Abstract
The brain is dependent on glucose as a primary energy substrate, but is capable of utilizing ketones such as β-hydroxybutyrate and acetoacetate, as occurs with fasting, starvation, or chronic feeding of a ketogenic diet. The relationship between changes in cerebral metabolic rates of glucose (CMRglc) and degree or duration of ketosis remains uncertain. To investigate if CMRglc decreases with chronic ketosis, 2-[18F]fluoro-2-deoxy-D-glucose in combination with positron emission tomography, was applied in anesthetized young adult rats fed 3 weeks of either standard or ketogenic diets. Cerebral metabolic rates of glucose (μmol/min per 100 g) was determined in the cerebral cortex and cerebellum using Gjedde–Patlak analysis. The average CMRglc significantly decreased in the cerebral cortex (23.0±4.9 versus 32.9±4.7) and cerebellum (29.3±8.6 versus 41.2±6.4) with increased plasma ketone bodies in the ketotic rats compared with standard diet group. The reduction of CMRglc in both brain regions correlates linearly by ∼9% for each 1 mmol/L increase of total plasma ketone bodies (0.3 to 6.3 mmol/L). Together with our meta-analysis, these data revealed that the degree and duration of ketosis has a major role in determining the corresponding change in CMRglc with ketosis.
Keywords: 2-deoxy-glucose, brain imaging, energy metabolism, glucose, positron emission tomography
Introduction
Researchers and clinicians have been interested in brain metabolism during starvation, fasting, or acute ketosis for many decades. Under physiologic blood glucose concentrations, the fractional contribution of ketone bodies to oxidative metabolism in adult brain has remained uncertain. During prolonged starvation, brain energy requirements have been traditionally accepted to be supplemented by ketone body oxidation.1, 2 The conviction was founded on the rationale that under glucose-sparing conditions, a large portion of oxidative energy must be derived from ketone bodies and thus resulting in reduced glucose consumption.1, 2, 3 Historically, there has been controversy among researchers whether there is a causal relationship between changes in CMRglc with degree and duration of ketosis. The inconsistencies across studies were revealed when the effects of short-term fasting (or acute ketosis) on changes in CMRglc were further explored.3, 4, 5, 6, 7
We deem that ketones are effective against pathology associated with altered glucose metabolism and inadequate regulation of salvation pathways. We hypothesize that ketone bodies are neuroprotective through the restoration in energy balance via suppression of glucose oxidation and stabilization of ATP supply. Ketone bodies, such as β-hydroxybutyrate (BHB) and acetoacetate (AcAc), are alternative energy substrates to glucose especially important during development and glucose-sparing conditions, such as with fasting, starvation, and diet-induced ketosis.1, 8, 9, 10, 11The relationship between energy supply and demand and the partitioning of substrate utilization between glucose and ketones in brain continues to be explored. The ketogenic diet (high-fat, very low-carbohydrate) to induce chronic ketosis has been successfully used in the clinical setting as a therapy for intractable seizures for nearly a century.9, 12, 13, 14, 15 However, the ‘mechanistic link' between the anticonvulsant effects and ketosis continues to be investigated and remains to be elucidated.16 Ketone bodies as neuroprotective agents appear to be related to the change in the regulation of the cell's stress responses,17 as well as changes in oxidative (glucose) metabolism.18, 19 Neuroprotection by ketosis is thought to be associated with improved mitochondrial function, decreased reactive oxygen species, apoptotic and inflammatory mediators, and protective pathways.9, 20
In the last few decades, the 2-deoxy-D-glucose or positron emission tomography (PET) approaches have been applied to ketotic studies, in both animal,18, 21, 22, 23 and humans.2, 5, 24, 25 Reports of altered CMRglc as a result of short-term fasting3, 4, 5, 6, 7or acute infusions of ketone bodies25, 26 had generated discrepancies. What remained to be clarified was (i) whether oxidation of ketone bodies can replace glucose proportionately during acute/mild ketosis under normoglycemia and (ii) the percent of glucose sparing with degree of ketosis. Some studies reported generalized, decreasing CMRglc with 3 to 5 days of fasting in humans2, 5, 24 while in other studies, there were no significant changes in CMRglc.3, 21, 27, 28
The goal of this study was to estimate CMRglc in chronic ketotic rats and to determine if ketosis induces a metabolic adaptation through changes in glucose phosphorylation rates. The effects of ketosis on CMRglc in intact brain during stabilized blood glucose conditions in diet-induced ketotic rats using PET and 2-[18F] fluoro-2-deoxy-D-glucose (18FDG) were determined. The rationale for using PET–FDG was based on the principle that the phosphorylation rate of 18FDG (a trapping tracer) can be used to estimate the phosphorylation rate of glucose. In support of our findings, a retrospective analysis of historical data (meta-analysis) to resolve the inconsistencies across studies was also performed.1, 2, 3, 5, 6, 7, 21, 22, 23, 24, 27, 28
Materials and methods
Animal Model and Diets
Young adult male Wistar rats were purchased from Charles River (Wilmington, MA, USA), 40 days old, and weighing ∼150 grams. All procedures were performed in strict accordance with the National Institutes of Health Guide for Care and were approved by Institutional Animal Care and Use Committee of Case Western Reserve University. Body weights were measured upon arrival and on the experimental day (Table 1). Littermates were housed three per cage in the Case Western Reserve University Animal Resource Center with 12:12 hours light/dark cycle. All rats were allowed to acclimate for 1 week before initiating dietary protocols. Standard rodent diet (STD) was fed to all rats during the acclimation period (Cincinnati Lab Supply, Cincinnati, OH, USA, Labdiet Prolab RMH3000 ANE) ad libitum. One week after their arrival, all rats were fasted overnight for 16 hours to deplete the liver glycogen stores and initiate ketosis. Rats were then randomly assigned to two diets, STD or ketogenic diet (ketogenic, KG; Research Diet, New Brunswick, NJ, USA, D12369b) and fed for 3 weeks ad libitum until FDG–PET experiments.29 The macro and micronutrient of the STD and KG diets is shown in Supplementary material 1. The original datasheets for the diets are included in Supplementary material 2 and 3.
