Abstract
N-arachidonoyl-L-serine (AraS) is a novel neuroprotective endocannabinoid. We aimed to test the effects of exogenous AraS on neurogenesis after traumatic brain injury (TBI). The effects of AraS on neural progenitor cells (NPC) proliferation, survival, and differentiation were examined in vitro. Next, mice underwent TBI and were treated with AraS or vehicle. Lesion volumes and clinical outcome were evaluated and the effects on neurogenesis were tested using immunohistochemistry. Treatment with AraS led to a dose-dependent increase in neurosphere size without affecting cell survival. These effects were partially reversed by CB1, CB2, or TRPV1 antagonists. AraS significantly reduced the differentiation of NPC in vitro to astrocytes or neurons and led to a 2.5-fold increase in expression of the NPC marker nestin. Similar effects were observed in vivo in mice treated with AraS 7 days after TBI. These effects were accompanied by a reduction in lesion volume and an improvement in neurobehavioral function compared with controls. AraS increases proliferation of NPCs in vitro in cannabinoid-receptor-mediated mechanisms and maintains NPC in an undifferentiated state in vitro and in vivo. Moreover, although given at 7 days post injury, these effects are associated with significant neuroprotective effects leading to an improvement in neurobehavioral functions.
Keywords: brain trauma, cannabinoids, immunohistochemistry, neural stem cells, neuroregeneration
Introduction
Neural stem cells are able to self-renew and to differentiate into neurons, astrocytes, or oligodendrocytes.1Many endogenous compounds regulate proliferation and differentiation of NPCs both in vitro and in vivo including growth factors,2, 3, 4 neurotrophic factors,5 cytokines, neurotransmitters, and hormones.6, 7
Endocannabinoids were reported to induce proliferation of NPCs in a process that involves both CB1 and CB2 receptors.8, 9, 10 The naturally occurring endocannabinoids anandamide and 2-arachidonoylglycerol inhibit differentiation of NPCs.11 The novel endocannabinoid-like compound N-arachidonoyl-L-serine (AraS) was isolated by Milman et al12 from bovine brain and was found to bind weakly to CB1 and CB2 receptors and to TRPV1 channels. However, it produces endothelium-dependent vasodilation and stimulates phosphorylation of p44/42 (pERK) mitogen-activated protein kinase and protein kinase B/Akt in cultured endothelial cells. It also prevents the attenuation in pERK levels and increases Akt levels after traumatic brain injury (TBI).12, 13 AraS also blocks the N-arachidonoyl glycine-induced migration of BV-2 microglia cells as well as the N-arachidonoyl glycine-induced migration of HEK293-GPR18-transfected cells in a GPR18-mediated manner.14 After TBI, AraS has neuroprotective effects manifested as larger motor and cognitive improvements and reduction in lesion volume and apoptotic death mediated by a CB2 mechanism, although it binds very weakly to this receptor.13, 15 However, the effects of AraS on proliferation and differentiation of NPCs after TBI remain unknown.
Traumatic brain injury leads to an increase in cell proliferation starting in the first two weeks after injury and is evident up to one year later, followed by recruitment of NPCs to the injured cortex.16, 17, 18, 19, 20, 21, 22 Brain injury also induces differentiation of the subventricular zone cells towards a glial fate, which is the default for postnatal differentiation, resulting in the formation of a glial scar in the injured cortex.18, 23, 24, 25 As the effects of cannabinoids are central to cell survival26 and may also influence differentiation, the present study was designed to investigate the effect of AraS on proliferation and differentiation of NPCs and to explore whether AraS has long-term effects after TBI.
Methods
Animals
The study was performed according to Institutional Animal Use and Care Committee guidelines and was approved by the institution's Animal Care and Use Committee. Male Sabra mice (strain of the Hebrew University, Harlan; Jerusalem, Israel) aged 6 to 7 weeks and weighing 35 to 45 g was used in this study. No variations in dominant/submissive characteristics were observed with this strain.
Cell Culture
Cerebral cortical cultures of NPCs were prepared from 14-day mouse embryos. Brains were removed and the cortices were separated from the hemispheres and added to 0.025% trypsin solution for 10 minutes in 37°C humidified 5% CO2 incubator. After removal of trypsin, tissues were added with MEM supplemented with 10% horse serum, 2 mmol/L L-glutamine, 0.35% glucose, and 0.5% penicillin—streptomycin and triturated with a pasteur pipette until clear solution was obtained. Cells were centrifuged for 10 minutes at 1,100 r.p.m. and re-suspended in N2 media (Dulbecco's modified eagle medium/F12 supplemented with 0.5 mg/mL bovine serum albumin, 100 μg/mL apo-transferrin, 300 ug/mL L-glutamine, 0.11 mg/mL sodium pyruvate, 2.5 μg/mL gentamycin sulfate, 25 μg/mL bovine insulin, 6 ng/mL progesterone, 16 ug/mL putrescine, 5.2 ng/mL sodium selenite, and 10 ng/mL D-biotin). The culture was added with 20 ng/mL basic fibroblast growth factor and 10 ng/mL epidermal growth factor daily to avoid differentiation. In vitro, single neural progenitor cells proliferate to form clonally derived floating sphere colonies (neurospheres), which contain cells that, upon dissociation into single cells, give rise to new sphere colonies (self-renewal) and cells that can differentiate into neurons or glia (multipotentiality).27 These neurospheres were cultured for 3 days before collected and re-cultured in the presence of AraS for incubation and further evaluation of their size or counting of dead cells.
Size of Neurospheres
After 3 days in vitro, neurospheres were collected, centrifuged for 5 minutes at 1,100 r.p.m. and dismounted to single cells by triturating with a 200 μL tip. Cells were re-cultured in a 96-well plate containing 300 μL N2 +basic fibroblast growth factor+epidermal growth factor media, in a concentration of less than 10 cells/well (single cell). Each 4 wells were added with a different concentration of AraS (0.05, 0.5, 5, and 10 μm) alone or 10 μmol/L together with 1 μmol/L CB1 antagonist (SR141716A), 1 μmol/L CB2 antagonist (SR144528), or 1 μmol/L TRPV1 antagonist (capsazepine). 1 μmol/L SR141716A, 1 μmol/L SR144528, or 1 μmol/L capsazepine alone were added also to the cultures. 100 μL of media from each well were replaced every day at the same time with a fresh 100 μL N2+basic fibroblast growth factor+epidermal growth factor. Four days after addition of AraS (7 days in vitro), the neurospheres in each well were photographed using a light inverted microscope (Zeiss, Goetingen, Germany) and the area of each sphere was measured using Image ProPlus 6.0 software (Media Cybernetics, Warrendale, PA, USA).
Propidium Iodide Uptake
Four days after addition of 10 μmol/L AraS to culture, neurospheres were collected, centrifuged for 5 minutes at 1,100 r.p.m. and dismounted to single cells by triturating with a 200 μL tip. Cells were re-suspended in phosphate-buffered saline before the addition of propidium iodide (1 μg/mL) and analyzed by flow cytometry (FACSCalibur and Cellquest Software Becton Dickinson, Istanbul, Turkey) using the FL3 channel. Propidium iodide-positive cells are considered dead cells.
