Summary
Short-term sleep curtailment associated with activation of the stress system in healthy, young adults has been shown to be associated with decreased leptin levels, impaired insulin sensitivity and increased hunger and appetite.
To assess the effects of one night of sleep loss in a less stressful environment on hunger, leptin, adiponectin, cortisol, and blood pressure/heart rate and whether a 2-hour mid-afternoon nap reverses the changes associated with sleep loss, 21 young healthy individuals (10 men, 11 women) participated in a 7-day sleep deprivation experiment (4 consecutive nights followed by a night of sleep loss and 2 recovery nights). Half of the subjects were randomly assigned to take a mid-afternoon nap (1400–1600) the day following the night of total sleep loss. Serial 24-hour blood sampling and hunger scales were completed on the fourth (pre-deprivation) and sixth day (post-deprivation).
Leptin levels were significantly increased after one night of total sleep loss, whereas adiponectin, cortisol levels, blood pressure/heart rate, and hunger were not affected. Daytime napping did not influence the effects of sleep loss on leptin, adiponectin or hunger.
Acute sleep loss, in a less stressful environment, influences leptin levels in an opposite manner from that of short-term sleep curtailment associated with activation of the stress system. It appears that sleep loss associated with activation of the stress system but not sleep loss per se may lead to increased hunger and appetite and hormonal changes which ultimately may lead to increased consumption of “comfort” food and obesity.
Keywords: Sleep loss, stress, leptin, hunger
Introduction
Over the last decade, several epidemiological studies have demonstrated an association between obesity and self-reported short sleep duration suggesting that sleep curtailment may be a novel factor contributing to the increasing prevalence of obesity (Kripke et al., 2002; Kohatsu et al., 2006; Vorona et al., 2005). It has been postulated that several appetite-regulating peptides, i.e., leptin, might mediate the relationship between shortened sleep, increased hunger and eventually, obesity (Knutson et al., 2007).
Following the first experimental studies that showed that partial sleep deprivation is associated with decrease of the appetite-inhibiting hormone leptin, increase of the appetite-stimulating hormone ghrelin and increased hunger in young, healthy men (Spiegel et al., 2004a; Spiegel et al., 2004b), several experimental studies have failed to confirm these initial findings (Bosy-Westphal et al., 2008; Mullington et al., 2003; Nedeltcheva et al., 2009; Schmid et al., 2008; Simpson et al., 2008). Interestingly, in the study by Spiegel et al., sleep loss was associated with activation of the stress system which is known to lower leptin levels and increase the appetite for “comfort food” (Dallman et al., 2003; Rayner and Trayhurn, 2001; Spiegel et al., 2004b) whereas in the studies that failed to confirm the findings of Spiegel et al., no data were provided in regard to the status of the stress system. Thus, it remains open to further investigation whether sleep loss per se or activation of the stress system are mediators of the sleep deprivation effects on leptin, and hunger levels.
In addition, it has been shown that short-term sleep curtailment leads to a significant decrease in glucose-tolerance and reduced insulin release and low-grade inflammation (Spiegel et al., 1999; Vgontzas et al., 2004; Spranger et al., 2003). However, whether short-term partial or total sleep deprivation affects adiponectin peripheral levels, an insulin sensitivity-enhancing adipokine, is not known.
Furthermore, napping in the afternoon has been suggested to promote longevity and to protect from cardiovascular risks (Naska et al., 2007). Also, we have shown that napping after a night of total sleep loss decreases sleepiness, improves performance and causes beneficial changes in cortisol and IL-6 secretion (Vgontzas et al., 2007). Whether napping corrects the effects of acute total sleep deprivation on hunger and peripheral levels of leptin and adiponectin is not known.
The goals of this study were to assess: 1) the effects of one night of total sleep loss in a less stressful environment on peripheral levels of leptin, adiponectin and hunger; and 2) whether a 2-hour mid-afternoon post-deprivation nap reverses the changes associated with sleep loss.
