Abstract
The therapeutic index of highly conformal radiotherapy (RT) depends on adequate selection and delineation of the gross tumor volumes (GTVs), the clinical target volumes (CTVs), and the tissues and organs whose sparing is likely to gain clinical benefit. Decisions about target and tissue selection and delineation affect the balance of reward and risk of highly conformal RT. Some of these issues relating to head and neck (HN) cancer, including target delineation after tumor shrinkage by induction chemotherapy or at mid-radiotherapy, are discussed in this paper.
Introduction
The ability of highly conformal radiotherapy (RT) to limit the prescribed high doses to the targets and reduce doses to organs and tissues in their vicinity enables an improvement of the therapeutic index. As dosimetric compromises between high target doses and minimizing adjacent organ doses are inevitable, maximizing the therapeutic index relies on optimal selection and delineation of both targets and organs. Marginal tumor recurrence is the highest risk associated with inadequate target volume delineation, and individual differences in addressing this risk and balancing it with organ sparing goals may account for some of the differences among physicians in how conservatively they delineate the targets. This issue is especially important in head and neck (HN) cancer, where inter-observer differences in both selecting and outlying the targets are larger than physical target dose coverage deficiencies or dose uncertainties due to set-up deviations (1). To date, clinical data gathered from series of intensity modulated RT (IMRT) may help address certain risk/benefit issues in an evidence-based approach. This review will attempt to use such an approach where data is available. It will examine separately the delineation of the gross tumor volumes (GTVs), the clinical target volumes (CTVs), and the critical organs, emphasizing the importance of the tissue embedded within the target volumes, which is often neglected in IMRT planning.
The GTVs
GTV determines the volume containing the highest tumor cell density and receiving the highest prescribed dose. Its accurate delineation, therefore, has the highest reward/risk ratio. If we could delineate the “true” GTV and avoid delineating inflammation and other artifacts, we would be able to spare adjacent tissue from unnecessarily high radiation doses. Imaging and physical examination are the basis for the delineation of the primary tumor GTV. The contrast-enhanced CT is the basis for imaging-derived GTV; MRI is essential for the delineation of the GTV near the base of skull in cases of advanced nasopharyngeal or paranasal sinus cancers, where the bony anatomy interferes with CT-based assessment of soft tissue details (2). Using FDG-PET to determine the GTV has been studied extensively in recent years (3). Relying on imaging alone and striving to find the best modality which tells us the “truth” is tempting. Many published studies assessed the reproducibility of GTV delineation among different physicians and reported large inter-observer differences (1, 4–7). None of the participating physicians in these studies had an opportunity to examine the patient, depriving the participants of an important piece of information. This may explain in part the reported variability in GTV delineation. However, after providing basic delineation guidelines are given to physicians, reduced variability was observed, emphasizing the importance of training and familiarity with delineation criteria (8). Adding additional imaging, such as FDG-PET, to the contrast-enhanced planning CT scan, may reduce somewhat the inter-observer variability but is not likely to completely abolish it (9–11).
The limits of defining the GTV based on imaging alone are illustrated by the study of Daisne et al (12). They performed pretreatment CT, MRI, and FDG-PET on patients with laryngeal and oropharyngeal cancers, nine of whom subsequently underwent total laryngectomy, and the surgical specimens were compared to the registered radiographic images. There was no difference between total CT and MR volumes, but GTVs obtained from FDG-PET were smaller than the other two modalities and the surgical specimen GTVs were even smaller, indicating overestimation of the GTVs by all 3 imaging modalities. However, when examined in detail, despite overestimating in most dimensions, all 3 imaging modalities underestimated the mucosal extent of disease. Thus, the mucosal tumor extent is the one likely to be better assessed by physical examination, including palpation and fiberoptic endoscopy, than by imaging.
A clinical example includes examination of base of tongue cancer, whose mucosal extent can easily be appreciated by palpation, while its extent on imaging can be hampered by the uncertainties of CT and the non-specific, physiologic FDG-PET uptake in the non-involved base of tongue. In cases of locally advanced tonsillar cancer, involvement of the palate, and its extent - whether to the lateral palate, or more extensively toward the midline, are better appreciated by careful observation than by any imaging modality. Similarly, palpation of the glosso-tonsillar sulcus can verify better than imaging whether the tonsillar tumor extends towards the tongue and base of tongue or not. Determining these tumor extents is crucial in order to avoid GTV underestimation or over-estimation resulting in unnecessary inclusion of non-involved parts of the oral cavity in the high-does volumes. A detailed recording of the physical examination is likely to improve the consistency of GTV delineation among observers in a study of delineation reproducibility. More importantly, it is an essential step in GTV delineation in the clinic.
When targets are outlined on FDG-PET and registered with the planning CT, the PET-GTV is often smaller than the CT-based GTV (12–14). If we could assume that PET showed us the “true” target and relied on the PET-based GTV alone, as suggested by some authors, we would be able to reduce the GTV in many cases and allow better sparing of neighboring tissues (13, 15). Does this reward balance the risk of missing tumor potentially observed on CT but not on PET? Matching the surgical specimens to the PET resulted in on average a 13% mismatch between the specimen and the PET-GTV (12). Superficial tumor extension to the contralateral larynx or the subglottis, as well as small extralaryngeal extension were indeed missed. But interestingly, although overestimating the GTV, CT and MR also missed similar tumor extension, illustrating the limit in spatial resolution of current imaging modalities, and emphasizing again the need to integrate the clinical examination in the target volume delineation.
The mismatch between the PET-defined GTV and the true extent of the tumor is likely to be affected by the specific algorithm used for PET image reconstruction, display windowing, image segmentation, threshold levels for contouring, and other issues detailed elsewhere (16–17). Despite these limitations, some authors have validated objective and user-independent methods for automatic segmentation of FDG-PET images (12, 18). Notwithstanding the intrinsic limitation in spatial resolution of PET imaging, such methods should be preferred to subjective methods, in order to homogenize as consistently as possible the functional target volume delineation.
Provided that the FDG-PET images are segmented properly, it is still unknown how to integrate functional imaging with anatomic imaging (CT or MRI) for GTV delineation. The debate regarding this issue is reflected in the somewhat different views among the authors of this article. Based on the study of Daisne and Geets, Gregoire has proposed to use the FDG-PET images to automatically segment GTV, around which a CTV is delineated (19). A prospective multicenter phase II study is ongoing to validate this concept. In contrast, Eisbruch has proposed using the union between the CT-based and PET-based GTVs, as done at the University of Michigan and other institutions (20–22). This argument is supported by studies that reported recurrences within the CT-based GTV but outside the PET-based GTV (20, 23). Such policy results in a larger target than using each modality alone, which might have consequences on the irradiation of non-target tissues, but it may be the safest option taking into account marginal miss risks. To reemphasize, irrespective of the strategy for using FDG-PET GTV, an additional necessary step is the integration of the physical examination results to obtain the final composite GTV. An expansion of the GTV by a small margin, typically 0.5 cm, accounts for a very high concentration of tumor cells that receives the full prescribed dose aimed at the gross disease.