Differentiation of Neural Progenitor Cells In Vitro
After 6 days in vitro, neurospheres were collected, centrifuged for 5 minutes at 1,100 r.p.m. and re-suspended in N2 media. Neurospheres were cultured on poly-ornithine+fibronectin-coated coverslips in a N2 media containing AraS or vehicle but without basic fibroblast growth factor and epidermal growth factor. Five days after addition of AraS (11 days in vitro), the neurospheres were fixed using ice-cold 5% acid alcohol (5% acetic acid in ethanol) in −20°C for 8 minutes, washed five times with Dulbecco's modified Eagle medium/F12 and blocked using 1% normal goat serum for 15 minutes. Blocking solution was removed and the neurospheres were subjected to primary antibody (nestin, Abcam, Cambridge, MA, USA, 1:100, glial fibrillary acidic protein, Dako, Glostrup, Denmark, 1:200, or class III beta tubulin (TUJ1), Sigma, Rehovot, Israel, 1:2,000) for 45 minutes followed by washing and incubation for additional 45 minutes with the appropriate secondary fluorescent antibodies. The cells were washed again and 4′6-diamido-2-phenylindole solution was added for 5 minutes followed by mounting of the coverslips. The stained cultures were photographed using a fluorescent microscope (BX51 Olympus, Center Valley, PA, USA) equipped with a digital camera (DXM1200F Nikon, Tokyo, Japan) and analyzed for differentiation using Image ProPlus 6.0 softwere.
Trauma Model
Mice were subjected to closed head injury (CHI) under isoflurane (2%) anesthesia, using a weight-drop device that falls over the left hemisphere, as described elsewhere.28 In brief, after a longitudinal scalp incision, mice were immobilized under a cylindrical calibrated weight-drop device. A tipped teflon cone was placed (upside down) 2 mm lateral to the midline and 1 mm caudal to the left coronal suture, and a metal rod (94 g) was dropped down on the cone from a height of 11 to 14 cm (adjusted to body weight, to ensure the severity of injury28 required to produce CHI). Less than 10% of the injured mice were excluded from the study, mostly because of death by apnea within minutes of injury. After recovery from anesthesia, the mice were returned to their home cages with free access to food and water.29, 30
Evaluation of Functional Outcome
One hour after CHI, the functional status of the mice was evaluated according to a set of 10 neurobehavioral tasks (neurologic severity score, NSS), which examine reflexes, alertness, coordination, motor abilities, and balancing.31 Failure to perform a task scores 1 point and a success scores 0. Hence, normal animals score 0, reflecting healthy mice, whereas a score of 10 reflects maximal neurologic impairment. Only mice with NSS 6 to 8 at 1 hour after injury (NSS 1 hour) were included in the study. The extent of improvement (ΔNSS) was calculated by subtracting the NSS at a specific time point from that achieved 1 hour after CHI. A NSS follow-up was performed weekly for 91 days.
Drug Application
Treatment at 1 hour
The dose selected for treatment was based on our previous studies.13 Mice were injected intraperitoneally with 3 mg/kg AraS dissolved in ethanol:cremophor:saline 1:1:18 or vehicle immediately after NSS evaluation at 1 hour. Based on the NSS at 1 hour, mice were assigned to treatment groups such that similar severity of injury (NSS 1 hour) was ensured in all groups (n=6 to 9 mice per group). NSS was re-evaluated on days 1, 2, 3, 7 and in 1-week intervals up to 91 days after CHI.
Treatment at 7 Days
After CHI and NSS evaluation at 1 hour, NSS was re-evaluated 7 days after the injury and immediately afterwards, the mice were assigned to treatment groups such that similar neurologic status (NSS 7 days) was ensured in all groups (n=6 to 7 mice per group). After assignment, mice were injected intraperitoneally with 3 mg/kg AraS dissolved in ethanol: cremophor:saline 1:1:18 or vehicle. Bromodeoxyuridine (BrdU) 50 mg/kg was injected intraperitoneally twice daily to the mice from 7 to 12 days after the trauma. NSS was re-evaluated in 1-week intervals up to 91 days after CHI. The NSS assessments were performed by an investigator that was masked to treatment.
In Vivo Proliferation and Differentiation
At 90 days post TBI, mice were perfused with 4% paraformaldehyde, decapitated, and the brains were frozen immediately on dry ice and kept at −80°C until further use. Before staining, brains were cut to 10 μm slices from bregma 0 to bregma −2 mm using a Leica CM1850 Cryostat (Leica Microsystems GmbH, Wetzlar, Germany). Slices were thawed, fixed with 4% paraformaldehyde solution for 20 minutes, washed, and incubated in 2 N HCl solution for 25 minutes (excluding staining for doublecortin) in a 37°C oven. Slices were washed and blocked for 2 hours at room temperature in 5% normal donkey serum followed by an overnight incubation at 4°C with the primary antibodies (BrdU 1:100 (Sigma-Aldrich, St Louis, MO, USA)+nestin 1:100 (Abcam), glial fibrillary acidic protein 1:200 (Dako, San Diego, CA, USA) or NeuN 1:100 (Millipore, Billerica, MA, USA)). After washes, the slices were incubated for 2 hours at room temperature with the appropriate secondary fluorescent antibodies (donkey antirat 488, donkey anti-mouse 488, donkey anti-mouse 555, donkey anti-rabbit 488, or donkey anti-rabbit 555; Invitrogen, Carlsbad, CA, USA) followed by incubation with 4′6-diamido-2-phenylindole solution (Invitrogen) for 5 minutes and mounting. A group of five sections was counted every 150 μm (n=5 to 6 mice/group). Each slice was divided into 6 regions: 2 in each side of the lateral ventricle and 2 at the traumatized cortex. All the marked cells in these fields were counted. The quantitative measurements of the markers were performed using a fluorescent microscope (Olympus BX51) equipped with a digital camera (Nikon DXM1200F).
