Abstract
Evidence indicates a critical role for cerebrovascular dysfunction in Alzheimer's disease (AD) pathophysiology. We have shown that fibrin(ogen), the principal blood-clotting protein, is deposited in the AD neurovasculature and interacts with beta-amyloid (Aβ), resulting in increased formation of blood clots. As apolipoprotein E (ApoE), a lipid-transporting protein with three human isoforms (E2, E3, and E4), also binds to Aβ, we hypothesized that ApoE and fibrin(ogen) may have a combined effect on the vascular pathophysiology in AD. We assessed whether APOE genotype differentially influences vascular fibrin(ogen) deposition in postmortem brain tissue using immunohistochemistry. An increased deposition of fibrin(ogen) was observed in AD cases compared with non-demented controls, and there was a strong correlation between cerebral amyloid angiopathy (CAA) severity and fibrin(ogen) deposition. Moreover, brains from AD cases homozygous for APOE ɛ4 showed increased deposition of fibrin(ogen), specifically in CAA- and oligomeric Aβ-positive vessels compared with AD APOE ɛ2 and ɛ3 allele carriers, an effect that was not directly linked to CAA severity and cerebrovascular atherosclerosis. These data further support a role for fibrin(ogen) in AD pathophysiology and link the APOE ɛ4/ɛ4 genotype with increased thrombosis and/or impaired fibrinolysis in the human AD brain.
Keywords: Alzheimer's disease, APOE, beta-amyloid, cerebral amyloid angiopathy, fibrin(ogen), neurovasculature
Introduction
There is considerable evidence that vascular diseases, such as ischemic stroke and atherosclerosis, have an important role in dementia and late-onset Alzheimer's disease (AD).1, 2, 3, 4, 5 Moreover, cerebrovascular dysfunction, including disruption of microvascular integrity and reduced cerebral blood flow, is often observed in AD patients.6, 7 One proposed mechanism underlying these changes is the accumulation of the beta-amyloid (Aβ) peptide in the AD vasculature, a condition known as cerebral amyloid angiopathy (CAA).8 Cerebral amyloid angiopathy can severely disrupt the integrity of blood vessels,9 thus predisposing the brain to hemodynamic disturbances and thrombosis.10 Cerebral amyloid angiopathy has also been strongly associated with cognitive impairment in humans.11
Another molecule involved in the vascular changes associated with AD pathophysiology is fibrinogen,12 the precursor of fibrin and the principal protein component of blood clots. Elevated levels of plasma fibrinogen have been linked to cardiovascular disease,13 as well as AD14 and dementia.15 Fibrinogen and fibrin (designated as fibrin(ogen)) accumulate in the AD neurovasculature12, 16 and physically interact with Aβ,17 which affects fibrin clot formation and degradation. Fibrin clots formed in the presence of Aβ show an abnormal structure and are more resistant to degradation by fibrinolytic enzymes both in vitro18 and in vivo.12 Moreover, depletion of fibrinogen lessens CAA pathology and reduces cognitive impairment in mouse models of AD.12 Together, these data support a role for vascular deposition of fibrin(ogen) in AD pathophysiology.
Apolipoprotein E (APOE) genotype is a major risk factor for both late-onset AD and cardiovascular disease.4, 19 The APOE gene encodes the lipid transporting protein ApoE, which is mostly expressed by the liver and by glial cells in the brain.20 The three common isoforms of ApoE in humans, ApoE2, ApoE3, and ApoE4, differ by only one or two amino acids, but these differences alter their structural and functional properties21 and thus their role in pathologic conditions. In AD, the APOE ɛ4 allele is associated with increased progression and severity of the disease,19 while the APOE ɛ2 allele may be protective.22 The mechanisms behind the effect of ApoE in AD pathophysiology remain to be fully elucidated, but a substantial body of evidence has implied an important role for ApoE in Aβ metabolism. ApoE can physically interact with Aβ in vitro23 and in vivo.24 This interaction is isoform specific,23 which may explain the different effects of the three isoforms on Aβ clearance and deposition in the brain parenchyma and neurovasculature.25, 26, 27 Although the APOE ɛ4 allele is associated with increased arteriolar and capillary CAA,25, 26, 27 little is known about how the different APOE genotypes affect other factors involved in vascular pathophysiology in AD.
In the present study, we assessed whether APOE genotype differentially affects vascular fibrin(ogen) deposition in the brains of AD patients and found that APOE ɛ4/ɛ4 is associated with increased deposition of fibrin(ogen) in amyloid-laden vessels. These results support a link between the APOE ɛ4/ɛ4 genotype and increased thrombosis and/or impaired fibrinolysis in the AD brain.
Materials and methods
Human Brain Tissue
Postmortem brain tissue was obtained from 58 cases at the Joseph and Kathleen Price Bryan Alzheimer Disease Research Center, Duke University, North Carolina, as described previously.28 The Duke University Medical Center Institutional Review Board approved the procedures for enrollment. After death, consent for autopsy was obtained from the next of kin and the autopsy was performed according to institutional guidelines. Cases were defined as non-demented (ND) (n=22) or AD (n=36) according to the Consortium to Establish a Registry for Alzheimer's Disease (CERAD)29 and by the National Institute on Aging and Reagan Institute (NIA-R)30 criteria. All cases were scored for the level of atherosclerosis in the Circle of Willis arteries by macroscopic inspection. Overall CAA severity was assessed in 15 different brain regions (e.g., mid frontal lobe, parietal lobe, superior lobe, middle temporal lobe, hippocampal formation, inferior temporal lobe, amygdala, entorhinal cortex, basal ganglia (insula, putamen, caudate, and basal forebrain), midbrain, medulla, cerebellum (medial and lateral), occipital lobe (Brodmann areas 17 and 18), cingulate gyrus, and corpus callosum), and scored as described previously.31 Due to the high incidence of AD in cases with the APOE ɛ4/ɛ4 genotype, there were no ND controls with this genotype. The mean postmortem delay was 8 hours (range of 1 to 23 hours), and the mean age of the cases was 76 years (range of 53 to 90 years). Diagnosis, APOE genotype, demographic information, Braak stage, overall CAA severity, and severity of atherosclerosis in the Circle of Willis arteries for each case are shown in Table 1. Human brain samples were handled in accordance with The Rockefeller University's Institutional Review Board.