IMRT typically delivers a non-uniform dose to the GTVs. The deliberate delivery of “hot spots” within the gross tumor can achieve steeper dose gradients outside the tumor, with better sparing of neighboring tissue (24), while potentially increasing tumor control probability (TCP). Tome and Fowler calculated that boost dose ratios of 1.2–1.3 are optimal if a substantial proportion of the GTV (60–80%) could be boosted, hypothetically resulting in significant gains in TCP (25). A partial GTV boost may be even more appealing if we can determine the parts of the GTV at highest risk of failure, due to high clonogen density, proliferation rate, hypoxic content or low cellular radiosensitivity, rather than boosting a random part of the tumor. Defining such a sub-target would be expected to improve the reward/risk ratio of the boost if the additional dose were high enough to overcome the cellular resistance, but of sufficiently small volume to prevent damage of critical tissue embedded within the boost volume (discussed below).
This approach, termed “dose painting” by Ling et al (26), has thus far been mostly investigated in head and neck cancer using FDG-PET or hypoxia imaging to define the sub-targets thought to be resistant and therefore worth boosting. A phase I study of boosting the pre-therapy FDG-PET-delineated sub-volumes within the GTV has been carried out the Ghent University hospital group in Belgium (23). Interestingly, a substantial number of local failures occurred within the boosted sub-volumes, attesting to both the validity of the underlying hypothesis regarding the local tumor resistance, and the limits of the added doses to overcome the resistance. Issues related to the choice of image intensity threshold for the FDG-PET sub-volume, as well as to the best way in which escalation should be made, for example, delivering dose proportional to the image intensity (“dose painting by numbers”), are subject to current research (27). An even more appealing use of FDG-PET for “dose painting” is using the imaged residual metabolically active tumor after part of the treatment course has been delivered, as was suggested by the Brussels group (28). The residual activity may represent parts of the tumor that are more radioresistant than the parts that ceased to be PET-avid after some radiation has been delivered (29). If it indeed represents a resistant sub-volume, notwithstanding inflammation that may interfere with defining the target in the latter phase of therapy, using the during-therapy FDG-PET-avid tumor as the target for dose escalation is likely to improve the reward/risk ratio compared with the pre-therapy metabolic image.
Similar to FDG-PET, tumor sub-volumes defined by hypoxia imaging have been proposed as targets for integrated boost as “hypoxia guided radiotherapy” (30–33). Compared with FDG-PET, which is spatially stable within the tumor before and throughout radiotherapy, hypoxia imaging shows significant spatial variability when repeated imaging is performed either pre- (34) or during therapy (35), representing reoxygenation and changes in the locations of tumoral hypoxia. These findings reduce the enthusiasm for using hypoxia-imaged sub-volumes as targets. Unless they are better characterized, they imply that targeting pre-therapy hypoxia may be close in some patients to randomly targeting any non-specific tumor sub-volume.
The CTVs
The selection of tissue volumes at risk of harboring sub-clinical disease and that should be irradiated prophylactically (the CTVs) relies on clinical knowledge of the pattern of the local spread and lymphatic metastases from each tumor site and stage, as no available imaging modality can demonstrate microscopic disease. The decisions in the IMRT setting are in general similar to those made in conventional RT. However, the use of highly conformal RT may occasionally affect these decisions. Foe example, a decision as to whether to include or exclude the nasopharynx from the CTVs in cases of neck metastases of unknown primary was more critical in conventional RT, where it would determine the risk of life-long xerostomia, compared with IMRT where a substantial portion of the salivary glands can be spared even if the nasopharyngeal mucosa in included in the CTV. Thus, the use of highly conformal RT may tilt the decision about the choice of targets in borderline cases toward inclusiveness.
Nodal CTVs
Some of the decisions about selecting neck lymph nodes requiring irradiation are identical in IMRT to those made in conventional RT. Examples include decisions as to whether or not the contralateral neck needs to be treated prophylactically in cases of tonsillar (36) or buccal squamous cell carcinoma (37). If the tumor does not invade organs with bilateral lymphatic drainage like the palate or base of tongue, and there is no or early ipsilateral lymphatic metastasis, exclusion of the contralateral neck may be adequate; in other cases, the risk to the contralateral neck may be high enough to justify its inclusion. The decisions in IMRT often need to be more precise: essentially, the inclusion or exclusion of each neck level, at each neck side. The decision-making process for CTV selection relies on data of pathological results and recurrence patterns accumulated over many years of surgical experience, while data about the exact sites of local/regional recurrences after RT has completely been lacking until the initial reports of recurrences after highly conformal RT have emerged in recent years (38–42). The mostly surgically originated data has been summarized in recent years by several publications aiming to convey this accumulated knowledge into decision-making for selecting the targets for prophylactic treatment for highly conformal RT (43–46). The first step in this decision-making process takes into account the risk of spread of disease locally and regionally, and the second step is a decision as to whether the perceived risk justifies the inclusion of the volume at risk in the CTV. This decision-making process is of course not unique to highly conformal RT; similar decisions are frequently made in conventional RT where in general a risk of sub-clinical disease below 10% is regarded as not worth addressing by prophylactic RT.
The other side of the coin is the need to identify the high-risk targets much more carefully and accurately while using highly conformal RT. The first echelon nodes in the bilateral upper neck (level II), as well as the retropharyngeal nodes, are included by default in the high-dose boost volumes of most upper aerodigestive tract cancers treated with lateral opposed boost fields. Using highly conformal RT, these nodes will be excluded if not specifically selected, or they may be under-dosed if their risk will not be defined as higher than the risk of subclinical disease in other nodal areas.
An accurate choice of the targets and the exclusion of very-low-risk targets determines the ability to spare neighboring crucial tissues. An example includes the selection of the target in the high neck in the non-nasopharyngeal N0 neck. While the radiological upper border of level II is the jugular foramen at the base of skull (47), the upper-most node in the jugular nodal chain (in essence, the first echelon node) for almost all HN cancers is the jugulodigastric (JD, subdigastric node) which lies below the crossing of the jugular vein and the posterior belly of the digastric muscle, except for nasopharyngeal cancer which may drain to the junctional nodes which are more cranial (48). Thus, outlining the upper-most border of level II such that it will include the JD node, but not more cranially, in the N0 neck of non-nasopharyngeal cancer, has a profound benefit regarding the ability to spare the parotid gland on that side of the neck. This selection is emphasized in an atlas of the targets for the N0, non-nasopharyngeal cancer (45), where the upper-most edge of level II is the bottom of the transverse process of C1, an edge that provides adequate irradiation of the JD nodes. This selection of the targets in the upper neck resulted in no out-of-field recurrences at level II (41).
