Abstract
Increased glutamatergic input in the hypothalamic paraventricular nucleus (PVN) plays an important role in the development of hypertension. Group II metabotropic glutamate receptors (mGluRs) are expressed in the PVN, but their involvement in regulating synaptic transmission and sympathetic outflow in hypertension is unclear. Here we show that the group II mGluRs agonist (2S,2'R,3'R)-2-(2',3'-dicarboxycyclopropyl)glycine (DCG-IV) produced a significantly greater reduction in the frequency of spontaneous and miniature excitatory postsynaptic currents and the amplitude of electrically evoked excitatory postsynaptic currents in retrogradely labeled spinally projecting PVN neurons in spontaneously hypertensive rats (SHRs) than in normotensive control rats. DCG-IV similarly decreased the frequency of GABAergic inhibitory postsynaptic currents of labeled PVN neurons in the two groups of rats. Strikingly, DCG-IV suppressed the firing of labeled PVN neurons only in SHRs. DCG-IV failed to inhibit the firing of PVN neurons of SHRs in the presence of ionotropic glutamate receptor antagonists. Lowering blood pressure with celiac ganglionectomy in SHRs normalized the DCG-IV effect on excitatory postsynaptic currents to the same level seen in control rats. Furthermore, microinjection of DCG-IV into the PVN significantly reduced blood pressure and sympathetic nerve activity in SHRs. Our findings provide new information that presynaptic group II mGluR activity at the glutamatergic terminals increases in the PVN in SHRs. Activation of group II mGluRs in the PVN inhibits sympathetic vasomotor tone through attenuation of increased glutamatergic input and neuronal hyperactivity in SHRs.
Keywords: hypertension, hypothalamus, sympathetic nervous activity, synaptic transmission, glutamate receptors
Introduction
The paraventricular nucleus (PVN) of the hypothalamus contributes to the development of hypertension 1–3. A population of PVN neurons projects to preganglionic sympathetic neurons in the spinal cord and rostral ventrolateral medulla and is likely involved in regulating sympathetic outflow and blood pressure 4–7. Elevated sympathetic nerve activity has been shown in patients with essential hypertension 8–10, and the hyperactivity of PVN presympathetic neurons has been demonstrated in animal models of essential hypertension, such as spontaneously hypertensive rats (SHRs) 11–15. However, the precise mechanisms underlying hyperactivity of PVN presympathetic neurons in hypertension are not fully known.
Glutamate is the major excitatory neurotransmitter in the PVN, and increased glutamatergic input into PVN presympathetic neurons leads to elevated sympathetic vasomotor tone in SHRs 3,12. In addition to fast-acting ionotropic glutamate receptors, G protein–coupled metabotropic glutamate receptors (mGluRs) are also activated by glutamate. Eight different mGluRs have been cloned and classified into three groups 16. Group I mGluRs (mGluR1 and mGluR5) are coupled to Gq/11 proteins to activate phospholipase C and protein kinase C, which increase neuronal firing activity and synaptic transmission. In contrast, group II (mGluR2 and mGluR3) and group III (mGluR4, mGluR6, mGluR7, and mGluR8) mGluRs are coupled to Gi/o proteins, which reduce neuronal excitability and synaptic transmission 17. Although mGluR2/3 are widely distributed in the brain, including the PVN 18,19 and median preoptic nucleus 20, the functional significance of group II mGluRs in the PVN in the control of glutamatergic input and sympathetic vasomotor tone in hypertension has not been determined.
In the present study, we tested the hypothesis that group II mGluRs control the excitability of PVN presympathetic neurons in hypertension by regulating glutamatergic input. In addition, we determined the role of group II mGluRs in the PVN in the regulation of sympathetic outflow in hypertension. Our findings provide new information that the activity of group II mGluRs at the glutamatergic terminals in the PVN increases in hypertension, which may provide a mechanism to dampen excessive glutamate release. Activation of group II mGluRs restrains the excitability of PVN presympathetic neurons and sympathetic vasomotor tone in hypertension.
Methods
Male Wistar-Kyoto (WKY) rats and SHRs (13 weeks old; Harlan, Indianapolis, IN) were used in this study. We used 89 SHRs and 79 WKY rats for the entire study. The surgical procedures and experimental protocols were approved by the Institutional Animal Care and Use Committee and conformed to the National Institutes of Health guidelines for the ethical use of animals. The arterial blood pressure (ABP) of the rats was measured every day for 1 week before the final experiments, using a non-invasive tail-cuff system. The systolic ABP was significantly higher in SHRs (208 ± 3.01 mmHg, n = 25) than in age-matched WKY rats (122.18 ± 2.56 mmHg, n = 25).
The detailed methods for retrograde labeling of spinally projecting PVN neurons, electrophysiological recordings of glutamatergic excitatory postsynaptic currents (EPSCs) and inhibitory postsynaptic currents (IPSCs) in brain slices, PVN microinjections and lumbar sympathetic nerve recording in vivo, and data analysis are described in Online Supplements.