Table 1. Physiologic parameters.
| STD diet (n=9) | KG diet (n=10) | |
|---|---|---|
| Age (days) | 72±6 | 77±7 |
| Weight (g) | 358±33 | 360±26 |
| Blood gas parametersa | ||
| pH | 7.35±0.04 | 7.32±0.03 |
| PaO2 (mm Hg) | 112±22 | 104±20 |
| PaCO2 (mm Hg) | 53±5 | 50±5 |
| Physiologic Parametersb | ||
| Breath rate (br/min) | 63±3 | 63±5 |
| Hematocrit (%) | 48±2 | 47±2 |
| Heart rate (beats/min) | 378±61 | 365±37 |
| Plasma metabolic parametersb | ||
| BHB (mM) | 0.36±0.10 | 3.30±0.85* |
| AcAc (mM) | 0.21±0.11 | 0.61±0.31* |
| BHB+AcAc (mM) | 0.51±0.25 | 3.92±1.04* |
| BHB/AcAc ratio | 1.74±0.58 | 4.78±1.19* |
| L-Lactate (mM) | 1.00±0.10 | 0.77±0.18* |
| D-Glucose (mM) | 11.51±1.08 | 10.71±1.87 |
AcAc, acetoacetate; BHB, β-hydroxybutyrate; CMRglc, cerebral metabolic rate for glucose; FDG, 2-[18F] fluoro-2-deoxy-D-glucose; KG, ketogenic; n, number of rats; STD, standard rodent diet; VOI, volume of interest. *P<0.05 compared with STD-diet group.
Physiologic parameters and concentrations of metabolites in plasma in rats fed standard or ketogenic diets. Values are the means±s.d. n, number of rats. Young adult rats were fed either standard (STD) or ketogenic diet (KG) for 3 weeks before measurements of CMRglc.
Measured at 45 minutes post 18FDG injection
Measured 60 minutes post 18FDG injection.
Anesthesia and Surgery
On the experimental day (post 3 weeks of diets) rats were morning fasted for 6 hours before PET imaging. Rats were then anesthetized with vaporized 2.5% isofluorane balanced with pure oxygen delivered through a nose cone during the surgical placement of arterial and venous catheters: right jugular catheter (MRE, 0.035 mm ID and 0.084 mm OD, Braintree Scientific, Braintree, MA, USA) was advanced towards the atrium for 18FDG injection and the tail artery (PE-50, 0.58 mm I.D., and 0.965 mm O.D. Stoelting Co. Wood Dale, IL, USA) was cannulated for blood sampling during the PET imaging period.30 Rats were then transported to the Inveon PET (Siemens, Knoxville, TN, USA) bed and maintained with a mixture of vaporized isofluorane, pure oxygen, and room air. Anesthesia level (1% to 2%), oxygen flow rate (0.05 to 0.2 liters per minute) and air flow rate (0.5 to 0.6 liters per minute) were adjusted to achieve a consistent physiologic status across animals. Absence of hind leg pinch reflex was monitored throughout the PET scan to ensure depth of anesthesia. Heart rate, respiratory rate (breaths/min), plethysmography, and oxygen saturation (%) were monitored (via hind leg sensor) and recorded throughout the experiment using a pulse oximeter system (MouseOx, Starr life sciences, Oakmont, PA, USA) (Table 1). To maintain breath rates (∼70 per minute) and normal blood gases throughout the 1.5-hour imaging process, isofluorane was adjusted, as well as the oxygen percentage and flow rates.
Physiological Parameters
Plasma glucose, lactate, and total ketone bodies (BHB +acetoacetate) concentrations were measured pre- and post imaging (t=0, 60 minutes) from a blood sample collected (0.1 mL) from the tail artery catheter. The whole blood samples were centrifuged and the plasma separated and immediately frozen in dry ice; the end-of-imaging hematocrits were also recorded. Plasma D-glucose and L-lactate were later measured by YSI 2700 Biochemistry Analyzer (YSI, Yellow Springs, OH, USA) and the plasma total ketone bodies were measured by gas chromatography-mass spectrometry, as previously described.31 Arterial blood gas parameters (pH, PaO2, and PaCO2) were measured at t=0, 45 minutes (ABL5 Radiometer, Copenhagen, Denmark); 45 minutes was considered the end point where CMRglc reached steady state.32, 33 Arterial blood glucose was measured at t=15, 30, and 45 minutes to verify the steady-state plasma glucose concentration during the experiment (Precision Xtra Meter, Abbott Diabetes Care Inc., Alameda, CA, USA). The breath and heart rates were also recorded throughout the imaging process and were used as indicators for physiologic status.