In contrast to the N0 neck, the selection of the targets in the clinically involved neck needs to address a higher risk in both upstream and downstream nodal levels, due to a higher tendency of the tumor to metastasize to that side of the neck, and due to potential obstruction of the lymphatic flow by a large metastases causing lymphatic backflow to nodal levels that are otherwise not at risk (46). Examples include the need to extend the CTV to the base of skull, as well as the need to include levels IB and V, in cases in which the JD node is clinically involved. Another example is the need to include both levels V and VI (paratracheal and paralaryngeal nodes) in the targets when low-neck, level IV is clinically involved. As level V and VI drain into the lower jugular lymphatic chain, the obstruction of these chains by low neck metastases may cause backflow into level V–VI. The inclusion of additional levels in the clinically involved neck, beyond the levels that drain the tumor sites directly and which are included in the N0 neck, carries an obvious price in the risk/reward balance. Cases of nasopharyngeal, oropharyngeal, and laryngeal cancer would usually not require irradiation of level IB if level II is not grossly involved, allowing partial sparing of the submandibular gland on that side of the neck, which should preserve partly the mucin-rich submandibular salivary flow and help reduce xerostomia (49). Similarly, the inclusion of level VI due to gross involvement of level IV will reduce markedly the ability to shield the larynx or reduce its dose.
The process of selecting the targets to be included in the CTVs contains inherent risks of missing potential disease sites. These risks can be divided into expected and unexpected ones. An example of an unexpected risk is retropharyngeal nodal (RPN) recurrences at the level of the clivus, which we have encountered in cases where the upper border of the RPN CTV was delineated at the top of C1 (41). This delineation was made following Rouviere’s description of the RPNs being in front of C1 (48), and our clinical experience showed that relying on this description was mistaken. However, most of the risks are expected and are related to the conscious decision to exclude targets whose risk is low. An example for a risk taken knowingly is the sparing of the ipsilateral parotid gland in nasopharyngeal cancer. The Sloan Kettering group has recently reported recurrences within the spared parotid glands in two patients treated with IMRT for locally and regionally advanced nasopharyngeal cancer (50). Such recurrences are expected according to known anatomical/physiogical factors. The Eustachian tube has several patterns of drainage, one of which is to superficial parotid lymph nodes (48, 51.). Thus, it is expected that some nasopharyngeal cancer cases involving the Eustachian tube in whom the ipsilateral parotid nodes where spared would result in recurrent disease.
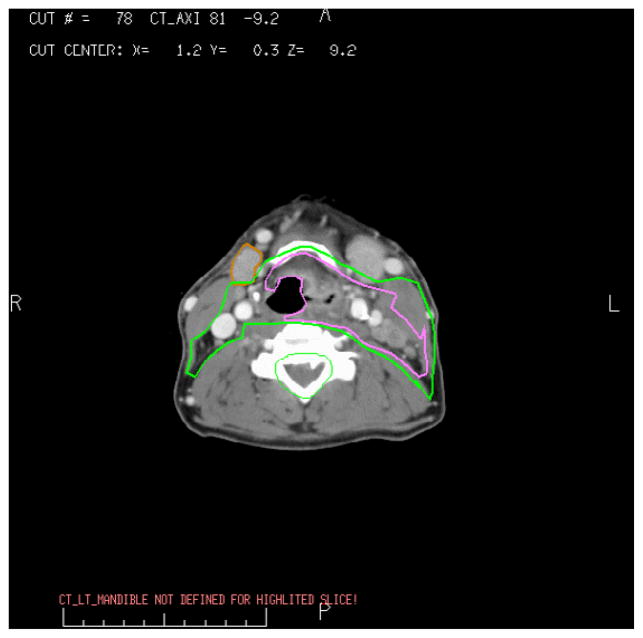
Another factor, that is likely even more important, is the risk of retrograde lymphatic flow to parotid lymph nodes in patients with significant involvement of level II. The occurrence of parotid lymph nodes metastases at diagnosis in nasopharyngeal cancer has been reported to be 3% (52), and a similar or higher risk is likely to be encountered if large enough numbers of patients with heavy involvement of level II are treated sparing the parotid gland on the heavily involved side of the neck (Fig. 1). Thus, both the known radiological experience at presentation, as well as the reports about the small number of recurrences within the parotid glands, are consistent with some risk of sparing the parotid gland in nasopharynx cancer on the side of the neck with significant nodal involvement at level II. There is little utility in looking for subclinical involvement by biopsies of small parotidean nodules, as suggested by Cannon et al; the only practical step is making the necessary clinical decision, weighting the reward vs risk of sparing the ipsilateral parotid gland; if the ipsilateral upper neck is heavily involved with metastatic disease, this reward/risk ratio is low.
Fig. 1.
Nasopharyngeal cancer presenting with multiple ipsilateral level II and V nodes, as well as involvement of ipsilateral parotidean nodes.
Another example of calculated risk-taking aimed at achieving specific tissue sparing goals is omitting the medial RPNs from the CTVs, including only the lateral RPNs in cases where the RPNs are at risk, in order to partially spare the pharyngeal constrictors and reduce dysphagia (53, 54). The basis for avoidance of the medial RPNs has been the information reported in many series demonstrating that in almost all cases of RPN metastases, the lateral, and not the medial nodes, were involved (54). While the risk in avoiding the posterior pharyngeal wall in most cases is calculated to be low, careful clinical follow-up is required to validate it. In addition, other factors may tilt this risk to higher level, including situations in which the risk is unknown and therefore should not be taken. Examples include cases in which the lateral RPNs are radiologically/clinically involved, in which the risk of sub-clinical involvement of the medial RPN nodes may be higher, and cases like advanced tonsillar cancer involving the posterior tonsillar pillar and the posterior pharyngeal wall (Fig. 2). In such cases, there is a risk of mucosal and submucosal tumor extension across the posterior pharyngeal wall. As mucosal tumor extension is poorly characterized by all imaging studies (12), the risk in defining the targets too tightly near the GTV in order to spare the superior/middle pharyngeal constrictors may be too high.
Fig. 2.
Advanced tonsillar cancer involving the lateral posterior pharyngeal wall (GTV, pink). The CTV in this case does not spare the pharyngeal constrictors.
As the confidence in our ability to appropriately select the targets diminishes, the reward/risk ratio for highly conformal RT decreases. Examples include cases where induction chemotherapy was delivered and there is no reliable data about the pre-chemotherapy extent of the tumor (see section below). Another example is the case of the patient who had a neck dissection in the past (a year or more before RT). After neck dissection, collateral lymphatics develop that circumvent the lymphatic drainage to the non-dissected parts of the neck. These lymphatics are frequently sub-cutaneous, their sites are unpredictable, and they are fully developed more than one year after surgery (55). Due to the unpredictability of the sites of these lymphatics, the adequacy of the CTVs in these cases is markedly reduced and highly conformal RT would be associated with reduced reward/risk ratio.
Reward/risk considerations in split-neck vs whole-neck IMRT
Another example of balancing reward/risk is the approach to IMRT of the low neck. In tumors that do not involve the larynx/hypopharynx, IMRT may be used for the targets in the upper neck while the lower neck is treated with an anterior field, typically matched at the thyroid notch, containing a larynx block similar to low-neck treatment in conventional RT. The lower neck is typically prescribed 45–50 Gy to 1–3 cm depth with higher-risk sub-volumes boosted with an anterior or anterior/posterior field. This method results in lower glottic larynx doses compared with IMRT which treats the whole neck, even if the glottis is specified as an avoidance structure (56–58). In addition to lower glottic larynx doses, this technique offers somewhat shorter target delineation time and treatment time, reduced delivered monitor units, and avoidance of potential set-up deviations in the low neck. The price is reduced certainty in the doses delivered to the lower neck compared with IMRT in which the targets in the lower neck are delineated and their doses are specified. There is also a risk of over- or under-dose at the level of the junction between the IMRT fields and the lower anterior fields.