Results
Group II mGluR activity is increased to regulate glutamate release to PVN presympathetic neurons in hypertension
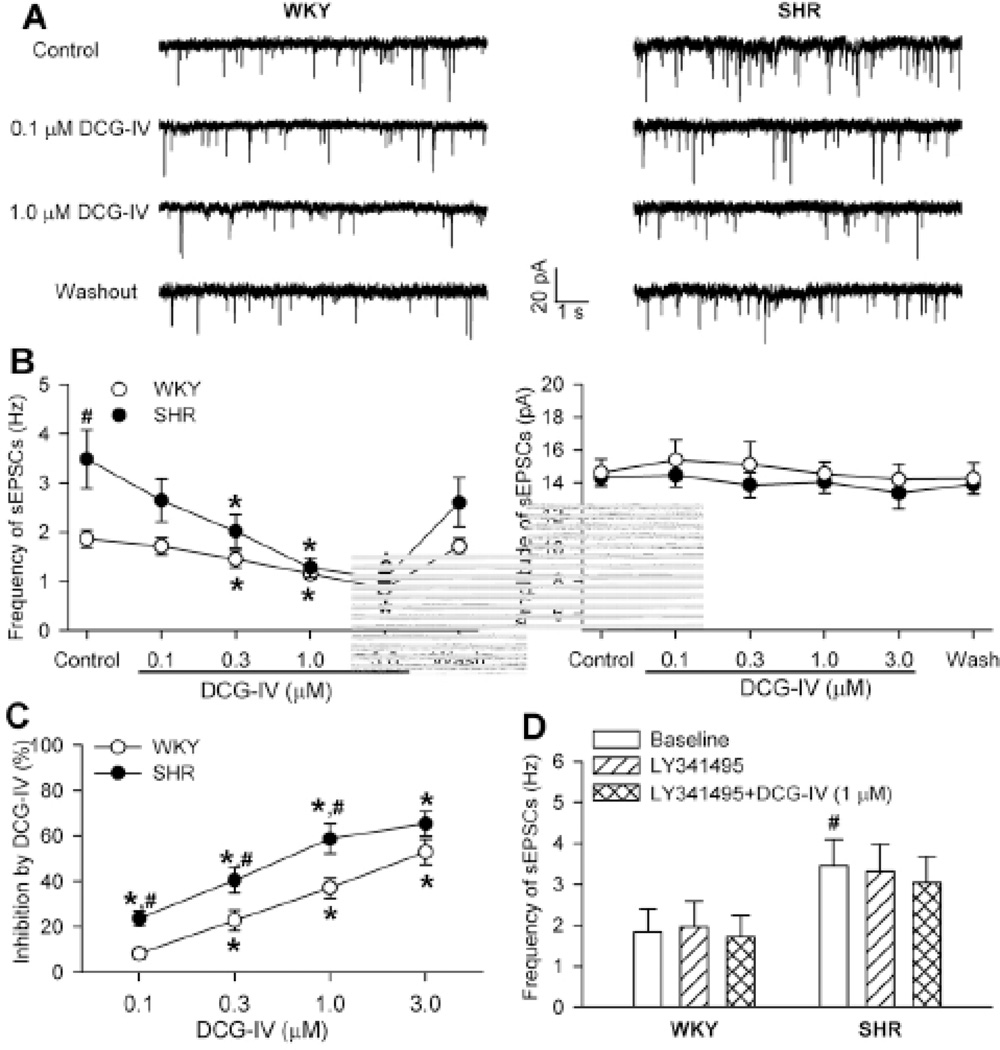
To determine the role of group II mGluRs in controlling glutamatergic input to PVN presympathetic neurons in hypertension, we examined the effect of DCG-IV, a highly specific group II mGluR agonist 21,22, on spontaneous EPSCs (sEPSCs) of spinally projecting PVN neurons in SHRs and WKY rats. The mean basal frequency of sEPSCs in labeled PVN neurons was significantly higher in SHRs than in WKY rats (Fig. 1, A,B). Bath application of DCG-IV (0.1, 0.3, 1.0, and 3.0 µM; each applied for about 4 min) significantly decreased the frequency, but not the amplitude, of sEPSCs in a concentration-dependent manner in both WKY rats and SHRs (Fig. 1, A–C). Notably, DCG-IV caused a significantly greater reduction in the frequency of sEPSCs in SHRs than in WKY rats (Fig. 1C). Bath application of 100 nM LY341495, a highly selective group II mGluR antagonist 22,23, had no significant effect on the basal frequency of sEPSCs. LY341495 abolished the inhibitory effect of DCG-IV (1 µM) on the frequency of sEPSCs in labeled PVN neurons in both WKY rats and SHRs (Fig. 1D).
Figure 1. DCG-IV inhibition of sEPSCs of spinal projecting PVN neurons is greater in SHRs than in WKY rats.
A, Representative traces show sEPSCs recorded from labeled PVN neurons during control and application of DCG-IV (0.1 and 1 µM) in 1 SHR and 1 WKY rat. B, Summary data show the concentration-dependent inhibitory effect of DCG-IV on the frequency and amplitude of sEPSCs in WKY rats (n = 8) and SHRs (n = 7). C, Comparison of the inhibitory effect of DCG-IV on the frequency of sEPSCs of PVN neurons (shown in B) in WKY rats and SHRs. D, Group data show that LY341495 (100 nM) blocked the effect of DCG-IV (1 µM) on the frequency of sEPSCs of labeled PVN neurons in SHRs and WKY rats (n = 7 neurons in each group). * P < 0.05, compared with the respective baseline control. # P < 0.05, compared with the corresponding value in WKY rats.
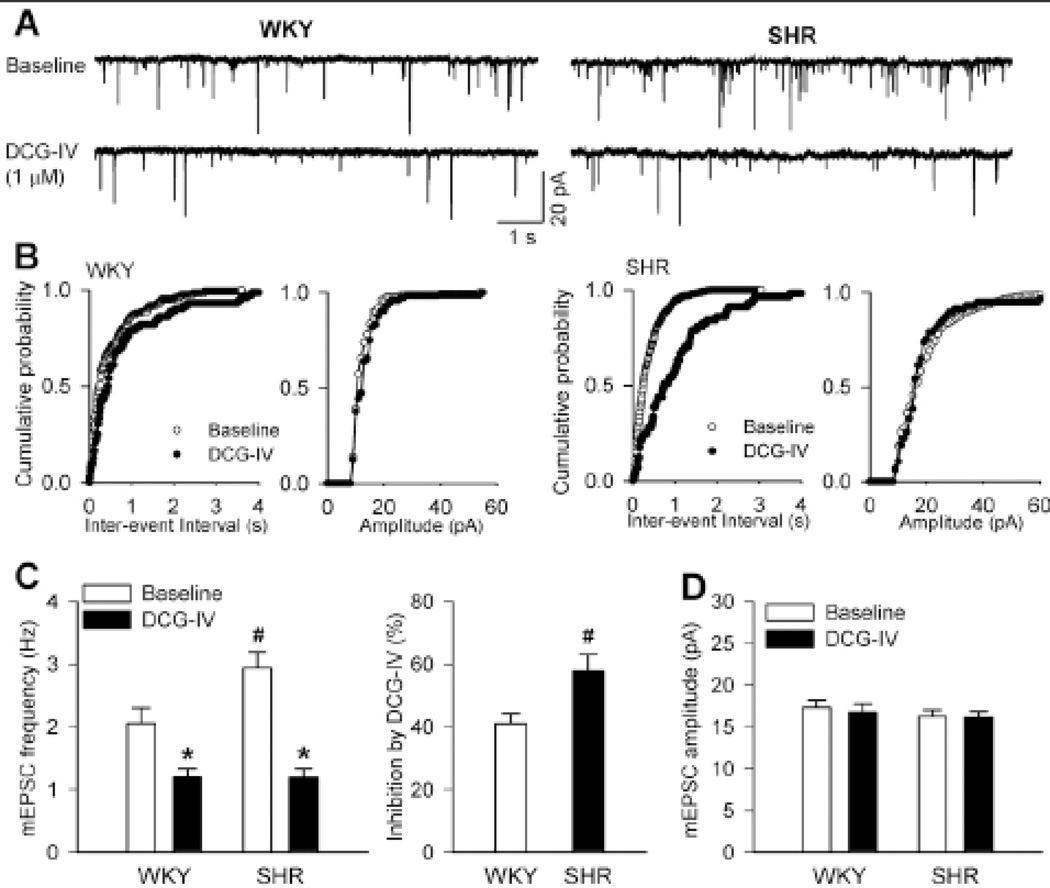
To further determine the activity of presynaptic group II mGluRs at glutamatergic terminals in hypertension, we examined the effect of DCG-IV on mEPSCs of spinally projecting PVN neurons in SHRs and WKY rats. Bath application of 1 µM DCG-IV significantly inhibited the frequency, but not the amplitude, of mEPSCs in labeled PVN neurons in both WKY rats and SHRs (Fig. 2, A–D). The cumulative probability analysis of mEPSCs revealed that the distribution pattern of the inter-event interval of mEPSCs was shifted toward the right in response to DCG-IV (Fig. 2, B,C). The inhibition of the frequency of mEPSCs by DCG-IV in labeled PVN neurons was significantly greater in SHRs than in WKY rats (Fig. 2C).
