinical situation and improve assessment of new therapies. The most significant risk factors (hypertension, abdominal obesity, diet, physical inactivity, and current smoking) account for 80% of the global stroke risk, with self-reported history of hypertension being the strongest of these.7 In this study we used spontaneously hypertensive stroke-prone rats (SHRSP) that display stroke comorbidities of hypertension, insulin resistance, and inflammation.
We chose to target two key pathways underlying stroke pathogenesis (oxidative stress and apoptosis) through neuroglobin (Ngb) upregulation in combination with c-jun N-terminal kinase (JNK) inhibition. Neuroglobin, a neuronal specific O2 binding protein, is generally considered to mediate neuroprotection in preclinical stroke studies after overexpression;8, 9, 10, 11 however, it is worth noting that, to date, neuroprotection has been shown in normal rodent strains but not in any strain exhibiting comorbidity. Administration of adeno-associated virus (AAV)-overexpressing Ngb significantly reduced lesion size and improved Bederson's 5-point neurologic score in young, normotensive rats after middle cerebral artery occlusion (MCAO).10 Transgenic Ngb overexpression in mice reduced infarct volume after transient MCAO (tMCAO),9, 11 but sensorimotor function remained unchanged.11 Intravenous delivery of Ngb linked to a fusion protein 2 hours before tMCAO reduced infarct volume and neurologic deficit score in mice.8 Adenovirus, AAV, and lentivirus have been used as viral vectors to overexpress neuroprotective genes that have resulted in reduced infarct volume and improved functional outcome in permanent and tMCAO models.12 Here, canine adenovirus type 2 (CAV-2 vectors), a vector that preferentially transduces neurons and has been shown to transduce a greater volume of brain tissue through retrograde transport from the site of injection,13, 14 was used.
To provide antiapoptotic protection, we targeted JNK, a downstream mediator of extrinsic and intrinsic apoptosis. A peptide inhibitor of JNK administered 6 hours after occlusion reduced lesion volume and improved functional outcome after tMCAO in mice15 and reduced infarct volume when delivered 3 hours after permanent MCAO in mice.16 After tMCAO in spontaneously hypertensive rats, however, no such neuroprotection was seen.17 This was attributed, in part, to the predominance of AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid)- rather than NMDA (N-methyl-D-aspartate)-activated ischemic brain injury in the SHR.17 One further reason may be the more severe ischemic insult induced by MCAO in SHR (and SHRSP), leading to a greater contribution of necrotic to apoptotic cell death. This could result in reduced efficacy of drugs targeting the JNK signaling pathway and highlights once again, the importance of including stroke associated comorbidities in preclinical studies. We used SP600125, an ATP-competitive inhibitor of JNK, shown previously to dose-dependently reduce infarct volume when administered intravenously <2 hours after tMCAO in mice.18 The efficacy of these agents alone has not previously been determined in preclinical stroke models displaying clinically relevant comorbidities, and the novel combination of an antiapoptotic and an antioxidant represents a further advance.
Materials and Methods
Virus Production
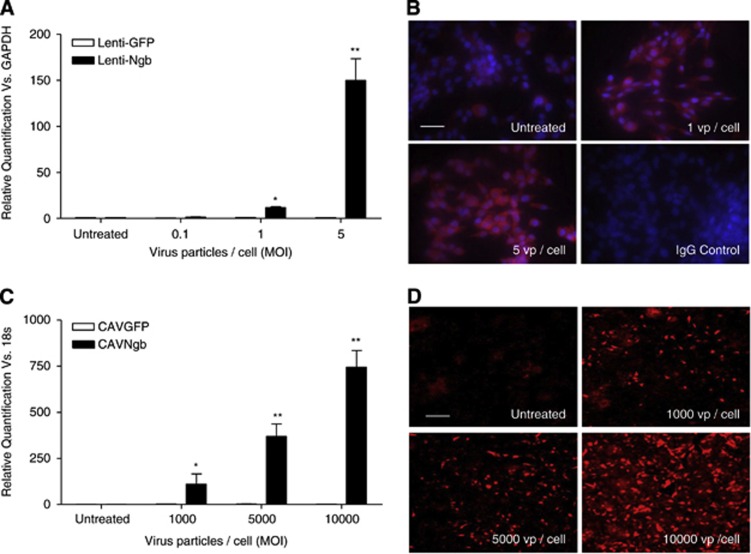
Because the expression of coxsackievirus and adenovirus receptor, the primary receptor for CAV-2, was negligible on B50 rat neuronal cells used for in vitro studies (Supplementary Figure S1A), a Ngb-expressing lentivirus was generated with functional overexpression (mRNA and protein) confirmed (Figures 1A and 1B). Lentiviral vectors were produced by triple transient transfection of HEK293T cells with a packaging plasmid (pCMVΔ8.74), a plasmid encoding the envelope of vesicular stomatitis virus (Plasmid Factory, Bielefeld, Germany) and pHR-SIN-SFFV-Ngb, using polyethylenimine (Sigma-Aldrich, Poole, UK) as previously described.19 For CAVNgb production, the Ngb complementary DNA was cloned from pET3a_Ngb into pTCAV-12VK and recombined with pTG5412 as previously described20 and propagated in DKZeo cells.21 CAVGFP,20 a CAV-2 vector overexpressing green fluorescent protein (GFP) as a reporter gene, was used as a control.
Figure 1.
Confirmation of neuroglobin (Ngb) overexpression from viral vectors. Functional overexpression of Ngb was assessed by TaqMan quantitative real-time PCR (qRT-PCR; mRNA) and immunocytochemistry (ICC; protein) in B50 neuronal (lenti-Ngb) or HepG2 (CAVNgb) cells 3 days after transduction. (A, C) The Ngb mRNA levels were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH; B50 cells, lenti-Ngb) or 18S (HepG2 cells, CAVNgb) after viral transduction. Relative quantification (RQ) was calculated from ΔΔCt (cycle threshold) and compared with green fluorescent protein (GFP)-expressing virus levels. RQ±RQmax/RQmin shown and analyzed by Student's unpaired t-test with Bonferroni's post hoc correction. *P<0.01 and **P<0.001 versus GFP-expressing virus, n=3. (B, D) Representative photomicrographs of Ngb protein expression determined by ICC in cells transduced with Ngb-expressing viruses using an α-Ngb antibody (B: nuclei=blue, 4',6-diamidino-2-phenylindole (DAPI); both: Ngb=red, TRITC). Negative staining in isotype-matched immunoglobulin G (IgG) controls shown for lenti-Ngb; untreated cells represent basal expression levels of neuroglobin expression. MOI, multiplicity of infection; TRITC, tetramethyl rhodamine isothiocyanate; vp/cell, virus particle/cell. Scale bar, 100 μm.
Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted from lysed cells 3 days after virus transduction using the Qiagen (Crawley, UK) miRNeasy kit as per the manufacturer's instructions. Qiagen RNAse-free DNAse was added during the on-column phase. RNA concentration and purity was determined by NanoDrop spectrophotometry (NanoDrop Technologies, Wilmington, DE, USA) and diluted to 200 ng/μL. Complementary DNA was synthesized from 1 μg total RNA using the Taqman mRNA reverse transcription kit (Applied Biosystems, Paisley, UK) with random hexamer primers. The reactions underwent sequential incubation in a 96-well plate at 25 °C (10 minutes), 48 °C (30 minutes), 95 °C (5 minutes), and held at 12 °C. Simplex reactions were performed for qRT-PCR with a specific Ngb expression probe (Rn 00583724_m1, FAM-labeled, Applied Biosystems) or appropriate housekeeper expression probes (glyceraldehyde 3-phosphate dehydrogenase (GAPDH) or 18S, VIC-labeled, Applied Biosystems). Duplicates of each sample were incubated at 95 °C (10 minutes) followed by 40 cycles of 95 °C (15 seconds) and 60 °C (1 minute) using a Taqman 7900HT Fast Real-time PCR System (Applied Biosystems). Samples were normalized to housekeeper and relative quantification calculated from ΔΔCt (cycle threshold) versus GFP-virus-treated cells.