The considerations of the reward/risk ratios in this case include the fact that the doses to the glottic larynx using whole-neck IMRT, while higher than in split-neck IMRT, are typically in the range of 30–45 Gy delivered over 32–35 fractions, at low daily fraction doses, if the larynx is defined as an avoidance structure, and median larynx doses of 20–25 Gy are achievable in some cases. These doses are not expected to cause long-term speech or swallowing difficulties. However, if the risk of subclinical disease in the low neck is low, such as in cases with no clinical involvement of neck levels III or IV, an anterior neck field is adequate. On the other hand, if the risk is higher, we recommend whole neck IMRT in order to increase the precision of the dose distributions in the low neck. Including the low neck in the IMRT plan allows the delivery of high doses to gross disease in the low neck while partly sparing the roots of the brachial plexus, the larynx, and esophagus. It may allow partial shielding of the glottic larynx while encompassing level VI, which is at risk of sub-clinical disease if there is a substantial involvement of level IV nodes (Fig. 3.).
Fig. 3.
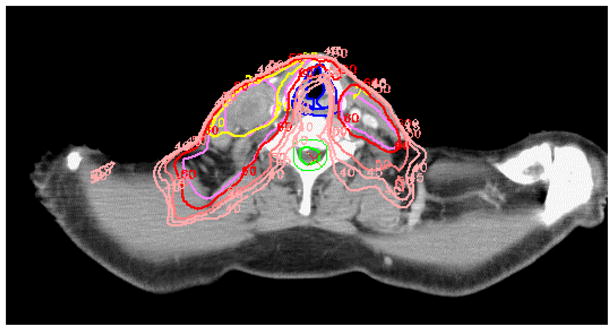
Whole-neck IMRT in a case of oropharyngeal cancer with level III–IV lymph node metastases. Ipsilateral level VI (pre-laryngeal nodes) is at risk, and is encompassed within the CTV while partially sparing the glottis. Such sparing would not be safely done using split-neck IMRT.
The clinical experience at Washington University suggested that treating the low neck with an anterior field was associated with a relatively high incidence of low neck failures, which was reduced substantially when the treatment plans were changed to whole-neck IMRT (39, 59). An adequate selection of patients for whole-neck or split-neck IMRT according to the risk of disease in the low neck will optimize the risk/benefit ratio, reducing low neck recurrence rates while preserving the simplicity of split neck IMRT for most patients.
The CTV around the primary tumor GTV
Recommendations for the selection of the CTV for the primary tumor on a 3D basis (receiving a sub-clinical RT dose) is an almost unexplored area. General operational guidelines for the major head and neck sites have been proposed (44). The general principles that should guide the selection of the CTV around the primary tumor is that the microscopic spread of squamous cell carcinomas follows anatomical compartments (e.g. para-laryngeal, para-pharyngeal, pre-epiglottic spaces) bounded by anatomical barriers (e.g. bone cortex, muscular fascia, ligaments). Using anatomical compartments to define CTV boundaries seems to us to be more adequate than using any arbitrary uniform expansion around the GTV, unless such boundaries are not clear, such as the anterior boundary in cases of base of tongue cancer.
The critical organ/tissue
On the reward side of the reward/risk equation, there are the organs whose sparing may achieve tangible gains in acute and late side effects and in improving quality of life. Some of the candidate organs for sparing have been obvious, like the mandible and the parotid glands. The potential gains from sparing other organs was not intuitive, but became apparent after better understanding of function had been gained. For example, sparing the parts of the oral cavity that are outside the targets is expected to reduce acute mucositis, and its neglect may lead to increased severity of mucositis during IMRT compared with conventional RT (60). In addition, sparing of the non-involved oral cavity reduces damage of the minor salivary glands, that are dispersed in the oral cavity. The need to spare the mucin-producing minor and submandibular salivary glands became apparent following the realization that parotid sparing alone achieves modest patient-reported gains in xerostomia (49, 61). Similarly, clinical experience with dysphagia after chemo-RT motivated a search for the most important swallowing structures and initial trials to spare these structures (53, 54). As dose-effect relationships for many of these structures are being accumulated, we will gain better ability to predict the rewards from their partial sparing. These issues are beyond the scope of this paper. Other tissues and organs whose sparing may achieve specific gains are the skin (62), pharyngeal mucosa (63), posterior neck musculature, and all non-specified tissue, whose irradiation is associated with increased risk of second malignancies. Sparing the non-involved glottic larynx is an obvious goal aimed at avoiding voice abnormalities, but if the laryngeal dose is very high because its sparing was not included in the cost functions of the IMRT plans, an additional sequela has been significant dysphagia (64). In many cases such decisions are not straightforward and demand a weighing of risk-benefit ratios using knowledge of dose-response relationships for both organ and target. Considerations of dosimetric trade-offs should be made not only between targets and spared organs, but also among the spared organs. For example, substantial sparing of the contralateral submandibular gland may be achieved where contralateral level I is not part of the targets, however, such sparing is associated with a modest rise in the doses to other organs like the parotid and swallowing structures (49). An optimal balance between these competing goals can only be achieved if we understand the clinical implications of the various trade-offs in the doses to the various organs.
As more organs are added to the sparing list, steeper fall-off of the doses at the boundary of the targets and the organs are expected. This is especially apparent in the oral cavity, where the margins of the CTVs and the spared mucosa may be quite arbitrary, and in the vicinity of the swallowing structures, which lie between the targets in the bilateral neck. A steep dose fall-off may increase the risk of marginal recurrences if the targets are not accurately delineated, because the usual “spillover” of dose outside the targets will be absent. This may reduce the reward/risk ratio as we add more organs to spare, unless the targets are delineated conservatively.
Within the targets in the HN there is embedded mucosa, submucosa, blood vessels, and nerves, whose recovery and integrity after therapy will determine the risk of long-term local ulceration and dysfunction. Very high doses delivered to the targets, either incidentally due to excessive “hot spots” within the targets, or intentionally, in an effort to escalate the doses to the targets or to parts of the targets expected to be resistant to standard doses, will affect these tissues. High dose per fraction could have an impact on toxicity. Full re-epithelization of the site of GTV in HN cancer after eradication of the tumor is a prerequisite for preservation of function and QOL. Severe acute mucositis leading to consequential fibrosis or necrosis of non-specific tissue within the targets requires consideration in the reward/risk calculations.