Materials and Methods
Subjects
Twenty-one normal sleepers (10 men, 11 women), 18–30 years of age were recruited from the community and from the medical and technical staff and students of Penn State’s Hershey Medical Center. They were in good general health and physically active, but not excessively so. Specifically, the subjects that were jogging > 45 miles per week were excluded from the study because of potential effects on the HPA axis (Luger et al., 1987). They had no sleep complaints or circadian abnormalities, were not taking any medications, and were screened in the laboratory for sleep disordered breathing, nocturnal myoclonus and other primary sleep disorders. Also, a battery of clinical tests (including a complete cell blood count, urinalysis, thyroid indexes, electrocardiogram, and urine screen for drug use) were negative for abnormal findings. Subjects were randomly assigned to take a mid-afternoon nap or not the day following the night of total sleep loss. Men and women were equally distributed in the two groups (“nap” vs. “no nap” group). The two groups were very similar in terms of age and BMI (23.5 ± 3.3 vs. 24.6 ± 2.9 years and 23.2 ± 2.8 vs. 25.0 ± 2.1 kg/m2)
Protocol
Each subject participated in a sleep deprivation experiment that lasted seven days. Adequate sleep time and regular sleep schedules were verified with a sleep log and actigraphy for two weeks before the study. After four consecutive nights in the sleep laboratory (one adaptation and three baseline nights), the subjects were deprived of sleep during the entire fifth night, while they were allowed to sleep again on nights six and seven (recovery nights). The subjects stayed awake in the presence of nursing or technical staff and total wake time before the first recovery night was 40 hours. Half of the subjects were randomly assigned to take a mid-afternoon nap (1400–1600) the day following the night of total sleep loss. Sleep laboratory recordings were carried out in a sound-attenuated, light- and temperature-controlled room that had a comfortable bedroom-like atmosphere. Each subject was monitored with EEG, electrooculograph, electromyography recordings continuously for 8h (2230–0630). The sleep schedule in the laboratory at baseline was similar to the subjects’ normal sleep schedule. Specifically, the subjects’ habitual times to go to bed and rise in the morning were no different than by an hour from their sleep schedule in the laboratory. Twenty-four hour blood sampling was performed serially every 30 minutes on the fourth day and night (pre-deprivation) and on the sixth day (the day following sleep deprivation) and the recovery night. In women, both periods of blood sampling took place during the follicular phase, as evidenced by low progesterone levels obtained on the fourth and sixth days of the experiment. An indwelling catheter was inserted in the antecubital vein about 30 minutes before the first blood sample. The catheter was kept patent with small amounts of heparin (the total amount of heparin did not exceed 800 units/24h period). During the sleep recording period, blood was collected outside the subjects’ room through a perforation in the wall, via extra tubing in order to decrease sleep disturbance from the blood sampling technique. During the night of sleep deprivation, as well as throughout the entire sampling period, the subjects were ambulatory, allowed to watch television, play computer and table games, go to the bathroom, etc. Also, they were instructed not to change their diet and were allowed to choose food items with consistent portion sizes from the hospital cafeteria menu for their three daily meals, at about 0700, 1200 and 1800. The brightness of the light in the room in which the blood drawing took place was, on average, about 800 lux, ranging from a minimum of 200 to a maximum of 1400 lux (Minolta III F digital light meter Konica, Minolta, Tokyo, Japan). Blood pressure was measured in the evening the days before and after sleep deprivation using a pneumoelectric microprocessor-controlled instrument with the appropriate sized cuffs. The accuracy of this monitor is reported to be ± 3 m Hg; in addition, internal calibration was performed before each use, and the machine was checked against a mercury sphygmomanometer at least annually. The recorded blood pressure was the average of 3 consecutive readings during a 5-min period following 10 min of sitting. The protocol was approved by the Institutional Review Board, and each subject signed a written consent form.
Assessment of Hunger and Food Consumption
Hunger/Fullness Scale
During the fourth and sixth days (days of blood sampling) the subjects’ levels of hunger were evaluated 15–30 minutes prior to each meal at about 0700, 1200 and 1800. The subjects rated the hunger levels on a hunger/fullness scale compiled and used clinically by the Department of Nutrition at Penn State University. The scale ranges from 0 (empty/starving) to 10 (full/want to throw-up). Also, the subjects noted the type and number of food items consumed at each meal. Food items were classified as sweets (such as cake, candy, cookies, ice cream, and pastry); salty foods (such as chips, salted nuts, pickles, and olives); starchy foods (such as bread, pasta, cereal, and potatoes); fruits and fruit juices; vegetables; meats, poultry, fish and eggs; and dairy products (such as milk, cheese, and yogurt) as described by Spiegel et al. (2004b).