Figure 2. DCG-IV reduces the frequency of mEPSCs of spinally projecting PVN neurons more in SHRs than in WKY rats.
A, Original recordings show mEPSCs recorded from labeled PVN neurons during control and application of DCG-IV (1 µM) in 1 WKY rat and 1 SHR. B, Cumulative probability plots from the same neuron in A show the distribution of interevent interval and amplitude of mEPSCs during control and DCG-IV application. C and D, Summary data show the effect of DCG-IV (1 µM) on the frequency and amplitude of mEPSCs of labeled PVN neurons in WKY rats and SHRs (n = 8 neurons in each group). * P < 0.05, compared with the respective baseline value. # P < 0.05, compared with the corresponding value in WKY rats.
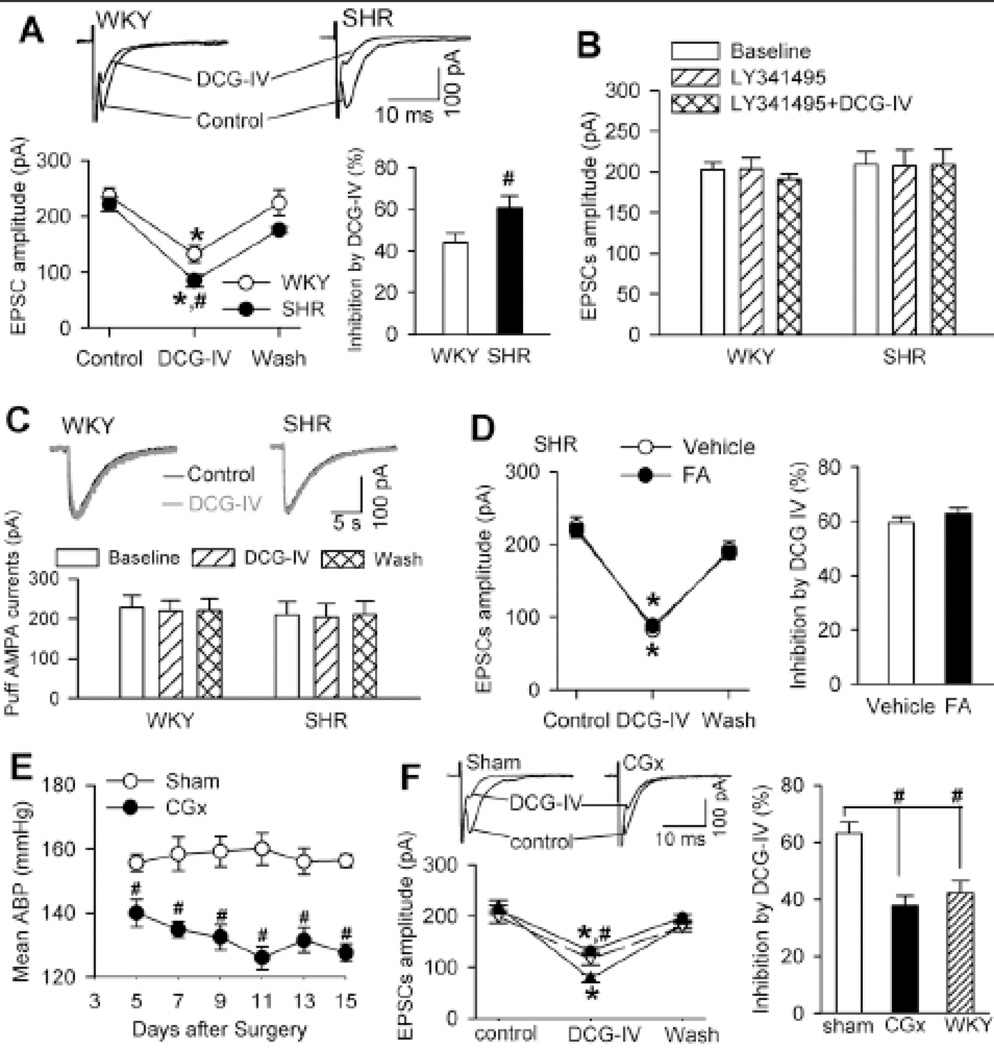
We next confirmed the presynaptic effects of DCG-IV on evoked EPSCs by including a general G protein inhibitor, GDP-β-s (1 mM), in the pipette solution to block the postsynaptic effect of DCG-IV 22. In WKY rats, bath application of 1 µM DCG-IV significantly decreased the amplitude of evoked EPSCs in the labeled PVN neurons (44.09 ± 4.23% reduction, n = 8). In SHRs, DCG-IV caused a much larger reduction in the amplitude of evoked EPSCs (60.74 ± 5.68% reduction, n = 8, P < 0.05; Fig. 3A). Bath application of 100 nM LY341495 abolished the inhibitory effects of DCG-IV on evoked EPSCs of PVN neurons in both WKY rats and SHRs (Fig. 3B). In addition, bath application of 1 µM DCG-IV had no significant effect on the amplitude of currents elicited by puff AMPA (100 µM) application onto labeled PVN neurons in WKY rats and SHRs (Fig. 3C).
Figure 3. DCG-IV inhibits synaptically evoked EPSCs of spinally projecting PVN neurons more in SHRs than in WKY rats.