Image Acquisition and Blood Sampling
A dual-modal PET-CT device, Inveon (Siemens), was used to image the 18FDG activities in the brain. The rats' eyes were placed at the center of the field of view for the best spatial resolution. First, a 10-minute transmission scan was performed before the 18FDG tracer injection. The transmission scan generates tissue attenuation map for attenuation correction in the PET images. Then 10±2 Mbq/100 g of 18FDG was injected through the jugular line at time 0. Simultaneously, a 60-minute listmode PET emission scan was started along with the automatic arterial sampling using a customized blood acquisition module. The blood acquisition module device acquires the whole blood radioactivity in the first 2.5 minutes post injection, at a rate of 0.2 mL/minute, specified by a connected syringe pump (Harvard Pump 11 plus, Holliston, MA, USA). The pump was stopped at 2.5 minutes post injection. Manual sampling for arterial blood activity was performed at 3.5, 5, 7.5, 10, 15, 25, 40, 50, 60 minutes, using heparin-coated, microcapillary tubes (HT9H, Statspin, Westwood, MA, USA) with each tube's volume no more than 9 μL. On the experimental day, the total blood sample volumes were noted from each rat, which was less than 1.4 mL.
After the PET emission scan, the manually sampled bloods were centrifuged (RH12, Statspin). The volume inside the microhematocrit tubes were premeasured as 8.3 μL/37 mm, therefore by measuring the length of the whole blood portion, whole blood activity per volume (Cwb*), was obtained by converting the counts from a Gamma counter (LKB1282 Compugamma, LKB Instruments, Mt Waverley, Vic, Australia) and time correct to time 0. Hematocrit tubes were then broken and the plasma radioactivity (Cp*) was also counted and corrected for the decay. The hematocrits at 3.5 (when manual sampling begins) and 60 minutes were recorded.
The first 2.5 minutes of input function Cwb*, was converted to Cp* by a factor R. This follows
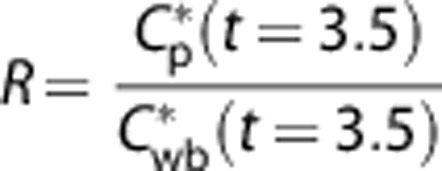 |
The manually sampled plasma radioactivity data were time corrected to the 18FDG injection time and the half-life (107 minutes). The blood acquisition module and manually sampled data were combined and saved in a text file for the Matlab (The MathWorks, Natick, MA, USA) program analysis. Factor R was not different between diet groups (1.7±0.10 versus 1.6±0.10; STD, KG, respectively).
Image Processing: Region and Volumes of Interest
The list mode emission data were binned to 34 frames: 6 × 10, 6 × 20, 4 × 30 seconds, 3 × 1, 2 × 2, 2 × 4, and 5 × 8 minutes. The reconstruction algorithm on the scanner was set to OSEM2D with a ramp filter supplied by the vendor of the scanner. The final images were saved as coronal, transversal, and sagittal images with 128 × 128 pixels, and the resolution was 0.78 mm in the sagittal, transversal sections and 0.79 mm in the coronal section. The value of each voxel in the reconstructed PET image sets is converted to radioactivity per volume.
The processed PET radioactivity image data were analyzed using Carimas 2 (Turku PET centre,Turku, Finland), to generate the region of interest (ROI) and volume of interest (VOI) data. Both the left and right eyes were identified as the landmarks. A rat brain atlas (Paxinos and Watson, Academic Press) was used to guide the selection of the region of interest and VOI. Starting from the rear of the eyecup, with slice thickness 0.125 mm, the left and right entire cortical hemispheres were encircled and two separate VOIs were generated. Similarly, the whole volumetric cerebellum was selected as one VOI. A separate PET CT image set for a rat of the same range (P60 to P80) of age was overlaid to verify the cortical and cerebellar regions (see Supplementary Material 4). The two hemispheres, VOI and the cerebellum VOI, were saved to text files and made importable to the Matlab program as the time activity curve format.
Parameter Estimation and Calculation of CMRglc
We developed a MatLab program to perform the parameter estimation and calculation of CMRglc. The plasma input function was interpolated to render a time resolution of 0.1 second. Then the 34-frame time activity curve was matched with the input function for each of the time points. The Gjedde–Patlak plots were generated and only the last six matching points, namely the time after 25 minutes data were used to generate the parameter Ki, which follows:
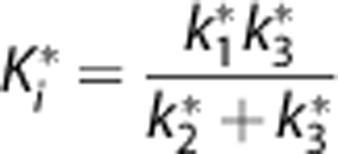 |
While k1* is the 18FDG transport rate constant (/minute) to the brain tissue, k2* is the 18FDG reverse transport rate constant from tissue to the plasma (/minute) and k3* is the 18FDG phosphorylation rate constant (/minute).
