Abstract
After stroke, brain inflammation in the ischemic hemisphere hampers brain tissue reorganization and functional recovery. Housing rats in an enriched environment (EE) dramatically improves recovery of lost neurologic functions after experimental stroke. We show here that rats housed in EE after stroke induced by permanent occlusion of the middle cerebral artery (pMCAO), showed attenuated levels of proinflammatory cytokines in the ischemic core and the surrounding peri-infarct area, including a significant reduction in the stroke-induced chemokine receptor CXCR4 and its natural ligand stromal cell-derived factor-1 (CXCL12). To mimic beneficial effects of EE, we studied the impact of inhibiting CXCL12 action on functional recovery after transient MCAO (tMCAO). Rats treated with the specific CXCL12 receptor antagonist 1-[4-(1,4,8,11-tetrazacyclotetradec-1-ylmethyl)phenyl]methyl]-1,4,8,11-tetrazacyclo-tetradecan (AMD3100) showed improved recovery compared with saline-treated rats after tMCAO, without a concomitant reduction in infarct size. This was accompanied by a reduction of infiltrating immune cells in the ischemic hemisphere, particularly cluster of differentiation 3-positive (CD3+) and CD3+/CD4+ T cells. Spleen atrophy and delayed death of splenocytes, induced by tMCAO, was prevented by AMD3100 treatment. We conclude that immoderate excessive activation of the CXCL12 pathway after stroke contributes to depression of neurologic function after stroke and that CXCR4 antagonism is beneficial for the recovery after stroke.
Keywords: brain ischemia, chemokine, enriched environment, inflammation, regeneration and recovery, T-cells
Introduction
Stroke is the main cause of adult disability worldwide. During stroke, brain function is rapidly lost due to ischemia, and the subsequent recovery of function is slow and incomplete due to neuronal death and the limited functional remodeling of remaining neuronal circuitry. While acute thrombolytic therapy is available to treat brain ischemia and prevent subsequent acute cell death, there are currently no pharmacological treatments that enhance lost neurologic functions after stroke.1 During recent years advanced sensorimotor and language training protocols alone or in combination with magnetic or electrical stimulation of the brain, have highlighted the capacity of the injured brain to recover by stimulating its plasticity, i.e., ability to modulate synaptic function and remodel neural networks. In contrast to the relative narrow therapeutic window of neuroprotection, clinical evidence shows that targeting brain plasticity can be effective when initiated days, weeks, and even years after stroke.2 Importantly, and in contrast to the many failed clinical trails using neuroprotective strategies, pharmacological enhancement of brain plasticity has shown efficacy in small clinical trials.3 Hence, elucidating the fundamental processes regulating or affecting brain plasticity after stroke could provide new therapies that would enhance recovery after stroke for the benefit of a majority of stroke patients.
Brain plasticity is a composite of multiple processes affecting all brain cells, notably neurons, and is particularly prominent during development.4 In the injured brain, additional processes influence brain remodeling, particularly the inflammatory response to injury.5 Brain ischemia and the progressing tissue infarction after stroke trigger the release of inflammatory mediators that activate resident microglia cells or attract invading immune cells to the injured tissue, processes that may be ongoing for several months after the insult.6 In the acute phase after stroke, inflammation is thought to induce and enhance acute or delayed cell death. Once cell death in the brain has subsided, inflammation may be either beneficial or detrimental: beneficial by stimulating the removal of debris from degenerated tissue in order to stimulate the axonal growth, or detrimental in processes of brain repair and reorganization by hampering the axonal growth.7 In addition to local inflammatory processes in the brain, stroke also activates an immune response in peripheral lymphoid organs such as the spleen and thymus.8 The peripheral immune response is preferentially mediated by the sympathetic nervous systems, leading to apoptosis and atrophy of the spleen and thymus and a subsequent immunodepression.8, 9, 10
Since immunomodulators such as cytokines and chemokines are also neuromodulators, brain cells and the immune system may cross-talk and influence brain function and plasticity.11 This is apparent when rodents are housed in an enriched environment (EE) that induces a strong multisensory stimulation to the brain, affecting multiple plasticity processes including the regulation of inflammatory mediators.12 Likewise, enriched housing initiated days after experimental stroke enhances long-term functional recovery, which occurs without decreasing infarct size. Here, the beneficial processes stimulate glia cells to encapsulate the infarct13, 14, 15 or enhance neuronal plasticity.16 Importantly, enriched housing has a profound effect on the inflammatory reponse after brain injury14 and modulates cell genesis.17 Means to optimize the inflammatory response in order to balance the removal of debris and to stimulate brain plasticity in the injured hemisphere are indeed warranted.18
While a general anti-inflammatory strategy would affect both repair and degenerative processes, targeting processes that modulate detrimental lymphocyte infiltration into the injured brain can be envisaged to support beneficial processes. Among the chemoattractants regulated in the brain, the chemokine CXCL12 acting on its receptors CXCR4 and CXCR719 has a recognized role in brain inflammation and neuromodulation.20 Among others, CXCL12 reduces spontaneous firing in Cajal-Retzius cells21 demonstrating its ability for neuro-inflammatory cross-talk.22 Moreover, CXCL12 is involved in migration and mobilization of stem cells in the brain after stroke.23 After experimental stroke, CXCL12 expression is elevated for several days24 and closely associated with the infiltration of CXCR4 expressing cells.25 The action of CXCL12 is not unambiguous. CXCL12 is instrumental in attracting CXCR4-expressing immune cells to tissues, aggravating tissue injury,26 while in demyelinating inflammatory conditions27 or infectious disease such as West Nile virus encephalitis, CXCL12 prevents the entry of cluster of differentiation 8-positive (CD8+) cells into the brain at the brain–endothelial interface and therefore appears to have a protective function.28
This study was undertaken to analyze how multisensory stimulation of the brain that improves brain function after experimental stroke, affect inflammatory processes in the brain. We demonstrate that brain activation by enriched housing downregulates the inflammatory response in the ischemic hemisphere after experimental stroke, including CXCL12 and CXCR4. We also show that attenuation of the inflammatory response by inhibiting the action of CXCL12 enhances recovery of sensorimotor function after stroke and prevents spleen atrophy. We conclude that targeting CXCL12 after stroke may provide new pharmacological treatments for improvement of recovery after stroke.
Materials And Methods
Permanent Rat Middle Cerebral Artery Occlusion
All animal experiments were approved by the Malmö-Lund Ethical Committee and were performed according to the ARRIVE guidelines. Male, spontaneously hypertensive rats (17 weeks old) were anaesthetized with 60 mg sodium pentobarbital (60 mg/ml per kg BW). Marcain (1.25 mg/kg) was used for local anesthesia. After dissection of the right temporal muscle, a small hole was drilled into the scull bone. The right middle cerebral artery (MCA) was localized and ligated.14 After surgery, animals were kept on a warm blanket to maintain body temperature at 37°C. The MCA was exposed but not ligated in sham-operated animals.
Transient Rat Middle Cerebral Artery Occlusion
Transient MCAO (tMCAO) was induced as described previously.14 Male Wistar rats (325 to 350 g, HsdBrlHan, Harlan Scandinavia, Horst, The Netherlands) were housed under diurnal light conditions and were fasted for 12 hours before surgery. Physiologic parameters—arterial blood pressure and gases after tail artery cannulation and insertion of a catheter (SIMS Portex, Ashford, UK) for rectal body temperature—were measured and controlled within physiologic limits during surgery (Supplementary Tables 1 and 2). Body temperature was also measured at 1 and 2 hours of occlusion and at 1 and 2 hours of recirculation. Rats were anesthetized (initially with 4% fluothane in N2O/O2 (70:30), and during surgery with 2% fluothane in N2O/O2, Astra Zeneca, Södertälje, Sweden) and the right common carotid artery and the external carotid artery were occluded permanently, the internal carotid artery was exposed and ligated. A nylon filament (top diameter 0.3 to 0.4 mm) was introduced into the internal carotid artery via a small incision into the distal end of the common carotid artery and advanced to occlude the origin of the MCA for 2 hours. Regional cerebral blood flow in the MCA was monitored by a flexible optical fiber connected to a laser Doppler (PeriFlux System 5000, Perimed, Järfälla, Sweden). Two hours after occlusion, a neurologic score was assessed and only rats showing rotational asymmetry and dysfunctional limb placement were included into the study.