A, Original traces and group data show the effects of DCG-IV (1 µM) on electrically evoked EPSCs of labeled PVN neurons recorded with intracellular dialysis of 1 mM GDP-β-s in SHRs and WKY rats (n = 8 neurons in each group). B, Summary data show LY341495 blocked the effect of DCG-IV on the amplitude of evoked EPSCs in WKY rats (n = 7) and SHRs (n = 8). C, Original recordings and summary data show the effect of DCG-IV on AMPA receptor currents elicited by AMPA puff application in WKY rats and SHRs (n = 7 in each group). D, Summary data show the effect of DCG-IV on evoked EPSCs of labeled PVN neurons in vehicle- and fluoroacetate (FA)-treated brain slices obtained from SHRs (n = 7 neurons in each group). E, Group data show the time course of changes in mean ABP of SHRs subjected to sham surgery or CGx (n = 5 rats in each group). F, Original traces and summary data comparing DCG-IV–induced inhibition of evoked EPSCs in SHRs undergoing CGx (n = 8 neurons) and sham controls (n = 7 neurons). The effect of DCG-IV on evoked EPSCs in WKY rats (n = 8 neurons) was also plotted as the control. * P < 0.05, compared with the respective baseline control. # P < 0.05, compared with the corresponding value in WKY rats or sham controls.
It has been shown that group II mGluRs are present on glia in some brain regions 24. To determine the possible contribution of these group II mGluRs located on glia to the inhibitory effect of DCG-IV on glutamatergic input to PVN neurons in SHRs, we treated brain slices with fluoroacetate, a selective inhibitor of glial metabolism 25. Incubating brain slices with 5 mM fluoroacetate for 30–50 min did not significantly change the inhibitory effect of DCG-IV on evoked EPSCs of PVN neurons in SHRs (Fig. 3D). Collectively, these results strongly suggest that the activity of presynaptic group II mGluRs at glutamatergic terminals is increased in the PVN in hypertension.
Increased presynaptic group II mGluR activity in the PVN in hypertension is an adaptive response to increased ABP
To determine whether the increased group II mGluR activity is the cause of hypertension or a secondary adaptive response to increased ABP, we examined the effects of DCG-IV on evoked EPSCs (recorded with GDP-β-s included in the pipette solution) of spinally projecting PVN neurons in SHRs two weeks after lowering the ABP with CGx 14. Compared with the sham surgery, CGx significantly lowered the mean ABP, measured with radiotelemetry, for at least 2 weeks in SHRs (Fig. 3E). CGx had no effects on the food or water intake and body weight in all rats studied. DCG-IV (1 µM) produced a significantly smaller inhibitory effect on evoked EPSCs in labeled PVN neurons in SHRs undergoing CGx than in the sham group (Fig. 3F). Notably, the effect of DCG-IV on evoked EPSCs of labeled PVN neurons in SHRs subjected to CGx was not significantly different from that in WKY rats.
Group II mGluR activity controlling GABAergic input to PVN presympathetic neurons does not change in hypertension
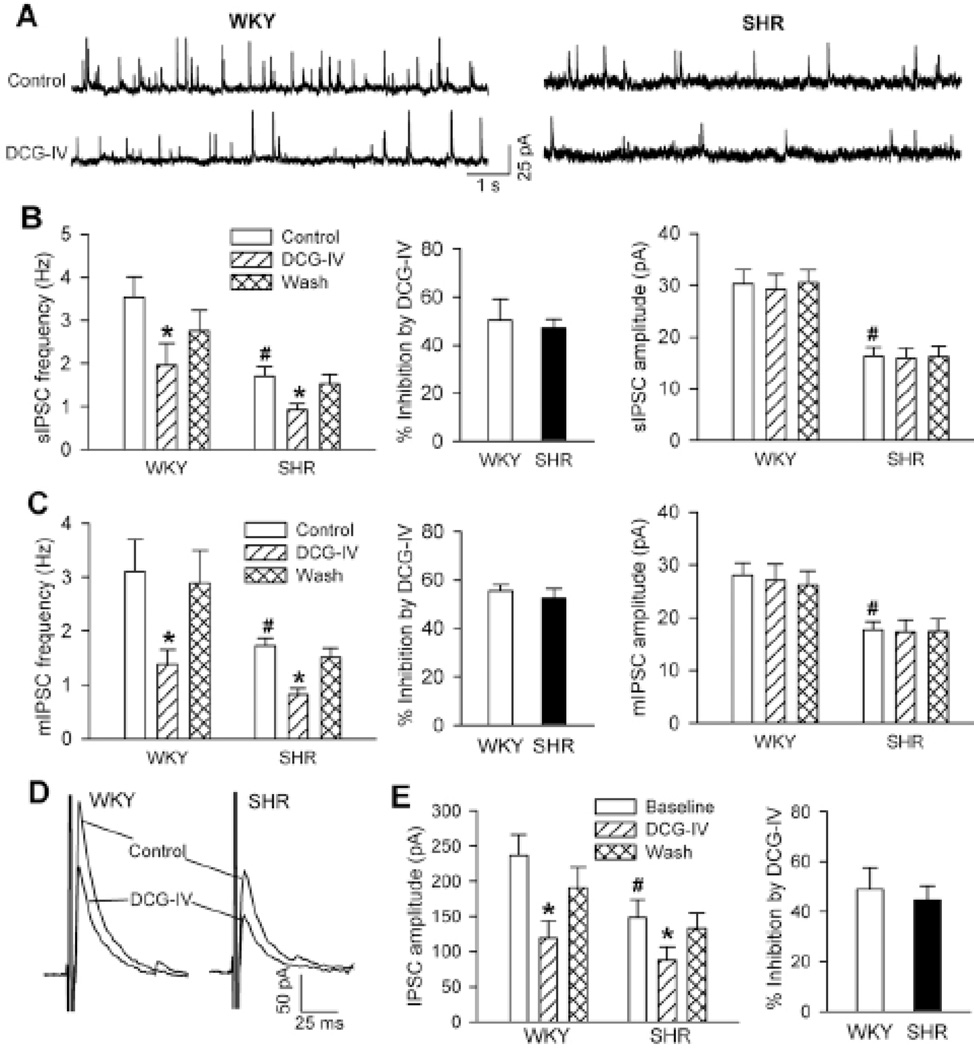
The activity of PVN presympathetic neurons is finely tuned by both excitatory glutamatergic and inhibitory GABAergic inputs 12,26,27. To determine whether the control of GABAergic input by the activity of group II mGluRs in the PVN is altered in hypertension, we compared the effect of DCG-IV on GABAergic IPSCs of spinally projecting PVN neurons in WKY rats and SHRs. The basal frequency of spontaneous IPSCs (sIPSCs) was significantly lower in the PVN in SHRs than in WKY rats (Fig. 4, A,B), and the amplitude of sIPSCs was significantly smaller in SHRs than in WKY rats (Fig. 4, A,B). Bath application of DCG-IV (1 µM) significantly decreased the frequency, but not the amplitude, of sIPSCs in both WKY rats and SHRs (Fig. 4, A,B). The inhibitory effect of DCG-IV on the frequency of sIPSCs did not differ significantly between WKY rats and SHRs (Fig. 4B).