The lumped constant in both the KG-and the STD-diet rats were assumed to be 0.71. 34. The 60-minute plasma glucose level, Cp, was used to generate the final CMRglc, which follows:35, 36
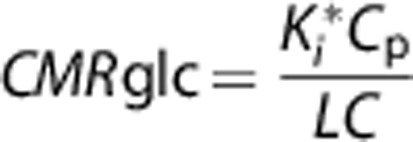 |
Results
Physiologic Parameters
There were no significant differences in body weights, blood gases, physiologic parameters, and plasma glucose concentrations between KG- and STD-diet groups after 3 weeks of feeding the diets (Table 1). As expected, plasma ketone (BHB, acetoacetate) concentrations were statistically higher and the plasma lactate concentrations were lower in the KG rats compared with the STD group.29 Ketosis ranged between 0.4 to 6.2 mmol/L as measured by total plasma ketone bodies (Figure 1); the STD group was mildly ketotic (0.3 to 0.9 mmol/L, plasma total ketone bodies) after a 6-hour fast before imaging. Lactate concentrations in the plasma were significantly lower in the KG-diet group (0.77±0.18 mmol/L), Table 1.
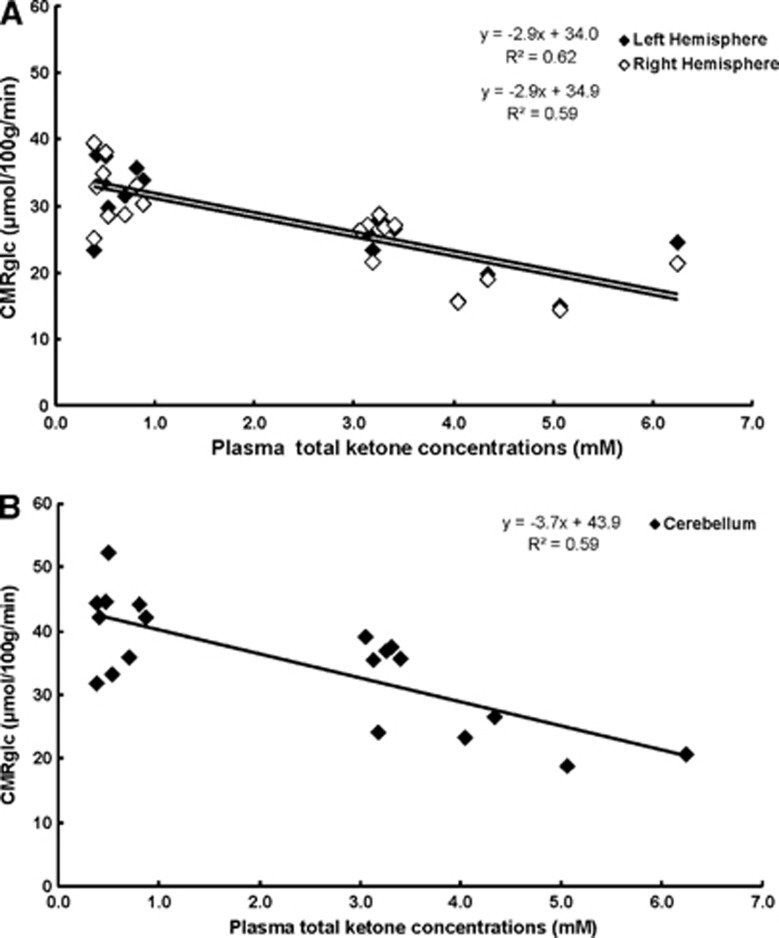
Figure 1.
Decreased cerebral metabolic rate for glucose (CMRglc) with increasing plasma ketone body concentrations in rats fed with ketogenic (KG) diet compared with standard diet (STD). Volumes of interest (VOIs) were defined as the left and right hemispheres (A; open circle, left and closed circle, right) and cerebellum (B). The CMRglc in each region was calculated with Gjedde–Patlak analysis and plotted as a function of the measured plasma total ketone body concentrations; the equation CMRglc=(slope × plasma total ketone concentrations+CMRglc at non-ketogenic state) corresponds to the linear correlation; ‘goodness of fit' was represented as the coefficient of determination, R2, which reflected ∼0.61 for each VOI. The STD diet group (n=9) total plasma ketone bodies were less than 0.87 mmol/L and the KG diet group (n=10) was greater than 3.0 mmol/L. These results demonstrate that CMRglc decreased ∼9% for each 1 mmol/L increase in total plasma ketone body concentration in ketotic rats induced by 3 weeks of KG diet.
Cerebral Glucose Metabolic Rates
The averages of the CMRglc (μmol/100 g per minute) measured in both the cerebral hemispheres and cerebellum are shown in Table 2. There were no significant differences in CMRglc between the left and right hemispheres. The PET analysis revealed that diet-induced ketosis resulted in a significant decrease in the average CMRglc in both the cerebral hemispheres and cerebellum compared with STD group. Cerebral metabolic rates of glucose was significantly lower in the left and right cerebral hemispheres compared with the cerebellum, in both dietary groups.