Randomization and Treatment Protocols
Loss of sensorimotor function was assessed by the rotating pole test at 48 hours after transient or pMCAO. Only animals that were not able to cross the rotating pole were randomly assigned into treatment groups.15 Subsequently, starting on day 2 after pMCAO, rats were housed in standard laboratory cages or EE cages for 3 days. Neurologic score was assessed by the rotating pole test at postoperative day 5 as described previously14 and brain tissue from the same set of animals has been used for analyses in the present study.
The same inclusion criteria applied for the injection studies. After inclusion, every other rat received (1,1′-[1,4-phenylenebis(methylene)]bis[1,4,8,11-tetraazacyclotetradecane], AMD3100, Tocris Bioscience, Bristol, UK), an antagonist on the CXCR4 receptor, which was dissolved in saline (final concentration 0.5 mg/kg body weight) and injected intraperitoneally every 12 hours for 3 days starting on day 2 after tMCAO. Neurologic score was obtained by the rotating pole test at postoperative day 5 and rats were killed on postoperative day 6. Mortality rate during the first 48 hours before treatment start was 8 out of 33 rats in injection study 1, and 2 out of 30 in injection study 2. Thereafter, none of the rats died.
Post Stroke Housing Conditions-Enriched Environment
The method has been described earlier.15 Rats were either housed in standard cages (cage size: 370 × 220 × 190 mm+one cage mate) or in large cages with multilevel platforms connected with beams, tubes, and ladders (cage size 600 × 600 × 1,200 mm together with several cage mates). The positions of moveable parts in the cage were changed every 3-4 days. After pMCAO, rats were kept for 2 days in their home cage, and randomly selected for housing in EE or standard housing for 3 days (even numbers—standard housing; uneven numbers–EE). Importantly, all animals showed severe neurologic deficit (test score on the rotating pole was below 2) before differential housing. Neurologic function was assessed by the rotating pole test that measures coordination and integration of movement and has been described previously.15 Infarct volumes were determined as decribed previously.14
Serum and Collection of Cerebrospinal Fluid
Rats were anaesthetized and blood was sampled into heparinized syringes by intracardiac puncture. After incubation for 30 minutes at room temperature (rt), blood was centrifuged at 1,000 g for 10 minutes. Supernatant serum was used for cytokine analyses. Cerebrospinal fluid was collected by puncture of the cisterna magna using a capillary (top diameter 0.2 mm). After transfer into a microtube, cells were separated by centrifugation at 1,000 g for 10 minutes.
Measurement of Postischemic Cytokine Production
Tissue samples from different brain regions were collected as described previously.14 Cytokine levels were measured from serum, cerebrospinal fluid, and brain tissue homogenates by a multiplex immunoassay kit according to manufacturer's instructions using a SECTOR Imager 6000 reader (Mesoscale, Gaithersburg, MD, USA).
Immune Cell Preparation from Brain
Rats were lightly anesthetized with isoflurane and perfused with saline for 4 minutes to remove circulating blood cells. Brains were removed, hemispheres dissected and freed from meninges. Hemispheres were dissociated by a dounce homogenizer in icecold Hank's balanced salt solution, supplemented with 0.2% bovine serum albumin, and 0.01% EDTA and passed through a 40 μm nylon cell strainer (BD Biosciences, Stockholm, Sweden). After centrifugation at 400 g at rt for 10 minutes, the pellet was resuspended in a Percoll solution (30% in Hank's balanced salt solution) and overlayed on a gradient of a 37% and 70% Percoll solution. Subsequent centrifugation was performed at 500 × g at rt for 20 minutes. Immune cells were collected at the 37% to 70% interface and washed with Hank's balanced salt solution containing 10% fetal bovine serum. After centrifugation (400 × g, rt, 10 minutes), cells were resuspended in 400 μL phosphate-buffered saline (PBS) containing 0.2% bovine serum albumin. Immediately after, cells were stained with trypan blue and counted.
Immune Cell Preparation from Blood and Spleens
During anesthesia, blood was obtained into a heparinized syringe and 200 μL was incubated with 5 mL red blood cell (RBC) lysis buffer (eBioscience cat# 00-4333-57, San Diego, CA, USA) for 5 minutes. Lysis was stopped by adding 10 mL PBS containing 2% fetal bovine serum. After centrifugation for 5 minutes (300 × g, 4°C), the supernatant was discarded and the cells resuspended in PBS containing 2% fetal bovine serum. The spleens were removed, weighed, and a portion of 10% was homogenized through a 40 μm nylon cell strainer (BD Biosciences) and incubated with 5 mL red blood cell lysis buffer and further processed as blood cells.
Fluorescence-Activated Cell Sorting Analysis
Immune cells were incubated with the following antibodies at 4°C for 20 minutes: monoclonal anti-CD3 fluorescein isothiocyanate-conjugated, monoclonal anti-CD4 allophycocyanin-conjugated, monoclonal anti-CD8 phycoerythrin-conjugated, monoclonal anti-CD45R fluorescein isothiocyanate-conjugated (1:200, all from eBioscience), monoclonal anti-CD11b phycoerythrin-conjugated (1:200, Abcam, Cambridge, UK), and monoclonal anti-CD11c allophycocyanin-conjugated (1:200, AbD Serotec, Düsseldorf, Germany). After rinsing with fluorescence activated cell sorting (FACS) buffer (PBS containing 2% fetal bovine serum), cells were incubated with fixation buffer (BD Cytofix, BD Biosciences) at rt for 20 minutes and washed with BD Perm buffer. After centrifugation (1,000 g, rt, 1 minute), cells were resuspended in FACS buffer and analyzed with a FACScalib (BD Biosciences) using the CellQuest software (BD Biosciences) for acquisition after exclusion of duplets, and FlowJo 8.8.6 (Tree Star, Ashland, OR, USA) was used for further analysis. Progenitor cells in blood were evaluated by staining with monoclonal anti-CD3—fluorescein isothiocyanate, monoclonal anti-CD4—allophycocyanin (diluted 1:200; BD Biosciences), sheep anti-mouse/rat CD34 (diluted 1:100; R&D Systems, Abingdon, UK) and donkey anti-sheep—phycoerythrin (dilution 1:10; R&D Systems). Cells were fixed with fixation buffer (Cytofix, BD Biosciences). Unstained cells and fluorescence minus one controls were used in parallel. Cells were analyzed with a FACS AriaIII and using FACS Diva software (BD Biosciences). Morphologic analysis was performed by using the forward scatter and side scatter parameters (Supplementary Figure 1). Data analysis was performed by using the FlowJo (version 7.6.5) software. In all FACS experiments, cells were morphologically identified by forward scatter and side scatter, doublets were discriminated and further divided by antigen positivity.
Histopathological Analysis of Spleen Tissue
Fresh frozen spleen sections (thickness 10 μm) were stained with hematoxylin and eosin using a standard procedure, and tissues have been analyzed morphologically by light microscopy (Olympus BX51 microscope and the viewfinder 3.0.1 software, Solna, Sweden).
DNA Extraction and Quantification of DNA Fragmentation
DNA was extracted using a modified commercial Easy-DNA Kit (Life Technologies, Stockholm, Sweden). Two micrograms of DNA was separated on a 1.5% agarose gel and stained by ethidium bromide. DNA fragments were analyzed using a fluorescence imager (Typhoon 9400, GE Healthcare, Uppsala, Sweden).