Immunocytochemistry to Detect Neuroglobin Expression
Neuroglobin immunocytochemistry (ICC) was performed on 4% (w/v) paraformaldehyde-fixed cells 3 days after virus transduction. Briefly, cells were permeabilized in 0.1% (v/v) Triton/phosphate-buffered saline (PBS) for 15 minutes before incubation with primary antibody (10 μg/mL; 13C8, Abcam, Cambridge, UK) for 1 hour at room temperature (RT). Secondary antibody (4 μg/mL; goat anti-mouse Alexa Fluor 546, Invitrogen, Paisley, UK; A21424) was added for 1 hour at RT. Cells were mounted using Prolong Gold with 4',6-diamidino-2-phenylindole (DAPI; Invitrogen).
Hypoxia/reoxygenation
Rat B50 neuroblastoma cells (European Collection of Cell Cultures (ECACC), Salisbury, UK) were maintained in Dulbecco's minimal essential medium supplemented with 10% (v/v) fetal bovine serum (FBS), 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin in a 5% CO2 humidified atmosphere. Cells receiving lentivirus were transduced with 5 virus particles/cell (vp/cell) lenti-GFP or lenti-Ngb for 4 hours 1 day after plating. After 48 hours, cells were switched to FBS-free medium and incubated in a hypoxic chamber (1% O2, 5% CO2, balance N2) for 9 hours. At 10 minutes before hypoxia, cells receiving SP600125 were incubated with 20 μmol/L or volume-matched DMSO vehicle control. Cells were reoxygenated in complete media, with readministration of SP600125 or DMSO where necessary, for 24 hours before lysis.
In Vitro Oxidative Stress Assays
Electron paramagnetic resonance (EPR) spectroscopy for reactive oxygen species (ROS) detection (e-scan R; Bruker BioSpin GmbH, Rheinstetten, Germany) used the spin probe 1-hydroxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine (CPH; Noxygen, Elzach, Germany) as previously described.22 Cells were incubated in situ with Krebs buffer and 1 mmol/L CPH in a total volume of 1 mL for 60 minutes at 37 °C for the last hour of the 24-hour reoxygenation period. Instrument settings were as follows: centre field of 3,392 G, modulation amplitude of 5.08 G, sweep time of 10.49 seconds, sweep width of 120 G, and 30 scans. In the presence of ROS, CPH is oxidized to the nitroxide CP radical and the triple-line spectrum is read giving the EPR amplitude in proportion to the amount of CP• reflecting the interaction of ROS with CPH after 60 minutes, giving a rate of ROS production calculated in counts per minute. All readings were normalized for input protein using a BCA protein assay kit (Pierce, Northumberland, UK).
A spectrophotometric assay (Tebu-Bio, Peterborough, UK) for malondialdehyde (MDA) was used to determine lipid peroxidation levels after hypoxia/reoxygenation (H/R) as per the manufacturer's instructions. The MDA and hydroxyalkenal determination protocol was used with 200 μL cell lysate (in PBS+5 μmol/L butylated hydroxytoluene). Reaction mixtures were incubated for 60 minutes at 45 °C before absorbance was measured at 586 nm in a spectrophotometer. All readings were normalized for input protein using a BCA protein assay kit (Pierce).
Apoptosis Assays
Apoptosis was measured with a cell death ELISA (Roche, Burgess Hill, UK) as per the manufacturer's instructions. Caspase-3 ICC was performed on 4% (w/v) paraformaldehyde-fixed cells. Briefly, cells were permeabilized in 0.1% (v/v) Triton/ PBS for 15 minutes before incubation with primary antibody (1:30 dilution; AB32351, Abcam) for 1 hour at RT. Secondary antibody (4 μg/mL, goat anti-rabbit Alexa Fluor 488, Invitrogen; A11008) was added for 1 hour at RT. Cells were mounted using Prolong Gold with DAPI (Invitrogen).
Animals
Animal experiments were performed in accordance with the Animals Scientific Procedures Act 1986 and approved by the Ethics Review Committee of the University of Glasgow. Male, SHRSP (n=65, 270 to 310 g) were housed separately under a 12:12 hour light/dark cycle with food and water ad libitum. Studies were randomized and masked, preventing bias in selection and data analysis in accordance with STAIR and other guidelines.5, 6 For randomization, treatment groups were given a number (1 to 6) and animals randomly allocated to a treatment group by a colleague not involved in performing the study using R (www.r-project.org). Subject groups were: sham (n=6), control tMCAO (n=9), tMCAO+CAVGFP (n=9), tMCAO+CAVNgb (n=9), tMCAO+SP600125 (n=9), and tMCAO+CAVNgb+SP600125 (n=8). The design of the in vivo intervention study is shown in Supplementary Figure S2. Investigators and animal unit staff caring for the animals were masked to group allocation. Findings are reported in accordance with the ARRIVE guidelines.23
Virus and Drug Administration
Anesthetic was induced with 5% isoflurane in oxygen and animals intubated and ventilated throughout surgery (∼2.5% isoflurane/oxygen). Body temperature was maintained at 37±0.5 °C. Animals undergoing tMCAO had cranial burrhole surgery 5 days before tMCAO for virus administration or as a sham procedure. Interestingly, this prestroke surgery reduces subsequent stroke-related mortality.24 Briefly, the head was secured in a stereotactic frame, a 1 mm cranial burrhole made, and a 24G needle connected to a Hamilton syringe used to pierce the dura and administer virus into the cortex. After a 2-minute rest period, 2.1 μL virus suspension was injected for 5 minutes, with a subsequent 2-minute rest period before slow needle retraction. A reporter gene-expressing lentivirus and CAV-2 were compared for transduction levels to determine which was the most efficient vector for this study. The CAV-2 vectors (3 × 109 physical particles) were injected at coordinates: AP +1.2 mm, ML +3 mm, and DV −2 mm relative to bregma; or two injections of 2 × 107 vp lentivirus injected at coordinates: AP −0.4 mm, ML +3 mm, and DV −2 mm, and AP −2.4 mm, ML+4 mm, and DV −2 mm relative to bregma. For the intervention study, the site of CAV-2 injection (3 × 109 physical particles) was AP −0.7 mm, ML +3 mm, and DV −2 mm relative to bregma. The burrhole was then sealed with dental cement (Wright Cottrell, Glasgow, UK). Animals randomized to SP600125 received an intravenous injection (1 mg/kg in PPCES vehicle—30% (w/v) PEG 400, 20% (v/v) polypropylene glycol, 15% (v/v) cremophor EL, 5% (v/v) ethanol, and 30% (v/v) saline) 15 minutes before and 3 hours after tMCAO as previously described in mice.18
Neurologic Assessments
Each animal was trained on the neurologic assessments before MCAO to ensure reproducibility at performing tasks. Animals were assessed on a single occasion 3 days before tMCAO to ascertain a baseline score. The 32-point neurologic score used was developed from a series of 10 tests that assess limb function, mobility, and general health; the lower the score, the greater the neurologic deficit. Animals were further assessed by the tapered beam walk test, quantifying the average number of footfalls as a percentage of the total number taken from 3 crossings of a 130-cm tapered beam. The animals were assessed at baseline 3 days before tMCAO and longitudinally on days 1, 2, 3, 7, 10, and 14 after MCAO. Where possible, behavioral measures were completed masked to surgery and treatment groups and were video recorded for assessment of footfalls and to allow for further validation of scores by a second masked observer.