Table 2. CMRglc in the VOI.
| VOI | STD diet (n=9) | KG diet (n=10) |
|---|---|---|
| Left hemisphere | 33.5±4.9 | 23.2±4.8* |
| Right hemisphere | 32.3±4.7 | 22.8±5.2* |
| Hemispheres average | 32.9±4.7 | 23.0±4.9* |
| Cerebellum | 41.2±6.4** | 29.3±8.6*,** |
CMRglc, cerebral metabolic rate for glucose; FDG, n, number of rats; 2-[18F] fluoro-2-deoxy-D-glucose; KG, ketogenic; PET, positron emission tomography; STD, standard rodent diet; VOI, volume of interest. *P<0.05 compared to STD-diet group, **P<0.05 compared to hemispheres
CMRglc (μmol/100 g per minute)
Cerebral metabolic rate for glucose (CMRglc) by positron emission tomography and 2- [18F] fluoro-2-deoxy-D-glucose in rats fed standard or ketogenic diets. CMRglc (μmol/ 100 g minute), at t=60 minutes post 18FDG injection. Values are the means (s.d.); n, number of rats. *Significance P<0.05 relative to STD. **Significance P<0.05 relative to cortical hemisphere.
The CMRglc calculated with Gjedde–Patlak analysis was plotted as a function of the measured total plasma ketone body concentrations (BHB+acetoacetate; mmol/L) (Figure 1). These data showed that the cerebral (left and right hemispheres) and cerebellar CMRglc decreased with increasing ketosis. The calculated CMRglc in each region was represented by a linear decrease with increasing total plasma ketone concentrations. There were no significant differences between the left and right cerebral hemispheres, (CMRglcright= −2.9 × (BHB+AcAc)+34.9; R2=0.59); where as the cerebellar region was significantly higher (CMRglc= −3.7 × (BHB+AcAc)+43.9; R2=0.59) compared with cerebral cortex. These data highlight the proportional change in CMRglc with increasing ketosis; thus for every 1 mmol/L increase in total plasma ketone bodies, CMRglc decreases by ∼9%.
Meta-analysis of CMRglc in Ketotic Subjects
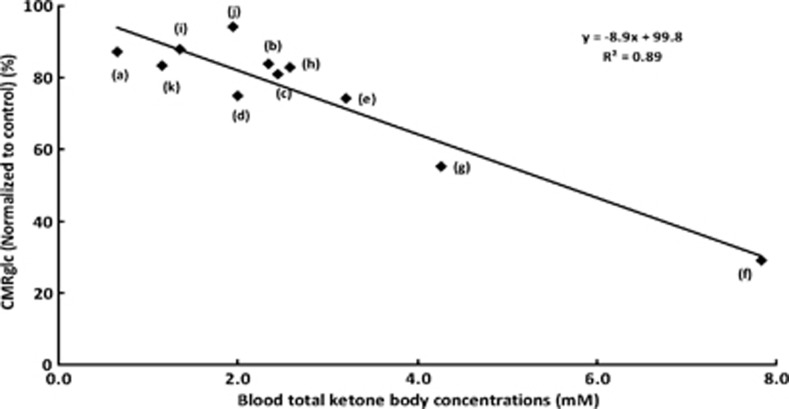
Meta-analysis of CMRglc reduction in ketotic subjects (human or rats) was shown in Figure 2. All data were collected from previously reported studies where CMRglc was measured and level of ketosis was reported. In each reported study, the CMRglc data from the ketotic subjects were normalized to the non-ketotic controls (%) and then graphed against the total blood ketone body concentrations (mmol/L). The normalization eliminated inconsistencies across studies due to anesthesia effects and species differences. The normalized glucose utilization rate decreased ∼9% for each 1 mmol/L increase of the total blood ketone bodies. A summary of these data collected from the various studies measuring CMRglc and blood ketone concentrations include (see Figure 2 legend for details): PET–FDG studies conducted in fasted humans showing a 27% decrease in CMRglc after 3.5 days of fasting,7 in humans that were fasted for 3 weeks, the authors reported a 46% decrease in CMRglc relative to the non-fasted baseline conditions.2 Other studies using different methodologies for assessing glucose utilization in ketotic rats also showed similar decreases. In one study where [6-14C]glucose and autoradiography were applied, glucose utilization decreased 12% in conscious 2-day fasted rats with mild ketosis.6
Figure 2.
Meta-analysis of cerebral metabolic rate for glucose (CMRglc) reduction in ketotic subjects (human or rats). All data were collected from previously reported studies where CMRglc was measured and level of ketosis was reported. Data were normalized (%) against control state (non-fasted, non-diabetic conditions) and graphed as a function of total blood ketone bodies level (mmol/L). The study, method, and reported outcome is noted for each point: (a) data from Al-Mudallal et al,21 ketosis by KG diet in rat, 2-DG method; no significant cortical change in CMRglc, (b) data from Corddry et al,22 3 days fasted rats, 2-DG method; frontal cortical change, not significant, (c) and (d) data from Dalquist et al,27 3 days fasted rats, A-V uptake method; no significant change, (e) data from Hasselbach et al,5 3.5 days fasted humans, PET–FDG imaging; significant reduction, (f) data from Owen et al,1 5 to 6 weeks fasted obese human subjects, A-V uptake method, CMRglc, was indirectly calculated by O2 consumption; significant change, (g) data from Redies et al,2 20 to 24 days fasted obese human subjects, PET–FDG and A-V uptake method; significant CMRglc reduction, (h) data from Ruderman et al,7 1–2 days fasted rats, A-V uptake method; trended significant, (i) Data from Mans et al,6 2 days fasted rats, compartmental modeling with non-trapping tracer (autoradiography); significant reduction, (j) data from Issad et al,28 2 days fasted rats, (autoradiography), no significant change, (k) data from Cherel et al,3 6 days fasted rats, modified 2-DG method, no significant change. The meta-analysis plot shows a linear relationship between CMRglc and level of ketosis in human or rat subjects. For each 1 mmol/L of total blood ketone concentration increase, there was approximately a 9% decrease in CMRglc.