Western Blotting
Proteins were extracted from brain tissue as described before.13 Human brain tissues were used with the approval of the Lund Ethical Review Board for research involving humans (Dnr 2011/80) and in compliance with the US Department of Health and Human Services. Ten micrograms of protein was separated on a 10% SDS polyacrylamide gel. Proteins were transferred onto polyvinyldifluoride membranes. Thereafter, unspecific protein binding sites were blocked in respective buffer (20 mmol/L Tris, 136 mmol/L NaCl, pH 7.6, 0.1% Tween 20, 5% nonfat dry milk) and proteins were detected using a primary polyclonal antibody against CXCR4 (Abcam, dilution 1:1,000). After incubation overnight at 4°C, signals were obtained by binding of a secondary anti-rabbit horse radish peroxidase linked antibody (1:50,000, Sigma-Aldrich, Deisenhofen, Germany) and visualized by exposing the membrane to a charge-coupled device camera (LAS1000, Fujifilm, Tokyo, Japan) using a chemiluminescence kit (Merck Millipore, Billerica, MA, USA). To detect CXCL12 proteins were separated in 10% to 20% gels (Novex Tricine system, Invitrogen, Paisley, UK). Transfer was performed onto polyvinyldifluoride membranes and immunoblotting was performed using the Snap i.d. system, according to the manufacturer's instructions (Merck Millipore, Darmstadt, Germany). We used a polyclonal anti-CXCL12 antibody (1:1,000, Torrey Pines Biolabs, Secaucus, NJ, USA) and a secondary antibody horse radish peroxidase-conjugate (1:5,000, Sigma-Aldrich, Deisenhofen, Germany). Membranes were stripped and reprobed for β-actin (Sigma-Aldrich, and diluted 1:50,000). After densitometric analysis using ImageJ software (National Institutes of Health, Bethesda, MD, USA), CXCL12 and CXCR4 expression were calculated as percentage of β-actin expression.
Immunofluorescence
Brain sections (thickness 30 μm) from 4% paraformaldehyde-perfused animals were washed in PBS. Blocking was performed with 5% normal donkey serum in PBS supplemented with 0.25% Triton X-100 (PBS-T) for 60 minutes. For colocalization, the following antibodies were used: rabbit polyclonal anti-CXCL12 (1:200, Santa Cruz Biotechnologies, Heidelberg, Germany), rabbit polyclonal anti-CXCR4 (1:200, Abcam), monoclonal directly Cy3 conjugated anti-glial fibrillary acidic protein, ( 1:5,000, Sigma-Aldrich, St. Louis, MO, USA), monoclonal antibody anti-Ox-42 (1:200, AbD Serotec), monoclonal anti-glutathione-S-transferase pi-isoform (GST-π, 1:200, BD Biosciences), monoclonal anti-neuronal nuclei (NeuN, 1:1,000, Merck Millipore, Watford, UK), monoclonal anti-CD3 (1:200, eBioscience), monoclonal anti-CD4 (1:200, eBioscience). After overnight incubation at 4°C, cells were incubated with appropriate secondary antibodies (Cy3 conjugated donkey anti-mouse or anti-rabbit antibody, both diluted 1:200, Jackson Laboratories) at rt for 90 minutes. Fluorescent signals were visualized using a confocal microscopy system (LSM510 Zeiss, Jena, Germany) and the AIM LSM 4.2 software (Zeiss).
Immunohistochemistry
Brain sections (thickness 30 μm) from 4% paraformaldehyde-perfused animals were gently washed three times in PBS, without Ca2+/Mg2+) and quenched (3% H2O2, 10% methanol) for 15 minutes. After washing in PBS-T, sections were blocked for 60 minutes (10% normal donkey serum and 1% bovine serum albumin in PBS-T) and incubated overnight at 4°C with anti-CXCL12 (Santa Cruz Biotechnologies, and diluted 1:200) or anti-CXCR4 (Abcam, and diluted 1:200) followed by a secondary biotinylated donkey anti-rabbit antibody (Vector Laboratories, Burlingame, CA, USA, diluted 1:200). Visualization was achieved via the Vectorstain ABC-horse radish peroxidase Elite kit using 3,3-diaminobenzidine/H2O2 (Vector). Omission of the primary antibody served as a negative control. Micrographs were aquired with an Olympus BX51 microscope and the viewfinder 3.0.1 software.
Statistics
Western blot results are presented as means±s.d. and analyzed by one-way analysis of variance using IBM SPSS Statistics 20.0 (IBM Svenska AB, Sweden). Behavioral data and immune cell counts are presented as median with the interquartile range (Q1, Q3) and 95% confidence interval. Post hoc multiple comparisons were performed as stated in the figure legends and P<0.05 was considered significant.
RESULTS
Enriched Environment Decreases Proinflammatory Cytokines in the Ischemic Hemisphere After Experimental Stroke
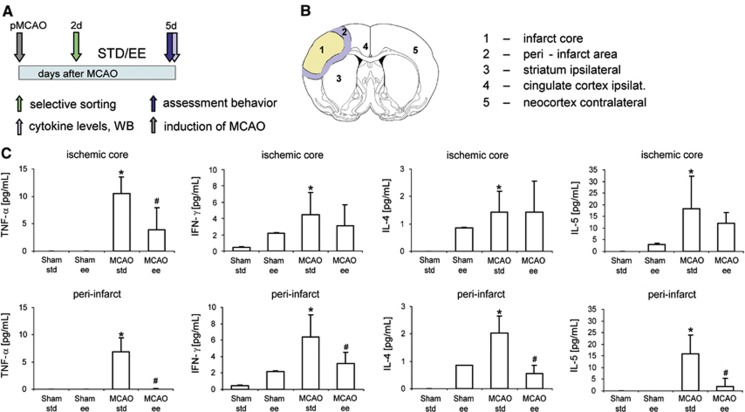
We subjected spontaneously hypertensive rats to pMCAO and then kept the animals in standard laboratory cages (n=8, STD) or EE (n=8, EE) for 3 days between postocclusion days 2 to 5 (Figure 1A), which significantly improved recovery of lost neurologic function.14 On day 5 after pMCAO, we analyzed levels of the proinflammatory cytokines tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), interleukin-4 (IL-4), IL-5, and IL-13 in different regions of the ischemic and contralateral hemisphere as markers for the level of inflammation (Figure 1B). The major proinflammatory cytokines TNF-α, IL-4, IL-5, and IFN-γ in pMCAO rats were generally upregulated in the ischemic core and the adjacent peri-infarct area compared with rats that underwent a sham operation; levels of these cytokines mainly in the peri-infarct area and for TNF-α also in the infarct core were significantly lower in EE rats after pMCAO compared with STD rats (Figure 1C). Downregulation of expression of proinflammatory molecules was also evident in rats housed for 12 days in EE after pMCAO (Supplementary Table 3).15
Figure 1.
Enriched environment (EE) suppressed the inflammatory response after experimental stroke. (A) Experimental design. Spontaneous hypertensive rats were subjected to permanent occlusion of the middle cerebral artery (pMCAO). Two days later, neurologic deficit was evaluated by the rotating pole test and only animals with substantial deficits (test score 2 or lower) were randomized either into housing in standard (STD) laboratory cages or EE for 3 consecutive days. At day 5, after pMCAO, neurologic score was evaluated and brains were analyzed for proinflammatory cytokines. (B) Brain areas analyzed for proinflammatory cytokines: (1) infarct core; (2) peri-infarct area; (3) striatum-injured hemisphere; (4) cingulate cortex-injured hemisphere; (5) neocortex-noninjured hemisphere; (C) Levels of the proinflammatory cytokines TNF-α, IFN-γ, IL-4, and IL-5 in the infarct core and the adjacent peri-infarct area of rats housed either in STD (n=8) or EE (n=8) for 3 consecutive days after pMCAO and respective sham-operated rats (sham STD, n=6; sham EE, n=6). Data are presented as means±standard deviation (s.d.). The asterisk denotes statistical difference compared with all other experimental groups (*P<0.05), and the hash denotes statistical difference (#P<0.05) to the MCAO STD group (one-way analysis of variance, Bonferroni correction, Fisher's least significant difference test for levels of IL-5 in the MCAO STD group compared with the sham EE group). Please note that levels of cytokines measured in brain regions 3 to 5 are displayed in Supplementary Figure 1.