Table 1. Diagnosis, APOE genotype, demographic information, Braak stage, overall CAA, and severity of atherosclerosis in the Circle of Willis arteries for each case included in the study.
| Case | Diagnosis | APOE genotype | Sex | PMD | Age | Braak stage | Overall CAA | Atherosclerosis |
|---|---|---|---|---|---|---|---|---|
| 1 | AD | ɛ2/ɛ3 | M | 3 | 81 | IV | Moderate | No |
| 2 | AD | ɛ2/ɛ3 | F | 16 | 90 | III | Moderate | Moderate |
| 3 | AD | ɛ2/ɛ3 | M | 6 | 60 | V | Mild | No |
| 4 | AD | ɛ2/ɛ3 | M | 4 | 69 | V | Mild | No |
| 5 | AD | ɛ2/ɛ3 | F | 4 | 81 | V | Severe | Severe |
| 6 | AD | ɛ2/ɛ3 | M | 8 | 83 | V | Mild | No |
| 7 | AD | ɛ2/ɛ3 | F | 10 | 87 | III | Mild | Mild |
| 8 | AD | ɛ2/ɛ3 | F | 16 | 90 | III | Moderate | Mild |
| 9 | AD | ɛ2/ɛ3 | F | 3 | 89 | VI | Mild | Moderate |
| 10 | AD | ɛ2/ɛ3 | F | 4 | 59 | VI | Mild | No |
| 11 | AD | ɛ3/ɛ3 | M | 3 | 73 | III | Mild | No |
| 12 | AD | ɛ3/ɛ3 | F | 1 | 77 | III | Moderate | Severe |
| 13 | AD | ɛ3/ɛ3 | F | — | 69 | III | Moderate | No |
| 14 | AD | ɛ3/ɛ3 | M | 7 | 68 | VI | Mild | Mild |
| 15 | AD | ɛ3/ɛ3 | M | — | 71 | III | Mild | Mild |
| 16 | AD | ɛ3/ɛ3 | F | 17 | 87 | VI | Severe | Moderate |
| 17 | AD | ɛ3/ɛ3 | M | 13 | 73 | VI | Moderate | No |
| 18 | AD | ɛ3/ɛ3 | F | 3 | 81 | V | Mild | Mild |
| 19 | AD | ɛ3/ɛ3 | M | 12 | 83 | V | Moderate | Moderate |
| 20 | AD | ɛ3/ɛ3 | F | 12 | 86 | III | Mild | Mild |
| 21 | AD | ɛ3/ɛ3 | M | 21 | 53 | VI | Mild | No |
| 22 | AD | ɛ3/ɛ3 | F | 5 | 87 | V | Moderate | No |
| 23 | AD | ɛ4/ɛ4 | M | 4 | 76 | V | Moderate | Moderate |
| 24 | AD | ɛ4/ɛ4 | F | 8 | 79 | V | Severe | Moderate |
| 25 | AD | ɛ4/ɛ4 | F | 4 | 79 | V | Mild | Severe |
| 26 | AD | ɛ4/ɛ4 | F | 3 | 80 | V | Moderate | Moderate |
| 27 | AD | ɛ4/ɛ4 | M | 7 | 80 | V | Moderate | No |
| 28 | AD | ɛ4/ɛ4 | F | 10 | 55 | VI | Severe | No |
| 29 | AD | ɛ4/ɛ4 | F | 18 | 80 | IV | Severe | Mild |
| 30 | AD | ɛ4/ɛ4 | F | 15 | 81 | IV | Severe | Mild |
| 31 | AD | ɛ4/ɛ4 | F | 14 | 81 | V | Severe | Moderate |
| 32 | AD | ɛ4/ɛ4 | M | — | 70 | III | Mild | No |
| 33 | AD | ɛ4/ɛ4 | F | 6 | 76 | III | Mild | Mild |
| 34 | AD | ɛ4/ɛ4 | F | 4 | 77 | III | Severe | Mild |
| 35 | AD | ɛ4/ɛ4 | F | 23 | 70 | VI | Moderate | Mild |
| 36 | AD | ɛ4/ɛ4 | F | 21 | 87 | VI | Severe | Severe |
| 37 | ND | ɛ2/ɛ3 | F | 10 | 82 | I | No | No |
| 38 | ND | ɛ2/ɛ3 | F | 4 | 90 | II | No | No |
| 39 | ND | ɛ2/ɛ3 | F | 1 | 67 | II | No | No |
| 40 | ND | ɛ2/ɛ3 | F | 2 | 70 | I | No | No |
| 41 | ND | ɛ2/ɛ3 | F | 5 | 90 | III | No | Mild |
| 42 | ND | ɛ2/ɛ3 | F | 5 | 90 | II | No | No |
| 43 | ND | ɛ2/ɛ3 | F | 20 | 88 | I | No | Mild |
| 44 | ND | ɛ2/ɛ3 | M | 17 | 88 | I | No | No |
| 45 | ND | ɛ2/ɛ3 | F | 6 | 90 | I | No | No |
| 46 | ND | ɛ2/ɛ3 | F | 3 | 67 | I | No | No |
| 47 | ND | ɛ3/ɛ3 | F | 2 | 73 | I | No | No |
| 48 | ND | ɛ3/ɛ3 | F | 6 | 78 | I | No | No |
| 49 | ND | ɛ3/ɛ3 | M | 6 | 71 | I | No | No |
| 50 | ND | ɛ3/ɛ3 | F | 8 | 67 | I | No | No |
| 51 | ND | ɛ3/ɛ3 | M | 1 | 69 | I | No | Moderate |
| 52 | ND | ɛ3/ɛ3 | M | 4 | 80 | I | No | No |
| 53 | ND | ɛ3/ɛ3 | M | 3 | 82 | I | No | Mild |
| 54 | ND | ɛ3/ɛ3 | M | 2 | 83 | I | No | No |
| 55 | ND | ɛ3/ɛ3 | M | 16 | 86 | I | No | No |
| 56 | ND | ɛ3/ɛ3 | M | 18 | 87 | I | No | Moderate |
| 57 | ND | ɛ3/ɛ3 | M | 11 | 56 | I | No | No |
| 58 | ND | ɛ3/ɛ3 | M | 19 | 87 | I | No | Mild |
AD, Alzheimer's disease; CAA, cerebral amyloid angiopathy; F, female; M, male; ND, non-demented; PMD, postmortem delay.