Hormone Assays
Blood collected from the indwelling catheter was transferred to an EDTA-containing tube and refrigerated until centrifugation (within three hours). The plasma was frozen at −70 C until assay. Plasma cortisol levels were measured by specific radio immunoassay techniques, and plasma leptin and adiponectin were measured by a commercially available RIA (Linco Research, Inc., St. Charles, MO) as previously described (Vgontzas et al., 2000; Vgontzas et al., 2004). The lower limit of detection for cortisol was 0.7 µg/dl, and the intra- and interassay coefficients of variation were, respectively, 4.6% and 6.0%. The lower detection limits for leptin and adiponectin were 0.05 ng/ml and 1.0 ng/ml, respectively. The intra- and interassay coefficients of variation were 5.8 and 4.9, respectively for leptin and 4.0 and 8.1 for adiponectin.
Statistical Analyses
To assess the effects of sleep deprivation and the post-deprivation 2-hour nap on leptin, adiponectin, cortisol and hunger, two types of analyses were performed. The first was to compare the mean differences between pre- and post- sleep deprivation 24-hour, daytime (0700–2200) and nighttime (2230–0630) leptin, adiponectin and cortisol levels using paired t-test. The same analysis was used to compare the differences between pre- and post- sleep deprivation hunger levels and number of food items consumed at each meal. Because there were no differences between the nap and no-nap conditions in terms of key variables, i.e., leptin, adiponectin, hunger, in the analysis, we combined the two groups. The second analysis was to compare the differences of leptin and adiponectin levels (pre-minus post- deprivation) between the nap and no-nap groups during and after the 2-hour nap. We used the analysis of covariance (ANCOVA) to adjust for the baseline (pre-deprivation) hormone values and baseline percent sleep time. Specifically, to assess the effects of the post-deprivation 2-hour nap on hormonal values, we compared the differences of the change of hormonal values before and after sleep loss between the nap vs. no nap groups for the nap (1400–1600) and the post-nap periods (1600–2230), respectively. Significance of the effect of gender and its interaction with the nap condition were also tested. Similar analyses contrasting the differences in the change in hunger levels after sleep loss and before (prior to breakfast and lunch) and after the nap (prior to dinner) periods were performed.
Data are presented as the mean ± SE for the purpose of group comparisons, except for demographic data and anthropometric data (age and body mass index), for which we used SD to describe variance.
Results
Effects of One Night of Total Sleep Deprivation on Leptin, Adiponectin, Cortisol, Blood Pressure/Heart Rate, and Hunger
Leptin
Twenty-four hour mean leptin levels were nonsignificantly higher in women vs men (17.8 ± 4.3 vs 13.5 ± 2.5, NS). One night of total sleep deprivation was associated with a significant overall increase in 24-hour secretion of leptin [difference of the mean 24-hour leptin levels (post deprivation minus baseline): 2.27 ± 0.71, P = 0.005]. This increase occurred primarily during daytime (0700–2200)[difference of the mean daytime leptin levels (post deprivation minus baseline): 3.43 ± 0.89, P = 0.001]. During the post-deprivation nighttime period (2230–0630), there was no significant difference in leptin levels compared to baseline [difference of the mean nighttime leptin levels (post deprivation minus baseline): 0.33 ± 0.74, P = NS)]. No gender effects were observed. Furthermore, at baseline, leptin demonstrated a clear circadian rhythm with the minimum levels occurring in the morning, a gradual rise during the rest of the day followed by the nocturnal rise with the maximum levels occurring during the first part of the nighttime sleep. One night of sleep loss resulted in the flattening of the diurnal leptin rhythm whereas maximum levels were observed again at nighttime.
Adiponectin
One night of total sleep deprivation did not significantly affect the 24-hour secretion of adiponectin [difference of the mean 24-hour adiponectin levels (post deprivation minus baseline): 3.23 ± 1.76, P = NS]. Furthermore, there were no differences in adiponectin levels compared to baseline both during the day and the night after one night of sleep loss [difference of the mean daytime adiponectin levels (post deprivation minus baseline): 2.96±1.86, P=NS and difference of the mean nighttime adiponectin levels (post deprivation minus baseline): 3.68 ± 2.20, P = NS]. No gender effects were observed.