Lee et al planned IMRT, prescribing 84 Gy to the hypoxic subvolumes of the pre-therapy GTVs, while non-hypoxic parts received 70 Gy, all in 33 fractions (31). The hypoxic sub-volumes were 30–80% of the GTVs in most patients (34). Dosimetric constraints for organs like the parotid glands, spinal cord, brain stem, etc. were not exceeded. Therefore the authors concluded that such a dose escalation was feasible. An organ that was not taken into account in this planning exercise was the tissue embedded within the tumor. If this tissue contains late-responding elements with a low alpha/beta ratio approaching 3 Gy, the nominal dose of 84 Gy delivered at 2.55 Gy/fraction would be biologically equivalent to 94 Gy at 2 Gy/fraction for these elements. Such a dose may not be tolerated even when delivered to a relatively small tissue volume.
Clinical trials of increasing GTV doses were reported for nasopharynx (65) and laryngeal cancer (66), reporting GTV doses of 65–67 Gy at daily fraction doses of 2.4 Gy, with acceptable toxicity. An interesting GTV dose escalation study was reported from the Medical College of Virginia (67). Patients were treated with IMRT without chemotherapy, receiving escalated GTV doses, keeping CTV doses fixed at 54–60 Gy, and addressing all dosimetric constraints to critical organs. The GTV dose escalation plan was 68.1, 70.8, and 73.8 Gy, all delivered over 30 fractions, at daily fraction doses of 2.27, 2.36, and 2.46 Gy, respectively. The results showed acceptable acute toxicity in the first two dose levels, while the patients at the third dose levels developed severe acute mucositis and dysphagia which prompted a reduction of their daily dose after the third treatment week. The authors concluded that the second dose level was the maximally tolerable dose. A statistically significant correlation was found between target volumes and severe toxicity, and it is possible, therefore, that a small subvolume of the GTV could have received safely a somewhat higher dose. This is not unlike the experience with brachytherapy of HN cancer, where even small implanted volumes in early oral cavity cancer may be associated with tissue necrosis if rules about total dose, dose rate, and dose inhomogeneities within the implant are neglected (68).
An example of dose escalation in which the volume receiving the high dose has been limited is the FDG-PET- guided phase I study from Ghent, Belgium, in which the GTV receiving the highest dose was limited to 10cc (23). Acute toxicity, including mucositis, dysphagia, and skin toxicity was higher in the dose escalated patient cohort, but did not reach maximally tolerated doses. An additional consideration is the role of concurrent chemotherapy, which improves tumor control rates but is expected to increase acute mucositis by an equivalent of 8 Gy (69). Concurrent chemotherapy with IMRT delivering GTV doses of 60 Gy at 2.4 Gy per fraction was reported to be prohibitive (70). Thus, increasing target fraction doses in this setting decreases, rather than increases, the reward/risk ratio.
Following induction chemotherapy or during RT: should we re-delineate a smaller GTV?
In recent years, a renewed interest in induction chemotherapy for head and neck cancer arose following studies demonstrating an advantage of induction regimens consisting of taxol, cisplatin, and 5-FU (TPF) over cisplatin and 5-FU (71–72). These results do not prove that any induction regimen followed by concurrent chemo-RT is superior to chemo-RT alone. They have, however, prompted increased use of induction chemotherapy in the community. The rate of tumor response after TPF is around 60%, and close to 10% of patients achieve complete responses (72). The consequence is that in many cases the radiation oncologist will face situations in which the GTV is significantly smaller after the completion of induction chemotherapy, before the start of IMRT, compared to its size at presentation. Delineating the new, smaller GTV may allow better sparing of neighboring organs and potentially better function and QOL compared with delineation of the original GTV. Proponents of induction chemotherapy have suggested that it helps the radiation oncologist to improve the therapeutic ratio (73).
What are the risks in delineating a post-induction, rather than the original, untreated GTV? Prior experience showed that induction chemotherapy following by RT did not improve local/regional tumor control despite achieving a substantial tumor response. This failure to affect outcome was predicted by Ian Tannock (74). If a 10-gr. tumor containing 1010 cells is treated with three cycles of induction chemotherapy, each of which kills 50–90% of the tumor cells, after three cycles, the number of viable cells is approximately 108 (<0.1 gr.), and the patient would be categorized as having achieved clinical and radiological complete response. However, tumor cell reduction has been trivial: only 2 out of 10 logs of tumor cells have been eliminated. Tissue volumes from which tumor shrank radiographically are still likely to contain large number of tumor cells that are below detection threshold. Another potential radiobiological-based explanation for the failure of induction chemotherapy to reduce local/regional recurrences is the recent findings that tumor stem cells, which represent a small percentage of the tumor, are more resistant to therapy compared with non-stem cells. Thus, clinical or radiological tumor regression may not represent the eradication of the stem cells that are the most important target of therapy (75). These considerations lend strong support for the definition and delineation of the pre-chemotherapy target.
Similar considerations exist regarding tumor shrinkage during therapy. After the delivery of 30–50 Gy, substantial shrinkage of tumor is often observed both anatomically and on metabolic evaluations like FDG-PET, prompting suggestions that this regression may allow a reduction in the GTV in order to improve organ sparing (76). However, the considerations regarding tumor cell kill after induction chemotherapy and after a partial course of chemo-RT are similar: both likely result in substantial numbers of tumor cells remaining in the tissue volumes that were previously occupied by the GTV. Clinical support for this assumption is found in studies which reported poor correlations between the radiologic (78) or metabolic (79) shrinkage of tumors after 4–5 weeks of chemo-RT and the existence of tumor cells on histological examinations of the surgical specimens after therapy. In this framework, giving an extra-boost dose to the shrinking GTV during treatment -as assessed by anatomic or functional imaging modalities- while maintaining the prescribed dose to the pre-treatment GTV, might be a reasonable option to validate (28).