Lesion Volume
Lesion volume was evaluated using Giemsa staining, as described elsewhere.25 Briefly, the mice were subjected to CHI followed by the different treatments at 7 days after the injury (n=5 mice per group). At 91 days after brain injury, the mice were anesthetized and perfused with 4% paraformaldehyde, decapitated, and the brains were frozen immediately on dry ice and kept at −80°C until further use. Brains were cut to 10 μm slices from bregma 0 to bregma −2 mm using a Leica CM1850 Cryostat (Leica Microsystems GmbH). Slices were stained with Giemsa stain-modified solution (1:1; Fluka, Sigma-Aldrich) and digitally photographed. ImageJ 1.40 g software (National Institutes of Health, Bethesda, MD, USA) was used to quantify lesion volume. A group of 5 sections 150 μm apart was counted for each brain. Lesion volume was calculated as the area of injured hemisphere divided by that of the contralateral hemisphere.32
Western Immunoblotting
To determine the levels of CB1 and CB2 in NPC, neurospheres were treated with AraS or vehicle and collected 4 days afterwards. The spheres were centrifuged (1,100 r.p.m. × 5 minutes) and soup was removed. Cells were lysed with cold lysis buffer (50 mmol/L Tris–HCl, pH 7.5, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L ethylene glycol tetraacetic acid, 1 mmol/L Na3VO4, 50 mmol/L NaF, 10 mmol/L sodium β-glycerophosphate, 10 mmol/L sodium pyrophosphate, 1 mmol/L phenylmethylsulfonyl fluoride, supplemented with 0.1% (v/v) NP-40, 0.1% (v/v) 2-β-mercaptoethanol and protease inhibitor cocktail). Protein content in the supernatant was determined according to Bradford method and the manufacturer's instructions. A total 15 μg of the protein extract from each sample was mixed with SDS-sample buffer and boiled at 95°C for 5 minutes before loading. The proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresisand transferred onto trans-blot nitrocellulose membranes. The membranes were blocked in 5% nonfat dry milk in TBS, pH 7.4, with 0.1% Tween 20 (TBS–Tween) for 1 hour at room temperature. Primary antibodies (antiCB1 1:500, antiCB2 1:500, β-actin 1:1000, Glyceraldehyde-3-phosphate dehydrogenase 1:2,000) were diluted in 5% bovine serum albumin solution, and the membranes were incubated overnight at 4°C. The primary antibody was removed, and the blots were washed in TBS–Tween and incubated for 1 hour at room temperature with horseradish peroxidase-conjugated secondary antibodies (1:5,000, Jackson Immunoresearch, Soham, Cambridgeshire, UK). Reactive proteins were visualized using enhanced chemiluminescence reagent (Biological Industries, Beit Haemek, Israel) by direct chemiluminescence using FUJIFILM Luminescent Image Analyzer camera LAS-3000 (Tokyo, Japan). The optical density was determined using ImageJ 1.40 g software (National Institutes of Health, USA).
Statistical Analysis
Statistical analysis was performed using SigmaStat 2.03 software (SPSS, Chicago, IL, USA). The data are presented as the mean±s.e.m. The statistical significance of differences between means was evaluated by student's t-test for NSS, ΔNSS, optical density measurements, differentiation of cells, and for lesion volume. Statistical analysis for the size of neurospheres was calculated with parametric one-way analysis of variance followed by the Tukey's post hoc test. Probability values (P) lower than 0.05 were considered statistically significant.
Results
AraS Increases the Size of Neurospheres of NPCs In Vitro
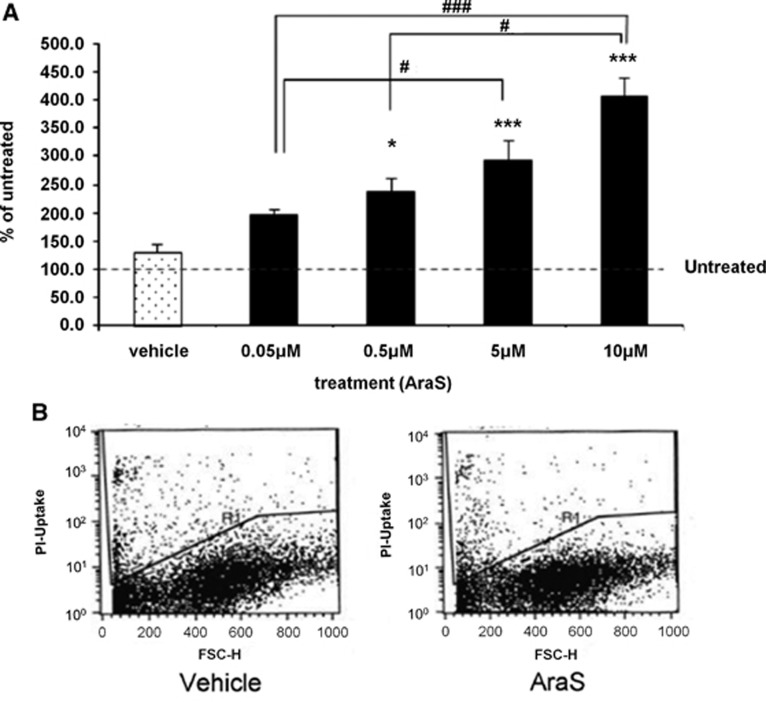
To determine the involvement of AraS in neurogenesis, the role of AraS in NPC proliferation was investigated using an in vitro model system. To this end, NPCs were isolated from embryonic cortical tissue and grown as neurospheres as described in the methods section. The cells were plated at clonal density to measure the size of neurospheres formed from each cell. AraS-treated neurospheres demonstrated a dose-dependent increase in size 4 days after treatment compared with vehicle-treated cells (Figure 1A). The diameter of neurospheres treated with 0.05 μmol/L AraS was almost 2-fold larger than the control group (61185±7690 pixles and 31217±4044 pixels, respectively), while the diameters of the subsequent AraS-treatment groups (0.5, 5, and 10 μm) were 2.4, 3, and 4-times larger (80500±8442, 88073±10881, and 126670±20948 pixels, respectively). This effect suggests either a proliferative effect or a prosurvival effect of AraS. However, no changes were detected in the number of dead cells between AraS and vehicle-treated cultures (Figure 1B) implying that the effect of AraS on sphere size is secondary to proliferative effects. Moreover, an increase in cell number was detected in AraS-treated group compared with vehicle 3 days after induction of treatment (data not shown).
Figure 1.
N-arachidonoyl-L-serine (AraS) induces proliferation of neural progenitor cells and does not influence cell survival. Neurospheres were treated with increasing doses of AraS. Four days after induction of treatment a dose-dependent increase in the size of the spheres was detected (A). Fluorescence-activated cell sorting analysis of dead cells (propidium iodide positive) are shown with no observed differences between the groups (B) (*P<0.05, ***P<0.001 versus vehicle, #P<0.05, ###P<0.001).