Figure 4. DCG-IV similarly reduces GABAergic IPSCs of spinally projecting PVN neurons in WKY rats and SHRs.
A, Original traces show sIPSCs of labeled PVN neurons during control and application of 1.0 µM DCG-IV in 1 WKY rat and 1 SHR. B, Summary data show the effect of DCG-IV on the frequency and amplitude of sIPSCs in WKY rats (n = 8) and SHRs (n = 11). C, Group data show the effect of DCG-IV on the frequency and amplitude of mIPSCs in WKY rats (n = 8) and SHRs (n = 7). D, Representative recordings of evoked IPSCs of labeled PVN neurons during control and the application of 1 µM DCG-IV in 1 WKY rat and 1 SHR. E, Summary data show the effect of DCG-IV on evoked IPSCs in WKY rats and SHRs (n = 8 in each group). * P < 0.05, compared with the respective baseline control. # P < 0.05, compared with the corresponding value in WKY rats.
The basal frequency and amplitude of mIPSCs in labeled PVN neurons were also significantly reduced in SHRs compared with WKY rats (Fig. 4C). Bath application of 1 µM DCG-IV significantly decreased the frequency, but not the amplitude, of mIPSCs in both WKY rats and SHRs. The inhibitory effect of DCG-IV on the frequency of mIPSCs in labeled PVN neurons did not differ significantly between WKY rats and SHRs (Fig. 4C).
We also examined the effect of DCG-IV on evoked IPSCs (recorded with GDP-β-s included in the pipette solution) of spinally projecting PVN neurons in WKY rats and SHRs. The basal amplitude of evoked IPSCs was significantly smaller in SHRs than in WKY rats (Fig. 4, D,E). DCG-IV (1 µM) produced a similar magnitude of reduction in the amplitude of evoked IPSCs in WKY rats and SHRs (Fig. 4E). These data suggest that the activity of presynaptic group II mGluRs at GABAergic terminals in the PVN is not altered in hypertension.
Group II mGluRs regulate the firing activity of PVN presympathetic neurons in hypertension
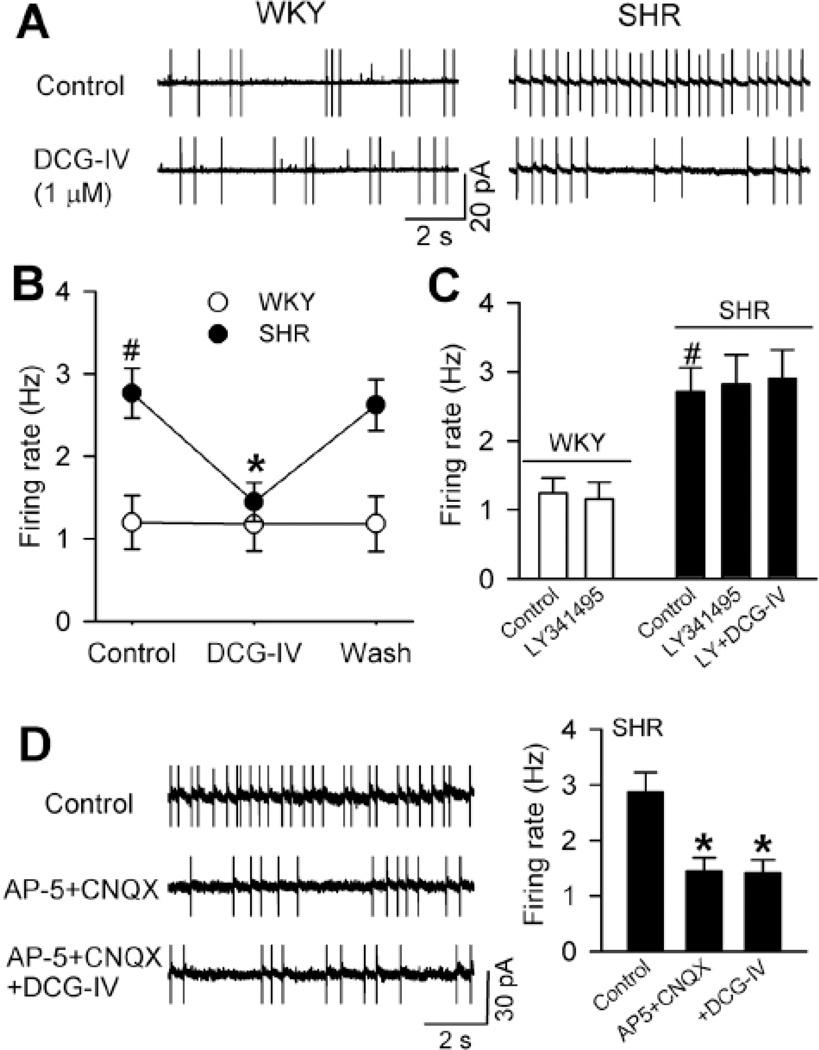
To determine the role of group II mGluRs in the control of the firing of PVN presympathetic neurons in hypertension, we tested the effect of DCG-IV on the firing activity of labeled PVN neurons with cell-attached recordings in SHRs and WKY rats. The basal firing rate of PVN neurons in SHRs was significantly higher than that in WKY rats. Bath application of DCG-IV (1 µM) had no significant effect on the firing activity of PVN neurons in WKY rats. In contrast, DCG-IV significantly decreased the firing rate of PVN neurons in SHRs (Fig. 5, A,B). The group II mGluR antagonist LY341495 abolished the inhibitory effect of DCG-IV on the firing activity in SHRs (Fig. 5C).
Figure 5. DCG-IV differentially inhibits the firing of spinally projecting PVN neurons in SHRs.
A, Original cell-attached recordings show the firing activity of labeled PVN neurons during control and application of 1 µM DCG-IV in 1 WKY rat and 1 SHR. B, Summary data show DCG-IV reduced the firing rate of labeled PVN neurons in SHRs but not in WKY rats (n = 10 in each group). C, Group data show LY341595 blocked the effect of DCG-IV on the firing activity of PVN neurons in SHRs (n = 8). LY341595 had no effect on the basal firing of PVN neurons in WKY rats (n = 6). D, Original traces and summary data show that DCG-IV had no further effect on the firing of PVN neurons of SHRs in the presence of AP-5 and CNQX (n = 6). * P < 0.05, compared with the baseline control. # P < 0.05, compared with the corresponding value in WKY rats.