Cortisol
One night of total sleep deprivation did not significantly affect the 24-hour secretion of cortisol [difference of the mean 24-hour cortisol levels (post deprivation minus baseline): −0.41 ± 0.26, P = NS]. Furthermore, there were no differences in cortisol levels compared to baseline both during the day and night after one night of sleep loss [difference of the mean daytime cortisol levels (post deprivation minus baseline): −0.30 ± 0.33, P = NS and difference of the mean nighttime cortisol levels (post deprivation minus baseline): −0.60 ± 0.22, P = NS]. No gender effects were observed.
Blood pressure and heart rate
There were no significant changes in heart rate or blood pressure in the evening before and post-sleep deprivation (71.1 ± 3.5 vs. 65.6 ±2.7 for heart rate, 114.2 ± 6.0 vs. 112.3 ± 4.1 for systolic blood pressure and 65.5 ± 3.1 vs. 61.0 ± 2.8 for diastolic blood pressure, all NS).
Hunger
There were no differences in hunger prior to breakfast, lunch and dinner the day after one night of sleep loss compared to baseline. Furthermore, there were no differences in the total number of food items at breakfast, lunch and dinner during the post-deprivation day compared to baseline [difference of the mean number of food items (post deprivation minus baseline) at breakfast, lunch and dinner −0.21 ± 0.49, −0.21 ± 0.39, −0.92 ± 0.46, respectively, all P = NS]. In addition, the number of items in the seven food categories (sweets; salty; starchy; fruits and fruit juices; vegetables; meat, poultry, fish and eggs; and dairy products) consumed at each meal were not different between the post-deprivation day and baseline. No gender effects were observed.
Effects of the Post-deprivation 2-hour Nap on Leptin, Adiponectin and Hunger
Leptin
Daytime napping following a night of total sleep loss did not affect significantly leptin secretion during both the nap and postnap periods [between-group difference, nap vs. no nap of the change in leptin levels before and after sleep loss was 1.00±1.97 for the nap period (1400–1600) (P=NS) and 1.10±2.10 for the postnap period (1600–2230) (P=NS), respectively].
Adiponectin
Daytime napping following a night of total sleep deprivation did not affect significantly both nap and postnap adiponectin plasma levels [between-group difference, nap vs. no nap of the change in adiponectin levels before and after sleep loss was −3.03±2.21 for the nap period (1400–1600) (P=NS) and −3.00±2.76 for the postnap period (1600–2230) (P=NS), respectively].
Hunger
Daytime napping following of night of total sleep loss did not affect significantly hunger levels during the postnap period [between-group difference, nap vs. no nap of the change in hunger levels before and after sleep loss was 0.20 ± 0.46 for the postnap period (P=NS)].
Discussion
This study shows that one night of less stressful total sleep deprivation in young healthy men and women is associated with a significant increase in daytime leptin levels and no change in hunger levels. In addition, a 2-hour mid-afternoon nap after one night of sleep loss has no effect on leptin or hunger levels.