AraS Reduces Differentiation of NPC In Vitro
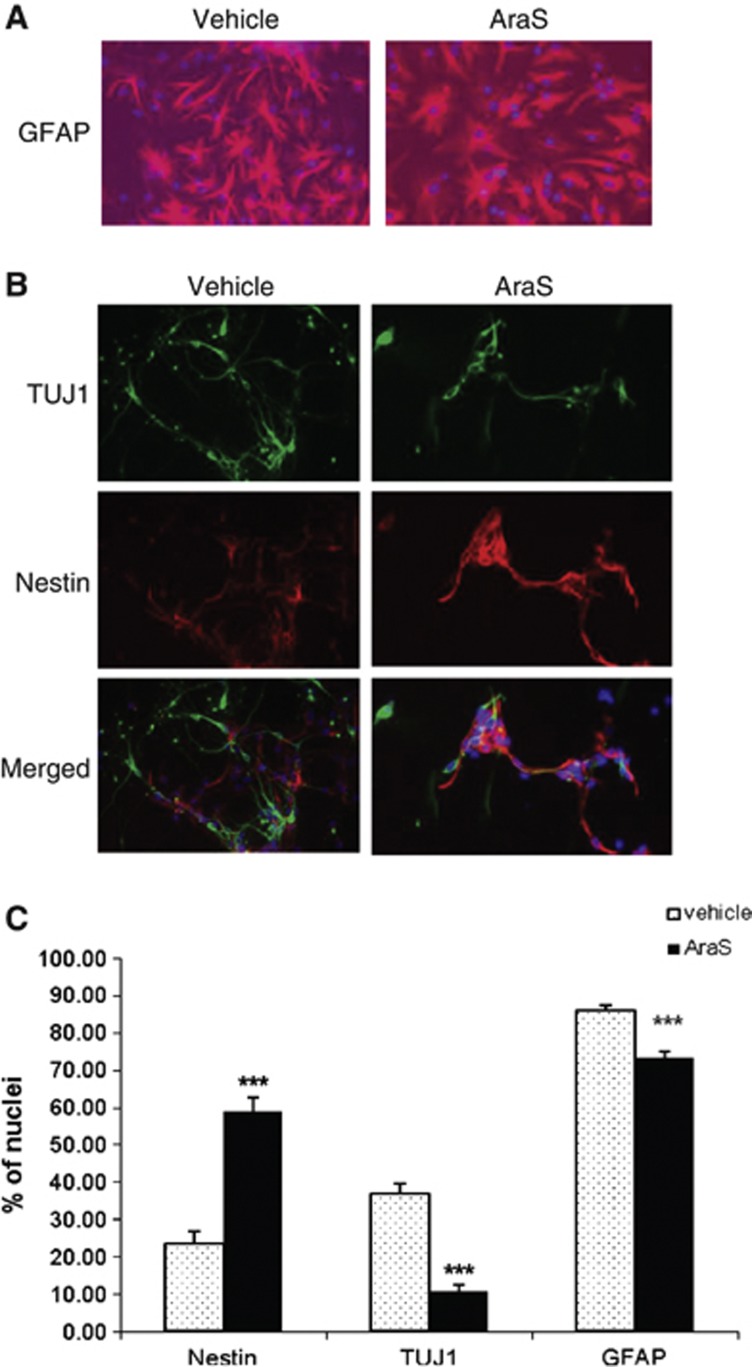
Neurospheres were treated with 10 μmol/L AraS or vehicle and were then let to differentiate for 5 days without addition of growth factors, after which they were stained for different neuronal and glial markers. A reduction in the expression of the astrocytic marker glial fibrillary acidic protein (73.26±1.60%) together with the neuronal marker TUJ1 (10.56±1.94%) was detected in NPCs treated with AraS compared with vehicle (86.11±1.26% and 36.79±2.69%, respectively). In contrast, a 2.5-fold increase in the expression of the NPC marker nestin was detected in the treated culture compared with control (58.88±3.65% and 23.40±3.59%, respectively; Figure 2). Stainings for the marker for oligodendrocytes galactocerebroside did not show any differentiation to these cells. Taken together, these results indicate that AraS maintains NPCs in an undifferentiated state and reduces their terminal differentiation in vitro.
Figure 2.
N-arachidonoyl-L-serine (AraS) reduces differentiation of neural progenitor cells into astrocytes and neurons and increases nestin+ cells. Neural progenitor cells were treated with AraS or vehicle. After 5 days in culture, NPCs were stained for the astrocytic marker glial fibrillary acidic protein (GFAP) (A), the neuronal marker TUJ1 and for the neuro-developmental marker nestin (B). A reduction in GFAP+ as well as in TUJ1+ cells was observed together with an increased number of nestin+ cells (C). (***P<0.001 versus vehicle). Scale bar, 100 μm.
CB1, CB2 and TRPV1 are Involved in the Proliferative Effect of AraS In Vitro
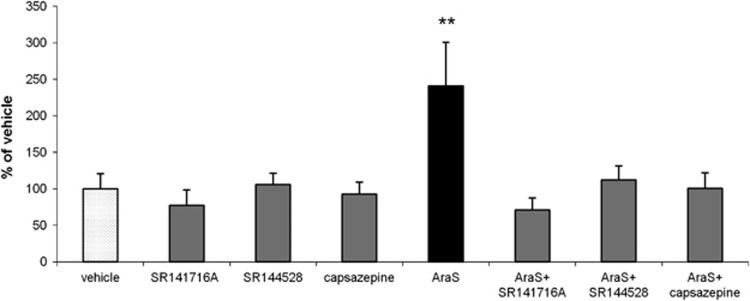
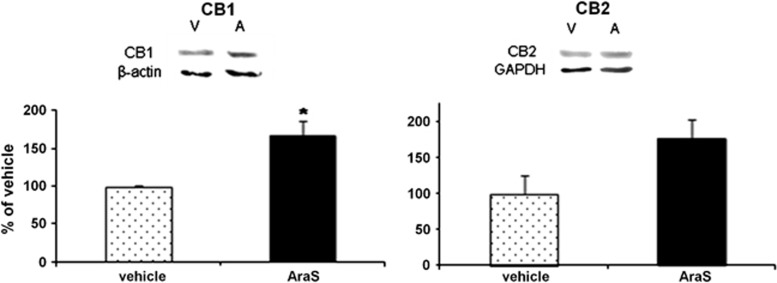
The mechanism involved in the proliferative effect of AraS was investigated in vitro to identify which of the cannabinoid-binding sites are involved. Addition of the CB1 antagonist SR141716A, the CB2 antagonist SR144528, or the TRPV1 antagonist capsazepine together with AraS, abolished the 3-fold increase in the size of neurospheres found in cultures treated with 10 μmol/L AraS (Figure 3). Another effect of AraS that may be related to its protective and proliferative potential is induction of the cannabinoid receptor expression. Both CB1 and CB2 levels were more than 60% higher in the AraS-treated neurospheres as compared with controls after 4 days in culture. (Figure 4). These results suggest that each of the endocannabinoid receptors may impact the proliferative effects of AraS on NPC.
Figure 3.
CB1, CB2, and TRPV1 antagonists abolish the proliferative effect induced by N-arachidonoyl-L-serine (AraS) in vitro. Treatment of neurospheres with 10 μmol/L AraS enhances the proliferation of the NPCs. Addition of CB1 (SR141716A) or CB2 (SR144528) antagonists or TRPV1 blocker (capsazepine) eliminates this effect demonstrating size similar to that achieved in vehicle-treated neurospheres (**P<0.01 versus vehicle). No pro/anti-proliferative effect was demonstrated in the cultures treated with the antagonists alone.
Figure 4.
N-arachidonoyl-L-serine (AarS) induces expression of the cannabinoid receptors in neural progenitor cells in culture. Quantitation of the levels of CB1 and CB2 receptors demonstrated a significant 66% increase in CB1 levels (left panel) and 75% increase in CB2 levels (right panel) in 10 μmol/L AraS-treated cultures compared with vehicle 4 days after induction of treatment (*P<0.05, **P<0.01 versus vehicle). A, AraS; V, vehicle. (n=3 to 5).