In additional labeled PVN neurons in SHRs, blocking NMDA and AMPA receptors with their respective antagonists, AP-5 (50 µM) and CNQX (20 µM), significantly reduced their basal firing activity (Fig. 5D). In the presence of AP5 and CNQX, DCG-IV failed to further inhibit the firing activity of these PVN neurons in SHRs (Fig. 5D). These results suggest that activation of group II mGluRs inhibits the firing of PVN presympathetic neurons through attenuation of glutamatergic input in hypertension.
Group II mGluRs in the PVN control sympathetic vasomotor tone in hypertension
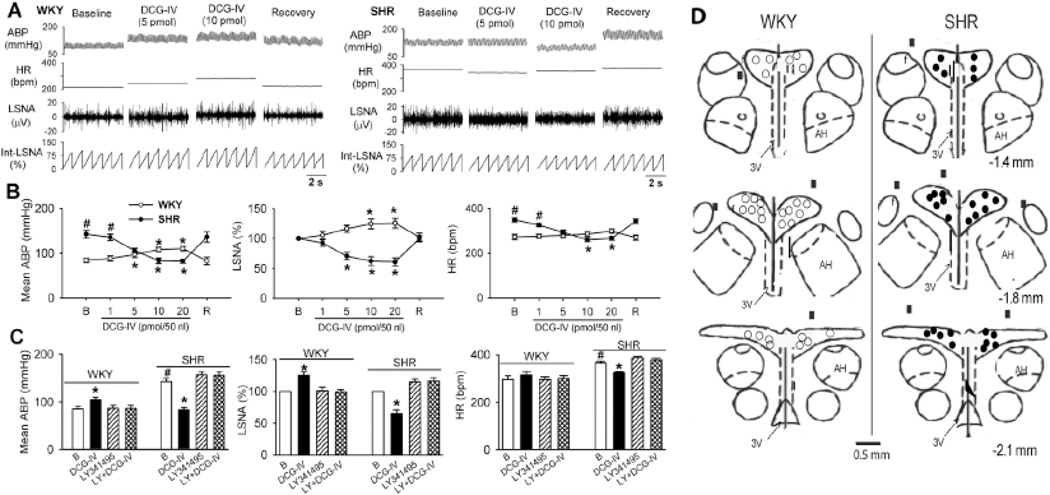
To determine the role of group II mGluRs in the PVN in controlling sympathetic outflow in hypertension, we microinjected DCG-IV directly into the PVN. Bilateral microinjection of DCG-IV (10–20 pmol/50 nl) slightly increased LSNA and mean ABP in WKY rats (n = 7 rats; Fig. 6, A,B). In contrast, in SHRs, microinjection of DCG-IV (5–20 pmol/50 nl) into the PVN significantly decreased LSNA, mean ABP, and HR in a dose-dependent manner (n = 7 rats). Bilateral microinjection of LY341495 (6 pmol/50 nl) had no significant effect on LSNA, mean ABP, and HR in WKY rats or SHRs (Fig. 6C). LY341495 abolished the effect of DCG-IV on LSNA, mean ABP, and HR in SHRs and WKY rats (Fig. 6C). These data suggest that activation of group II mGluRs in the PVN inhibits sympathetic outflow in hypertension.
Figure 6. Microinjection of DCG-IV into the PVN inhibits sympathetic vasomotor tone in SHRs.
A, Representative recordings show the effect of bilateral microinjection of DCG-IV (5 and 10 pmol/50 nl) into the PVN on ABP, HR, LSNA, and integrated LSNA (Int-LSNA) in 1 SHR and 1 WKY rat. B, Summary data show differential effects of microinjection of DCG-IV (1–20 pmol/50 nl) into the PVN on LSNA, mean ABP, and HR in SHRs and WKY rats (n = 7 in each group). C, Group data show that microinjection of LY341495 (6 pmol/50 nl) blocked the effect of DCG-IV on LSNA, mean ABP, and HR in WKY rats and SHRs (n = 7 in each group). B, baseline; R, recovery; LY, LY341495. * P < 0.05 compared with the corresponding baseline control. # P < 0.05, compared with the corresponding value in WKY rats. D, Schematic drawings show the microinjection sites in WKY rats and SHRs. Note that misplacement of microinjections was marked with filled squares. The distance posterior to the bregma is shown below the diagram in each panel. AH, anterior hypothalamus; 3V, the third ventricle.
During the course of this study, 2 WKY rats and 3 SHRs were excluded from the data analysis because of the misplacement of microinjections (Fig. 6D). Microinjection of DCG-IV (20 pmol/50 nl) had minimal effect on ABP (WKY: 85.8 ± 4.6 vs. 84.3 ± 3.7 mmHg; SHR: 138.5 ± 8.5 vs. 140.1 ± 6.2 mmHg), LSNA (WKY: 100% vs. 101.5 ± 0.5%; SHR: 100% vs. 101.4 ± 1.7%), and HR (WKY: 278.5 ± 10.5 vs. 282.0 ± 9.0 bpm; SHR: 340.8 ± 12.1 vs. 344.8 ± 12.3 bpm) in these 2 WKY rats and 3 SHRs.
Discussion
The major objective of our study was to determine the role of group II mGluRs in the control of synaptic input to PVN presympathetic neurons and sympathetic outflow in a commonly used animal model of essential hypertension. At the present time, there are no available methods to unequivocally identify PVN neurons involved only in ABP control in brain slices. We acknowledge that not all spinally projecting PVN neurons are exclusively involved in ABP regulation. Group II mGluRs are located at presynaptic terminals, postsynaptic neurons, and glia in several brain regions 28. In this study, we found that activation of group II mGluRs with DCG-IV significantly inhibited the frequency, but not the amplitude, of sEPSCs and mEPSCs in spinally projecting PVN neurons in both WKY rats and SHRs. DCG-IV also significantly reduced the amplitude of electrically evoked EPSCs when GDP-β-s was intracellularly dialyzed to inhibit the postsynaptic G proteins. Furthermore, DCG-IV had no significant effect on postsynaptic AMPA receptor currents elicited by puff AMPA application. In addition, we found that the inhibitory effect of DCG-IV on evoked EPSCs of PVN neurons was not altered brain slices were treated with fluoroacetate to inhibit glial function, which suggests that glial cells are not involved in the control of glutamatergic input by group II mGluRs in the PVN. Our findings indicate that activation of group II mGluRs inhibits glutamatergic input to PVN presympathetic neurons through a presynaptic mechanism.