Tissue Preparation
Brain tissue was dissected from the frontal cortex at autopsy. Tissue was immediately fixed by immersion in 10% phosphate-buffered formalin. Tissue was then washed in distilled water, dehydrated through successive alcohol washes, cleared in butyl acetate, embedded in paraffin, and blocked out in a suitable mold. Tissue blocks were cut into 7-μm thick sections and mounted.
Immunohistochemistry
Slides were deparaffinzed and treated with proteinase K (Dako, Gostrup, Denmark) for antigen retrieval, washed in 0.05 mol/L Tris, and immersed in 3% H2O2 in methanol for 30 minutes to quench endogenous peroxidase activity. For immunohistochemical staining of ApoE, slides were pretreated with 70% formic acid for 30 minutes. Before incubation with primary antibodies for 1 hour, slides were blocked with 0.1 mol/L Tris buffer containing 2% donkey/horse serum (DHS, 1:1). The following primary antibodies were used: rabbit anti-human fibrin(ogen) (Dako), mouse anti-oligomeric Aβ (NAB61, kindly provided by Dr Virginia MY Lee, University of Pennsylvania),32 and mouse anti-CD68 (Abcam, Cambridge, MA, USA). After washing in 0.1 mol/L Tris, slides were blocked with Tris/2% DHS and incubated with secondary antibodies: biotinylated anti-rabbit (Vector Laboratories, Burlingame, CA, USA), biotinylated anti-mouse (Vector Laboratories), or donkey anti-mouse Alexa 555 antibody (Life Technologies, Carlsbad, CA, USA) antibody for 1 hour. Subsequently, slides were washed in 0.1 mol/L Tris and developed with ImmPACT DAB (Vector Laboratories) according to the manufacturer's instructions. After washing in 0.1 mol/L Tris buffer, slides were co-stained with 1% Thioflavin S (Sigma-Aldrich, St Louis, MO, USA) in 70% ethanol, dehydrated in an ascending series of ethanol, and mounted using VectaMount medium (Vector Laboratories). To test for specificity of the immunohistochemical reactions, control sections were incubated with normal mouse or rabbit IgG serum instead of primary antibodies.
Microscopy and Quantitative Analyses
Thirty non-overlapping image fields per tissue slide sample were acquired at 20 × magnification under a constant predefined light setting using an inverted Zeiss Axiovert 200 microscope (Zeiss, Thornwood, NY, USA). Cortical vessels ⩾20 μm in diameter were included in the analyses, whereas leptomeningeal vessels were excluded. To quantify the number of cortical vessels positive for CAA, oligomeric Aβ, or fibrin(ogen), a ratio of immunopositive vessels versus the total number of vessels analyzed was determined. Vessels regarded as fibrin(ogen)-positive showed intense immunoreactivity against fibrin(ogen) in the lumen, along the vessel wall, and/or in the tunica media. Vessels with intense Thioflavin S staining or immunoreactivity against oligomeric Aβ in the tunica media and in the basement membrane were considered CAA- or oligomeric Aβ-positive, respectively. NIH ImageJ software (NIH, Bethesda, MD, USA) was used to analyze the intraluminal area that stained positive for fibrin(ogen) and the vessel wall area that stained positive for CD68. At least two tissue slides per subject were examined, and all stainings were performed on two separate occasions.
Statistical Analyses
Values are presented as mean±s.e.m., and P⩽0.05 is considered statistically significant. Differences between groups were evaluated by unpaired Student's t-test or one-way analysis of variance (ANOVA). When one-way ANOVA indicated a significant overall effect, post-hoc analysis was performed by Tukey's test. Correlation analyses were performed by simple and multiple linear regression. The SPSS software for Macintosh (IBM, Armonk, NY, USA) was used for all statistical calculations.
Results
Increased Vascular Fibrin(ogen) Deposition in Alzheimer's Disease Brains
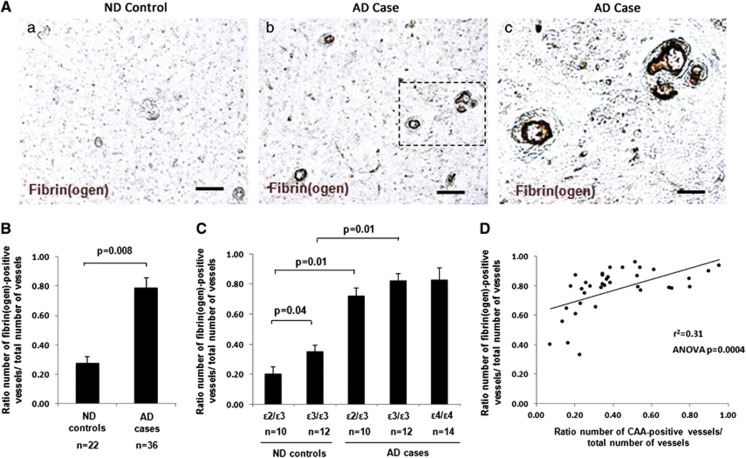
In all AD cases, regardless of APOE genotype or disease severity, we confirmed the presence of fibrin(ogen) deposition in the lumen, along the vessel wall, and in the tunica media of cortical vessels ⩾20 μm (Figure 1A). Areas of diffuse parenchymal fibrin(ogen) immunoreactivity were also observed in association with larger vessels in AD brains. In line with previous findings,12, 16 AD brains presented with significantly more fibrin(ogen)-positive vessels compared with ND brains (Figures 1A and 1B).