Tail-Cuff Plethysmography
Conscious systolic blood pressure monitoring was performed by noninvasive computerized tail-cuff, based on the plethysmographic method. Rats were preheated to ∼39 °C for ∼20 minutes and restrained by wrapping in a surgical sheet, before a pneumatic pressure sensor was attached to the tail distal to a pneumatic pressure cuff, both under the control of a Programmed Electro-Sphygmomanometer (Harvard Apparatus, Kent, UK). Systolic blood pressure values from each animal were determined by averaging a minimum of six separate indirect pressure measurements.
Stroke Model
Animals were anesthetized as before and underwent tMCAO (45 minutes) by advancing a silicone-coated monofilament (Doccol, Sharon, MA, USA) through the internal carotid artery, blocking the origin of the MCA.25 Animals were maintained under anesthesia during the ischemia and then anesthetic was withdrawn and the animals allowed to recover consciousness after removal of the filament. The duration of occlusion was determined to reflect a reproducible infarct volume and accompanying neurologic deficit but with an acceptably low mortality rate (60 minutes of MCAO resulted in 100% mortality in SHRSP within 2 to 3 days of experimental stroke, LM Work, unpublished observation).
Infarct Analysis
At 14 days after tMCAO, rats were killed by transcardiac perfusion fixation. Formalin-fixed, paraffin-embedded tissue sections (6 μm) were stained with hematoxylin and eosin. Infarct volume was assessed at seven coronal levels throughout the MCA territory.26 Briefly, areas of tissue infarction were identified and the location transcribed onto scale line diagrams from the rat brain atlas.27 Line diagrams were then scanned and infarct areas were measured by image analysis (ImageJ, NIH, Bethesda, MD, USA). The infarct volume (mm3) for each brain was calculated by plotting the area (mm2) of damage at each coronal level against the known anterior/posterior stereotaxic coordinates from Bregma and calculating the area under the curve. Infarct measures were made masked to group allocation.
Immunohistochemistry for Virus Transduction
For determination of virus transduction efficiency and spread from the injection site, rats were killed by transcardiac perfusion fixation. Brains were then placed in a 30% (w/v) sucrose gradient and subsequently embedded in optimal cutting temperature (OCT). Frozen sections (40 μm) were permeabilized in 0.1% (v/v) Triton-X for 10 minutes and blocked for 1 hour at RT. Primary antibody or immunoglobulin G (IgG) control were diluted (1:500, GFP, Abcam, Ab6556; 1:500, NeuN, Millipore, Watford, UK, MAB377) and incubated on sections overnight at 4 °C. Fluorescent secondary antibody (4 μg/mL; goat anti-rabbit (GFP) or goat anti-mouse (NeuN) Alexa Fluor 488, Invitrogen, UK; A11008 or A11001, respectively) was incubated on the slides for 1 hour at RT before mounting with ProLong Gold with DAPI. Areas of virus transduction (GFP expression) were identified on brain sections and their location transcribed onto scale line diagrams at seven predetermined coronal levels. The area of GFP transduction of three sections at each coronal level were delineated onto the appropriate coronal line diagrams, and measured using image analysis (ImageJ, NIH).
To determine levels of Ngb overexpression after virus delivery before tMCAO, formalin-fixed, paraffin-embedded tissue sections (6 μm) were rehydrated, endogenous peroxidise quenched (30 minutes in 3% (v/v) H2O2 in methanol), followed by citrate buffer antigen retrieval. Sections were blocked for 1 hour at RT and incubated overnight at 4 °C in primary antibody (1:200, Ngb, Sigma, Poole, UK; 1:200 NeuN, Abcam) or IgG control. Fluorescent secondary antibody (4 μg/mL; goat anti-mouse Alexa Fluor 546, Invitrogen, UK; A21424) was incubated on the slides for 1 hour at RT before mounting with ProLong Gold with DAPI. Images were taken on a Zeiss confocal imaging system LSM 510 (Carl Zeiss, UK) using settings DAPI (405) pinhole 61 μm and TRITC (546) pinhole 71 μm and objective C-apochromat 40x/1.2W at five matched locations across the cortex and striatum at two coronal levels (coordinates (mm): 5 sites at AP −0.12 from Bregma −ML +3.5, DV −1; ML+1, DV −1; ML +2, DV −4; ML +4, DV −4; ML +3.5, DV −6; and 5 sites at AP −2.28 from Bregma −ML +4.5, DV −1; ML +1, DV −1; ML +4, DV −4; ML +4.5, DV −6; ML 1.5, DV −4) to reflect the areas of high virus transduction and infarct. To quantify the extent of Ngb expression, Image Pro (Media Cybernetics, Basingstoke, UK) was used. The area for quantification of Ngb cell count in peri-infarct tissue was selected using the Area of Interest Macro and pixel values were transformed to optical density units. The number of cells positive for Ngb was then determined as a percentage of the total number of DAPI-stained nuclei within each given field. Image acquisition and subsequent quantifications were performed masked to group allocation.
Statistics
Data are presented as mean±s.e.m. In vitro experiments were performed in triplicate on ⩾3 independent occasions and analyzed by unpaired Student's t-test. In vivo groups were compared using repeated measures analysis of variance (ANOVA). Survival rates were compared using Fisher's exact test. Bonferroni's or Tukey's post hoc test was used for multiple comparisons.
Results
Neuroglobin Overexpression Combined with c-Jun N-Terminal Kinase Inhibition Protects Against Hypoxia/Reoxygenation In Vitro
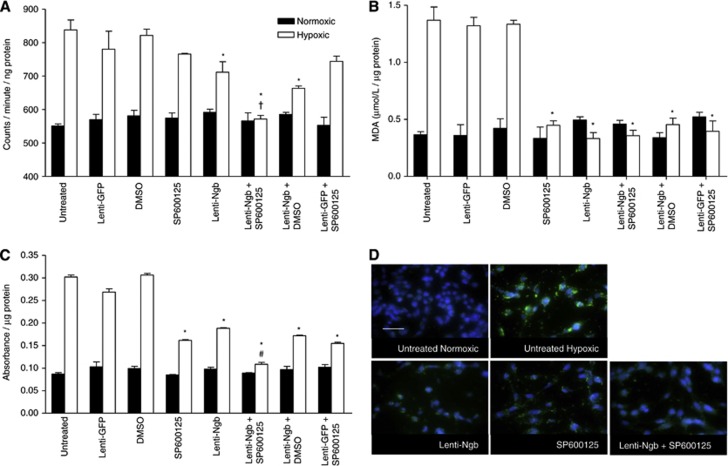
B50 neuronal cells subjected to 9 hours of hypoxia and 24 hours of reoxygenation produced significant increases in ROS generation (Figure 2A), MDA (Figure 2B), DNA fragmentation (Figure 2C), and caspase-3 nuclear translocation (Figure 2D). Lenti-Ngb pretreatment significantly reduced ROS generation versus controls (Figure 2A). SP600125 treatment alone did not significantly reduce ROS levels but potentiated the lenti-Ngb-mediated reduction (Figure 2A). The MDA levels appeared maximally reduced in cells pretreated with lenti-Ngb or SP600125. Consequently, no further benefit of combined therapy was observed (Figure 2B). Lenti-Ngb or SP600125 pretreatment significantly reduced levels of apoptosis, with a further significant reduction from that seen with lenti-Ngb after combined intervention (Figure 2C). In all assays, in cells pretreated with the lenti-Ngb or SP600125 and the appropriate control treatments (DMSO or lenti-GFP), the same effect as the single active agent (either lenti-Ngb or SP600125) was found, showing no confounding effect attributable to either vehicle or virus. Caspase-3 nuclear translocation was assessed qualitatively by ICC (Figure 2D). Lenti-Ngb or SP600125 pretreatment reduced the extent of nuclear translocation of caspase-3 with combined treatment returning the cellular distribution pattern of caspase-3 to that of normoxic cells (Figure 2D).