An important finding of our study is that the activity of group II mGluRs on glutamatergic terminals is increased in the PVN in hypertension. This conclusion is supported by our data showing that the effects of DCG-IV on the frequency of sEPSCs and mEPSCs and the amplitude of evoked EPSCs in the PVN were significantly greater in SHRs than in WKY rats. Interestingly, lowering ABP in SHRs with CGx normalized the inhibitory effect of DCG-IV on evoked EPSCs of PVN neurons to the same level observed in WKY rats. This finding suggests that the increased activity of group II mGluRs at the presynaptic glutamatergic terminals in the PVN in SHRs is a secondary adaptive response to increased ABP. Although it is not clear whether this increased activity of presynaptic group II mGluRs in SHRs results from an increased receptor number or from signal coupling, the increased activity of group II mGluRs at the glutamatergic terminals may constrain synaptic glutamate release to PVN presympathetic neurons in hypertension.
The firing activity of PVN presympathetic neurons is finely tuned by both glutamatergic and GABAergic inputs 3,26,27,29. We have shown that the inhibitory GABAergic input to PVN presympathetic neurons is diminished in SHRs 11,15,30. In the present study, we found that DCG-IV significantly reduced the amplitude of evoked IPSCs of spinally projecting PVN neurons in SHRs and WKY rats. DCG-IV treatment also significantly inhibited the frequency, but not the amplitude, of GABAergic sIPSCs and mIPSCs of PVN neurons in both groups. These data suggest that the activation of group II mGluRs also inhibits GABAergic transmission through a presynaptic mechanism. However, the degree of presynaptic effects of DCG-IV in the PVN did not differ significantly between SHRs and WKY rats. These findings suggest that the activity of group II mGluRs at GABAergic terminals is not altered in the PVN in hypertension.
The increase in excitatory glutamatergic input to PVN presympathetic neurons in SHRs is caused by augmentation of pre- and postsynaptic NMDA receptor activity by the protein kinase CK2 12,31. Interestingly, we found that DCG-IV inhibited the firing activity of spinally projecting PVN neurons only in SHRs, although DCG-IV inhibited synaptic glutamate release in both SHRs and WKY rats. The lack of an inhibitory effect of DCG-IV on the firing of PVN neurons in WKY rats is probably due to the fact that group II mGluR activation also removed inhibitory GABAergic input to PVN neurons in WKY rats. Because of augmented excitatory glutamatergic input and diminished inhibitory GABAergic input in the PVN in SHRs 3,11,12,30, activation of group II mGluRs could reduce the excitability of PVN presympathetic neurons by attenuation of increased glutamatergic input in hypertension. This hypothesis is supported by our finding that the inhibitory effect of DCG-IV on the firing activity of PVN neurons in SHRs was abolished when NMDA and AMPA receptors were blocked. Although the presynaptic glutamatergic neuronal soma may not be present within the PVN, group II mGluRs present on the presynaptic terminals are directly involved in controlling the firing activity of postsynaptic (spinally projecting) neurons in the PVN in SHRs.
Another significant finding of our study is that activation of group II mGluRs in the PVN inhibited sympathetic vasomotor tone only in SHRs. Microinjection of DCG-IV into the PVN induced a significant and dose-dependent reduction in the ABP and LSNA in SHRs. These results are consistent with our brain slice recordings showing an inhibitory effect of DCG-IV on the firing activity of PVN neurons only in SHRs. However, we unexpectedly found that microinjection of DCG-IV into the PVN slightly increased the ABP and LSNA in WKY rats at high doses. It is not clear how DCG-IV in the PVN increases ABP and sympathetic nerve activity in WKY rats. It is unlikely that DCG-IV produces a non-specific effect, because blocking group II mGluRs with LY341495 abolished the DCG-IV effect in both SHRs and WKY rats. Because GABAergic inhibition of PVN neuronal excitability is more pronounced in WKY rats than in SHRs 11,15,30, removal of GABAergic input by the group II mGluR agonist may disinhibit PVN presympathetic neurons in vivo, leading to a small increase in sympathetic outflow in WKY rats. Although we did not observe a stimulatory effect of DCG-IV on neuronal firing in brain slices of WKY rats, it is possible that certain inhibitory interneurons might have been removed in our thin brain slice preparation.
Perspectives
We found that activation of group II mGluRs inhibits both glutamatergic and GABAergic transmission at a presynaptic site in the PVN. The activity of group II mGluRs at glutamatergic terminals is increased in hypertension, which may limit excessive glutamate release through a negative feedback mechanism. This finding provides further evidence of synaptic and neuronal plasticity in the PVN associated with hypertension. We also show that activation of group II mGluRs inhibits the hyperactivity of PVN presympathetic neurons and sympathetic vasomotor tone only in SHRs and not in normotensive rats. On the basis of these findings, group II mGluRs may represent a potential therapeutic target for the treatment of neurogenic hypertension. It should be noted that because the group II mGluR agonist was microinjected into the PVN, it can inhibit sympathetic outflow by affecting neurons other than just spinally projecting neurons. Further studies are also needed to determine changes in presynaptic group II mGluRs in the PVN in other models of hypertension and to examine the long-term effect of the group II mGluR agonist in conscious animal models of hypertension.
Supplementary Material
Novelty and Significance.
- What Is New:
- Presynaptic group II mGluRs regulate excitatory and inhibitory synaptic input to presympathetic neurons in the hypothalamus
- Group II mGluR stimulation inhibits hyperactivity of hypothalamic presympathetic neurons in hypertension by reducing glutamatergic input
- Group II mGluR activation in the hypothalamus reduces sympathetic nerve discharges and arterial blood pressure in hypertension
- What Is Relevant:
- Increased glutamatergic input and excitability of presympathetic neurons in the hypothalamus contribute to elevated sympathetic output in hypertension
- Group II mGluRs may be targeted for treatment of hypertension by reducing the sympathetic vasomotor tone.
- Summary:
- Activation of group II mGluRs in the hypothalamus inhibits sympathetic drive and hyperactivity of presympathetic neurons by reducing synaptic glutamate release.
Acknowledgments
Source(s) of Funding
This study was supported by the National Institutes of Health (grant HL077400), a postdoctoral fellowship from the American Heart Association–South Central Affiliate (to Z.-Y.Y), and the N.G. and Helen T. Hawkins Endowment (to H.-L.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict(s) of Interest/Disclosure(s)
None.