The consistent depression of proinflammatory cytokines seen in the ischemic hemisphere of the EE groups compared with the STD groups were not observed in the contralateral hemispheres except the homeotopic neocortex with a significant reduction of TNF-α levels in rats housed in EE after pMCAO (Supplementary Figure 2). Likewise, there were no differences of proinflammatory cytokines in the serum of rats subjected to pMCAO and afterward housed in STD compared with rats housed in EE (data not shown). Cytokines were not detectable in the cerebrospinal fluid at 5 days after tMCAO of any of the animals (data not shown). Furthermore, IL-13 was not regulated in any of the brain areas (data not shown). Together, the results demonstrate that EE profoundly suppressed tissue inflammation in the ischemic hemisphere after experimental stroke.
Enriched Environment Downregulates CXCL12 and CXCR4 in the Ischemic Hemisphere
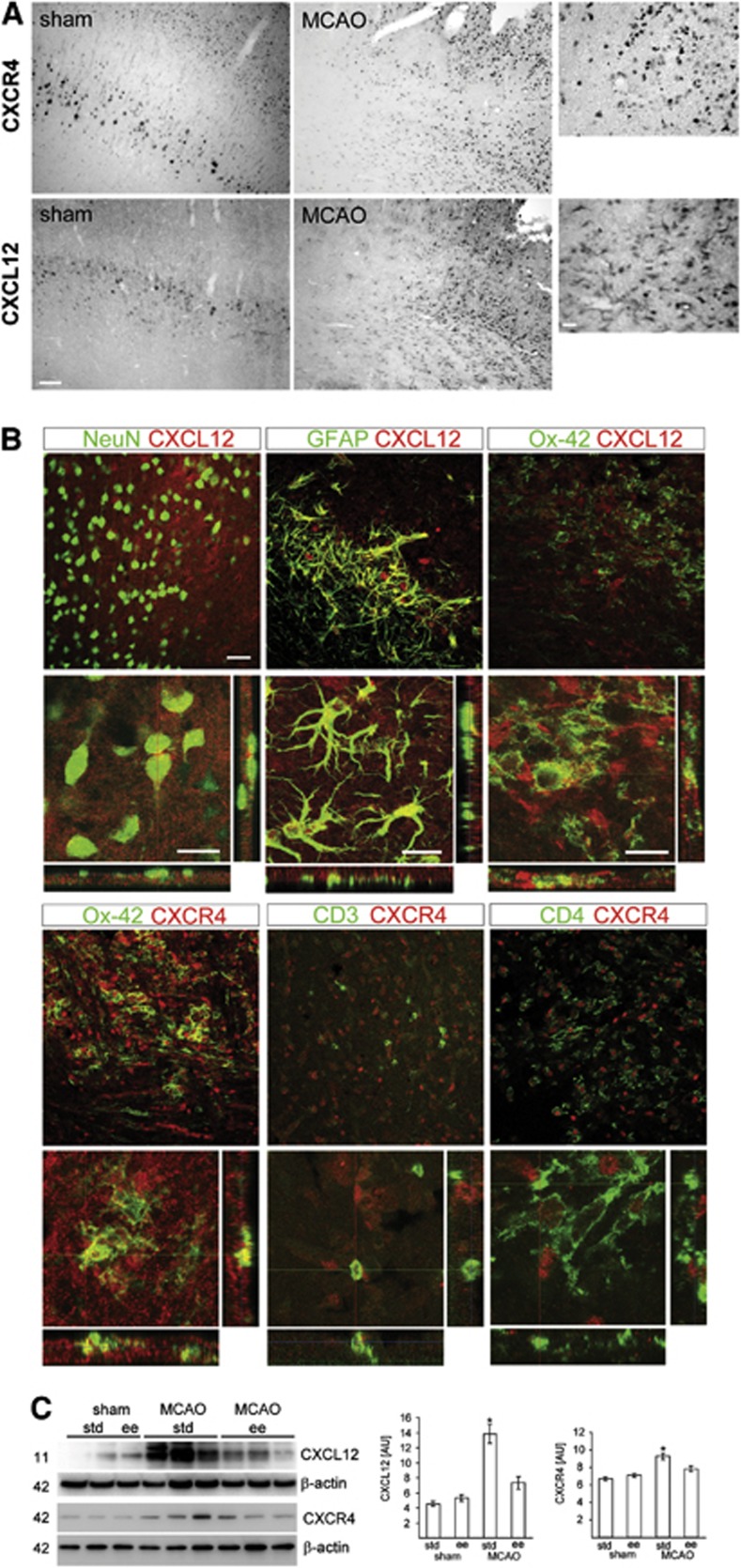
The inflammatory response to stroke, including the regulation of proinflammatory cytokines, is perpetuated by resident microglia and infiltrating leukocytes.7 This implies that the attraction of these cells into the ischemic territory is mediated by specific regulatory mechanisms. Previous studies have shown that the chemokine receptor CXCR4 and its natural ligand stromal cell-derived factor 1 (CXCL12) are upregulated in the ischemic hemisphere after experimental stroke.25 In sham-operated rats, we observed that basal expression of CXCL12 and CXCR4 was mainly located in neuron-like cells based on their appearance and localization in the neocortex (Figure 2A) after pMCAO, CXCL12, and CXCR4 levels increased in cells in the peri-infarct area (Figure 2A). Immunofluorescence analysis revealed increased expression of CXCL12 in NeuN+ neurons, glial fibrillary acidic protein+ astrocytes, and OX-42+ microglia/macrophages, and CXCR4 was found in OX-42+, CD3+, and CD4+ cells (Figure 2B and Supplementary Figure 3–5). Importantly, housing rats in EE had significantly decreased the levels of CXCL12 and CXCR4 in the ischemic core/peri-infarct border zone (Figure 2C). Taken together, these results show the CXCL12 and CXCR4 are upregulated in brains of rats. Importantly, the benefical effects of EE on recovery after pMCAO in rats are correlated with decreased levels of these molecules that are critically involved in immune cell attraction.
Figure 2.
Chemokine receptor CXCL12 pathway was suppressed by enriched environment (EE) after permanent middle cerebral artery occlusion (pMCAO). (A) Expression of CXCL12 and CXCR4 in the neocortex of sham-operated rats and the peri-infarct area of the ischemic hemisphere after pMCAO. Scale bars, low magnification, 100 μm; high magnification, 20 μm. (B) Identification of CXCL12- and CXCR4-expressing cells in the ischemic hemisphere. CXCL12 (Cy3, red) is expressed in NeuN+ (Cy5, green) neurons, GFAP+ (Cy5, green) astrocytes, and Ox-42+ (Cy5, green) microglia/macrophages. Colocalization of CXCR4 (Cy3, red) with Ox-42 (Cy5, green), CD3 (Cy5, green), CD4 (Cy5, green). Scale bars, 50 μm. (C) Levels for CXCL12 and CXCR4 at the infarct border zone from rats housed in standard (STD) (n=8) or EE (n=8) for 3 days after pMCAO or sham operation (sham STD, n=5; sham EE, n=5). Values are presented as means±s.d. and were calculated as percentage of β-actin expression. *P<0.05 versus all other experimental groups. One-way analysis of variance, Bonferroni correction.