Figure 2.
Neuroglobin (Ngb) overexpression combined with c-jun N-terminal kinase (JNK) inhibition protects against hypoxia/reoxygenation (H/R) injury in vitro. Normoxic control cells (solid bars) received the same treatment as cells exposed to 9 hours of hypoxia with 24 hours of reoxygenation (hypoxic; open bars). Oxidative stress assays for H/R injury: (A) reactive oxygen species (ROS) generation detected by electron paramagnetic resonance (EPR); (B) lipid peroxidation levels detected by malondialdehyde (MDA) assay; and apoptosis by (C) apoptotic cell death enzyme-linked immunosorbent assay (ELISA) and (D) caspase-3 (green) immunocytochemistry (ICC; nuclei=blue, 4',6-diamidino-2-phenylindole (DAPI). Scale bar, 100 μm. Data presented as mean±s.e.m., analyzed using unpaired Student's t-test and Bonferroni's post hoc correction, representative of n=3. *P<0.01 versus untreated hypoxic cells, †P<0.001 versus lenti-Ngb- or SP600125-treated hypoxic cells, #P<0.01 versus lenti-Ngb-treated hypoxic cells. DMSO, dimethyl sulfoxide; GFP, green fluorescent protein.
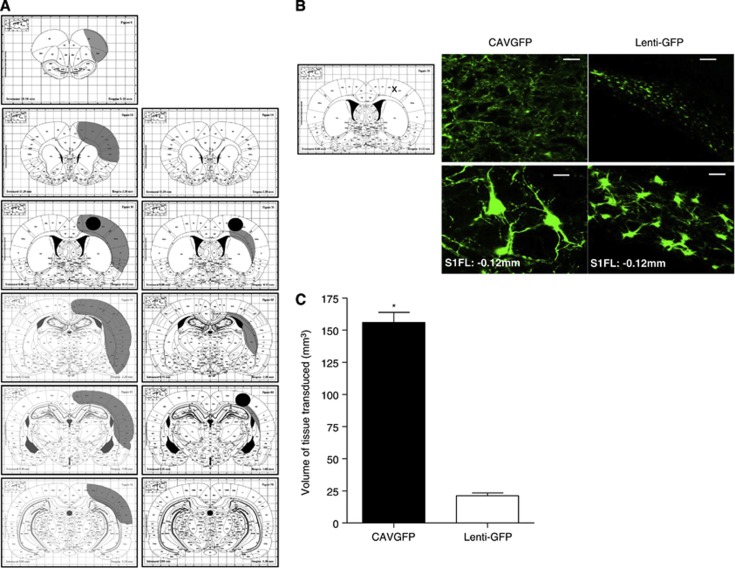
Canine Adenovirus Type 2 Vectors More Efficiently Transduce the Adult Rat Cortex Than Lentivirus
Different levels and distribution of reporter gene, GFP, expression were found for the reporter gene-expressing lentivirus and CAV-2 (Figures 3A and 3B). One week after a single cortical injection of CAV-2, the rostro-caudal spread from the injection site was in excess of 7 mm with spread through the cortical parenchyma. In contrast, after double cortical injection of lentivirus, the spread was minimal (< 2.5 mm) from the site(s) of injection (Figures 3A and 3B). Determination of the tissue volume expressing the reporter gene, GFP, showed that seen with the CAV-2 vector was significantly greater than that resulting from a double cortical injection of lentivirus (156±11.1* versus 20±3.8 mm3; *P<0.0002 versus lentivirus; Figure 3C). Therefore, a Ngb-expressing CAV-2 virus was generated for in vivo studies and functional Ngb overexpression (mRNA and protein) confirmed in vitro (Figures 1C and 1D, respectively). Expression of CAR mRNA, the primary receptor for CAV-2 virus, in the brain of SHRSP was confirmed (Supplementary Figure S1B).
Figure 3.
Comparative transduction levels from canine adenovirus type 2 (CAV-2) and lentivirus in adult rat brain. (A) Rostro-caudal gene transduction (grey) after single injection of 3 × 109 virus particle (vp) CAVGFP (n=4; left) and double injection of 2 × 107 vp lenti-GFP (n=4; right) determined from the median animal in each virus group. Black dots represent cortical injection sites. The staining pattern of green fluorescent protein (GFP) expression was transcribed onto stereotaxic atlas plates.27 (B) Representative cortical images of GFP epifluorescence from lentivirus or CAV-2 transduction 7 days after stereotactic injection; scale bar, 100 μm (top) and 20 μm (bottom). Images taken from the primary somatosensory cortex (S1FL) at coronal level Bregma −0.12 mm (indicated with an X on atlas plate image). (C) Volume of tissue transduced by each viral vector quantified from area under the curve analysis across seven coronal levels. Data presented as mean±s.e.m.; *P<0.0002 versus lentivirus using Student's unpaired t-test.
Neuroglobin Overexpression Combined with c-Jun N-Terminal Kinase Inhibition Protects In Vivo Against Cerebral Ischemia/Reperfusion
Virus-mediated overexpression of neuroglobin
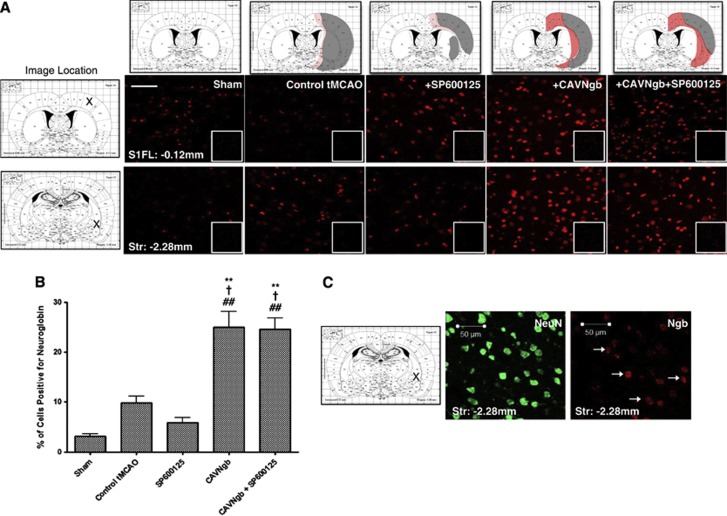
Neuroglobin expression was determined 14 days after tMCAO across all groups (Figure 4A), 19 days after the CAV-2 vector was administered. In control tMCAO animals, there was a small, nonsignificant increase in the number of cells expressing Ngb in the peri-infarct cortical and striatal regions (9.9±1.3%) compared with sham animals (3.1±0.5% Figure 4B). Similarly, no significant change in the number of cells positive for Ngb expression was seen in animals randomly allocated to receive SP600125 (5.8±1.1% Figures 4A and 4B). The endogenous expression of Ngb in these groups was largely restricted to the peri-infarct region (Figure 4A). In animals receiving CAVNgb alone (25±3.1%) or in combination with SP600125 (24.6±2.2%), there was a marked and significant increase in the number of cells positive for Ngb expression within the peri-infarct cortex and striatum 19 days after virus administration (Figure 4A) compared with sham-, control tMCAO-, or SP600125-treated animals (Figure 4B). The increased Ngb expression in CAVNgb-treated groups was evident in both cortical and striatal regions around the infarct (Figure 4A). The rostro-caudal spread in enhanced Ngb expression after tMCAO appeared consistent with that seen for CAVGFP (Figure 3), with increased expression evident over a rostro-caudal distance of ∼7 mm from the site of injection. Ngb expression was primarily colocalized with NeuN-positive cells indicating a neuronal expression. Note that not all NeuN-positive cells appear positive for Ngb (Figure 4C).