References
- 1.Ciriello J, Kline RL, Zhang TX, Caverson MM. Lesions of the paraventricular nucleus alter the development of spontaneous hypertension in the rat. Brain Res. 1984;310:355–359. doi: 10.1016/0006-8993(84)90159-8. [DOI] [PubMed] [Google Scholar]
- 2.Eilam R, Malach R, Segal M. Selective elimination of hypothalamic neurons by grafted hypertension-inducing neural tissue. J Neurosci. 1994;14:4891–4902. doi: 10.1523/JNEUROSCI.14-08-04891.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li DP, Pan HL. Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension. 2007;49:916–925. doi: 10.1161/01.HYP.0000259666.99449.74. [DOI] [PubMed] [Google Scholar]
- 4.Kannan H, Hayashida Y, Yamashita H. Increase in sympathetic outflow by paraventricular nucleus stimulation in awake rats. Am J Physiol. 1989;256:R1325–R1330. doi: 10.1152/ajpregu.1989.256.6.R1325. [DOI] [PubMed] [Google Scholar]
- 5.Martin DS, Haywood JR. Hemodynamic responses to paraventricular nucleus disinhibition with bicuculline in conscious rats. Am J Physiol. 1993;265:H1727–H1733. doi: 10.1152/ajpheart.1993.265.5.H1727. [DOI] [PubMed] [Google Scholar]
- 6.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 7.Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience. 2000;100:549–556. doi: 10.1016/s0306-4522(00)00283-9. [DOI] [PubMed] [Google Scholar]
- 8.Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1989;14:177–183. doi: 10.1161/01.hyp.14.2.177. [DOI] [PubMed] [Google Scholar]
- 9.Greenwood JP, Stoker JB, Mary DA. Single-unit sympathetic discharge : quantitative assessment in human hypertensive disease. Circulation. 1999;100:1305–1310. doi: 10.1161/01.cir.100.12.1305. [DOI] [PubMed] [Google Scholar]
- 10.Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension. 1999;34:724–728. doi: 10.1161/01.hyp.34.4.724. [DOI] [PubMed] [Google Scholar]
- 11.Li DP, Pan HL. Plasticity of GABAergic control of hypothalamic presympathetic neurons in hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H1110–H1119. doi: 10.1152/ajpheart.00788.2005. [DOI] [PubMed] [Google Scholar]
- 12.Li DP, Yang Q, Pan HM, Pan HL. Pre- and postsynaptic plasticity underlying augmented glutamatergic inputs to hypothalamic presympathetic neurons in spontaneously hypertensive rats. J Physiol. 2008;586:1637–1647. doi: 10.1113/jphysiol.2007.149732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li DP, Byan HS, Pan HL. Switch to glutamate receptor 2-lacking AMPA receptors increases neuronal excitability in hypothalamus and sympathetic drive in hypertension. J Neurosci. 2012;32:372–380. doi: 10.1523/JNEUROSCI.3222-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye ZY, Li DP, Li L, Pan HL. Protein kinase CK2 increases glutamatergic input in the hypothalamus and sympathetic vasomotor tone in hypertension. J Neurosci. 2011;31:8271–8279. doi: 10.1523/JNEUROSCI.1147-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye ZY, Li DP, Byun HS, Li L, Pan HL. NKCC1 upregulation disrupts chloride homeostasis in the hypothalamus and increases neuronal activity-sympathetic drive in hypertension. J Neurosci. 2012;32:8560–8568. doi: 10.1523/JNEUROSCI.1346-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- 17.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 18.Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J Comp Neurol. 1993;335:252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- 19.Gu G, Lorrain DS, Wei H, Cole RL, Zhang X, Daggett LP, Schaffhauser HJ, Bristow LJ, Lechner SM. Distribution of metabotropic glutamate 2 and 3 receptors in the rat forebrain: Implication in emotional responses and central disinhibition. Brain Res. 2008;1197:47–62. doi: 10.1016/j.brainres.2007.12.057. [DOI] [PubMed] [Google Scholar]
- 20.Kolaj M, Renaud LP. Metabotropic glutamate receptors in median preoptic neurons modulate neuronal excitability and glutamatergic and GABAergic inputs from the subfornical organ. J Neurophysiol. 2010;103:1104–1113. doi: 10.1152/jn.00808.2009. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi Y, Sekiyama N, Nakanishi S, Jane DE, Sunter DC, Birse EF, Udvarhelyi PM, Watkins JC. Analysis of agonist and antagonist activities of phenylglycine derivatives for different cloned metabotropic glutamate receptor subtypes. J Neurosci. 1994;14:3370–3377. doi: 10.1523/JNEUROSCI.14-05-03370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou HY, Chen SR, Chen H, Pan HL. Functional plasticity of group II metabotropic glutamate receptors in regulating spinal excitatory and inhibitory synaptic input in neuropathic pain. J Pharmacol Exp Ther. 2011;336:254–264. doi: 10.1124/jpet.110.173112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson H, Neale SA, Salt TE. Activation of Group II and Group III metabotropic glutamate receptors by endogenous ligand(s) and the modulation of synaptic transmission in the superficial superior colliculus. Neuropharmacology. 2004;47:822–832. doi: 10.1016/j.neuropharm.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- 25.Hulsmann S, Oku Y, Zhang W, Richter DW. Metabolic coupling between glia and neurons is necessary for maintaining respiratory activity in transverse medullary slices of neonatal mouse. Eur J Neurosci. 2000;12:856–862. doi: 10.1046/j.1460-9568.2000.00973.x. [DOI] [PubMed] [Google Scholar]
- 26.Li DP, Chen SR, Pan HL. Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J Neurosci. 2003;23:5041–5049. doi: 10.1523/JNEUROSCI.23-12-05041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li DP, Chen SR, Pan HL. VR1 receptor activation induces glutamate release and postsynaptic firing in the paraventricular nucleus. J Neurophysiol. 2004;92:1807–1816. doi: 10.1152/jn.00171.2004. [DOI] [PubMed] [Google Scholar]
- 28.Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- 29.Li DP, Chen SR, Finnegan TF, Pan HL. Signalling pathway of nitric oxide in synaptic GABA release in the rat paraventricular nucleus. J Physiol. 2004;554:100–110. doi: 10.1113/jphysiol.2003.053371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li DP, Pan HL. Role of gamma-aminobutyric acid (GABA)A and GABAB receptors in paraventricular nucleus in control of sympathetic vasomotor tone in hypertension. J Pharmacol Exp Ther. 2007;320:615–626. doi: 10.1124/jpet.106.109538. [DOI] [PubMed] [Google Scholar]
- 31.Ye ZY, Li L, Li DP, Pan HL. Casein kinase 2-mediated synaptic GluN2A up-regulation increases N-methyl-D-aspartate receptor activity and excitability of hypothalamic neurons in hypertension. J Biol Chem. 2012;287:17438–17446. doi: 10.1074/jbc.M111.331165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.