Treatment with AMD3100 Improves Lost Neurologic Function and Reduces the Number of Immune Cells in the Ischemic Hemisphere
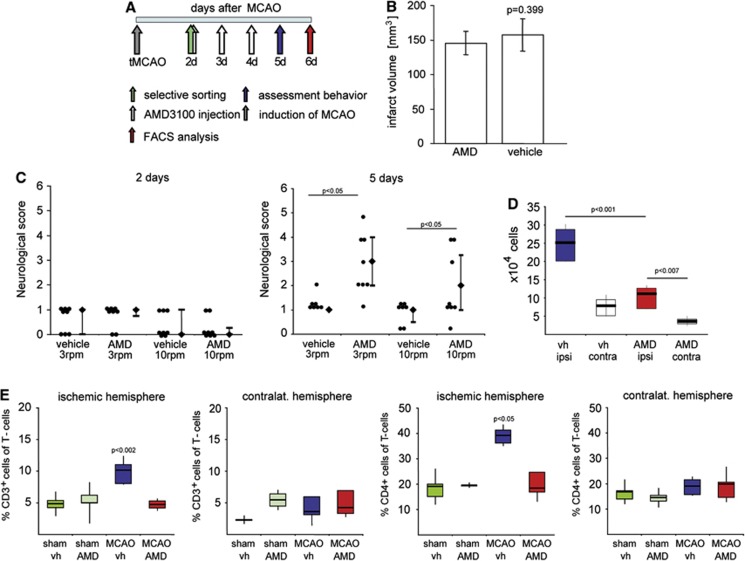
We reasoned that if the multimodal stimulation provided by the EE depresses the CXCL12 pathways, an antagonist of CXCR4 would enhance functional recovery after stroke. Therefore, male Wistar rats subjected to tMCAO (120 minutes) were treated with AMD3100 (0.5 mg/kg intraperitoneally twice daily) starting on day 2 after stroke (Figure 3A). Animals having the same neurologic deficit upon start of treatment were selected (Figure 3C). On day 5 after tMCAO, 50% of the AMD3100-treated rats traversed the rotating pole at 3 rotations per minute (rpm), while none of the vehicle-treated rats passed the test. This improvement was also evident when the rats were tested at 10 rpm (Figure 3C). Importantly, no difference in infarct volume was observed between the treatment groups (vehicle: 157.07±23.37 mm3, AMD3100: 145.51±17.01 mm3) (Figure 3B).
Figure 3.
Treatment with the specific CXCR4 antagonist AMD3100 suppressed immune cell invasion in the ischemic hemisphere and improved functional recovery after experimental stroke. (A) Experimental design. Male Wistar rats were subjected to transient occlusion of the middle cerebral artery (tMCAO). Two days later, neurologic deficit was evaluated by the rotating pole test and only animals with a test score of 2 or lower were randomized into treatment groups receiving either AMD3100 (0.5 mg/kg intraperitoneal twice daily) or saline (vehicle (vh), intraperitoneal twice daily) for 3 consecutive days. On day 5 after tMCAO, neurologic score was evaluated, and on day 6, brains were analyzed for the invasion of immune cells. (B) Infarct volumes of vehicle (n=5) and AMD3100-treated rats (n=5) at day 6 after tMCAO. Values are presented as mean±s.d., Student's t-test. (C) Rat sensorimotor score obtained on the rotating pole (at 3 and 10 rotations per minute to the right) 2 and 5 days after tMCAO after treatment with AMD3100 (n=8) or saline (n=8). Difference in functional recovery was significant between the two treatments (Mann–Whitney). The values are presented as the median with the 25th to75th percentiles (bars), individual scores are presented to the left. (D) Number of immune cells in the ischemic (ipsi) and contralateral (contra) hemisphere after treatment with AMD3100 (AMD) or vehicle after tMCAO. Results are presented as the median with the 25th to75th percentiles (bars) and the 95% confidence interval (CI), one-way analysis of variance (ANOVA), Bonferroni correction. (E) Effect of AMD3100 treatment on CD3+ and CD3+/CD4+ cells in the injured and contralateral hemisphere 6 days after tMCAO or sham operation. The values are presented as the median with the 25th to 75th percentiles and 95% CI, one-way ANOVA, Bonferroni correction versus MCAO AMD. FACS, fluorescence-activated cell sorting.
The AMD3100 treatment resulted in a significant reduction in invading immune cells (AMD3100: median 112,000 cells; vehicle treatment: 252,500 cells) in the ischemic hemisphere. The number of immune cells in the noninjured contralateral hemisphere did not differ (AMD3100: median 36,000 cells; vehicle treatment: 79,300 cells) (Figure 3D). We next examined the subpopulations of invading immune cells in the ischemic and contralateral hemispheres by FACS analysis. We found that the numbers of invading CD3 single positive (CD3+, 4.71%, and 10.1% of the T-cell population in AMD3100- and vehicle-treated rats, respectively) and CD3+/CD4+ cells (18.4% and 39.2% of T cells in AMD3100- and vehicle-treated rats, respectively) were significantly reduced in the ischemic hemisphere (Figure 3E), but were unchanged in the contralateral hemisphere (Figure 3E). We did not detect any differences between the two treatment groups after tMCAO with regard to the numbers of invading CD45R+, CD11b+, CD11c+, and CD3+/CD8+ cells (Supplementary Figure 6), or CD11b+/CD11c+ cells (Supplementary Figure 7). From this set of experiments, we conclude that systemic inhibition of the CXCL12 pathway substantially reduces the number of invading CD3+ and CD3+/CD4+ cells and improves recovery of function after experimental stroke.
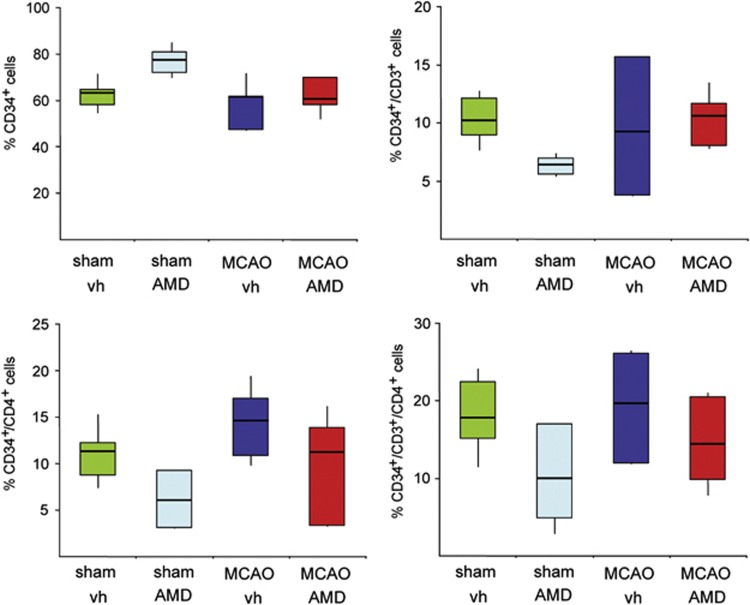
Moreover, AMD3100 has been reported to mobilize CD34+ hematopoetic progenitor cells from the bone marrow.29 We sought to assess if the recovery-enhancing effects obtained in rats treated with AMD3100 correlated with an increase of CD34+ cells in the blood after tMCAO. Rats either received vehicle (n=7) or AMD3100 (0.5 mg/kg twice daily, n=7) for 5 consecutive days with treatment initiation on day 2 after tMCAO occlusion for 105 minutes. As shown in Figure 4, AMD3100 treatment did not increase the number of CD34 single positive cells or the number of CD34+/CD3+, CD34+/CD4+, and CD34+/CD3+/CD4+ cells.
Figure 4.
Effect of AMD3100 on CD34+ cell mobilization after transient middle cerebral artery occlusion (tMCAO). Effect of AMD3100 treatment on CD34+, CD34+/CD3+, CD34+/CD4+, and CD34+/CD3+/CD4+ cells in the blood 6 days after tMCAO or sham operation. Values are presented as percentage of total CD34+ cells as the median with the 25th to 75th percentiles and 95% confidence interval.