AraS Improves Neurobehavioral Function, Increases Proliferation and Decreases Differentiation of NPC Into Astrocytes After Traumatic Brain Injury
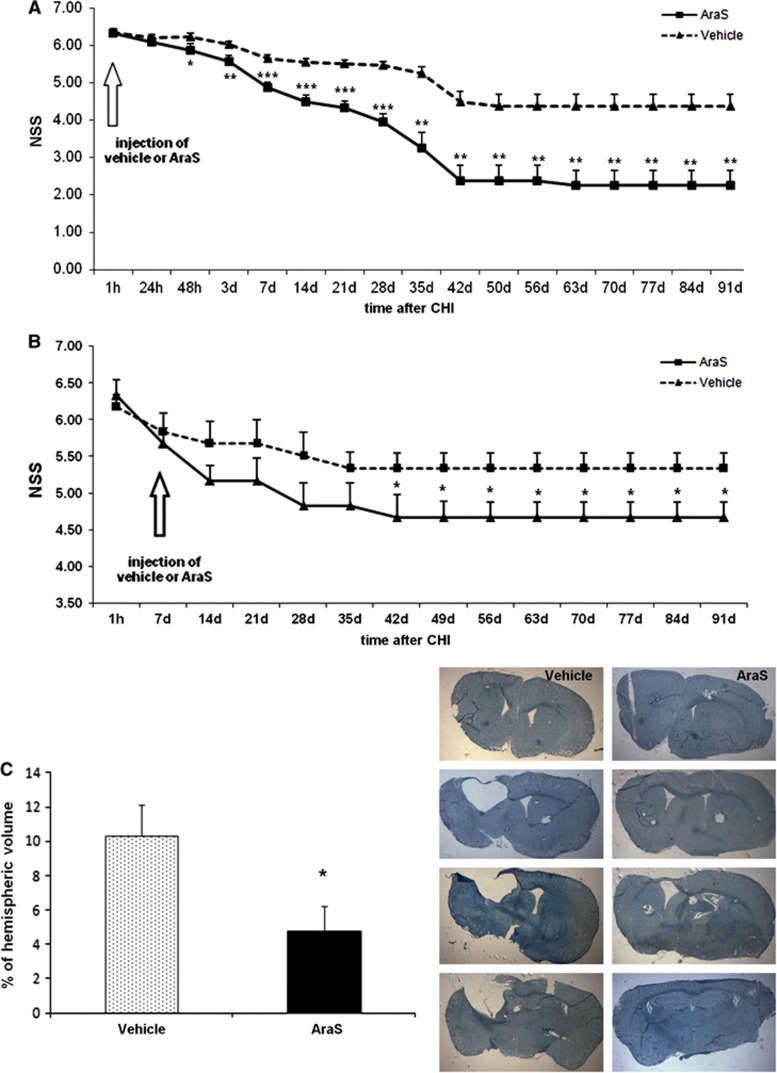
To further investigate the neurogenic properties of AraS, and to discriminate between the early neuroprotective phase that was shown in our previous study13, 15 and the later proneurogenic phase, AraS was injected in the current series of experiments 7 days after TBI. The beneficial effect of AraS, namely reduction of the behavioral deficits as evaluated by NSS, was only evident from 5 weeks after treatment and sustained up to 3 months thereafter (Figure 5B). A sham group was included in our previous experiments and showed no clinical deficits in the NSS test over time.13, 33 It should be noted that when AraS was given 1 hour after TBI, the effect reached significance 48 hours later, and its extent was more pronounced (Figure 5A).
Figure 5.
Injection of N-arachidonoyl-L-serine (AraS) after closed head injury (CHI) improves functional outcome and reduces the volume of the lesioned tissue for up to 3 months. AraS or vehicle were injected to mice 1 hour (A) or 7 days (B) after CHI. A single injection of AraS significantly improved neurobehavioral function 48 hours (A) or 5 weeks (B) after treatment (42 days after CHI) in AraS-treated group compared with vehicle. The improvement lasted up to 91 days after the injury (*P<0.05, **P<0.01, ***P<0.001 versus vehicle). (n=5 to 9). Note the difference in the scale at the Y-axis. (C) Lesion volume of the brains of mice, treated with AraS or vehicle, was calculated from serial brain slices stained with Giemsa. A significant decrease in the lesion volume was observed in the brains of mice treated with AraS compared with vehicle (*P<0.05).
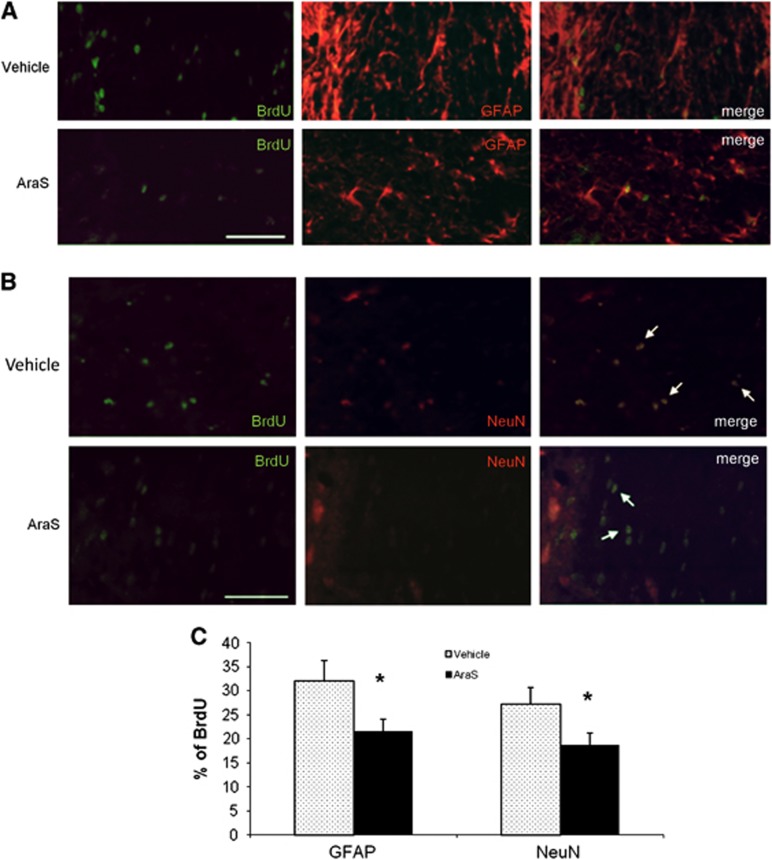
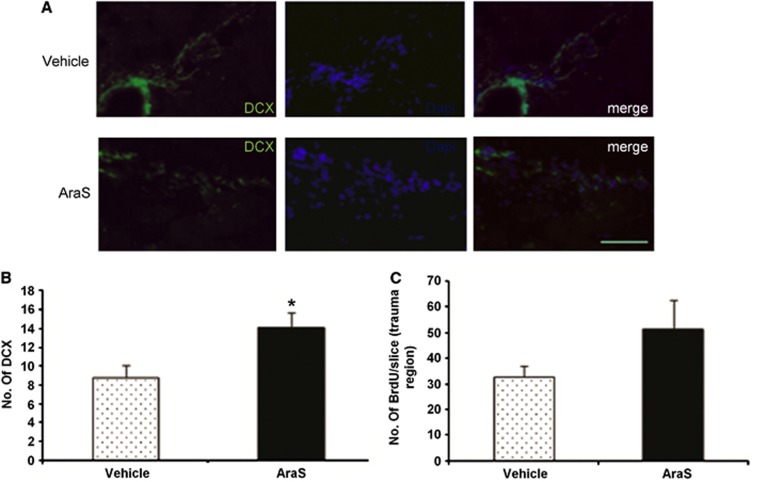
The improved function may imply an induction of neurogenesis and remodeling processes. Indeed, treatment with AraS led to a 1.8-fold increase in the levels of proliferating cells (BrdU+) (10.94±2.40 and 6.10±0.77, respectively; Figure 6). Staining with the astrocytic marker glial fibrillary acidic protein together with the proliferation marker BrdU demonstrated a decrease in the differentiation of proliferating cells into astrocytes in the AraS-treated group compared with vehicle (21.5±1.9% versus 32.8±4.1% respectively; P<0.05; Figures 6A and 6C). Furthermore, a decrease in the differentiation of proliferating cells into neurons was also demonstrated in the AraS-treated group compared with vehicle (18.68±2.52% versus 27.26±3.33%, respectively; P<0.05; Figures 6B and 6C). In contrast, a 1.6-fold increase in the expression of the newborn neuroblastic marker doublecortin was detected in the treated group compared with vehicle (14.08±1.61 versus 8.81±1.32, respectively; P<0.05; Figures 7A and 7B). However, no significant change was detected in the levels of the proliferation marker BrdU after the injury although a trend toward significant results was observed Figures 7A and 7C.