Figure 4.
Canine adenovirus type 2 (CAV-2)-mediated overexpression of neuroglobin (Ngb) after stereotactic delivery and subsequent transient middle cerebral artery occlusion (tMCAO). (A) Infarct location (grey) and representative distribution of neuroglobin expression (red) for each group (top panel) determined by immunohistochemistry (IHC) 19 days after CAV-2 delivery and 14 days after tMCAO. Representative images showing levels of neuroglobin expression at two distinct peri-infarct sites (Bregma −0.12 mm, cortical site, top panel or Bregma −2.28 mm, striatal site, bottom panel) after tMCAO. Inset panels representative of isotype-matched immunoglobulin G (IgG) control staining. Scale bar, 50 μm. Image location indicated by X on atlas plate—primary somatosensory cortex (S1FL) or striatum (Str). (B) Quantification of neuroglobin-positive cells expressed as a percentage of total cells (4',6-diamidino-2-phenylindole (DAPI) nuclear stain) within five matched locations across the cortex and striatum at two coronal levels. Data presented as mean±s.e.m. and analyzed by analysis of variance (ANOVA) using Tukey's post hoc correction. **P<0.001 versus sham, †P<0.01 versus control tMCAO, and ##P<0.001 versus tMCAO+SP600125. (C) Representative double-labeled images of immunopositive neurones (NeuN) and neuroglobin-positive (Ngb) cells at coronal level Bregma −2.28 mm in the striatum (Str). Representative dual-labeled cells indicated by arrows. Scale bar, 50 μm.
Survival and systolic blood pressure
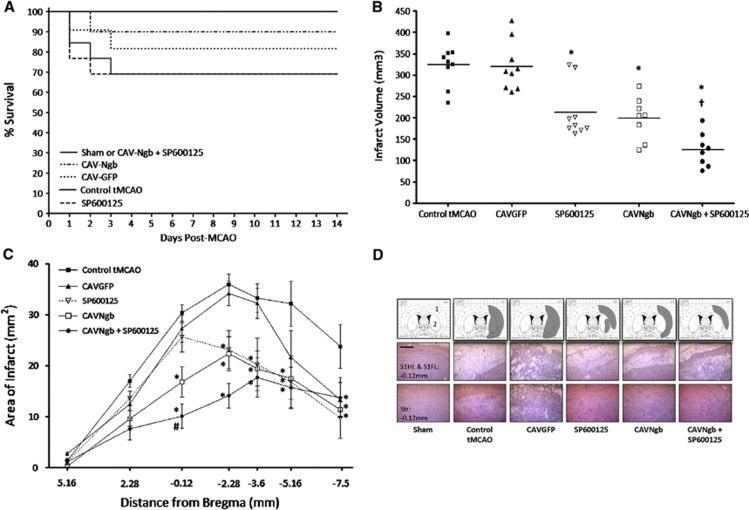
No intervention affected systolic blood pressure (SBP) at 7 or 14 days after tMCAO (Table 1). Furthermore, there was no significant difference in the starting/final SBP values between groups. The associated weight loss after tMCAO was similar between groups (Table 1). Survival over the duration of the study was 100% in the sham (6/6) and tMCAO with combined intervention (8/8) groups only. Although mortality occurred in the other groups, survival rates were not significantly different between groups: tMCAO, 9/13; tMCAO+CAVNgb, 9/10; tMCAO+SP600125, 9/13; and tMCAO+CAVGFP, 9/11 (Figure 5A).
Table 1. Physiologic parameters.
| Baseline SBP (mm Hg) | Day 7 SBP (mm Hg) | Before killing SBP (mm Hg) | Weight loss after tMCAO (g) | |
|---|---|---|---|---|
| Sham | 177.5±5.6 | 175.3±5.1 | 180.7±5.8 | 6.83±2.7 |
| tMCAO | 173.2±3.8 | 177.8±4.2 | 175.4±4.5 | 15.22±3.0 |
| tMCAO+CAVGFP | 171.4±4.3 | 181.3±4.5 | 186.6±3.3 | 12.59±1.7 |
| tMCAO+SP600125 | 179.3±3.1 | 179.3±2.8 | 179.3±4.2 | 18.11±3.5 |
| tMCAO+CAVNgb | 174.7±4.2 | 182.8±2.8 | 177.7±4.1 | 13.13±2.5 |
| tMCAO+CAVNgb+SP600125 | 173.5±5.4 | 176.8±4.3 | 182.6±2.9 | 18.11±3.5 |
CAVGFP, canine adenovirus type 2 vector overexpressing green fluorescent protein; Ngb, neuroglobin; SBP, systolic blood pressure; tMCAO, transient middle cerebral artery occlusion.
SBP measured 3 days before (baseline) and 7 and 14 days after tMCAO (before killing) and the change in body weight over the corresponding period. Data presented as mean±s.e.m, n=6 to 9.
Figure 5.
Neuroglobin (Ngb) overexpression combined with c-jun N-terminal kinase (JNK) inhibition does not affect mortality but reduces infarct volume after ischemia/reperfusion in vivo. (A) Kaplan–Meier curves showing comparable survival rates between sham, transient middle cerebral artery occlusion (tMCAO), and intervention groups using Fisher's exact test. (B) Infarct volume determined 14 days after tMCAO. Individual data points for each animal, with line representing the mean. (C) Rostro-caudal distribution of infarct. Data presented as mean±s.e.m. and analyzed by one-way analysis of variance (ANOVA) with Bonferroni's post-hoc correction; P<0.01 versus *tMCAO, †tMCAO+SP600125 or tMCAO+CAVNgb, and #tMCAO+SP600125. (D) Representative transcribed infarct (top panel) and hematoxylin and eosin (H&E) images (middle and bottom panels) from the median animal in each group at coronal level Bregma −0.12 mm. Middle panel indicates primary somatosensory cortex (S1HL and S1FL, indicated with 1 on atlas plate image) and bottom panel indicates striatum (Str, indicated with 2 on atlas plate image). Scale bar, 50 μm.
Infarct volume
Infarct volumes were comparable in untreated tMCAO control (324±16.4 mm3, n=9) and tMCAO+CAVGFP (314±22.2 mm3, n=9) rats (Figure 5B). Treatment with SP600125 and CAVNgb (n=9 per group) significantly reduced infarct volume by 33.8% (215±34.2 mm3*) and 38.6% (199±14.4 mm3*), respectively compared with control tMCAO (*P<0.01 versus tMCAO). Importantly, combined intervention (n=8) produced a greater reduction in infarct volume than single treatments (57% 137±20.7 mm3, *†P<0.01 versus *tMCAO or †SP600125 and CAVNgb). The rostro-caudal extent of infarction in each group is shown in Figure 5C. Morphologic analysis showed that in animals receiving CAVNgb, SP600125, or CAVNgb+SP600125, the extent of infarct in the primary motor cortex and striatum was reduced versus control tMCAO or CAVGFP (Figure 5D). However, there was a region in the primary somatosensory forelimb and hindlimb cortex that was consistently infarcted irrespective of intervention (Figure 5D, middle panel).