A recent consensus meeting of medical and radiation oncologists summarized the recommendations for delineating the targets after induction chemotherapy (80). The consensus was that using the pre-chemotherapy targets, regardless of tumor response, is the prudent recommended approach in order to avoid risking marginal recurrences. To this end, several principles were recommended: Prior to chemotherapy the patient should be evaluated by the radiation oncologist and high-quality imaging should be performed to gain maximal information regarding the extent of the tumor. It was recommended that the patient be simulated before induction, but it is likely that a new immobilization device will be necessary after chemotherapy, approximating the pre-chemotherapy head position as closely as possible. The pre-induction primary tumor and nodal GTVs should be used for planning and the post-induction targets should correspond as closely as possible to the original disease extent in all dimensions. All structures involved by tumor prior to induction chemotherapy should be included in the targets even if they are not grossly involved after induction chemotherapy. Fusion or image registration of the pre-chemotherapy simulation CT data set with a post-induction simulation CT may improve target delineation. These recommendations may change or be modified in the future, as clinical experience and knowledge of tumor recurrence patterns after induction chemotherapy are gained.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hong TS, Chappell RJ, Harari PM. Variations in target delineation for head and neck IMRT: An international multi-institutional study. Int J Rad Onc Biol Phys. 2004;60:S157. [Google Scholar]
- 2.Som PM. The present controversy over the imaging method of choice for evaluating the soft tissues of the neck. AJNR. 1997;18:1869–1872. [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn PH, Garg MK. Positron emission tomography/computed tomography for target delineation in head and neck cancers. Semin Nucl Med. 2008;38:141–8. doi: 10.1053/j.semnuclmed.2007.11.002. Review. [DOI] [PubMed] [Google Scholar]
- 4.Rasch C, Eisbruch A, Reimeijer P, et al. Irradiation of paranasal sinus tumors, a delineation and dose comparison study. Int J Radiat Oncol Biol Phys. 2002;52:120–7. doi: 10.1016/s0360-3016(01)01751-5. [DOI] [PubMed] [Google Scholar]
- 5.Chao KS, Bhide S, Chen H, et al. Reduce in Variation and Improve Efficiency of Target Volume Delineation by a Computer-Assisted System Using a Deformable Image Registration Approach. Int J Rad Onc Biol Phy. 2007;68:512–1521. doi: 10.1016/j.ijrobp.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 6.Jeanneret-Sozzi W, Moeckli R, Valley JF, et al. The reasons for discrepancies in target volume delineation: A SASRO study on head-and-neck and prostate cancers. Strahlenther Onkol. 2006;182:450–457. doi: 10.1007/s00066-006-1463-6. [DOI] [PubMed] [Google Scholar]
- 7.Steenbakkers RDJ, Fitton I, Deurloo K, et al. Observer variation in delineation of nasopharyngeal carcinoma for radiotherapy A 3-D analysis. Int J Radiat Oncol Biol Phys. 2004;60:S160. [Google Scholar]
- 8.Geets X, Daisne JF, Arcangeli S, Coche E, Poel MD, Duprez T, Nardella G, Gregoire V. Inter-observer variability in the delineation of pharyngo-laryngeal tumor, parotid glands and cervical spinal cord: Comparison between CT-scan and MRI. Radiother Oncol. 2005;77:25–31. doi: 10.1016/j.radonc.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Ashamalla H, Rafla S, Parikh K, et al. The contribution of integrated PET/CT to the evolving definition of treatment volumes in radiation treatment planning in lung cancer. Int J rad Onc Biol Phys. 2005;63:1016–1023. doi: 10.1016/j.ijrobp.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Ciernik IF, Dizendorf E, Baumert BG, et al. Radiation treatment planning with integrated CT/PET. Int J Rad Onc Biol Phys. 2003;57:853–863. doi: 10.1016/s0360-3016(03)00346-8. [DOI] [PubMed] [Google Scholar]
- 11.Syed R, Bomanji JB, nagabhushan N, et al. Impact of combined FDG PET/CT in head and neck tumors. Br J cancer. 2005;92:1046–50. doi: 10.1038/sj.bjc.6602464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daisne JF, Duprez T, Weynand B, et al. Accuracy of CT scan, MRI and FDG-PET in delineating the tumor volume in pharyngo-laryngeal squamous cell carcinomas treated by radiotherapy: validation with the macroscopic tumor specimen used as reference. Radiology. 2004;233:93–100. doi: 10.1148/radiol.2331030660. [DOI] [PubMed] [Google Scholar]
- 13.Geets X, Daisne JF, Tomsej M, et al. Impact of the type of imaging modality on target volumes delineation and dose distribution in pharyngo-laryngeal squamous cell carcinoma: comparison between pre- and per-treatment studies. Radiother Oncol. 2006;78:291–297. doi: 10.1016/j.radonc.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Paulino AC, Koshy M, Howell R, et al. Comparison of CT- and FDG-PET-defined gross tumor volume in intensity-modulated radiotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61:1385–92. doi: 10.1016/j.ijrobp.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz DL, Ford EC, Rajendran J, et al. FDG-PET/CT-guided intensity modulated head and neck radiotherapy: a pilot investigation. Head Neck. 2005;27:478–87. doi: 10.1002/hed.20177. [DOI] [PubMed] [Google Scholar]
- 16.Schingal DA, Vogel WV, Hoffmann AL, et al. Comparison of five segmentation tools for 18F-fluoro-deoxy-glucose-positron emission tomography-based target volume definition in head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;69:1282–9. doi: 10.1016/j.ijrobp.2007.07.2333. [DOI] [PubMed] [Google Scholar]
- 17.Gregoire V, daisne JF, Geets X. Comparison of CT- and FDG-PET-defined GTV. Int J Radiat Oncol Biol Phys. 2005 Sep 1;63:308–9. doi: 10.1016/j.ijrobp.2005.05.038. (letter) [DOI] [PubMed] [Google Scholar]
- 18.Geets X, Lee J, Bol A, et al. A gradient-based method for segmenting FDG-PET images: methodology and validation. Eur J Nucl Med Mol Imaging. 2007;34:1427–38. doi: 10.1007/s00259-006-0363-4. [DOI] [PubMed] [Google Scholar]
- 19.Grégoire V, Daisne JF, Geets X, Levendag P. Selection and delineation of target volumes in head and neck tumors. Rays. 2003;28:217–224. [PubMed] [Google Scholar]
- 20.Soto D, Piert M, Eisbruch A. Correlation between pretreatment FDG-PET biological target volume and anatomical location of failure after radiation therapy of head and neck cancer. Radiother Oncol. doi: 10.1016/j.radonc.2008.05.021. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scarfone C, Lavely WC, Cmelak AJ, et al. Prospective feasibility trial of radiotherapy target definition for head and neck cancer using 3-dimensional PET and CT imaging. J Nucl Med. 2004;45:543–52. [PubMed] [Google Scholar]
- 22.Wang D, Schultz CJ, Jursinic PA, et al. Initial experience of FDG-PET/CT guided IMRT of head-and-neck carcinoma. Int J Radiat Oncol Biol Phys. 2006;65:143–51. doi: 10.1016/j.ijrobp.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 23.Madani I, vanderstraeten B, Bral S, et al. Positron emission tomography-guided, focal-dose escalation using intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;68:126–35. doi: 10.1016/j.ijrobp.2006.12.070. [DOI] [PubMed] [Google Scholar]