Figure 6.
N-arachidonoyl-L-serine (AraS) decreases differentiation of NPCs into glial fibrillary acidic protein positive (GFAP+) and NeuN+ cells after closed head injury (CHI) in vivo. Brains of mice, treated with AraS or vehicle 7 days after CHI, were stained for the proliferative marker BrdU and the astrocytic marker GFAP or the neuronal marker NeuN 91 days after injury. A significant decrease in the proliferating cells expressing GFAP (A) or NeuN (B) was detected in brains of AraS-treated mice compared with vehicle (*P<0.05 versus vehicle). Scale bar, 100 μm. (C) Bar graph summarizing the effect of AraS on differentiation of neural progenitor cells.
Figure 7.
N-arachidonoyl-L-serine (AraS) increases differentiation of neural progenitor cells (NPCs) into doublecortin positive (DCX+) cells but does not alter proliferation after closed head injury (CHI) in vivo. Brains of mice, treated with AraS or vehicle 7 days after CHI, were stained for the migrating neuroblast marker DCX or for the proliferative marker BrdU 91 days after the injury. A significant increase in neuroblasts expressing DCX was detected in brains of AraS-treated mice compared with vehicle (*P<0.05 versus vehicle; left panel), however, no significant change was detected in the levels of the proliferative marker after the injury (right panel) (A). Scale bar, 100 μm. Bar graph summarizing the effect of AraS on differentiation of NPCs into DCX+ cells (B) and the effect of AraS on proliferation (C) (n=5 to 6 mice/group).
These results show that similar to its effects in vitro, AraS reduces terminal differentiation of NPC although an increase in neuroblasts was detected.
AraS Reduces Lesion Volume Three Months After Brain Trauma
To further explore the effects of delayed (7 days post injury) administration of AraS, the lesion volume was measured 91 days after injury. While lesion hemispheric volume of 10.34±1.79% was found in the vehicle-treated mice, it was only 4.76±1.43% in the brains of AraS-treated mice (P<0.05; Figure 5C).
Discussion
This study is focused on the long-term recovery and rehabilitative effects of the endocannabinoid AraS, given at a single dose after injury. When the drug was given 1 hour post injury the neurobehavioral function continuously improved during 6 weeks. This time is long beyond the cellular prosurvival signaling, which we have reported earlier,13 suggesting the involvement of other mechanisms, including activation of neural stem/progenitor cells. To dissociate the neuroprotective from the proliferative effects, mice were treated with AraS 7 days after injury and their functional outcome was followed for 3 months. In addition, NPC were prepared from 14-day mouse embryos and exposed to AraS in vitro. In culture, AraS-induced dose-dependent increase in NPC proliferation, reduced differentiation into astrocytes and neurons, and preserved them in an undifferentiated state. In vivo, the delayed (7 days post TBI) treatment led to improved functional outcome, reduced infarct volume and, similar to its effects in vitro, reduced terminal differentiation of NPC.
At different embryonic and postnatal stages of brain development, the endocannabinoid system is involved in the regulation of NPC differentiation, which occurs in parallel with CB1 receptor expression.9, 11 CB2 receptor is present in progenitor cells from embryo origin and from adult brain. It mediates acceleration of neurogenesis and stimulates neural progenitor proliferation.8, 34 In the present study, we found that the proliferative effect of AraS is mediated via the cannabinoid receptors CB1, CB2, and TRPV1, as treatment of neurospheres in vitro with specific antagonists to each of these individual receptors abolished the AraS-induced increase in the size of the neurospheres. Interestingly, AraS also induced increased expression of the CB1 and CB2 receptors 4 days after exposure of NPC to AraS, which may indicate involvement of gene transcription.
AraS previously demonstrated neuroprotective effects in a CB2-related mechanism,13 although it binds very weakly to this receptor.12 As CB2 is a known direct mediator of NPC proliferation and indirectly mediates neurogenesis,8, 34 it may be suggested that the increase in the levels of the neuroepithelial marker nestin induced by AraS is mediated, indirectly, by CB2. However, this suggested the mechanism needs to be elucidated.
AraS is shown here to induce proliferation of NPCs in vitro and also in vivo, (although not significant) thus, the better functional outcome (lower NSS and infarct volume) seen in AraS-treated mice may be also secondary to these effects. However, a reduction in terminal differentiation of NPCs was observed both in vitro and in vivo after treatment with AraS, while an increase in the neuroepithelial cells/newborn neuroblasts was detected. The effect in vitro may indicate a ‘prostemness' effect of AraS, favoring the multipotent progeny state of the cells over the differentiated form. However, after brain injury, the more committed cells fail to fully differentiate and survive as detected in vivo. It may be speculated that the role of AraS is similar to that reported for anandamide, namely, to preserve the progenitor pool in the brain encouraging proliferation over differentiation.9, 11 Given the effects of AraS on terminal differentiation of NPC, it is clear that the functional gains in treated animals cannot be explained by an increase in the number of newborn neurons and replacement of dead cells by newborn fate and site-appropriate cells. Rather, these undifferentiated cells can by themselves lead to facilitated recovery by secreting a plethora of growth and survival factors including vascular endothelial growth factor, brain derived growth factor, cilliary neurotrophic growth factor and others.35, 36 Furthermore, young undifferentiated cells also possess an immunomodulatory effect that may also improve outcome.37 Alternatively, AraS may induce plasticity and synaptogenesis. Indeed, a synthetic CB2 agonist was recently found in our lab to enhance synaptophysin levels 25 days after CHI (M Algali, MSc thesis, personal communication).