Figure 1.
Increased cerebrovascular deposition of fibrin(ogen) in Alzheimer's disease (AD) brains. Immunohistochemical staining of fibrin(ogen) was performed on cortical brain sections, and a ratio of the number of fibrin(ogen)-positive vessels versus the total number of vessels quantified was determined. (A) Representative images of fibrin(ogen) staining in non-demented (ND) (a) and AD (b) brains. Scale bar, 100 μm. (c) Higher magnification of fibrin(ogen)-positive vessels marked with dashed line in (b). Scale bar, 20 μm. (B) AD cases presented with significantly more fibrin(ogen)-positive vessels compared with ND controls (P=0.008). (C) In ND brains, apolipoprotein E (APOE) ɛ3/ɛ3 cases presented with significantly more fibrin(ogen)-positive vessels compared with APOE ɛ2/ɛ3 cases (P=0.04). In AD brains, no significant difference was observed in the number of fibrin(ogen)-positive vessels between the different APOE genotypes. Differences between groups were evaluated by unpaired Student's t-test (B) or one-way analysis of variance (ANOVA) and Tukey's post-hoc test (C). Values are presented as mean±s.e.m. (D) Simple linear regression analysis revealed a significant correlation between the number of fibrin(ogen)-positive vessels and the number of cerebral amyloid angiopathy (CAA)-positive vessels in the frontal cortex of AD cases (r2=0.31, ANOVA P=0.0004).
To determine if APOE genotype influences vascular fibrin(ogen) deposition, we compared the total number of fibrin(ogen)-positive vessels in the frontal cortex between the different APOE genotype groups. In ND brains, significantly more fibrin(ogen)-positive vessels were observed in the APOE ɛ3/ɛ3 group compared with the APOE ɛ2/ɛ3 group (Figure 1C). However, there was no significant difference in the number of fibrin(ogen)-positive vessels between AD cases of different APOE genotype (Figure 1C). Therefore, APOE genotype does not appear to influence the total number of fibrin(ogen)-positive vessels in the AD cerebrovasculature, though AD brains overall show significantly more fibrin(ogen)-positive vessels than ND brains. No significant association was found between the number of fibrin(ogen)-positive vessels and Braak stage or postmortem delay (data not shown).
Significant Association Between Fibrin(ogen) Deposition and Severity of Cerebral Amyloid Angiopathy in Alzheimer's Disease Brains
Microvascular abnormalities associated with AD, such as CAA,8 may influence vascular fibrin(ogen) deposition. Severity of CAA in AD brains was determined using two independent analyses: (1) quantification of the number of CAA-positive vessels in the frontal cortex, and (2) neuropathological scoring of overall CAA in 15 different brain regions performed at the Joseph and Kathleen Price Bryan Brain Bank. AD cases with the ɛ4/ɛ4 genotype exhibited significantly more CAA-positive vessels in the frontal cortex (Supplementary Figure 1) and increased severity of overall CAA (Table 1; Student's t-test, P=0.04, ɛ2/ɛ3 versus ɛ4/ɛ4, and P=0.01, ɛ3/ɛ3 versus ɛ4/ɛ4). This result is consistent with previous reports,25, 26 and demonstrates that the APOE ɛ4/ɛ4 genotype is associated with elevated CAA severity in the AD brain.
To further examine the relationship between vascular fibrin(ogen) deposition, severity of CAA, and APOE genotype, regression analysis was performed. A significant correlation was observed between the number of fibrin(ogen)-positive vessels and CAA severity in the frontal cortex (Figure 1D; r2=0.31, ANOVA P=0.0004) and overall CAA severity (data not shown; r2=0.20, ANOVA P=0.006). However, multiple regression analysis did not reveal any correlation between number of fibrin(ogen)-positive vessels, CAA severity, and APOE genotype (data not shown).