Discussion
We report here, in diet-induced ketotic rats, decreases in CMRglc highly correlate with both the level and the duration of the ketosis. These data revealed that the degree and duration of ketosis have a major role in determining corresponding changes in CMRglc with ketosis. We also present a retrospective analysis of historical data (meta-analysis) that appears to reconcile the inconsistencies from previous studies, which supports our conclusion.
The brain's ability to switch from glucose oxidation towards ketone bodies requires a type of ‘cerebral metabolic adaptation'. This process is not well understood but is thought to be highly associated with the duration and level of ketosis.10, 19, 37, 38 Ketones are considered to supply up to 70% of the total energy demands once maximal metabolic adaptation occurs.1 Blood ketones become elevated during prolonged fasting or with a ketogenic diet reaching a state ketosis and glucose sparing. During this process, monocaboxylic transporters upregulate at the blood–brain barrier with increasing demand for ketone utilization by brain.37, 38 Recently, investigators have recognized additional therapeutic properties of ketosis, such as neuroprotection after stroke or injury.19, 20 What remains unclear is whether the neuroprotective or therapeutic properties of ketosis is as a result of changes in the regulation of metabolic signaling pathways. These would include those associated with enzyme-catalyzed steps involved with glucose regulation39 or glucose independent pathways, such as the Nrf2 pathway (a responder to cellular stress).17 In this study, we questioned whether cerebral metabolism of ketone bodies (CMRket) replaces CMRglc after 3 weeks of diet-induced ketosis.
Previous studies measuring CMRglc in ketotic subjects report either changes in CMRglc with ketosis or failure to detect significant changes.1, 2, 3, 5, 6, 7, 21, 22, 27, 28 Historically, it has been established that brain can utilize ketone bodies under ketotic conditions.1, 4, 10, 13, 14, 15, 16, 39 However, corresponding changes in CMRglc during ‘metabolic adaptation' to ketosis has not been clearly described. Using PET–FDG imaging, the focus of this study was to determine if CMRglc decreases with increasing ketosis in adult-anesthetized diet-induced ketotic rats. CMRglc in the cerebral hemispheres and cerebellum decreased with increasing ketosis (0.3 to 6.3 mmol/L) in rats fed either STD or KG diets for 3 weeks. These data are consistent with the conclusions described in the classic human study by Owen et al.1 Their study was the first to highlight that the brain can switch from glucose oxidation to ketone body oxidation with chronic ketosis. Most revealing to us was a previous study using a similar rat model of diet-induced ketosis to measure changes CMRglc.21 The study failed to detect significant changes in CMRglc even though the duration and method of induction of ketosis was similar. However, the level of ketosis was four-fold lower making it difficult to detect a corresponding change in CMRglc with ketosis.
The most striking information obtained from our study was the correlative finding that CMRglc decreased 9% with every 1 mmol/L increase in total plasma ketones. Although not previously reported as such, the results of this study are consistent with previous studies measuring CMRglc in ketotic subjects, as our meta-analysis (Figure 2) also showed the same linear association between level of ketosis and corresponding changes in CMRglc. The meta-analysis supports our current findings and has brought new insight into previous studies (as authors' interpretations led to discrepancies or incomplete conclusions). One explanation for the discrepancies may be the difficulty to distinguish small changes in CMRglc with a small degree of ketosis. This was the case with our previous study in diet-induced ketotic rats where CMRglc was assessed using 2-DG.21 The level of ketosis was less than 1 mmol/L, making it difficult to detect a less than 9% decrease in glucose utilization using a non-imaging compartment modeling method.
Another consideration is the induction of ketosis through acute ketone body infusions. The main difficulty to this approach is the lack of metabolic adaptation to ketosis.29, 38 We have previously shown metabolic adaptation to ketosis is directly associated with duration of ketosis and level of ketosis.18, 29 Thus, in some studies using acute infusions of ketones to mimic ketotic conditions, the outcome failed to show decreases (or consistency) in glucose utilization.25 An exception might be in studies where low doses were given after short-term fasting, but the analytical approach often requires a higher degree of sensitivity for detecting small changes in CMRglc.40 Variabilities in experimental models such as, physiologic status, level of ketosis via metabolic adaptation, and analytical approach have a key role in the measured outcome. The emphasis of our current study was to use PET imaging together with our diet-induced rat model of ketosis to measure detectable changes in CMRglc.