It is important to mention that the lack of change in cell death in vitro may suggest a prosurvival effect for AraS instead of the proproliferative effect suggested here, however, if so, we may have expected a decrease in mortality in the AraS-treated group and not a lack of change in mortality as was actually observed.
To summarize this part of the study, AraS possesses properties, which may contribute towards remodeling the tissue using the existing unharmed cells, while maintaining the population of progenitors in the brain. This assumption may also explain, at least in part, the relatively late improvement in neurobehavioral function since such processes take a long time to establish.25, 38
Behavioral function of injured mice, assessed during 3 months after trauma, was less impaired in the AraS-treated groups compared with vehicle. This functional improvement was achieved already at 48 hours, after a single dose of AraS was given 1 hour post injury and could be attributed to the neuroprotective processes induced by AraS. These involve increased levels of pAkt and the prosurvival factor Bcl-xL as well as reduced activation of the proapoptotic enzyme caspase 3.13 These neuprotective effects may also account for the decrease in lesion volume reported in our previous study, from 14.4% in the vehicle-treated mice to 8.00% in the mice treated with AraS at 1 hour.13 However, the neuroprotective cellular effects are short-term (2 to 24 hours) and cannot account for those achieved when AraS is given 7 days after injury. Thus, the late-onset benefits described here as improved functional outcome, yet much less pronounced than in the group treated 1 hour post injury, and reduced lesion volume, exclude the period described as the window for neuroprotection and could be derived from another property of the endocannabinoids, including AraS.
Handling, anesthesia and stress may affect neurogenesis and may have impacted our results. However, since similar manipulations were used in all groups including vehicle, and since sham-operated animals demonstrated no abnormalities in the NSS or neurogenesis,13 these factors were not likely to impact the results shown in this work.
One of the secondary insults after TBI is cerebral vasospasm, which occurs in more than one-third of patients with TBI (for a review, see Werner and Engelhard39). The temporal profile and extent of hypoperfusion with posttraumatic vasospasm shows an onset, which varies from posttraumatic day 2 to 15 as a result of chronic depolarization of vascular smooth muscle.40 In our earlier studies, (for a review see Shohami et al.15) we have demonstrated that human brain endothelial cells express CB1, CB2, and TRPV1 receptors, and that 2-arachidonoylglycerol, the most abundant endocannabinoid in the brain, functions as a vasorelaxant, that may counteract the powerful vasoconstrictor ET-1.41, 42 These observations, along with those of Milman et al12 on the vasorelaxant properties of AraS support the hypothesis that AraS, even given beyond the ‘classical' therapeutic window may still contribute towards improved functional outcome and reduced infarct volume by counteracting hypoperfusion.
In conclusion, we showed here for the first time that the novel endocannabinoid AraS, even when given as late as 7 days after injury, may still improve neurobehavioral function and reduce infarct volume. It exerts regenerative effects in vitro and in vivo in a mechanism not related to its neuroprotective effects. The regenerative effects include proliferation of NPCs in a CB1–CB2 and TRPV1-mediated mechanism. AraS also reduces differentiation of NPC and increases their self-renewal, which may imply its involvement in the maintenance of NPC in the brain. We also pointed to its endothelium-dependent vasodilatior properties as an additional mechanism of neuroprotection. These observations encourage further studies aiming at the development of AraS or other analogs for clinical application in the setting of TBI.
The authors declare no conflict of interest.
Footnotes
This study was supported by NIH Grant No. DA-9789, a grant from the Brettler Center for Research in Molecular Pharmacology and Therapeutics, the HU School of Pharmacy, The Peritz and Chantal Scheinberg Cerebrovascular Research Fund and the Sol Irwin Juni Trust Fund. ES is the incumbent of the Dr. Leon and Mina Deutch Chair in Psychopharmacology at the Hebrew University.
References
- Merkle FT, Alvarez-Buylla A. Neural stem cells in mammalian development. Curr Opin Cell Biol. 2006;18:704–709. doi: 10.1016/j.ceb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, et al. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell. 2003;2:175–183. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachyankar MB, Condon PJ, Quesenberry PJ, Litofsky NS, Recht LD, Ross AH. Embryonic precursor cells that express Trk receptors: induction of different cell fates by NGF, BDNF, NT-3, and CNTF. Exp Neurol. 1997;144:350–360. doi: 10.1006/exnr.1997.6434. [DOI] [PubMed] [Google Scholar]
- Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, Geary C, et al. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–120. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- Karishma KK, Herbert J. Dehydroepiandrosterone (DHEA) stimulates neurogenesis in the hippocampus of the rat, promotes survival of newly formed neurons and prevents corticosterone-induced suppression. Eur J Neurosci. 2002;16:445–453. doi: 10.1046/j.1460-9568.2002.02099.x. [DOI] [PubMed] [Google Scholar]
- Palazuelos J, Aguado T, Egia A, Mechoulam R, Guzmán M, Galve-Roperh I. Non-psychoactive CB2 cannabinoid agonists stimulate neural progenitor proliferation. FASEB J. 2006;20:2405–2407. doi: 10.1096/fj.06-6164fje. [DOI] [PubMed] [Google Scholar]
- Aguado T, Monory K, Palazuelos J, Stella N, Cravatt B, Lutz B, et al. The endocannabinoid system drives neural progenitor proliferation. FASEB J. 2005;19:1704–1706. doi: 10.1096/fj.05-3995fje. [DOI] [PubMed] [Google Scholar]
- Jin K, Xie L, Kim SH, Parmentier-Batteur S, Sun Y, Mao XO, et al. Defective adult neurogenesis in CB1 cannabinoid receptor knockout mice. Mol Pharmacol. 2004;66:204–208. doi: 10.1124/mol.66.2.204. [DOI] [PubMed] [Google Scholar]