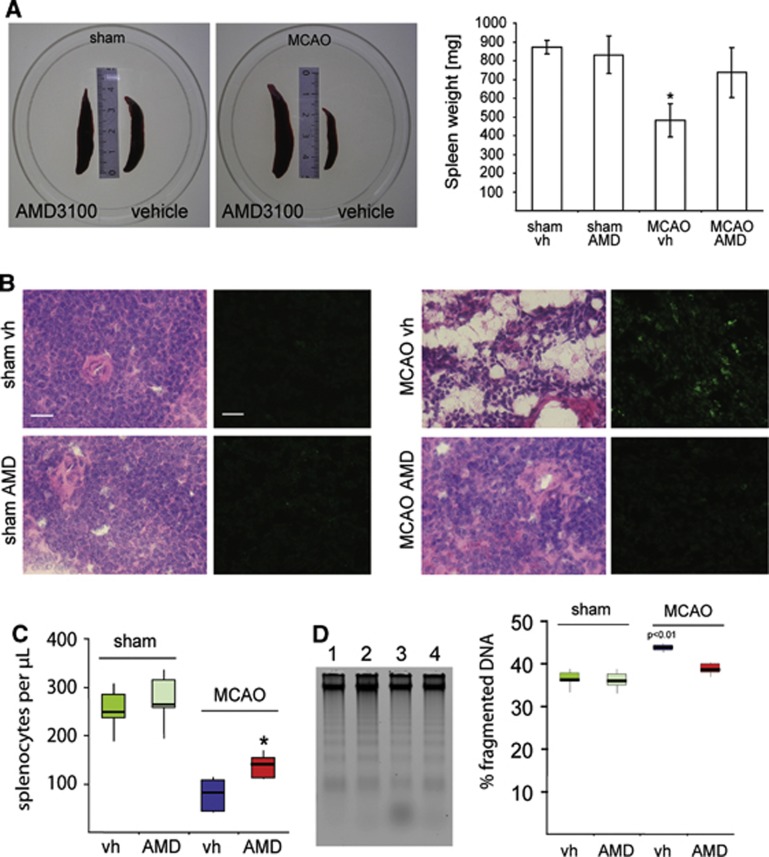
AMD310 Treatment Prevents Spleen Atrophy After Experimental Stroke
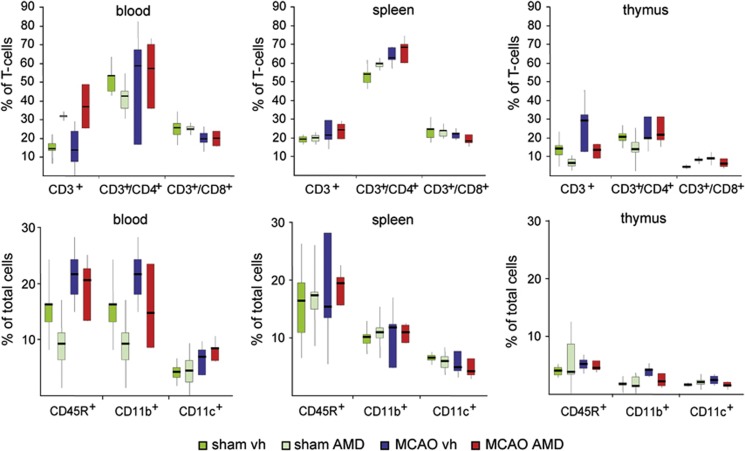
Stroke induces a profound downregulation of the peripheral immune system, including a loss of lymphocyte populations and atrophy of secondary lymphatic organs such as the spleen and thymus.9 We confirm that spleen atrophy is induced after stroke and found that administration of AMD3100 for 3 days after tMCAO starting at 2 days of recovery prevented spleen atrophy (736.4±131.8 mg; vehicle: 481.7±89.1 mg) (Figure 5A). In vehicle-treated rats after tMCAO substantial loss of cells, irregularly formed apoptotic splenocytes, appearance of trabecular structures in the spleen, and an increase in 7-aminoactinomycin D–positive cells were found (Figure 5B). In contrast, in AMD3100-treated animals, the spleen tissue had a homogenous texture similar to that of sham-operated rats, and low numbers of 7-aminoactinomycin D–positive cells were observed after tMCAO (Figure 5B). Similarly, the spleens of AMD3100-treated rats had a higher number of splenocytes per microliter compared with vehicle-treated rats (AMD3100: 139.9 splenocytes/μL, Q1 111.25, Q3 154.75; vehicle: 81.0 splenocytes/μL, Q1 41.75, Q3 107.5) (Figure 5C) and a significantly lower level of fragmented DNA (Figure 5D). Despite changes in absolute cell numbers, we observed no shift in the distribution of leukocyte subpopulations in the spleens of rats in any of the treatment groups (Figure 6). In addition, analysis of blood leukocytes revealed an increase of CD3+ cells in rats treated with AMD3100, which suggests mobilization of double negative (CD4−/CD8−) immature thymocytes from the thymus (Figure 6). The levels of all other cell populations analyzed were unaffected by the treatment (Figure 6).
Figure 5.
Effects of AMD3100 treatment on the spleens after transient middle cerebral artery occlusion (tMCAO). (A) Representative pictures of rat spleens of sham-operated animals (left) and animals subjected to tMCAO (right) and treated either with AMD3100 (0.5 mg/kg intraperitoneal) or vehicle (vh) (saline intraperitoneal) for 3 consecutive days. Spleen weight (mg) from rats treated with sham vehicle (sham vh, n=4), sham AMD3100 (sham AMD, n=4), MCAO vehicle (MCAO vh, n=8), or MCAO AMD3100 (MCAO AMD, n=8) 6 days after surgery (mean±s.d., one-way analysis of variance, Bonferroni correction; *P<0.05). (B) Histopathological analysis of the spleen tissue. Adjacent sections were stained either with hematoxylin and eosin (H&E) or 7-aminoactinomycin-D (7-AAD) to estimate the number of apoptotic cells. Scale bars, H&E 20 μm, 7-AAD 20 μm. (C) Number of splenocytes after different treatments. Cells were calculated as number of cells/μL and normalized to the spleen weight. (D) Percentage of lower molecular weight fragmented DNA in the spleens after: (1) sham vehicle; (2) sham AMD3100; (3) MCAO vehicle; or (4) MCAO AMD3100 treatment and respective quantification. Data are presented as median with the 25th to 75th percentiles and 95% confidence interval.
Figure 6.
Effects of AMD3100 treatment on peripheral immune cell populations after transient middle cerebral artery occlusion. Effect of AMD3100 treatment on CD3+, CD3+/CD4+, CD3+/CD8+, CD45R+, CD11b+, and CD11c+ cells in the spleen, blood, and thymus 6 days after tMCAO or sham operation. Values are presented as the median with the 25th to 75th percentiles and 95% confidence interval.
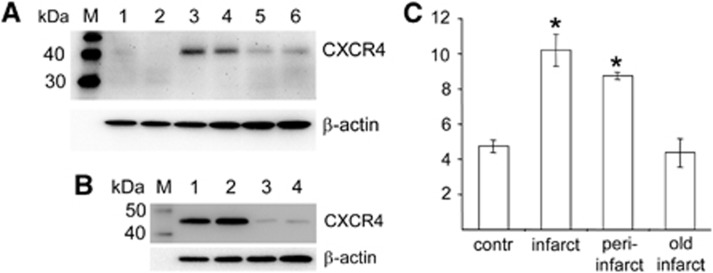
To evaluate if the CXCL12 pathway is regulated after clinical stroke in a proof-of-principle study, we analyzed different brain areas from postmortem tissue from patients who had suffered a stroke. As shown in Figure 7, increased levels of CXCR4 were detected in the infarct core and adjacent peri-infarct area of the ischemic hemisphere. In addition, levels of CXCR4 declined to levels found in the corresponding nonischemic areas of the contralateral hemisphere (Figure 7). Together, evidence of CXCR4 in the ischemic hemisphere support the idea of an CXCR4 antagonist therapy to modulate the brain inflammatory response after stroke.
Figure 7.
Upregulation of CXCR4 in the infarct border zone in stroke patients. (A) Levels of CXCR4 in unaffected brain tissue of the noninjured hemisphere (lanes 1 and 2), infarct core (lanes 3 and 4), and proximal peri-infarct area (lanes 5 and 6) after clinical stroke. (B) Levels of CXCR4 in the infarct core (lanes 1 and 2) and old infarcts (lanes 3 and 4) after clinical stroke. (C) Values are means±s.d. and were obtained from the indicated brain regions of six patients and were calculated as percentage of β-actin expression. *P<0.05 versus noninfarcted control tissue of the contralateral hemisphere (contr) and old infarct tissue (infarcted areas older than 2 months). Statistical analysis was performed by one-way analysis of variance and Bonferroni correction. M, protein marker.