Our findings of elevated daytime leptin levels following a night of sleep loss are in agreement with several recent studies showing elevation or no change of leptin secretion following total or partial sleep restriction in young men and women (Bosy-Westphal et al., 2008; Simpson et al., 2008; Mullington et al., 2003; Nedeltcheva et al., 2009; Schmid et al., 2008). These findings contrast the earlier findings from two studies in which leptin levels were decreased following restriction of sleep to 4 hours for six days and to 4 hours for two days (Spiegel et al., 2004a; Spiegel et al., 2004b). One clear difference between our study and that by Spiegel et al. is that in the latter study, sleep loss was associated with stressful conditions, i.e., bed rest for 60 hours, vs. ambulatory conditions, dim-light vs. ordinary room light and intravenous glucose infusions vs. regular meals. That the experimental conditions were stressful is supported by that, in the study by Spiegel et al., there was a 24-hour increase of sympathovagal balance and an increase in late afternoon and evening cortisol following restriction of sleep to 4 hours for six days (Spiegel et al., 2004b). In contrast, in our study, there was no change in 24-hour, daytime, including evening, and nighttime peripheral cortisol levels or evening levels of blood pressure/heart rate following one night of sleep loss. The observed elevation of daytime leptin levels following one night of sleep loss in our study is consistent with the observed lower activity of the HPA axis, which has an inhibiting effect on leptin levels (Bornstein et al., 1997; Licinio et al., 1997). However, the regulation of leptin levels by HPA axis appears to be more complex as other studies have reported increased leptin levels in depressed patients with hypercortisolemia or following the administration of dexamethasone in normal subjects and appropriate for the BMI levels in patients with Cushing’s syndrome (Papaspyrou-Rao et al., 1997; Antonijevic et al., 1998; Torpy et al., 1998). Furthermore, increased sympathetic activity may lead to decreased leptin levels (Spiegel et al., 2004b; Rayner and Trayhurn, 2001; Schafroth et al., 2001). Notably, the majority of total sleep deprivation studies have shown a heart rate reduction and/or decreased muscle sympathetic nerve activity (Bond et al., 1986; Chen et al., 1991; Holmes et al., 2002; Kato et al., 2000; Ogawa et al., 2003; Vaara et al., 2009). Finally, the increase of leptin after less stressful sleep loss is consistent with the potential role of leptin as a novel antidepressant (sleep deprivation improves temporarily mood of depressed individuals) and with its thermogenic and energy expenditure promoting effects since sleep loss is associated with decreased body temperature and increased energy expenditure (Horne, 1983; Horne and Pettite, 1985; Lu, 2007; Mastorakos and Zapanti, 2004; Tuominen et al., 1997).
Early studies of the physiological effects of total or REM sleep deprivation anecdotally reported increased feelings of hunger in some subjects but not all (Dement, 1960; Horne and Pettitt, 1985). Also, Spiegel at al. reported increased hunger following restriction of sleep to 4 hours for two days (Spiegel et al., 2004a). In contrast, in this study, despite a significant increase of plasma leptin levels, hunger levels did not change after a night of sleep loss which is consistent with the view that decrease but not increase of leptin levels has more acute and greater impact on appetite (Korbonits et al., 1997; Leibel, 2002; Kolaczynski et al., 1996). Moreover, in our study there was no difference in the amount of food consumed at each meal before and after a night of sleep loss suggesting that the observed rise in leptin secretion following total sleep deprivation is not a result of additional food intake. Interestingly, elevation of the activity of the stress system leads to increased hunger and intake of “comfort food” which in turn decrease the activity of the stress system (Dallman et al., 2003).
Several sleep deprivation experiments have shown that total sleep loss is associated with a decrease in glucose tolerance (Van Helder et al., 1993; Spiegel et al., 1999). However, adiponectin does not appear to be affected by acute total sleep deprivation suggesting that this adipokine may not be a sensitive marker of acute changes in glucose metabolism.
We have previously demonstrated that a 2-hour mid-afternoon nap after one night of sleep loss has beneficial effects on cortisol, IL-6 levels, sleepiness and performance (Vgontzas et al., 2007). In contrast to the observed changes in cortisol and IL-6 levels, leptin and adiponectin secretion were not affected during and after 2-h mid-afternoon nap following a night of sleep loss. That deep sleep did not affect leptin and adiponectin levels during or after its termination suggests that these two adipokines do not meet the criteria as sleep factors in humans (Krueger et al., 1989).
In summary, in young healthy men and women, one night of sleep loss in a less stressful environment is associated with increased leptin levels and no change in hunger and adiponectin levels. One possible explanation of the discrepant findings between our study and the study by Spiegel at al. is that in their study, in contrast to ours, sleep restriction was associated with increased cortisol and sympathetic activity (Spiegel et al., 2004b; Vgontzas et al., 2007). Thus, it appears that it is the combination of sleep loss and activation of the stress system but not sleep loss per se that may result in decreased leptin levels and increased hunger. If this speculation is correct, then a critical question arises: is sleep loss in the “real world”, associated with activation of the stress system? Since most people after sleep loss in the “real world” in contrast to the comfortable and quiet environment of GCRC, must carry out daily responsibilities (work, school and family), it appears that stressful sleep loss is the norm and not the exception, which in turn may result in increased hunger, increased consumption of comforting food, and eventually obesity. Further studies are needed to test this hypothesis by mimicking sleep loss in “real life” situations.