Alzheimer's Disease Cases with the APOE ɛ4/ɛ4 Genotype Present with Increased Fibrin(ogen) Deposition Specifically in Cerebral Amyloid Angiopathy- and Oligomeric Aβ-Positive Vessels
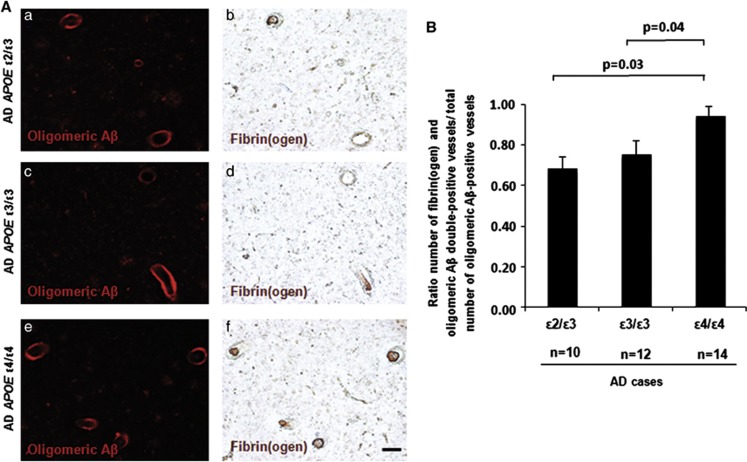
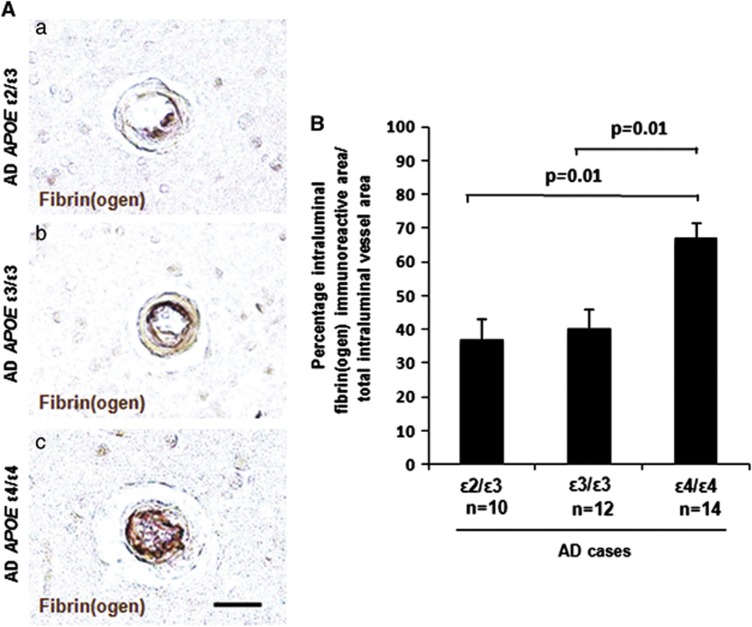
ApoE has been shown to bind to all forms of Aβ, including oligomers, in an isoform-dependent manner.23 Therefore, we investigated whether there is a genotype-specific effect of APOE on vascular deposition of fibrin(ogen) in CAA- and oligomeric Aβ-positive vessels in the AD brain. Significantly more vessels stained positive for both fibrin(ogen) and CAA (Figure 2) and fibrin(ogen) and oligomeric Aβ (Figure 3). No significant association was found between the number of fibrin(ogen) and CAA/oligomeric Aβ double-positive vessels and Braak stage or postmortem delay (data not shown). To investigate whether the degree of vascular fibrin(ogen) deposition is influenced by APOE genotype in the AD brain, we determined the percentage of intraluminal fibrin(ogen) deposition in CAA-positive vessels (Figure 4A). A significantly larger intraluminal area showed immunoreactivity against fibrin(ogen) in AD cases with the APOE ɛ4/ɛ4 genotype compared with the APOE ɛ2/ɛ3 or ɛ3/ɛ3 genotype (Figure 4B).
Figure 2.
Alzheimer's disease (AD) apolipoprotein E (APOE) ɛ4/ɛ4 cases present with significantly more fibrin(ogen) and cerebral amyloid angiopathy (CAA) double-positive vessels. Immunostaining of fibrin(ogen) and CAA was performed on cortical sections from AD brains. (A) Representative images of CAA (Thioflavin S staining, ThioS) (a, c, e) and fibrin(ogen) (b, d, f) in AD APOE ɛ2/ɛ3 (a, b), AD APOE ɛ3/ɛ3 (c, d), and AD APOE ɛ4/ɛ4 (e, f). Scale bar, 100 μm. (B) Significantly more fibrin(ogen) and CAA double-positive vessels were observed in AD APOE ɛ4/ɛ4 cases compared with AD APOE ɛ3/ɛ3 (P=0.02) or ɛ2/ɛ3 (P=0.03) cases. Differences between groups were evaluated by one-way analysis of variance and Tukey's post hoc test. Values are presented as mean±s.e.m.
Figure 3.
Alzheimer's disease (AD) apolipoprotein E (APOE) ɛ4/ɛ4 cases present with significantly more fibrin(ogen) and oligomeric Aβ double-positive vessels. Immunostaining of fibrin(ogen) and oligomeric Aβ was performed on cortical sections from AD brains. (A) Representative images of oligomeric Aβ (a, c, e) and fibrin(ogen) (b, d, f) in AD APOE ɛ2/ɛ3 (a, b), AD APOE ɛ3/ɛ3 (c, d), and AD APOE ɛ4/ɛ4 (e, f) cases are shown. Scale bar, 100 μm. (B) Significantly more fibrin(ogen) and oligomeric Aβ double-positive vessels were observed in AD APOE ɛ4/ɛ4 cases compared with AD APOE ɛ3/ɛ3 (P=0.04) or ɛ2/ɛ3 (P=0.03) cases. Differences between groups were evaluated by one-way analysis of variance and Tukey's post-hoc test. Values are presented as mean±s.e.m.
Figure 4.
Increased intraluminal deposition of fibrin(ogen) in cerebral amyloid angiopathy (CAA)-positive vessels in Alzheimer's disease (AD) apolipoprotein E (APOE) ɛ4/ɛ4 cases. Immunostaining of fibrin(ogen) and CAA was performed on cortical sections from AD brains, and the percentage of intraluminal vessel area staining positive for fibrin(ogen) relative to the total intraluminal vessel area was quantified. (A) Representative images of intraluminal fibrin(ogen) deposition in AD APOE ɛ2/ɛ3 (a), AD APOE ɛ3/ɛ3 (b), and AD APOE ɛ4/ɛ4 (c) cases. Scale bar, 20 μm. (B) A significantly higher percentage of the total intraluminal area was immunoreactive for fibrin(ogen) in AD APOE ɛ4/ɛ4 cases compared with AD APOE ɛ3/ɛ3 (P=0.01) or ɛ2/ɛ3 (P=0.01) cases. Differences between groups were evaluated by one-way analysis of variance and Tukey's post-hoc test. Values are presented as mean±s.e.m.