In summary, CMRglc decreased ∼9% in both the cortex and the cerebellum for each 1 mmol/L increase in blood ketone bodies, which is consistent with diet-induced ketosis, as well as long- and short-term fasted ketosis. We attribute previous discrepancies to (1) the failure to detect significant differences within and across studies, (2) inadequate metabolic adaptation to ketosis, and (3) difficulty in establishing and/or maintaining a higher degree of ketosis. Our work puts historical data into a current perspective by reconciling the inconsistencies from previous studies where little or no change in CMRglc with ketosis was reported. Nevertheless, the maximum percent ketone bodies that can replace glucose oxidation still need to be determined. A quantitative understanding of CMRglc and CMRket under different durations and degrees of ketosis would elucidate the energy balance between glucose and ketone bodies.
Acknowledgments
We would thank the CASE Mouse Metabolic Phenotyping Center (MMPC; U24 DK76174) for assisting with GC–MS assays.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This research has been supported by the National Institutes of Health, R01 HL092933-01, R21 NS062048-01.
Supplementary Material
References
- Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF., Jr. Brain metabolism during fasting. J Clin Invest. 1967;46:1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redies C, Hoffer LJ, Beil C, Marliss EB, Evans AC, Larviere F, et al. Generalized decrease in brain glucose metabolism during fasting in humans studied by PET. Am J Physiol. 1989;256:E805–E810. doi: 10.1152/ajpendo.1989.256.6.E805. [DOI] [PubMed] [Google Scholar]
- Cherel Y, Burnol AF, Leturque A, Le MY. In vivo glucose utilization in rat tissues during the three phases of starvation. Metabolism. 1988;37:1033–1039. doi: 10.1016/0026-0495(88)90063-7. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Crone C. Induction processes in blood brain transfer of ketone bodies during starvation. A J Physiol. 1975;229:1165–1169. doi: 10.1152/ajplegacy.1975.229.5.1165. [DOI] [PubMed] [Google Scholar]
- Hasselbalch SG, Knudsen GM, Jakobsen J, Hageman LP, Holm S, Paulson OB. Brain metabolism during short-term starvation in humans. J Cereb Blood Flow Metab. 1994;14:125–131. doi: 10.1038/jcbfm.1994.17. [DOI] [PubMed] [Google Scholar]
- Mans AM, Davis DW, Hawkins RA. Regional brain glucose use in unstressed rats after two days of starvation. Metab Brain Dis. 1987;2:213–221. doi: 10.1007/BF00999693. [DOI] [PubMed] [Google Scholar]
- Ruderman RB, Ross PS, Berger M, Goodman MN. Regulation of glucose and ketone-body metabolism in brain of anaesthetized rats. Biochem J. 1974;138:1–10. doi: 10.1042/bj1380001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RA, Williamson DH, Krebs HA. Ketone-body utilization by adult and suckling rat brain in vivo. Biochem J. 1971;122:13–18. doi: 10.1042/bj1220013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo TM, Nehlig A, Sonnewald U. Neuronal-glial interactions in rats fed a ketogenic diet. Neurochem Int. 2006;48:498–507. doi: 10.1016/j.neuint.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Nehlig A. Brain uptake and metabolism of ketone bodies in animal models. Prostaglandins Leukot Essent Fatty Acids. 2004;70:265–275. doi: 10.1016/j.plefa.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids. 2004;70:309–319. doi: 10.1016/j.plefa.2003.09.007. [DOI] [PubMed] [Google Scholar]
- DiVivo DC, Pagliara AS, Prensky AL. Ketotic hypoglycemia and the ketogenic diet therapy. Neurology. 1973;23:640–649. doi: 10.1212/wnl.23.6.640. [DOI] [PubMed] [Google Scholar]
- Kinsman SL, Vining EPG, Quaskey SA, Mellits D, Freeman JM. Efficacy of the ketogenic diet for intractable seizure disorders: review of 58 cases. Epilepsia. 1992;33:1132–1136. doi: 10.1111/j.1528-1157.1992.tb01770.x. [DOI] [PubMed] [Google Scholar]
- Lennox WB. Ketogenic diet in the treatment of epilepsy. New Engl J Med. 1928;199:74–75. [Google Scholar]
- Swink TD, Vining EPG, Freeman JM. The ketogenic diet: 1997. Adv Pediatr. 1997;44:297–329. [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Melo TM, Nissim I, Sonnewald U, Nissim I. The ketogenic diet and brain metabolism of amino acids: relationship to the anticonvulsant effect. Annu Rev Nutr. 2007;27:415–430. doi: 10.1146/annurev.nutr.27.061406.093722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010;40:238–244. doi: 10.1016/j.nbd.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins ML, Hovda DA. The effects of age and ketogenic diet on local cerebral metabolic rates of glucose after controlled cortical impact injury in rats. J Neurotrauma. 2009;26:1083–1093. doi: 10.1089/neu.2008.0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchowicz MA, Zechel JL, Valerio J, Emancipator DS, Xu K, Pundik S, et al. Neuroprotection in diet-induced ketotic rat brain after focal ischemia. J Cereb Blood Flow Metab. 2008;28:1907–1916. doi: 10.1038/jcbfm.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins ML. Cerebral metabolic adaptation and ketone metabolism after brain injury. J Cereb Blood Flow Metab. 2008;28:1–16. doi: 10.1038/sj.jcbfm.9600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mudallal AS, Levin BE, Lust WD, Harik SI. Effects of unbalanced diets on cerebral glucose metabolism in the adult rat. Neurology. 1995;45:2261–2265. doi: 10.1212/wnl.45.12.2261. [DOI] [PubMed] [Google Scholar]