Figure 1.
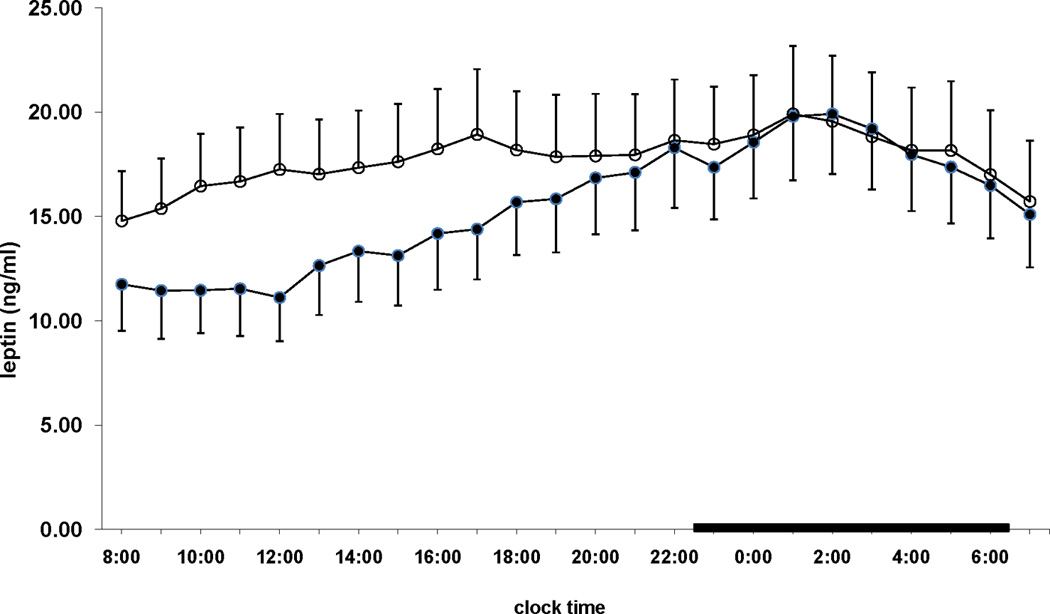
24-hour leptin values pre- (●) and post- (○) sleep deprivation. The thick black lines in the abscissa indicate the nighttime sleep recording period.
Table 1.
Sleep deprivation – nap protocol in young men and women
| Days |
|||||||
|---|---|---|---|---|---|---|---|
| Baseline |
Sleep Loss |
Recovery |
|||||
| Sleep laboratory | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Nighttime | x | x | x | x | x | x | |
| Hunger Scale | x | x | |||||
| 2h nap* (14.00–16.00) | x | ||||||
| Blood drawing | x | x | |||||
50% of the subjects completed the 2 hours nap after the night of total sleep loss
Table 2.
Summary of hunger levels before and after one night of total sleep loss
| Pre-deprivation | Post-deprivation | ∆ (pre-post) | P-value | |
|---|---|---|---|---|
| Hunger – breakfast time (0700) | 3.67 ± 0.2 | 3.33 ± 0.2 | 0.34 ± 0.3 | 0.268 |
| Hunger – lunch time (1200) | 4.00 ± 0.2 | 3.95 ± 0.2 | 0.05 ± 0.2 | 0.790 |
| Hunger – dinner time (1800) | 4.05 ± 0.3 | 4.35 ± 0.2 | −0.30 ± 0.2 | 0.385 |
Acknowledgments
This study was partially funded by the National Institutes of Health Grant R01 64415.