Discussion
In this investigation, we demonstrated that (1) multi-sensory stimulation of the brain by enriched housing depresses the poststroke inflammatory response including the levels of CXCL12 and its receptor CXCR4; (2) pharmacological inhibition of CXCL12 action with AMD3100 improves sensorimotor function after stroke without affecting infarct size; (3) this is accompanied by a decrease in CD4+ T lymphocytes into the brain; (4) spleen atrophy associated with experimental stroke was prevented by blocking CXCL12 action. In the following, we will discuss the results in view of the influence of inflammation in general and the CXCL12 system in particular on brain plasticity after stroke at a time when the development of infarction has subsided.
After experimental stroke, brain responds by synthesizing chemoattractants that mobilize cells from blood and peripheral lymphoid organs.30 The invading cells create havoc in the injured tissue enhancing cell death, but at later stages after stroke, when cell death has subsided, the inflammatory cells may influence brain plasticity and recovery of the remaining neural networks affecting functional outcome.31 By 2 days after stroke, neutrophils invade the brain tissue and after 3 to 4 days, the number of T-lymphocytes increases.32 These events in the central nervous system are followed by a marked spleen atrophy causing a long lasting immunodepression that appears to be due to activation of the sympathoadrenergic system.8, 9, 10
Our investigation was designed to assess the influence of multi-sensory stimulation of recovery of brain function, i.e., on brain plasticity. Hence, treatment with AMD3100 or housing animals in an EE was initiated at a time when the contribution of inflammation on acute cell death has subsided.
Importantly, inhibition of the CXCL12 pathway with AMD3100 depresses peripheral immune cell invasion into the ischemic territory, which promotes recovery of function. Specifically, we found that AMD3100 treatment inhibited accumulation of CD3+/CD4+ cells, which suggests that these cells could depress neuronal plasticity in the ischemic hemisphere,33 while the number of B cells, instrumental in recovery after MCAO,34 remained unaffected. In addition, reduced levels of transcripts of proinflammatory cytokines accompanied with protection of blood–brain barrier integrety and reduced infarct volumes have been observed in the acute phase after pMCAO in mouse.35 Also, after a stroke, the spleen releases immune cells diminishing its size by up to 80%, a process that is partly reversible.36 The prevention of poststroke spleen atrophy by AMD3100 strongly suggests that the CXCL12 pathway is instrumental in the release process ultimately leading to spleen atrophy. Our data suggest that blocking CXCL12 action may prevent the perpetuation of a vicious cycle that is initiated by release of proinflammatory compounds from the brain concomitant with a sympathoadrenergic surge mobilizing immune cells from the periphery and leading to atrophy of lymphoid organs. Our results also support the idea that CXCR4 antagonism may actively block programmed cell death in spleen.8 The mechanisms by which inhibiting the CXCL12 pathway improves recovery could be due to a direct action on CXCR4/7 expressing detrimental immune cells. Alternatively, CXCR4 antagonism may modulate neurotransmission by direct action on neurons21 or reactive astrocytes37 in the peri-infarct area, thereby promoting a milieu permissive for the formation of new neuronal circuits in the ischemic hemipshere.37
After experimental stroke in mice, CXCL12 promotes angiogenesis and migration of neuronal and endothelial progenitor cells (EPCs) in the ischemic territory.23, 38 Endothelial progenitor cells are thought to secrete growth factors and other neuroprotectants that in concert mediate beneficial actions in the ischemic hemsiphere.38 In that context, it can be envisaged that the CXCL12 pathway is important in homing of EPCs to the ischemic territory, and hence blockade of the CXCR4 by AMD3100 would be detrimental. It is, however, more likely that AMD3100 depresses the deletrious homing of inflammatory cells, while the importance of EPC in the context of our study may not be of prime significance for recovery. Indeed, neurogenesis and gliogenesis in the poststroke brain is markedly attenuated when animals are housed in an EE,17 in accord with the findings of a decrease in growth-promoting cytokines and chemokines in brains of animals housed in enriched cages. Also, the action of EPCs is observed in immune-deficient nude mice38 and therefore, the poststroke inflammatory response might have been critically attenuated contributing to the beneficial effects on recovery after MCAO, as observed in the present study. Hence, current data indicate that dependent on the spatiotemporal expression of CXCL12 and CXCR4, this chemokine pathway is involved in early neuroprotection but has adverse effects in the delayed inflammatory response after stroke.35
Further studies are required to determine the extent to which different CXCL12 isoforms contribute to the mechanisms of neuronal plasticity during reorganization of the ischemic hemisphere. Recently, it was demonstrated that CXCL12 affects the function of GABAergic neurons at the infarct boundary39 and upregulation of CXCL12 in glial cells promotes differentiation of oligodendrocyte precursor cells, which are important for remyelination in the ischemic hemisphere.40 While these potentially beneficial long-term processes require the activation of the CXCL12 pathway, our results show that immoderate excessive activation during the first week is detrimental for recovery of function after stroke supporting a dual role of this pathway after stroke.
In conclusion, we show that multimodal sensorimotor stimulation by EE depresses the inflammatory response occuring in the ischemic hemisphere after experimental stroke. Moreover, we provide evidences for the involvement of the CXCL12 pathway in migration of T-cell populations into the ischemic hemisphere that is detrimental in stroke-injured rats. Pharmacological inhibition of CXCL12 binding to its receptors during the first week after stroke promotes long-term recovery, importantly without affecting the infarct size. Hence, the CXCL12 pathway may be an attractive pharmacological target to modulate immune cell recruitment into the ischemic territory and improve functional recovery after stroke.
Acknowledgments
We thank Carin Sjölund, Gunilla Gidö, Kerstin Beirup, and Sol da Rocha Baez for excellent technical assistance.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by the European Union's Seventh Framework Program (FP7/2008-2013) under grant agreement No 201024 and No 202213 (European Stroke Network), the Swedish Research Council (2011-2652 and 2011-2684), the Pia Ståhl Foundation, the Swedish Brain Fund, The Hans-Gabriel och Alice Wachtmeisters stiftelse, the Kocks Foundations, the Crafoord Foundation, the European Neurological Society, Stiftung Felgenhauer, and the Royal Physiographic Society in Lund.