- Corddry DH, Rapoport SI, London ED. No effect of hyperketonemia on local cerebral glucose utilization in conscious rats. J Neurochem. 1982;38:1637–1641. doi: 10.1111/j.1471-4159.1982.tb06644.x. [DOI] [PubMed] [Google Scholar]
- LaManna JC, Salem N, Puchowicz M, Erokwu B, Koppaka S, Flask C, et al. Ketones suppress brain glucose consumption. Adv Exp Med Biol. 2009;645:301–306. doi: 10.1007/978-0-387-85998-9_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselbalch SG, Knudsen GM, Jakobsen J, Hageman LP, Holm S, Paulson OB. Blood–brain barrier permeability of glucose and ketone bodies during short-term starvation in humans. Am J Physiol. 1995;268:E1161–E1166. doi: 10.1152/ajpendo.1995.268.6.E1161. [DOI] [PubMed] [Google Scholar]
- Hasselbalch SG, Madsen PL, Hageman LP, Olsen KS, Justesen N, Holm S, et al. Changes in cerebral blood flow and carbohydrates metabolism during acute hyperketonemia. Am J Physiol. 1996;270:E746–E751. doi: 10.1152/ajpendo.1996.270.5.E746. [DOI] [PubMed] [Google Scholar]
- Linde R, Hasselbalch SG, Topp S, Paulson OB, Madsen PL. Global cerebral blood flow and metabolism during acute hyperketonemia in the awake and anesthetized rat. J Cereb Blood Flow Metab. 2006;26:170–180. doi: 10.1038/sj.jcbfm.9600177. [DOI] [PubMed] [Google Scholar]
- Dahlquist G, Persson B. The rate of cerebral utilization of glucose, ketone bodies, and oxygen: a comparative in vivo study of infant and adult rats. Pediatr Res. 1976;10:910–917. doi: 10.1203/00006450-197611000-00002. [DOI] [PubMed] [Google Scholar]
- Issad T, Penicaud L, Ferre P, Kande J, Baudon MA, Girard J. Effects of fasting on tissue glucose utilization in conscious resting rats. Major glucose-sparing effect in working muscles. Biochem J. 1987;246:241–244. doi: 10.1042/bj2460241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchowicz MA, Xu K, Sun X, Ivy A, Emancipator D, LaManna JC. Diet-induced ketosis increases capillary density without altered blood flow in rat brain. Am J Physiol Endocrinol Metab. 2007;292:E1607–E1615. doi: 10.1152/ajpendo.00512.2006. [DOI] [PubMed] [Google Scholar]
- Xu K, Puchowicz MA, Sun X, LaManna JC. Decreased brainstem function following cardiac arrest and resuscitation in aged rat. Brain Res. 2010;1328:181–189. doi: 10.1016/j.brainres.2010.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchowicz MA, Smith CL, Bomont C, Koshy J, David F, Brunengraber H. Dog model of therapeutic ketosis induced by oral administration of R,S-1,3-butanediol diacetoacetate. J Nutr Biochem. 2000;11:281–287. doi: 10.1016/s0955-2863(00)00079-6. [DOI] [PubMed] [Google Scholar]
- Gjedde A. Calculation of cerebral glucose phosphorylation from brain uptake of glucose analogs in vivo: a re-examination. Brain Res. 1982;257:237–274. doi: 10.1016/0165-0173(82)90018-2. [DOI] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- Tokugawa J, Ravasi L, Nakayama T, Schmidt KC, Sokoloff L. Operational lumped constant for FDG in normal adult male rats. J Nucl Med. 2007;48:94–99. [PubMed] [Google Scholar]
- Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol. 1979;6:371–388. doi: 10.1002/ana.410060502. [DOI] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, Rosiers MD, Patlak CS, Pettigrew KEA, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Leino RL, Gerhart DZ, Duelli R, Enerson BE, Drewes LR. Diet-induced ketosis increases monocarboxylate transporter (MCT1) levels in rat brain. Neurochem Int. 2001;38:519–527. doi: 10.1016/s0197-0186(00)00102-9. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Simpson IA. Developmental switch in brain nutrient transporter expression in the rat. Am J Physiol Endocrinol Metab. 2003;285:E1127–E1134. doi: 10.1152/ajpendo.00187.2003. [DOI] [PubMed] [Google Scholar]
- Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S, et al. Brain fuel metabolism, aging, and Alzheimer's disease. Nutrition. 2011;27:3–20. doi: 10.1016/j.nut.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Mason GF, Rothman DL, de Graaf RA, Behar KL. Cortical substrate oxidation during hyperketonemia in the fasted anesthetized rat in vivo. J Cereb Blood Flow Metab. 2011;31:2313–2323. doi: 10.1038/jcbfm.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.