References
- Antonijevic IA, Murck H, Frieboes R-M, Horn R, Brabant G, Steiger A. Elevated nocturnal profiles of serum leptin in patients with depression. J Psychiatr Res. 1998;32:403–410. doi: 10.1016/s0022-3956(98)00032-6. [DOI] [PubMed] [Google Scholar]
- Bond V, Balkissoon B, Franks BD, et al. Effects of sleep deprivation on performance during submaximal and maximal exercise. J Sports Med Phys Fitness. 1986;26:169–174. [PubMed] [Google Scholar]
- Bornstein SR, Uhlmann K, Haidan A, Ehrhart-Bornstein M, Scherbaum WA. Evidence for a novel peripheral action of leptin as a metabolic signal to the adrenal gland: leptin inhibits cortisol release directly. Diabetes. 1997;46:1235–1238. doi: 10.2337/diab.46.7.1235. [DOI] [PubMed] [Google Scholar]
- Bosy-Westphal A, Hinrichs S, Jauch-Chara K, et al. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts. 2008;1:266–273. doi: 10.1159/000158874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HI. Effects of 30-h sleep loss on cardiorespiratory functions at rest and in exercise. Med Sci Sports Exerc. 1991;23:193–198. [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, et al. Chronic stress and obesity: a new view of "comfort food". Proc Natl Acad Sci U S A. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dement W. The effect of dream deprivation. Science. 1960;131:1705–1706. doi: 10.1126/science.131.3415.1705. [DOI] [PubMed] [Google Scholar]
- Holmes AL, Burgess HJ, Dawson D. Effects of sleep pressure on endogenous cardiac autonomic activity and body temperature. J Appl Physiol. 2002;92:2578–2584. doi: 10.1152/japplphysiol.01106.2001. [DOI] [PubMed] [Google Scholar]
- Horne JA. Human sleep and tissue restitution: some qualifications and doubts. Clin Sci (Lond) 1983;65:569–577. doi: 10.1042/cs0650569. [DOI] [PubMed] [Google Scholar]
- Horne JA, Pettitt AN. High incentive effects on vigilance performance during 72 hours of total sleep deprivation. Acta Psychol (Amst) 1985;58:123–139. doi: 10.1016/0001-6918(85)90003-4. [DOI] [PubMed] [Google Scholar]
- Kato M, Phillips BG, Sigurdsson G, Narkiewicz K, Pesek CA, Somers VK. Effects of sleep deprivation on neural circulatory control. Hypertension. 2000;35:1173–1175. doi: 10.1161/01.hyp.35.5.1173. [DOI] [PubMed] [Google Scholar]
- Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohatsu ND, Tsai R, Young T, et al. Sleep duration and body mass index in a rural population. Arch Intern Med. 2006;166:1701–1705. doi: 10.1001/archinte.166.16.1701. [DOI] [PubMed] [Google Scholar]
- Kolaczynski JW, Ohannesian JP, Considine RV, Marco CC, Caro JF. Response of leptin to short-term and prolonged overfeeding in humans. J Clin Endocrinol Metab. 1996;81:4162–4165. doi: 10.1210/jcem.81.11.8923877. [DOI] [PubMed] [Google Scholar]
- Korbonits M, Trainer PJ, Little JA, et al. Leptin levels do not change acutely with food administration in normal or obese subjects, but are negatively correlated with pituitary-adrenal activity. Clin Endocrinol (Oxf) 1997;46:751–757. doi: 10.1046/j.1365-2265.1997.1820979.x. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Obal F, Jr, Johanssen L, Cady AB, Toth L. Endogenous slow-wave sleep substances: a review. In: Wauquier A, Dugovic C, Radulovacki M, editors. Slow-wave sleep. New York: Raven Press; 1989. pp. 75–90. [Google Scholar]
- Leibel RL. The role of leptin in the control of body weight. Nutr Rev. 2002;60:S15–S19. doi: 10.1301/002966402320634788. [DOI] [PubMed] [Google Scholar]
- Licinio J, Mantzoros C, Negrao AB, et al. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat Med. 1997;3:575–579. doi: 10.1038/nm0597-575. [DOI] [PubMed] [Google Scholar]
- Lu XY. The leptin hypothesis of depression: a potential link between mood disorders and obesity? Curr Opin Pharmacol. 2007;7:648–652. doi: 10.1016/j.coph.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger A, Deuster PA, Kyle SB, et al. Acute hypothalamic-pituitary-adrenal responses to the stress of treadmill exercise. Physiologic adaptations to physical training. N Engl J Med. 1987;316:1309–1315. doi: 10.1056/NEJM198705213162105. [DOI] [PubMed] [Google Scholar]