Supplementary Material
References
- Brewer L, Horgan F, Hickey A, Williams D. Stroke rehabilitation: recent advances and future therapies. QJM. 2012;106:11–25. doi: 10.1093/qjmed/hcs174. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Marchina S, Wan CY. The use of non-invasive brain stimulation techniques to facilitate recovery from post-stroke aphasia. Neuropsychol Rev. 2011;21:288–301. doi: 10.1007/s11065-011-9181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K, Fries W, Muller F, Koenig E. Effect of levodopa in combination with physiotherapy on functional motor recovery after stroke: a prospective, randomised, double-blind study. Lancet. 2001;358:787–790. doi: 10.1016/S0140-6736(01)05966-9. [DOI] [PubMed] [Google Scholar]
- Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci. 2010;30:14964–14971. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieloch T, Nikolich K. Mechanisms of neural plasticity following brain injury. Curr Opin Neurobiol. 2006;16:258–264. doi: 10.1016/j.conb.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hebert M, Phaneuf D, Soucy G, Weng YC, Kriz J. Live imaging of Toll-like receptor 2 response in cerebral ischaemia reveals a role of olfactory bulb microglia as modulators of inflammation. Brain. 2009;132:940–954. doi: 10.1093/brain/awn345. [DOI] [PubMed] [Google Scholar]
- Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147 (Suppl 1:S232–S240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Vandenbark AA, Hurn PD. Effect of experimental stroke on peripheral immunity: CNS ischemia induces profound immunosuppression. Neuroscience. 2009;158:1098–1111. doi: 10.1016/j.neuroscience.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajmo CT, Jr, Collier LA, Leonardo CC, Hall AA, Green SM, Womble TA, et al. Blockade of adrenoreceptors inhibits the splenic response to stroke. Exp Neurol. 2009;218:47–55. doi: 10.1016/j.expneurol.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewitus GM, Schwartz M. Behavioral immunization: immunity to self-antigens contributes to psychological stress resilience. Mol Psychiatry. 2009;14:532–536. doi: 10.1038/mp.2008.103. [DOI] [PubMed] [Google Scholar]
- Williamson LL, Chao A, Bilbo SD. Environmental enrichment alters glial antigen expression and neuroimmune function in the adult rat hippocampus. Brain Behav Immun. 2012;26:500–510. doi: 10.1016/j.bbi.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickhag M, Deierborg T, Patel S, Ruscher K, Wieloch T. Apolipoprotein D is elevated in oligodendrocytes in the peri-infarct region after experimental stroke: influence of enriched environment. J Cereb Blood Flow Metab. 2008;28:551–562. doi: 10.1038/sj.jcbfm.9600552. [DOI] [PubMed] [Google Scholar]
- Ruscher K, Johannesson E, Brugiere E, Erickson A, Rickhag M, Wieloch T. Enriched environment reduces apolipoprotein E (ApoE) in reactive astrocytes and attenuates inflammation of the peri-infarct tissue after experimental stroke. J Cereb Blood Flow Metab. 2009;29:1796–1805. doi: 10.1038/jcbfm.2009.96. [DOI] [PubMed] [Google Scholar]
- Ruscher K, Shamloo M, Rickhag M, Ladunga I, Soriano L, Gisselsson L, et al. The sigma-1 receptor enhances brain plasticity and functional recovery after experimental stroke. Brain. 2011;134:732–746. doi: 10.1093/brain/awq367. [DOI] [PubMed] [Google Scholar]
- Johansson BB, Belichenko PV. Neuronal plasticity and dendritic spines: effect of environmental enrichment on intact and postischemic rat brain. J Cereb Blood Flow Metab. 2002;22:89–96. doi: 10.1097/00004647-200201000-00011. [DOI] [PubMed] [Google Scholar]
- Nygren J, Wieloch T, Pesic J, Brundin P, Deierborg T. Enriched environment attenuates cell genesis in subventricular zone after focal ischemia in mice and decreases migration of newborn cells to the striatum. Stroke. 2006;37:2824–2829. doi: 10.1161/01.STR.0000244769.39952.90. [DOI] [PubMed] [Google Scholar]
- Endres M, Engelhardt B, Koistinaho J, Lindvall O, Meairs S, Mohr JP, et al. Improving outcome after stroke: overcoming the translational roadblock. Cerebrovasc Dis. 2008;25:268–278. doi: 10.1159/000118039. [DOI] [PubMed] [Google Scholar]
- Thelen M, Thelen S. CXCR7, CXCR4 and CXCL12: an eccentric trio. J Neuroimmunol. 2008;198:9–13. doi: 10.1016/j.jneuroim.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Murakami F. Chemokine CXCL12 and its receptors in the developing central nervous system: emerging themes and future perspectives. Dev Neurobiol. 2012;72:1349–1362. doi: 10.1002/dneu.22041. [DOI] [PubMed] [Google Scholar]
- Marchionni I, Takács VT, Nunzi MG, Mugnaini E, Miller RJ, Maccaferri G. Distinctive properties of CXC chemokine receptor 4-expressing Cajal-Retzius cells versus GABAergic interneurons of the postnatal hippocampus. J Physiol. 2010;588:2859–2878. doi: 10.1113/jphysiol.2010.190868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler MW, Geller EB, Chen X, Rogers TJ. Viewing chemokines as a third major system of communication in the brain. AAPS J. 2005;7:E865–E870. doi: 10.1208/aapsj070484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill WD, Hess DC, Martin-Studdard A, Carothers JJ, Zheng J, Hale D, et al. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol. 2004;63:84–96. doi: 10.1093/jnen/63.1.84. [DOI] [PubMed] [Google Scholar]
- Schonemeier B, Schulz S, Hoellt V, Stumm R. Enhanced expression of the CXCl12/SDF-1 chemokine receptor CXCR7 after cerebral ischemia in the rat brain. J Neuroimmunol. 2008;198:39–45. doi: 10.1016/j.jneuroim.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Blades MC, Ingegnoli F, Wheller SK, Manzo A, Wahid S, Panayi GS, et al. Stromal cell-derived factor 1 (CXCL12) induces monocyte migration into human synovium transplanted onto SCID Mice. Arthritis Rheum. 2002;46:824–836. doi: 10.1002/art.10102. [DOI] [PubMed] [Google Scholar]
- Meiron M, Zohar Y, Anunu R, Wildbaum G, Karin N. CXCL12 (SDF-1alpha) suppresses ongoing experimental autoimmune encephalomyelitis by selecting antigen-specific regulatory T cells. J Exp Med. 2008;205:2643–2655. doi: 10.1084/jem.20080730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandless EE, Zhang B, Diamond MS, Klein RS. CXCR4 antagonism increases T cell trafficking in the central nervous system and improves survival from West Nile virus encephalitis. Proc Natl Acad Sci USA. 2008;105:11270–11275. doi: 10.1073/pnas.0800898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liles WC, Broxmeyer HE, Rodger E, Wood B, Hubel K, Cooper S, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102:2728–2730. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ. Acute anti-inflammatory approaches to ischemic stroke. Ann N Y Acad Sci. 2010;1207:143–148. doi: 10.1111/j.1749-6632.2010.05761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vexler ZS, Yenari MA. Does inflammation after stroke affect the developing brain differently than adult brain. Dev Neurosci. 2009;31:378–393. doi: 10.1159/000232556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SL, Bao J, Hollis J, Lessov NS, Clark WM, Stenzel-Poore MP. The use of flow cytometry to evaluate temporal changes in inflammatory cells following focal cerebral ischemia in mice. Brain Res. 2002;932:110–119. doi: 10.1016/s0006-8993(02)02292-8. [DOI] [PubMed] [Google Scholar]
- Lewitus GM, Zhu J, Xiong H, Hallworth R, Kipnis J. CD4(+), CD25(-) effector T-cells inhibit hippocampal long-term potentiation in vitro. Eur J Neurosci. 2007;26:1399–1406. doi: 10.1111/j.1460-9568.2007.05788.x. [DOI] [PubMed] [Google Scholar]
- Ren X, Akiyoshi K, Dziennis S, Vandenbark AA, Herson PS, Hurn PD, et al. Regulatory B cells limit CNS inflammation and neurologic deficits in murine experimental stroke. J Neurosci. 2011;31:8556–8563. doi: 10.1523/JNEUROSCI.1623-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Li Y, Tang Y, Tang G, Yang GY, Wang Y. CXCR4 antagonist AMD3100 protects blood-brain barrier integrity and reduces inflammatory response after focal ischemia in mice. Stroke. 2012;44:190–197. doi: 10.1161/STROKEAHA.112.670299. [DOI] [PubMed] [Google Scholar]
- Seifert HA, Hall AA, Chapman CB, Collier LA, Willing AE, Pennypacker KR. A transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. J Neuroimmune Pharmacol. 2012;7:1017–1024. doi: 10.1007/s11481-012-9406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, et al. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- Fan Y, Shen F, Frenzel T, Zhu W, Ye J, Liu J, et al. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann Neurol. 2010;67:488–497. doi: 10.1002/ana.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya BJ, Banisadr G, Jung H, Ren D, Cronshaw DG, Zou Y, et al. The chemokine stromal cell-derived factor-1 regulates GABAergic inputs to neural progenitors in the postnatal dentate gyrus. J Neurosci. 2008;28:6720–6730. doi: 10.1523/JNEUROSCI.1677-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JR, McCandless EE, Dorsey D, Klein RS. CXCR4 promotes differentiation of oligodendrocyte progenitors and remyelination. Proc Natl Acad Sci USA. 2010;107:11062–11067. doi: 10.1073/pnas.1006301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.