Neurologic deficit
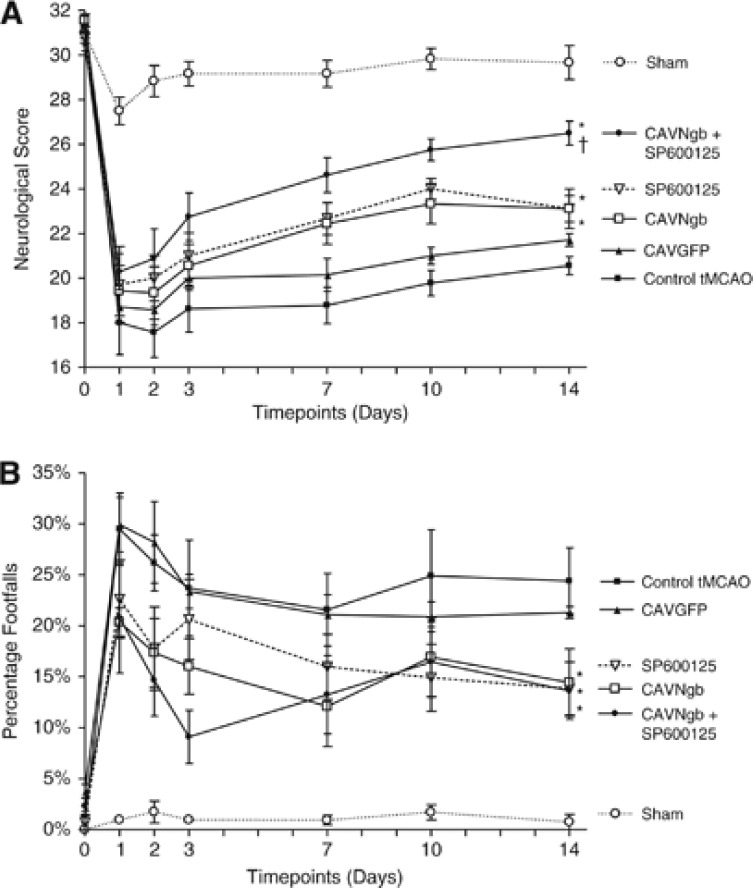
All tMCAO groups exhibited a significant reduction in neurologic score from baseline at 24 hours after tMCAO (Figure 6A). No significant difference in neurologic score was observed between groups over days 1 to 3. At day 7, a significant improvement in neurologic recovery was observed with combined therapy compared with control tMCAO. However, at day 10, there was a significant improvement with single treatment groups compared with control tMCAO, but no further improvement with combination therapy. By day 14, single treatments significantly improved neurologic score, with combined therapy producing a further significant improvement compared with single treatments (Figure 6A). All tMCAO groups exhibited a significant increase in percentage footfalls 24 hours after tMCAO on the tapered beam walk test (Figure 6B). By day 14, a significant improvement in total footfalls was evident in all treatment groups versus the control group. However, no additional improvement with combined therapy was observed (Figure 6B).
Figure 6.
Neuroglobin (Ngb) overexpression combined with c-jun N-terminal kinase (JNK) inhibition improves neurologic deficit. (A) A 32-point neurologic score measured general condition, righting reflex, grip strength, paw placement, circling, horizontal bar, inclined platform, visual forepaw reaching, rotation, and mobility. (B) Movement on tapered beam was tracked, footfalls onto an underhanging ledge recorded for both ipsi- and contralateral side, and expressed as a percentage of footsteps taken. Data presented as mean±s.e.m. and analyzed by analysis of variance (ANOVA) using Bonferroni's post hoc correction (n=6 to 9 per group). *P<0.01 versus transient middle cerebral artery occlusion (tMCAO) and †P<0.01 versus single treatment.
Discussion
We describe, for the first time, an improved beneficial effect (both reduced infarct volume and improved neurologic recovery determined longitudinally) through combined antiapoptotic and antioxidant intervention in a clinically relevant stroke model displaying stroke-associated comorbidities. Combined intervention mediated greater neuroprotection than either therapy alone, both in vitro using a model of H/R and in vivo after tMCAO. Furthermore, this study introduces neurotrophic CAV-2 vector as a platform that will allow better understanding of stroke pathophysiology through high levels of brain transduction. Together, this supports the potential for combined intervention strategies; in this case, targeting excessive oxidative stress and apoptosis after cerebral ischemia.
In vitro studies showed that Ngb overexpression combined with JNK inhibition mediated greater neuroprotection and less oxidative stress and apoptosis after H/R injury compared with individual treatments. Although each single treatment has been studied previously in neuronal cells in vitro, combination therapy has not. Immortalized HN33 hippocampal neurones transfected to overexpress Ngb exhibited improved cell viability, measured by MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, after 8 or 24 hours of anoxia.28 Additional mechanistic studies determined that there was no increase in oxygen consumption in Ngb-overexpressing cells compared with controls, and an involvement of nitric oxide scavenging was further ruled out through the failure of Ngb to protect against sodium nitroprusside-induced toxicity.28 It has since been shown that Ngb elicits protection from H/R insult in vitro through multiple mechanisms including improving mitochondrial function, maintenance of intracellular ATP levels, inhibition of calcium influx, binding to cytochrome c, and reduced caspase 3/7 and 9 activity in human neuroblastoma cells overexpressing Ngb.29, 30, 31 In this study, vector-mediated Ngb overexpression resulted in reduced ROS generation (Figure 2). Although Ngb was initially believed to act purely as an antioxidant, studies29, 31 including this study suggest antiapoptotic effects through an interaction with cytochrome c, reduced caspase 3/7 or 9 activity/nuclear localization, and DNA fragmentation (reviewed in Brittain32). It is impossible to distinguish unequivocally between apoptotic and oxidative stress pathways as they are inextricably linked with extensive crosstalk between them. However, Ngb directly activates the PI3K/AKT/mTOR (phosphatidylinositide 3-kinase/AKT/ mammalian target of rapamycin) pathway of cell survival.33 Furthermore, JNK may be involved in prooxidant pathways because the N-terminal Von Hippel–Lindau recognition site of hypoxia inducible factor-1α contains a JNK-binding domain.34
Notably, translation to an in vivo stroke model confirmed that combined Ngb upregulation and JNK inhibition was better than single treatment using infarct size and neurologic deficit as outcome measures (Figures 4, 5, 6). The greatest reduction in infarct size, detected in the combination treatment group, was accompanied by an improved neurologic score and reduction in footfalls at day 14. The tapered beam walk test failed to show any additional improvement with combined treatment, which likely reflects the conserved region of infarct within the primary somatosensory forelimb/hindlimb cortex in all groups (Figure 5D). There was a trend toward an increase in endogenous Ngb levels at 14 days after tMCAO in the SHRSP, although this was not significantly enhanced compared with sham (Figure 4). We cannot exclude the possibility that peak Ngb levels occurred earlier and returned toward basal levels 2 weeks after cerebral ischemia. Endogenous upregulation of Ngb in cells in the peri-infarct region agrees with other preclinical28 and clinical stroke studies35 where increased Ngb levels were reported. Importantly, virus-mediated overexpression of Ngb was sustained to 14 days after tMCAO (19 days after administration) in animals randomized to receive CAVNgb or the combined treatment. Interestingly, in SP600125-treated animals, which had a similar infarct volume to the CAVNgb single intervention group, there was no difference in the number of Ngb-positive cells compared with sham. This may reflect that with the smaller infarct resulting from SP600125 treatment, the stimulus for endogenous Ngb upregulation after tMCAO was reduced. Interestingly, in the intervention study, increased Ngb expression was detected in the striatum and cortex after virus delivery and tMCAO. Striatal gene expression was not evident from the CAVGFP administration in naive rats (Figure 3). Although we cannot distinguish between endogenous or virus-mediated upregulation in Ngb expression within the striatum, we can postulate that after tMCAO transduction profiles may differ or that there is an active transport process occurring similar to what has previously been described with CAV-2 vectors.36