- Mantzoros C, Flier JS, Lesem MD, Brewerton TD, Jimerson DC. Cerebrospinal fluid leptin in anorexia nervosa: correlation with nutritional status and potential role in resistance to weight gain. J Clin Endocrinol Metab. 1997;82:1845–1851. doi: 10.1210/jcem.82.6.4006. [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Zapanti E. The hypothalamic-pituitary-adrenal axis in the neuroendocrine regulation of food intake and obesity: the role of corticotropin releasing hormone. Nutr Neurosci. 2004;7:271–280. doi: 10.1080/10284150400020516. [DOI] [PubMed] [Google Scholar]
- Mullington JM, Chan JL, Van Dongen HP, et al. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol. 2003;15:851–854. doi: 10.1046/j.1365-2826.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- Naska A, Oikonomou E, Trichopoulou A, Psaltopoulou T, Trichopoulos D. Siesta in healthy adults and coronary mortality in the general population. Arch Intern Med. 2007;167:296–301. doi: 10.1001/archinte.167.3.296. [DOI] [PubMed] [Google Scholar]
- Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Kanbayashi T, Saito Y, et al. Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: a study with microneurographic technique. Sleep. 2003;26:986–989. doi: 10.1093/sleep/26.8.986. [DOI] [PubMed] [Google Scholar]
- Papaspyrou-Rao S, Schneider SH, Petersen RN, Fried SK. Dexamethasone increases leptin expression in humans in vivo. J Clin Endocrinol Metab. 1997;82:1635–1637. doi: 10.1210/jcem.82.5.3928. [DOI] [PubMed] [Google Scholar]
- Rayner DV, Trayhurn P. Regulation of leptin production: sympathetic nervous system interactions. J Mol Med. 2001;79:8–20. doi: 10.1007/s001090100198. [DOI] [PubMed] [Google Scholar]
- Schafroth U, Godang K, Ueland T, Bollerslev J. Leptin response to endogenous acute stress is independent of pituitary function. Eur J Endocrinol. 2001;145:295–301. doi: 10.1530/eje.0.1450295. [DOI] [PubMed] [Google Scholar]
- Schmid SM, Hallschmid M, Jauch-Chara K, Born J, Schultes B. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J Sleep Res. 2008;17:331–334. doi: 10.1111/j.1365-2869.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- Simpson N, Banks S, Dinges DF. Five nights of partial sleep restriction increased plasma leptin levels in healthy adults. Sleep. 2008;31:A128. [Google Scholar]
- Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, L'hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004a;89:5762–5770. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004b;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- Spranger J, Kroke A, Mohlig M, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226–228. doi: 10.1016/S0140-6736(03)12255-6. [DOI] [PubMed] [Google Scholar]
- Torpy DJ, Bornstein SR, Cizza G, Chrousos GP. The effects of glucocorticoids on leptin levels in humans may be restricted to acute pharmacologic dosing. J Clin Endocrinol Metab. 1998;83:1821–1822. doi: 10.1210/jcem.83.5.4821-1. [DOI] [PubMed] [Google Scholar]
- Tuominen JA, Ebeling P, Heiman ML, Stephens T, Koivisto VA. Leptin and thermogenesis in humans. Acta Physiol Scand. 1997;160:83–87. doi: 10.1046/j.1365-201X.1997.00102.x. [DOI] [PubMed] [Google Scholar]
- Vaara J, Kyrolainen H, Koivu M, Tulppo M, Finni T. The effect of 60-h sleep deprivation on cardiovascular regulation and body temperature. Eur J Appl Physiol. 2009;105:439–144. doi: 10.1007/s00421-008-0921-5. [DOI] [PubMed] [Google Scholar]
- Van Helder T, Symons JD, Radomski MW. Effects of sleep deprivation and exercise on glucose tolerance. Aviat Space Environ Med. 1993;64:487–492. [PubMed] [Google Scholar]
- Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–1158. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Pejovic S, Zoumakis E, et al. Daytime napping after a night of sleep loss decreases sleepiness, improves performance, and causes beneficial changes in cortisol and interleukin-6 secretion. Am J Physiol Endocrinol Metab. 2007;292:E253–E261. doi: 10.1152/ajpendo.00651.2005. [DOI] [PubMed] [Google Scholar]
- Vorona RD, Winn MP, Babineau TW, Eng BP, Feldman HR, Ware JC. Overweight and obese patients in a primary care population report less sleep than patients with a normal body mass index. Arch Intern Med. 2005;165:25–30. doi: 10.1001/archinte.165.1.25. [DOI] [PubMed] [Google Scholar]