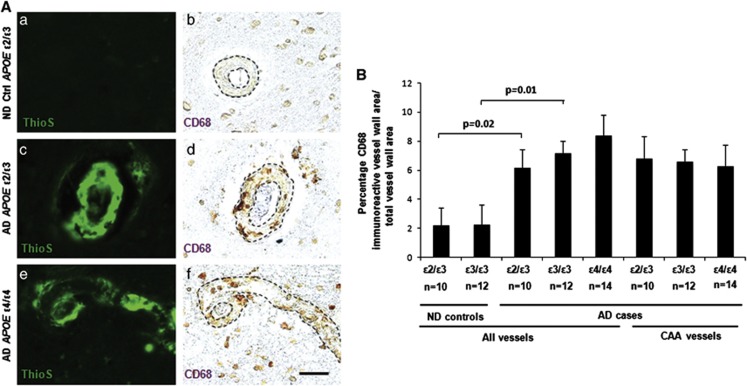
Increased Cerebrovascular Atherosclerosis in Alzheimer's Disease Brains
Atherosclerosis is associated with an increased risk of AD.2, 5 Since atherosclerotic changes may influence vascular fibrin(ogen) deposition, the severity of cerebrovascular atherosclerosis was determined using two independent analyses: (1) quantification of the degree of atherosclerosis in cortical vessels using a marker for perivascular macrophage infiltration, e.g., CD68,33 and (2) neuropathological scoring of atherosclerosis in the Circle of Willis arteries performed at the Joseph and Kathleen Price Bryan Brain Bank. A significant association was found between AD and severity of atherosclerosis in cortical vessels (Figure 5) and in the Circle of Willis arteries (Table 1; Student's t-test, P=0.0005). However, no significant difference in severity of atherosclerosis in cortical vessels was observed between AD cases of different APOE genotype (Figure 5). To further examine a possible relationship between vascular fibrin(ogen) deposition, severity of atherosclerosis, and APOE genotype in AD cases, multiple regression analysis was performed. No significant correlation was observed between the number of fibrin(ogen)-positive vessels, severity of atherosclerosis in cortical vessels, and APOE genotype (data not shown).
Figure 5.
Increased perivascular CD68 immunoreactivity in Alzheimer's disease (AD) brains. Cortical brain sections from non-demented (ND) and AD brains were analyzed for CD68, a marker for perivascular macrophage infiltration. (A) Representative images of cerebral amyloid angiopathy (CAA) (ThioS) (a, c, e) and CD68 (b, d, f) in ND apolipoprotein E (APOE) ɛ2/ɛ3 controls (a, b), AD APOE ɛ2/ɛ3 (c, d), and AD APOE ɛ4/ɛ4 (e, f) cases. Scale bar, 20 μm. (B) Significantly more of the vessel wall area was immunoreactive for CD68 in AD APOE ɛ3/ɛ3 and ɛ2/ɛ3 cases compared with ND cases in the respective control groups (P=0.01 and P=0.02, respectively). No significant difference in CD68 immunoreactivity was observed between AD cases of different APOE genotype when comparing all vessels or only CAA-positive vessels in the analyses. Differences between groups were evaluated by one-way analaysis of variance and Tukey's post hoc test. Values are presented as mean±s.e.m.
Discussion
The present study provides evidence that AD is associated with increased cerebrovascular deposition of fibrin(ogen) (Figures 1A and 1B), and that fibrin(ogen) deposition is linked with CAA severity (Figure 1D). Our data also show that the AD APOE ɛ4/ɛ4 genotype is associated with an increased accumulation of fibrin(ogen), specifically in CAA- and oligomeric Aβ-positive vessels (Figures 2, 3, 4), and that this genotype-specific effect is not directly linked to CAA severity or cerebrovascular atherosclerosis (Figure 5).
In line with the previous findings,12, 16 we observed an increased deposition of fibrin(ogen) in cortical vessels of AD brains compared with ND brains. Vascular fibrin(ogen) deposits were localized both in the vascular lumen, along the vessel wall, and in the tunica media, which is indicative of hemostatic disturbances and structural damages to the cerebrovasculature. Indeed, evidence implies that AD is associated with altered hemostasis34 and brain hypoperfusion,7 which may result from various microvascular abnormalities including CAA,8, 10 atherosclerosis,2, 5 thickening of the basement membrane,35 fibrinoid necrosis,36 and disruption of the neurovascular integrity.9 In the present study, a significant association was identified between CAA severity and fibrin(ogen) deposition, thus suggesting that the degree of CAA may influence the accumulation of fibrin(ogen) in the AD neurovasculature. On the contrary, the lack of association between severity of atherosclerosis and fibrin(ogen) deposition in AD cases indicates that atherosclerosis is not a major determinant of fibrin(ogen) accumulation. However, a more detailed analysis focusing on atherosclerotic changes would be useful in further defining a possible association between these variables. It also remains to be determined if accumulation of fibrin(ogen) in cerebral vessels is a result of increased CAA and/or if fibrin(ogen) itself can initiate and potentiate CAA pathology. We have previously demonstrated that fibrin(ogen), by its direct interaction with Aβ,17 influences cerebrovascular deposition of Aβ,12 and that fibrin clots formed in the presence of Aβ are more resistant to degradation by fibrinolytic enzymes.12, 18 Moreover, depletion of fibrin(ogen) reduces CAA pathology and cognitive impairment in transgenic mouse models of AD. Together, these data implicate fibrin(ogen) as a critical factor in the CAA pathology of AD.
On further investigation, we found an increased number of fibrin(ogen) and CAA/oligomeric Aβ double-positive vessels in the brains of AD APOE ɛ4/ɛ4 cases, thus demonstrating a genotype-specific effect of APOE on cerebrovascular accumulation of fibrin(ogen). Although the AD APOE ɛ4/ɛ4 genotype has been associated with increased severity of CAA,25, 26, 27 an association that was confirmed in the present study (Supplementary Figure 1), our data does not directly link the APOE ɛ4/ɛ4 genotype-dependent effect on fibrin(ogen) deposition to severity of CAA or atherosclerosis. However, there may be other morphologic differences between CAA-positive vessels in AD APOE genotype groups that could explain the genotype-specific effect. An alternative explanation for this observed association is that the binding of Aβ and fibrin(ogen)12, 17 and thus the fibrin clot degradation process12, 18 are differentially altered in the presence of ApoE isoforms. Evidence indicates that ApoE4 is less efficient than the other isoforms in binding to Aβ23 yet more potent in inducing fibrillization and aggregation of Aβ,37 all of which may have a potential impact on the Aβ-fibrin(ogen) interaction. This notion is supported by the present observation of an increased intraluminal accumulation of fibrin(ogen) in CAA-positive vessels in AD APOE ɛ4/ɛ4 cases compared with the other AD genotype groups.