Previous studies determining the neuroprotective effect of Ngb in vivo showed Ngb-overexpressing transgenic mice had lower levels of lipid peroxidation measured by MDA assay in the ipsilateral hemisphere after tMCAO.11 In addition, reduced numbers of TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling)-positive cells and increased numbers of neurones with a normal morphology (from cresyl violet histology) were seen after delivery of Ngb linked to a fusion protein before tMCAO in C57BL/6J mice.8 This shows that in spite of the multifaceted mechanism of action of Ngb (antioxidant and antiapoptotic), further protection can be afforded through combined JNK inhibition. All previous experimental stroke reports of neuroprotection afforded by Ngb have been determined in normal mice or rats or using transgenic overexpression.8, 9, 10, 11 In using SHRSP, which exhibit a number of stroke-related comorbidities, we have addressed STAIR guidelines5 as hypertension was reported as the strongest risk factor for stroke in a global case study.7 Furthermore, all analyses were performed under masked conditions with animals randomly assigned to experimental groups and blood pressure monitoring throughout, in accordance with the preclinical stroke study guidelines.6 Of the 142 publications describing combined therapy for stroke, only 3% have used hypertensive rats.3 The majority of these determined the potential for adjuvant therapy to extend the therapeutic window for rt-PA.4 Only one combination treatment study (antioxidant tempol and/or cannabinoid/NMDA antagonist dexanabinol after permanent MCAO) used hypertensive rats and this failed to identify any additional benefit of combined treatment, attributing this to a ceiling neuroprotection achieved by either single treatment.37 We designed our study to limit this possibility. The dose of SP600125 used (1 mg/kg) was selected based on previous studies in mice showing a significant, but submaximal (seen at 3 mg/kg), reduction in infarct volume after tMCAO18 in an attempt to avoid a similar ceiling effect. A recovery period of 14 days was set to ensure capture of final infarct size (shown previously to continue to evolve 48 to 72 hours after tMCAO in rats38) and allow serial assessment of neurologic outcome measures.
The utility of CAV-2 vectors for neurodegenerative diseases has previously been shown in dopamine-deficient mice where CAV-2-transduced neurons expressed tyrosine hydroxylase for >1 year from delivery.39 More recently, in a lysosomal storage disorder, neonatal delivery led to persistant transgene expression and normalization of the functional deficit for up to 20 weeks.40 A single cortical CAV-2 injection efficiently transduced neurons and resulted in an eightfold greater transduction volume and covered a greater rostro-caudal distance than lentivirus in the present study. In the first study using CAV-2 in a cerebral ischemia model, increased levels of Ngb transduction remained in neurons of peri-infarct cortical and striatal regions 2 weeks after tMCAO and 19 days after CAV-2 delivery, showing sustained and selective transgene expression. Gene- and cell based-therapies are now being clinically tested for a wide range of diverse disease pathologies. Although the success of the CAV-2 vector to directly deliver Ngb to the brain parenchyma overcomes limitations relating to the ability of pharmacological antioxidants to reach the brain by failure to cross the blood–brain barrier, translation of novel gene delivery vectors, such as CAV-2, will require further extensive preclinical research. A more feasible clinical prospect may be through pharmacological upregulation of Ngb to similar levels achieved with CAV-2 vectors. A recent study described the use of the prolyl hydroxylase inhibitor (PHI) deferoxamine and the short-chain fatty acids cinnamic and valproic acids to pharmacologically increase Ngb expression in HN33 cells in vitro.41 However, whether such an effect on Ngb expression is also evident in vivo has yet to be described. In addition, these agents are likely to affect a number of pathways in addition to merely upregulating Ngb—the PHIs, for example, enhance levels of hypoxia inducible factor-1α and -2α, resulting in neuroprotection through a plethora of target pathways.42
In conclusion, the combination of antiapoptotic JNK inhibition and antioxidant Ngb upregulation shows a significant neuroprotective potential when administered before tMCAO. This study shows that targeting these pathways in combination improves efficacy compared with a single target using an animal model exhibiting stroke-associated comorbidities. Further use of such a combined approach targeting excessive oxidative stress and apoptosis may be applicable in disease settings where these pathways are implicated, such as neurodegenerative disorders (Alzheimer's disease, Parkinson's disease), traumatic brain injury, or hypoxic insult (such as hypoxic–ischemic neonatal encephalopathy, a problem in perinatal medicine). The next step in stroke translation will be to assess if neuroprotection and improved outcome can be achieved when treatment is delivered in the acute poststroke period and if it can act additively or synergistically with thrombolysis. However, although many single and combination therapeutic approaches have proven successful in preclinical models, this has not led to successful translation in stroke patients, and thrombolysis within a tight time frame of the onset of acute cerebral ischemia remains the primary intervention at this time.
Acknowledgments
The authors thank Professor Andrew H Baker (University of Glasgow) for helpful discussion and advice, Charles Thomson for technical assistance, and Wendy Crawford for donating the SHRSP heart and liver RNA. The pET3a_Ngb was a gift from Professor David A Greenberg (Buck Institute for Research on Aging, USA).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by a PhD studentship through a Capacity Building Award in Integrative Mammalian Biology (BB/E527071/1; Biotechnology & Biological Science Research Council, British Pharmacological Society, Knowledge Transfer Network, Medical Research Council, and Scottish Funding Council); a British Heart Foundation project grant (PG/07/126/24223); a Region Languedoc Roussillon-Communauté de travail des Pyrénées grant (115277); and a Fondation de France grant (2008005416).