- 24.Vineberg KA, Eisbruch A, Coselmon MM, et al. Is uniform target dose possible in IMRT plans in the head and neck? Int J Rad Onc Biol Phys. 2002;52:1159–1172. doi: 10.1016/s0360-3016(01)02800-0. [DOI] [PubMed] [Google Scholar]
- 25.Tome WA, Fowler JF. Selective boosting of tumor subvolumes. Int J Radiat Oncol Biol Phys. 2000;48:593–9. doi: 10.1016/s0360-3016(00)00666-0. [DOI] [PubMed] [Google Scholar]
- 26.Ling CC, Humm J, Larson S, et al. Towards multidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int J Radiat Oncol Biol Phys. 2000;47:551–60. doi: 10.1016/s0360-3016(00)00467-3. Review. [DOI] [PubMed] [Google Scholar]
- 27.Bentzen SM. Theragnostic imaging for radiation oncology: dose-painting by numbers. Lancet Oncol. 2005;6:112–7. doi: 10.1016/S1470-2045(05)01737-7. [DOI] [PubMed] [Google Scholar]
- 28.Geets X, Tomsej M, Lee JA, et al. Adaptive biological image-guided IMRT with anatomic and functional imaging in pharyngo-laryngeal tumors: Impact on target volume delineation and dose distribution using helical tomotherapy. Radiother Oncol. 2007;85:105–115. doi: 10.1016/j.radonc.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Brun E, Kjellen E, Tennvall J, et al. FDG PET studies during treatment: prediction of therapy outcome in head and neck squamous cell carcinoma. Head Neck. 2002;24:127–35. doi: 10.1002/hed.10037. [DOI] [PubMed] [Google Scholar]
- 30.Chao KS, Bosch WR, Mutic S, et al. A novel approach to overcome hypoxic tumor resistance: Cu-ATSM-guided intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2001;49:1171–82. doi: 10.1016/s0360-3016(00)01433-4. [DOI] [PubMed] [Google Scholar]
- 31.Lee NY, Mechalakos JG, Nehmeh S, et al. Fluorine-18-labeled fluoromisonidazole positron emission and computed tomography-guided intensity-modulated radiotherapy for head and neck cancer: a feasibility study. Int J Radiat Oncol Biol Phys. 2008;70:2–13. doi: 10.1016/j.ijrobp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alber M, Paulsen F, Eschmann SM, Machulla HJ. On biologically conformal boost dose optimization. Phys Med Biol. 2003;48:N31–5. doi: 10.1088/0031-9155/48/2/404. [DOI] [PubMed] [Google Scholar]
- 33.Grosu AL, Souvatzoglou M, Rpoer B, et al. Hypoxia imaging with FAZA-PET and theoretical considerations with regard to dose painting for individualization of radiotherapy in patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;69:541–51. doi: 10.1016/j.ijrobp.2007.05.079. [DOI] [PubMed] [Google Scholar]
- 34.Nehmeh SA, Lee NY, Schroder H, et al. Reproducibility of intratumor distribution of (18)F-fluoromisonidazole in head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;70:235–42. doi: 10.1016/j.ijrobp.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thorwarth D, Eschmann SM, Holzner F, et al. A model of reoxygenation dynamics of head-and-neck tumors based on serial 18F-fluoromisonidazole positron emission tomography investigations. Int J Radiat Oncol Biol Phys. 2007;68:515–21. doi: 10.1016/j.ijrobp.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 36.O’Sullivan B, Warde P, Grice B, et al. The benefits and pitfalls of ipsilateral radiotherapy in carcinoma of the tonsillar region. Int J Radiat Oncol Biol Phys. 2001;51:332–43. doi: 10.1016/s0360-3016(01)01613-3. [DOI] [PubMed] [Google Scholar]
- 37.Lin CY, Lee LY, Huang SF. Treatment outcome of combined modalities for buccal cancers: Unilateral or bilateral neck radiation? IJROBP. 2007;68:750–757. doi: 10.1016/j.ijrobp.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 38.Dawson LA, Anzai Y, Marsh L, et al. Patterns of local-regional recurrence following parotid-sparing conformal and segmental intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2000;46:1117–26. doi: 10.1016/s0360-3016(99)00550-7. [DOI] [PubMed] [Google Scholar]
- 39.Chao KS, Wippold FJ, Ozygit G, et al. Determination and delineation of nodal target volumes for head-and-neck cancer based on patterns of failure in patients receiving definitive and postoperative IMRT. Int J Radiat Oncol Biol Phys. 2002;53:1174–84. doi: 10.1016/s0360-3016(02)02881-x. [DOI] [PubMed] [Google Scholar]
- 40.Lee N, Xia P, Fischbein NJ, et al. Intensity-modulated radiation therapy for head-and-neck cancer: the UCSF experience focusing on target volume delineation. Int J Radiat Oncol Biol Phys. 2003;57:49–60. doi: 10.1016/s0360-3016(03)00405-x. [DOI] [PubMed] [Google Scholar]
- 41.Eisbruch A, Marsh L, Dawson LA, et al. Recurrences near base of skull after IMRT for head-and-neck cancer: implications for target delineation in high neck and for parotid gland sparing. Int J Radiat Oncol Biol Phys. 2004;59:28–42. doi: 10.1016/j.ijrobp.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 42.Yao M, Chang K, Funk GF, et al. The failure patterns of oral cavity squamous cell carcinoma after intensity-modulated radiotherapy-the university of Iowa experience. Int J Radiat Oncol Biol Phys. 2007;67:1332–41. doi: 10.1016/j.ijrobp.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 43.Gregoire V, Coche E, Cosnard G, et al. Selection and delineation of lymph node target volumes in head and neck conformal radiotherapy. Proposal for standardizing terminology and procedure based on the surgical experience. Radiother Oncol. 2000;56:135–50. doi: 10.1016/s0167-8140(00)00202-4. Review. [DOI] [PubMed] [Google Scholar]
- 44.Eisbruch A, Foote RL, O’Sullivan B, et al. Intensity-modulated radiation therapy for head and neck cancer: emphasis on the selection and delineation of the targets. Semin Radiat Oncol. 2002;12(3):238–49. doi: 10.1053/srao.2002.32435. [DOI] [PubMed] [Google Scholar]
- 45.Grégoire V, Levendag P, Ang KK, et al. CT-based delineation of lymph node levels and related CTVs in the node-negative neck: DAHANCA, EORTC, GORTEC, NCIC, RTOG consensus guidelines. Radiother Oncol. 2003;69:227–236. doi: 10.1016/j.radonc.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 46.Grégoire V, Eisbruch A, Hamoir M, Levendag P. Proposal for the delineation of the nodal CTV in the node positive and the post-operative neck. Radiother Oncol. 2006;79:15–20. doi: 10.1016/j.radonc.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 47.Som PM, Curtin HD, Mancuso AA. An image-based classification for the cervical nodes designed as an adjunct to recent clinically based nodal classification. Arch Otolaryngol Head Neck Surg. 1999;125:388–396. doi: 10.1001/archotol.125.4.388. [DOI] [PubMed] [Google Scholar]
- 48.Rouviere H. In: lymphatic systems of the head and neck. Tobias MJ, translator. Ann Arbor, Mich: Edwards Brothers; 1938. [Google Scholar]