- Aguado T, Palazuelos J, Monory K, Stella N, Cravatt B, Lutz B, et al. The endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cells. J Neurosci. 2006;26:1551–1561. doi: 10.1523/JNEUROSCI.3101-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman G, Maor Y, Abu-Lafi S, Horowitz M, Gallily R, Batkai S, et al. N-arachidonoyl L-serine, an endocannabinoid-like brain constituent with vasodilatory properties. Proc Natl Acad Sci USA. 2006;103:2428–2433. doi: 10.1073/pnas.0510676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Yeshurun A, Trembovler V, Alexandrovich A, Ryberg E, Greasley PJ, Mechoulam R, et al. N-arachidonoyl-L-serine is neuroprotective after traumatic brain injury by reducing apoptosis. J Cereb Blood Flow Metab. 2011;31:1768–1777. doi: 10.1038/jcbfm.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D, Hu SS, Rimmerman N, Juknat A, Vogel Z, Walker JM, et al. N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor. BMC Neurosci. 2010;11:44. doi: 10.1186/1471-2202-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohami E, Cohen-Yeshurun A, Magid L, Algali M, Mechoulam R. Endocannabinoids and traumatic brain injury. Br J Pharmacol. 2011;163:1402–1410. doi: 10.1111/j.1476-5381.2011.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Bullock MR, Altememi N, Zhou Z, Hagood S, Rolfe A, et al. The effect of epidermal growth factor in the injured brain after trauma in rats. J Neurotrauma. 2010;27:923–938. doi: 10.1089/neu.2009.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindienst A, McGinn MJ, Harvey HB, Colello RJ, Hamm RJ, Bullock MR. Enhanced hippocampal neurogenesis by intraventricular S100B infusion is associated with improved cognitive recovery after traumatic brain injury. J Neurotrauma. 2005;22:645–655. doi: 10.1089/neu.2005.22.645. [DOI] [PubMed] [Google Scholar]
- Chen XH, Iwata A, Nonaka M, Browne KD, Smith DH. Neurogenesis and glial proliferation persist for at least one year in the subventricular zone following brain trauma in rats. J Neurotrauma. 2003;20:623–631. doi: 10.1089/089771503322144545. [DOI] [PubMed] [Google Scholar]
- Lu D, Mahmood A, Zhang R, Copp M. Upregulation of neurogenesis and reduction in functional deficits following administration of DEtA/NONOate, a nitric oxide donor, after traumatic brain injury in rats. J Neurosurg. 2003;99:351–361. doi: 10.3171/jns.2003.99.2.0351. [DOI] [PubMed] [Google Scholar]
- Chirumamilla S, Sun D, Bullock MR, Colello RJ. Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J Neurotrauma. 2002;19:693–703. doi: 10.1089/08977150260139084. [DOI] [PubMed] [Google Scholar]
- Urrea C, Castellanos DA, Sagen J, Tsoulfas P, Bramlett HM, Dietrich WD. Widespread cellular proliferation and focal neurogenesis after traumatic brain injury in the rat. Restor Neurol Neurosci. 2007;25:65–76. [PubMed] [Google Scholar]
- Ramaswamy S, Goings GE, Soderstrom KE, Szele FG, Kozlowski DA. Cellular proliferation and migration following a controlled cortical impact in the mouse. Brain Res. 2005;1053:38–53. doi: 10.1016/j.brainres.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Goings G, Sahni V, Szele F. Migration patterns of subventricular zone cells in adult mice change after cerebral cortex injury. Brain Res. 2004;996:213–226. doi: 10.1016/j.brainres.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Kernie SG, Erwin TM, Parada LF. Brain remodeling due to neuronal and astrocytic proliferation after controlled cortical injury in mice. J Neurosci Res. 2001;66:317–326. doi: 10.1002/jnr.10013. [DOI] [PubMed] [Google Scholar]
- Thau-Zuchman O, Shohami E, Alexandrovich AG, Leker RR. Vascular endothelial growth factor increases neurogenesis after traumatic brain injury. J Cereb Blood Flow Metab. 2010;30:1008–1016. doi: 10.1038/jcbfm.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Spatz M, Shohami E. Endocannabinoids and neuroprotection. Sci STKE. 2002;23:RE5. doi: 10.1126/stke.2002.129.re5. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Yatsiv I, Grigoriadis N, Simeonidou C, Stahel PF, Schmidt OI, Alexandrovitch AG, et al. Erythropoietin is neuroprotective, improves functional recovery, and reduces neuronal apoptosis and inflammation in a rodent model of experimental closed head injury. FASEB J. 2005;19:1701–1703. doi: 10.1096/fj.05-3907fje. [DOI] [PubMed] [Google Scholar]
- Shapira Y, Shohami E, Sidi A, Soffer D, Freeman S, Cotev S. Experimental closed head injury in rats: mechanical, pathophysiologic, and neurologic properties. Crit Care Med. 1988;16:258–265. doi: 10.1097/00003246-198803000-00010. [DOI] [PubMed] [Google Scholar]
- Shapira Y, Setton D, Artru AA, Shohami E. Blood–brain barrier permeability, cerebral edema, and neurologic function after closed head injury in rats. Anesth Analg. 1993;77:141–148. doi: 10.1213/00000539-199307000-00028. [DOI] [PubMed] [Google Scholar]
- Beni-Adani L, Gozes I, Cohen Y, Assaf Y, Steingart RA, Brenneman DE, et al. A peptide derived from activity-dependent neuroprotective protein (ADNP) ameliorates injury response in closed head injury in mice. J Pharmacol Exp Ther. 2001;296:57–63. [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Chen Y, Constantini S, Trembovler V, Weinstock M, Shohami E. An experimental model of closed head injury in mice: pathophysiology, histopathology, and cognitive deficits. J Neurotrauma. 1996;13:557–568. doi: 10.1089/neu.1996.13.557. [DOI] [PubMed] [Google Scholar]
- Palazuelos J, Ortega Z, Díaz-Alonso J, Guzmán M, Galve-Roperh I. CB2 cannabinoid receptors promote neural progenitor cell proliferation via mTORC1 signaling. J Biol Chem. 2012;287:1198–1209. doi: 10.1074/jbc.M111.291294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan L, Ourednik V, Ourednik J. Neural stem/progenitor cells initiate the formation of cellular networks that provide neuroprotection by growth factor-modulated antioxidant expression. Stem Cells. 2008;26:254–265. doi: 10.1634/stemcells.2007-0221. [DOI] [PubMed] [Google Scholar]
- Su H, Zhang W, Guo J, Guo A, Yuan Q, Wu W. Neural progenitor cells enhance the survival and axonal regeneration of injured motoneurons after transplantation into the avulsed ventral horn of adult rats. J Neurotrauma. 2009;26:67–80. doi: 10.1089/neu.2008.0656. [DOI] [PubMed] [Google Scholar]
- Ben-Hur T. Immunomodulation by neural stem cells. J Neurol Sci. 2008;265:102–104. doi: 10.1016/j.jns.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Leker RR, Lasri V, Chernoguz D. Growth factors improve neurogenesis and outcome after focal cerebral ischemia. J Neural Transm. 2009;116:1397–1402. doi: 10.1007/s00702-009-0329-3. [DOI] [PubMed] [Google Scholar]
- Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- Oertel M, Boscardin WJ, Obrist WD, Glenn TC, McArthur DL, Gravori T, et al. Posttraumatic vasospasm: the epidemiology, severity, and time course of an underestimated phenomenon: a prospective study performed in 229 patients. J Neurosurg. 2005;103:812–824. doi: 10.3171/jns.2005.103.5.0812. [DOI] [PubMed] [Google Scholar]
- Golech SA, McCarron RM, Chen Y, Bembry J, Lenz F, Mechoulam R, et al. Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Brain Res Mol Brain Res. 2004;132:87–92. doi: 10.1016/j.molbrainres.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Chen Y, McCarron RM, Ohara Y, Bembry J, Azzam N, Lenz FA, et al. Human brain capillary endothelium: 2-arachidonoglycerol (endocannabinoid) interacts with endothelin-1. Circ Res. 2000;87:323–327. doi: 10.1161/01.res.87.4.323. [DOI] [PubMed] [Google Scholar]