Supplementary Material
References
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Collins VE, Macleod MR, Donnan GA, Howells DW. Evaluation of combination therapy in animal models of cerebral ischemia. J Cereb Blood Flow Metab. 2012;32:585–597. doi: 10.1038/jcbfm.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang ZG, Chopp M. The neurovascular unit and combination treatment strategies for stroke. Trends Pharmacol Sci. 2012;33:415–422. doi: 10.1016/j.tips.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Feuerstein GZ, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the Stroke Therapy Academic Industry Roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod MR, Fisher M, O'Collins V, Sena ES, Dirnagl U, Bath PMW, et al. Good laboratory practice: preventing introduction of bias at the bench. Stroke. 2009;40:e50–e52. doi: 10.1161/STROKEAHA.108.525386. [DOI] [PubMed] [Google Scholar]
- O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- Cai B, Lin Y, Xue X, Fang L, Wang N, Wu Z. TAT-mediated delivery of neuroglobin protects against focal cerebral ischemia in mice. Exp Neurol. 2011;227:224–231. doi: 10.1016/j.expneurol.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Khan AA, Sun Y, Jin K, Mao XO, Chen S, Ellerby LM, et al. A neuroglobin-overexpressing transgenic mouse. Gene. 2007;398:172–176. doi: 10.1016/j.gene.2007.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Peel A, Mao XO, Xie L, Greenberg DA. Neuroglobin protects the brain from experimental stroke in vivo. Proc Natl Acad Sci USA. 2003;100:3497–3500. doi: 10.1073/pnas.0637726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu J, Zhu H, Tejima E, Tsuji K, Murata Y, et al. Effects of neuroglobin overexpression on acute brain injury and long-term outcomes after focal cerebral ischemia. Stroke. 2008;39:1869–1874. doi: 10.1161/STROKEAHA.107.506022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Miller JD, Heistad DD. Gene therapy for stroke: 2006 overview. Curr Hypertens Rep. 2007;9:19–24. doi: 10.1007/s11906-007-0005-7. [DOI] [PubMed] [Google Scholar]
- Soudais C, Laplace-Builhe C, Kissa K, Kremer EJ. Preferential transduction of neurons by canine adenovirus vectors and their efficient retrograde transport in vivo. FASEB J. 2001;15:2283–2285. doi: 10.1096/fj.01-0321fje. [DOI] [PubMed] [Google Scholar]
- Soudais C, Skander N, Kremer EJ. Long-term in vivo transduction of neurons throughout the rat central nervous system using novel helper-dependent CAV-2 vectors. FASEB J. 2004;18:391–393. doi: 10.1096/fj.03-0438fje. [DOI] [PubMed] [Google Scholar]
- Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, et al. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat. Med. 2003;9:1180–1186. doi: 10.1038/nm911. [DOI] [PubMed] [Google Scholar]
- Hirt L, Badaut J, Thevenet J, Granziera C, Regli L, Maurer F, et al. D-JNKI1, a cell-penetrating c-Jun-N-terminal kinase inhibitor, protects against cell death in severe cerebral ischemia. Stroke. 2004;35:1738–1743. doi: 10.1161/01.STR.0000131480.03994.b1. [DOI] [PubMed] [Google Scholar]
- Gow WR, Campbell K, Meade AJ, Watt PM, Milech N, Knuckey NW, et al. Lack of neuroprotection of inhibitory peptides targeting Jun/JNK after transient focal cerebral ischemia in spontaneously hypertensive rats. J Cereb Blood Flow Metab. 2011;31:e1–e8. doi: 10.1038/jcbfm.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Signore AP, Yin W, Cao G, Yin XM, Sun F, et al. Neuroprotection against focal ischemic brain injury by inhibition of c-Jun N-terminal kinase and attenuation of the mitochondrial apoptosis-signaling pathway. J Cereb Blood Flow Metab. 2005;25:694–712. doi: 10.1038/sj.jcbfm.9600062. [DOI] [PubMed] [Google Scholar]
- Demaison C, Parsley K, Brouns G, Scherr M, Battmer K, Kinnon C, et al. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther. 2002;13:803–813. doi: 10.1089/10430340252898984. [DOI] [PubMed] [Google Scholar]
- Kremer EJ, Boutin S, Chillon M, Danos O. Canine adenovirus vectors: an alternative for adenovirus-mediated gene transfer. J Virol. 2000;74:505–512. doi: 10.1128/jvi.74.1.505-512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soudais C, Boutin S, Kremer EJ. Characterization of cis-acting sequences involved in canine adenovirus packaging. Mol Ther. 2001;3:631–640. doi: 10.1006/mthe.2001.0263. [DOI] [PubMed] [Google Scholar]
- Greig JA, Shirley R, Graham D, Denby L, Dominiczak AF, Work LM, et al. Vascular-targeting anti-oxidant therapy in a model of hypertension and stroke. J Cardiovasc Pharmacol. 2010;56:642–650. doi: 10.1097/FJC.0b013e3181f8f19f. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ord ENJ, Shirley R, van Kralingen JC, Graves A, McClure JD, Wilkinson M, et al. Positive impact of pre-stroke surgery on survival following transient focal ischemia in hypertensive rats. J Neurosci Methods. 2012;211:305–308. doi: 10.1016/j.jneumeth.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi J, Yoshida Y, Nakazawa T, Ooneda G. Experimental studies of ischemic brain edema: a new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke. 1986;8:1–8. [Google Scholar]
- Osborne KA, Shigeno T, Balarsky AM, Ford I, McCulloch J, Teasdale GM, et al. Quantitative assessment of early brain damage in a rat model of focal cerebral ischaemia. J Neurol Neurosurg Psychiatry. 1987;50:402–410. doi: 10.1136/jnnp.50.4.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C.(eds).. The Rat Brain in Stereotaxic Coordinates6th edn.Academic Press; 2007 [Google Scholar]
- Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc Natl Acad Sci USA. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong TTH, Witting PK, Antao ST, Parry SN, Kennerson M, Lai B, et al. Multiple protective activities of neuroglobin in cultured neuronal cells exposed to hypoxia re-oxygenation injury. J Neurochem. 2009;108:1143–1154. doi: 10.1111/j.1471-4159.2008.05846.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Yu Z, Guo S, Lee S-R, Xing C, Zhang C, et al. Effects of neuroglobin overexpression on mitochondrial function and oxidative stress following hypoxia/reoxygenation in cultured neurons. J Neurosci Res. 2009;87:164–170. doi: 10.1002/jnr.21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri S, Skommer J, Henty K, Birch N, Brittain T. Neuroglobin protects nerve cells from apoptosis by inhibiting the intrinsic pathway of cell death. Apoptosis. 2010;15:401–411. doi: 10.1007/s10495-009-0436-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain T. The anti-apoptotic role of neuroglobin. Cells. 2012;1:1133–1155. doi: 10.3390/cells1041133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antao ST, Duong TT, Aran R, Witting PK. Neuroglobin overexpression in cultured human neuronal cells protects against hydrogen peroxide insult via activating phosphoinositide-3 kinase and opening the mitochondrial K(ATP) channel. Antioxid Redox Signal. 2010;13:769–781. doi: 10.1089/ars.2009.2977. [DOI] [PubMed] [Google Scholar]
- Antoniou X, Sclip A, Ploia C, Colombo A, Moroy G, Borsello T. JNK contributes to Hif-1alpha regulation in hypoxia. Molecules. 2009;15:114–127. doi: 10.3390/molecules15010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Mao Y, Mao X, Xie L, Greenberg DA. Neuroglobin expression in ischemic stroke. Stroke. 2010;41:557–559. doi: 10.1161/STROKEAHA.109.567149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas S, Bilsland LG, Henaff D, Weston AE, Keriel A, Schiavo G, et al. CAR-associated vesicular transport of an adenovirus in motor neuron axons. PLoS Pathog. 2009;5:e1000442. doi: 10.1371/journal.ppat.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichner A, Ovadia H, Lavie G, Leker RR. Combination of dexanabinol and tempol in focal cerebral ischemia: is there a ceiling effect. Exp Neurol. 2003;182:353–360. doi: 10.1016/s0014-4886(03)00083-9. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T, Kastrup A, de Crespigny A, Yenari MA, Ringer T, Sun GH, et al. Serial MRI after transient focal cerebral ischemia in rats: dynamics of tissue injury, blood-brain barrier damage, and edema formation. Stroke. 2000;31:1965–1973. doi: 10.1161/01.str.31.8.1965. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Perez FA, Scouras AD, Stoll EA, Gale SD, Luquet S, et al. Cre recombinase-mediated restoration of nigrostriatal dopamine in dopamine-deficient mice reverses hypophagia and bradykinesia. Proc Natl Acad Sci USA. 2006;103:8858–8863. doi: 10.1073/pnas.0603081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau AA, Hopwood JJ, Kremer EJ, Hemsley KM. SGSH gene transfer in mucopolysaccharidosis type IIIA mice using canine adenovirus vectors. Mol Genet Metab. 2010;100:168–175. doi: 10.1016/j.ymgme.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Jin K, Mao X, Xie L, John V, Greenberg DA. Pharmacological induction of neuroglobin expression. Pharmacology. 2011;87:81–84. doi: 10.1159/000322998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC. Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci. 2007;27:6320–6332. doi: 10.1523/JNEUROSCI.0449-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.