- 49.Murdoch-Kinch MA, Kim HM, Vineberg K, et al. Dose –response relationships for the submandibular salivary glands and implications for their sparing by intensity modulated radiotherapy. Int J Rad Onc Biol Phys. doi: 10.1016/j.ijrobp.2007.12.033. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cannon DM, Lee NY. Recurrence in region of spared parotid gland after definitive intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;70:660–5. doi: 10.1016/j.ijrobp.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 51.Proctor B. Embryology and anatomy of the Eustachian Tube. Arch otolaryngol. 1967;86:503–514. doi: 10.1001/archotol.1967.00760050505008. [DOI] [PubMed] [Google Scholar]
- 52.Ng SH, Chang JT, Chan SC, et al. Nodal metastases of nasopharyngeal carcinoma: Patterns of disease on MRI and FDG PET. Eur J Nucl Med Mol Imaging. 2004;31:1073–1080. doi: 10.1007/s00259-004-1498-9. [DOI] [PubMed] [Google Scholar]
- 53.Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration following chemo- irradiation of head and neck cancer: which anatomical structures are affected, and can they be spared by IMRT? Int J Rad Onc Biol Phys. 2004;60:1425–39. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 54.Feng F, Kim HM, Lyden T, et al. IMRT of head and neck cancer aiming at reducing dysphagia: Early Dose-volume-effect relationships for the swallowing structures. Int J Rad Onc Biol Phys. 2007;68:1289–98. doi: 10.1016/j.ijrobp.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 55.Fisch U. Cervical lymph flow following surgery in the neck. In: Fisch U, editor. Lymphography of the cervical lymphatic system. WB sauders; Philadelphia: 1968. pp. 112–141. [Google Scholar]
- 56.Amdur RJ, Li JG, Hinerman RW, et al. Unnecessary laryngeal irradiation in the IMRT era. Head Neck. 2004;26:257–63. doi: 10.1002/hed.10379. [DOI] [PubMed] [Google Scholar]
- 57.Dabaja BS, Suki D, Bonnen M, et al. Intensity-modulated radiation therapy (IMRT) of cancers of the head and neck: comparison of split-field and whole-field techniques. Int J Radiat Oncol Biol Phys. 2005;63:1000–5. doi: 10.1016/j.ijrobp.2005.03.069. [DOI] [PubMed] [Google Scholar]
- 58.Lee N, Mechalakos J, Puri DR, Hunt M. Choosing an intensity-modulated radiation therapy technique in the treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2007;68:1299–309. doi: 10.1016/j.ijrobp.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 59.Thorstad WL, et al. Recurrences in the low neck after IMRT of head and neck cancer. ASTRO 2008. Int J Rad Onc Biol Phys. 2008 (abstract, in press) [Google Scholar]
- 60.Khuntia D, et al. Comparisons of mucositis between IMRT and conventional RT in an RTOG study of post-operative chemotherapy or cetuximab-chemotherapy. ASTRO 08. Int J Rad Onc Biol Phys. (abstract, in press) [Google Scholar]
- 61.Eisbruch A. Reducing xerostomia by IMRT: what can, and cannot, be achieved. J Clin Oncol. 2007;25:4863–4. doi: 10.1200/JCO.2007.13.4874. [DOI] [PubMed] [Google Scholar]
- 62.Lee N, Chuang C, Quivey JM, et al. Skin toxicity due to intensity-modulated radiotherapy for head-and-neck carcinoma. Int J Radiat Oncol Biol Phys. 2002;53:630–7. doi: 10.1016/s0360-3016(02)02756-6. [DOI] [PubMed] [Google Scholar]
- 63.Sanguineti G, Endres EJ, Gunn BG, Parker B. Is there a “mucosa-sparing” benefit of IMRT for head-and-neck cancer? Int J Radiat Oncol Biol Phys. 2006;66:931–8. doi: 10.1016/j.ijrobp.2006.05.060. [DOI] [PubMed] [Google Scholar]
- 64.Fua TF, Corry J, Milner AD, et al. Intensity-modulated radiotherapy for nasopharyngeal carcinoma: clinical correlation of dose to the pharyngo-esophageal axis and dysphagia. Int J Radiat Oncol Biol Phys. 2007;67:976–81. doi: 10.1016/j.ijrobp.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 65.Koom WS, Kim TH, Shin KH, et al. SMART (simultaneous modulated accelerated radiotherapy) for locally advanced nasopharyngeal carcinomas. Head Neck. 2008;30:159–69. doi: 10.1002/hed.20667. [DOI] [PubMed] [Google Scholar]
- 66.Guerrero urbano T, Clark CH, Hansen VN, et al. A phase I study of dose-escalated chemoradiation with accelerated intensity modulated radiotherapy in locally advanced head and neck cancer. Radiother Oncol. 2007;85:36–41. doi: 10.1016/j.radonc.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 67.Lauve A, Morris M, Schmidt-Ullrich R, et al. Simultaneous integrated boost intensity-modulated radiotherapy for locally advanced head-and-neck squamous cell carcinomas: II--clinical results. Int J Radiat Oncol Biol Phys. 2004;60:374–87. doi: 10.1016/j.ijrobp.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 68.Mazeron JJ, Simon JM, Le Pechoux, et al. Effect of dose rate on local control and complications in irradiation of T1-2 carcinomas of tongue and floor of mouth with interstitial Iridium -192. Radiother Oncol. 1991;21:39–47. doi: 10.1016/0167-8140(91)90339-i. [DOI] [PubMed] [Google Scholar]
- 69.Lee I, Eisbruch A. The dose-equivalent increase of mucositis by adding concurrent chemotherapy to irradiation of head and neck cancer. ASTRO 2008. Int J Rad Onc Biol Phys. doi: 10.1016/j.ijrobp.2008.12.011. (abstract, in press) [DOI] [PubMed] [Google Scholar]
- 70.Amosson CM, The BS, Garg AK, et al. Accelerated fractionation for head and neck cancer using the SMART boost technique. Int J Rad Onc Biol Phys. 2003;57:S306. (abstract #1076) [Google Scholar]
- 71.Posner MR, Hershok DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–15. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 72.Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 73.Posner M. Evolving strategies for combined modality therapy for locally advanced head and neck cancer. Oncologist. 2007;12:967–74. doi: 10.1634/theoncologist.12-8-967. [DOI] [PubMed] [Google Scholar]
- 74.Tannock IF. Combined modality treatment with radiotherapy and chemotherapy. Radiother Oncol. 1989;16:83–101. doi: 10.1016/0167-8140(89)90025-x. [DOI] [PubMed] [Google Scholar]
- 75.Eisbruch A. Commentary: induction chemotherapy for head and neck cancer: hypothesis-based rather than evidence-based medicine. Oncologist. 2007;12:975–7. doi: 10.1634/theoncologist.12-8-975. [DOI] [PubMed] [Google Scholar]
- 76.Woodford C, yartsev S, Dar AR, et al. Adaptive radiotherapy planning on decreasing gross tumor volumes as seen on megavoltage computed tomography images. Int J Radiat Oncol Biol Phys. 2007;69:1316–22. doi: 10.1016/j.ijrobp.2007.07.2369. [DOI] [PubMed] [Google Scholar]
- 78.Klug C, Keszthelyi D, Ploder O, et al. Neoadjuvant radiochemotherapy of oral cavity and oropharyngeal cancer: evaluation of tumor response by CT differs from histopathologic response assessment in a significant fraction of patients. Head Neck. 2004;26:224–31. doi: 10.1002/hed.10373. [DOI] [PubMed] [Google Scholar]
- 79.Konski AA, Cheng JD, Goldberg M, et al. Correlation of molecular response as measured by 18-FDG positron emission tomography with outcome after chemoradiotherapy in patients with esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2007;69:358–63. doi: 10.1016/j.ijrobp.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salama JK, Hadad RI, Kies MS, et al. Clinical practice guidance for radiotherapy planning following induction chemotherapy for head and neck cancer. doi: 10.1016/j.ijrobp.2008.11.059. Submitted. [DOI] [PubMed] [Google Scholar]