It is notable that the APOE ɛ4 allele is associated with reduced blood flow in specific brain regions, including the prefrontal cortex, both in ND38 and AD cases.39 Due to the relative rarity of the APOE ɛ4/ɛ4 genotype among cognitively normal elderly individuals, a possible APOE genotype-specific effect on vascular fibrin(ogen) deposition in these individuals could not be tested in the present study. Additional studies regarding the relationship between APOE and cerebrovascular fibrin(ogen) deposition in vivo and in vitro would be of great interest.
In conclusion, our study further supports a role for fibrin(ogen) in AD pathogenesis and provides evidence for a relationship between the APOE ɛ4/ɛ4 genotype and increased deposition of fibrin(ogen) in the AD neurovasculature. Understanding the mechanism by which the different ApoE isoforms influence cerebrovascular health, thrombosis, and/or fibrinolysis will provide further insights into the pathophysiology of AD.
Acknowledgments
We gratefully thank the Joseph and Kathleen Bryan Alzheimer's Disease Research Center, Duke University Medical Center, funded by NIA Grant # P30 AG028377, for providing human brain tissue; Dr. Virginia M. –Y. Lee (University of Pennsylvania, Philadelphia) for providing the monoclonal antibody directed against oligomeric Aβ (NAB61); Dr. Joel C da Rosa (The Rockefeller University, NY) for advice regarding statistical analyses; and members of the Strickland Laboratory for helpful discussions regarding this study.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by the National Institutes of Health grant NS50537, Litwin Foundation, May and Samuel Rudin Family Foundation, Blanchette Hooker Rockefeller Fund, and the Mellam Family Foundation. KH was supported by the Swedish Research Council and the Sweden-America Foundation.
Supplementary Material
References
- Bangen KJ, Delano-Wood L, Wierenga CE, McCauley A, Jeste DV, Salmon DP, et al. Associations between stroke risk and cognition in normal aging and Alzheimer's disease with and without depression. Int J Geriatr Psychiatry. 2010;25:175–182. doi: 10.1002/gps.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Wilson JR, Sue LI, Newell A, Poston M, Cisneros R, et al. Circle of Willis atherosclerosis: association with Alzheimer's disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathol. 2007;113:13–21. doi: 10.1007/s00401-006-0136-y. [DOI] [PubMed] [Google Scholar]
- Brundel M, de Bresser J, van Dillen JJ, Kappelle LJ, Biessels GJ. Cerebral microinfarcts: a systematic review of neuropathological studies. J Cereb Blood Flow Metab. 2012;32:425–436. doi: 10.1038/jcbfm.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman A, Ott A, Breteler MM, Bots ML, Slooter AJ, van Harskamp F, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer's disease in the Rotterdam Study. Lancet. 1997;349:151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- Roher AE, Esh C, Kokjohn TA, Kalback W, Luehrs DC, Seward JD, et al. Circle of willis atherosclerosis is a risk factor for sporadic Alzheimer's disease. Arterioscler Thromb Vasc Biol. 2003;23:2055–2062. doi: 10.1161/01.ATV.0000095973.42032.44. [DOI] [PubMed] [Google Scholar]
- Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Staffen W, Bergmann J, Schonauer U, Zauner H, Kronbichler M, Golaszewski S, et al. Cerebral perfusion (HMPAO-SPECT) in patients with depression with cognitive impairment versus those with mild cognitive impairment and dementia of Alzheimer's type: a semiquantitative and automated evaluation. Eur J Nucl Med Mol Imaging. 2009;36:801–810. doi: 10.1007/s00259-008-1028-2. [DOI] [PubMed] [Google Scholar]
- Vinters HV. Cerebral amyloid angiopathy. A critical review. Stroke. 1987;18:311–324. doi: 10.1161/01.str.18.2.311. [DOI] [PubMed] [Google Scholar]
- Carrano A, Hoozemans JJ, van der Vies SM, van Horssen J, de Vries HE, Rozemuller AJ. Neuroinflammation and blood-brain barrier changes in capillary amyloid angiopathy. Neurodegener Dis. 2012;10:329–331. doi: 10.1159/000334916. [DOI] [PubMed] [Google Scholar]
- Thal DR, Capetillo-Zarate E, Larionov S, Staufenbiel M, Zurbruegg S, Beckmann N. Capillary cerebral amyloid angiopathy is associated with vessel occlusion and cerebral blood flow disturbances. Neurobiol Aging. 2009;30:1936–1948. doi: 10.1016/j.neurobiolaging.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Attems J, Quass M, Jellinger KA, Lintner F. Topographical distribution of cerebral amyloid angiopathy and its effect on cognitive decline are influenced by Alzheimer disease pathology. J Neurol Sci. 2007;257:49–55. doi: 10.1016/j.jns.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Cortes-Canteli M, Paul J, Norris EH, Bronstein R, Ahn HJ, Zamolodchikov D, et al. Fibrinogen and beta-amyloid association alters thrombosis and fibrinolysis: a possible contributing factor to Alzheimer's disease. Neuron. 2010;66:695–709. doi: 10.1016/j.neuron.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst E, Resch KL. Fibrinogen as a cardiovascular risk factor: a meta-analysis and review of the literature. Ann Intern Med. 1993;118:956–963. doi: 10.7326/0003-4819-118-12-199306150-00008. [DOI] [PubMed] [Google Scholar]
- van Oijen M, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Fibrinogen is associated with an increased risk of Alzheimer disease and vascular dementia. Stroke. 2005;36:2637–2641. doi: 10.1161/01.STR.0000189721.31432.26. [DOI] [PubMed] [Google Scholar]
- Xu G, Zhang H, Zhang S, Fan X, Liu X. Plasma fibrinogen is associated with cognitive decline and risk for dementia in patients with mild cognitive impairment. Int J Clin Pract. 2008;62:1070–1075. doi: 10.1111/j.1742-1241.2007.01268.x. [DOI] [PubMed] [Google Scholar]
- Ryu JK, McLarnon JG. A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer's disease brain. J Cell Mol Med. 2009;13:2911–2925. doi: 10.1111/j.1582-4934.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn HJ, Zamolodchikov D, Cortes-Canteli M, Norris EH, Glickman JF, Strickland S. Alzheimer's disease peptide beta-amyloid interacts with fibrinogen and induces its oligomerization. Proc Natl Acad Sci USA. 2010;107:21812–21817. doi: 10.1073/pnas.1010373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamolodchikov D, Strickland S. A β delays fibrin clot lysis by altering fibrin structure and attenuating plasminogen binding to fibrin. Blood. 2012;119:3342–3351. doi: 10.1182/blood-2011-11-389668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Boyles JK, Pitas RE, Wilson E, Mahley RW, Taylor JM. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest. 1985;76:1501–1513. doi: 10.1172/JCI112130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatters DM, Peters-Libeu CA, Weisgraber KH. Apolipoprotein E structure: insights into function. Trends Biochem Sci. 2006;31:445–454. doi: 10.1016/j.tibs.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr., et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- Manelli AM, Stine WB, Van Eldik LJ, LaDu MJ. ApoE and Abeta1-42 interactions: effects of isoform and conformation on structure and function. J Mol Neurosci. 2004;23:235–246. doi: 10.1385/JMN:23:3:235. [DOI] [PubMed] [Google Scholar]
- Navarro A, Del Valle E, Astudillo A, Gonzalez del Rey C, Tolivia J. Immunohistochemical study of distribution of apolipoproteins E and D in human cerebral beta amyloid deposits. Exp Neurol. 2003;184:697–704. doi: 10.1016/S0014-4886(03)00315-7. [DOI] [PubMed] [Google Scholar]
- Chalmers K, Wilcock GK, Love S. APOE epsilon 4 influences the pathological phenotype of Alzheimer's disease by favouring cerebrovascular over parenchymal accumulation of A beta protein. Neuropathol Appl Neurobiol. 2003;29:231–238. doi: 10.1046/j.1365-2990.2003.00457.x. [DOI] [PubMed] [Google Scholar]
- Trembath D, Ervin JF, Broom L, Szymanski M, Welsh-Bohmer K, Pieper C, et al. The distribution of cerebrovascular amyloid in Alzheimer's disease varies with ApoE genotype. Acta Neuropathol. 2007;113:23–31. doi: 10.1007/s00401-006-0162-9. [DOI] [PubMed] [Google Scholar]
- Thal DR, Papassotiropoulos A, Saido TC, Griffin WS, Mrak RE, Kolsch H, et al. Capillary cerebral amyloid angiopathy identifies a distinct APOE epsilon4-associated subtype of sporadic Alzheimer's disease. Acta Neuropathol. 2010;120:169–183. doi: 10.1007/s00401-010-0707-9. [DOI] [PubMed] [Google Scholar]
- Hulette CM, Welsh-Bohmer KA, Crain B, Szymanski MH, Sinclaire NO, Roses AD. Rapid brain autopsy. The Joseph and Kathleen Bryan Alzheimer's Disease Research Center experience. Arch Pathol Lab Med. 1997;121:615–618. [PubMed] [Google Scholar]
- Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer's disease: a commentary. Neurobiol Aging. 1997;18:S91–S94. doi: 10.1016/s0197-4580(97)00058-4. [DOI] [PubMed] [Google Scholar]
- Consensus recommendations for the postmortem diagnosis of Alzheimer's disease The national institute on aging, and reagan institute working group on diagnostic criteria for the neuropathological assessment of Alzheimer's disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- Attems J, Jellinger KA, Lintner F. Alzheimer's disease pathology influences severity and topographical distribution of cerebral amyloid angiopathy. Acta Neuropathol. 2005;110:222–231. doi: 10.1007/s00401-005-1064-y. [DOI] [PubMed] [Google Scholar]
- Lee EB, Leng LZ, Zhang B, Kwong L, Trojanowski JQ, Abel T, et al. Targeting amyloid-beta peptide (Abeta) oligomers by passive immunization with a conformation-selective monoclonal antibody improves learning and memory in Abeta precursor protein (APP) transgenic mice. J Biol Chem. 2006;281:4292–4299. doi: 10.1074/jbc.M511018200. [DOI] [PubMed] [Google Scholar]
- Uchikado H, Akiyama H, Kondo H, Ikeda K, Tsuchiya K, Kato M, et al. Activation of vascular endothelial cells and perivascular cells by systemic inflammation-an immunohistochemical study of postmortem human brain tissues. Acta Neuropathol. 2004;107:341–351. doi: 10.1007/s00401-003-0815-x. [DOI] [PubMed] [Google Scholar]
- Cortes-Canteli M, Zamolodchikov D, Ahn HJ, Strickland S, Norris EH. Fibrinogen and altered hemostasis in Alzheimer's disease. J Alzheimers Dis. 2012;32:599–608. doi: 10.3233/JAD-2012-120820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas E, De Jong GI, de Vos RA, Jansen Steur EN, Luiten PG. Pathological features of cerebral cortical capillaries are doubled in Alzheimer's disease and Parkinson's disease. Acta Neuropathol. 2000;100:395–402. doi: 10.1007/s004010000195. [DOI] [PubMed] [Google Scholar]
- Greenberg SM, Vonsattel JP. Diagnosis of cerebral amyloid angiopathy. Sensitivity and specificity of cortical biopsy. Stroke. 1997;28:1418–1422. doi: 10.1161/01.str.28.7.1418. [DOI] [PubMed] [Google Scholar]
- Castano EM, Prelli F, Wisniewski T, Golabek A, Kumar RA, Soto C, et al. Fibrillogenesis in Alzheimer's disease of amyloid beta peptides and apolipoprotein E. Biochem J. 1995;306 (Pt 2:599–604. doi: 10.1042/bj3060599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, et al. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- Hogh P, Knudsen GM, Kjaer KH, Jorgensen OS, Paulson OB, Waldemar G. Single photon emission computed tomography and apolipoprotein E in Alzheimer's disease: impact of the epsilon4 allele on regional cerebral blood flow. J Geriatr Psychiatry Neurol. 2001;14:42–51. doi: 10.1177/089198870101400110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.