Abstract
Reading difficulties appear to be related to a phonological deficit that has its origin in poor speech perception. As such, disabled readers may use contextual cues to compensate for their weak speech perception abilities. We compared good and poor readers, 7–13 years, on auditory perception of words varying in phonological contrast, in congruent vs. incongruent sentence contexts. Both groups did worse in the phonologically similar than in the phonologically dissimilar incongruent condition. Magnetoencephalography revealed differential activation between the groups as a function of phonological contrast in left superior temporal gyrus between 200–300 ms, suggesting that poor readers may have processed phonologically similar incongruent stimuli as congruent. The results are consistent with a phonological account of reading disability.
Keywords: Magnetoencephalography, PMN, N400, dyslexia, reading impairment
Introduction
Approximately 5–20% of children struggle to learn to read despite adequate intelligence, motivation, and schooling1. While the underlying cause of dyslexia is still debated, one prominent hypothesis implicates a deficit in phonological processing, such that direct access to, and manipulation of, phonemic language units retrieved from long-term memory is impaired2. In fact, individuals with reading disability frequently have problems on tasks of phonological awareness, nonword repetition, rapid naming, and verbal memory. These problems are believed to arise directly or indirectly from a deficit in speech perception rooted in poorly encoded phonological representations3.
In spite of their phonological processing problems, children with reading disability for the most part go on to develop normal spoken language and adequate reading comprehension abilities1,4. Disabled readers appear to rely on sight word knowledge and/or sentence context cues to compensate for their poor phonological decoding skills. Indeed, studies investigating higher-level cognitive influences on word recognition suggest that poor readers, more than good readers, take advantage of contextual cues provided by words or sentences to facilitate word recognition. Poor readers, thus, tend to draw on top-down influences in both visual and auditory word recognition5–7.
In electroencephalography (EEG) and magnetoencephalography (MEG) studies, the neural activity related to the effect of context on written and spoken word perception is evident in two evoked response components related to phonological and semantic processing, the phonological mismatch negativity (PMN) and the N400, respectively. The PMN is generally observed between 200–350 ms post-stimulus in auditory tasks that engage phonological processing, and is typically elicited when there is a phonological mismatch between a heard word and what was anticipated from context. The PMN is thought to reflect integration of phonological expectations with incoming acoustic information8, or early lexical and semantic influences on word recognition9. The N400 is believed to index semantic expectancy or ease of lexical integration and is elicited by all word-like stimuli10–12.
The aim of the present study was to investigate good and poor readers’ ability to perceive words of varying degrees of phonological similarity presented in sentence context. We used a semantic congruity judgment task in which the phonological contrast between sentence-terminal congruent and incongruent words was manipulated. We hypothesized that poor readers, due to their weak speech perception abilities, are more likely to be deceived by the semantically incongruent but phonologically similar than phonologically dissimilar target words and misperceive them as congruent with their phonological expectations, resulting in reduced activation in the PMN time range for the similar condition.
Methods
Participants
Two groups of children participated: 15 good readers (GR; 11 females, 7–13 years old, mean 9.7), and 15 poor readers (PR; 8 females, 8–13 years old, mean 10.4). There was no significant difference in age between the groups (p > 0.01). Informed consent was obtained from all subjects; the protocol was approved by MGH Human Research Committee. All children had English as their primary language, normal or corrected-to-normal vision, no neurological or psychological histories, and passed a standard hearing screening at 20 dB for 500 to 4000 Hz (ANSI Specification for Audiometers S3.6, 1989). In each group, 3 of the subjects were left-handed (based on the abbreviated form of the Annett Handedness Questionnaire).
Good and poor reader groups were selected on the basis of their performance on the Word Attack and/or Word Identification subtests of the Woodcock Reading Mastery Tests-Revised. Poor readers scored below the 25th percentile on one or both subtests, good readers were above the 39th percentile on both subtests. Additionally, children in the poor reader group were identified by the school system as reading below grade level, and were receiving reading remediation. All subjects scored in the normal range (85–120) on the Peabody Picture Vocabulary Test-R, a measure that correlates with verbal IQ, and on the Test of Nonverbal Intelligence-3; there were no significant group differences in any of these measures, except in reading (GR: 114 (9.7), PR: 87 (6.7), p < 0.05).
Stimuli and task
In all, 400 spoken sentences were constructed, consisting of a sentence stem (ranging from 5–10 words) followed by a sentence-final critical word. The critical words were created in pairs to be either phonologically similar, (PS; e.g., ball-doll), or phonologically dissimilar, (PD; e.g., ball-hall). Specifically, the first phoneme differed in one phonetic feature (voicing or place of articulation) for PS pairs and in two or more of the features (voicing, place, or manner of articulation) for the PD pairs. The words used were of high frequency according to Francis and Kucera and familiar to children reading at first or second grade level. Words in the PS and PD conditions were matched for word frequency (median: 23.5 occurrences/million), word length (median: 4 letters), and number of syllables (median: 1 syllable). The stimuli were recorded by a phonetically-trained native speaker with neutral intonation in a sound treated room at a 22 kHz sampling rate using WaveSurfer software.
The sentence stems and critical terminal words were combined to yield either semantically congruent (e.g., “The boy rolled the ball”), or semantically incongruent sentences (“The boy rolled the hall”). Each critical word was heard twice, in a semantically congruent context, and a semantically incongruent context. The critical words for the incongruent trials were categorized as either PS (e.g., “The boy rolled the doll”: congruent word ball), or PD (“The boy rolled the hall”: congruent word ball) to a congruent word. Ten adults using a five-point scale assessed the sentences to ensure that there was no difference in semantic incongruity between the PS and PD conditions. One hundred filler sentence stems were included to balance the number of ‘yes’ and ‘no’ responses.
Subjects had to indicate whether a sentence they heard made sense or not by pressing one of two buttons on appearance of a question mark at the end of each sentence. Reaction times, measured from the onset of the question mark, were calculated only for correct responses. Response times <200 ms or >3000 ms were rejected as errors, and accounted for 8% of the data. The average sentence duration was 3500 ms. Subjects were instructed to fixate on a cross to reduce eye movement-related contamination in MEG data. A pseudorandomized order of sentences (with at least twenty trials between consecutive presentations of the same sentence stem) identical for all subjects was used. There were a total of eight runs (50 sentences per run), with two-minute breaks between runs.
Data acquisition
MEG and EEG were recorded with a 306-channel (204 planar gradiometers, 102 magnetometers) VectorView MEG system (Elekta-Neuromag, Helsinki, Finland), and 19 EEG electrodes. (For details on recordings and data processing see Ref.13). The signals were filtered at 0.03–200 Hz and sampled at 601 Hz. At the beginning of each run, the head position with reference to the MEG sensors was determined using four marker coils. Epochs from 100 ms before to 800 ms after the onset of the critical word were averaged. Trials containing blinks or other artifacts (>150 µV in EOG, >500 fT/cm in gradiometers) were rejected. The good readers had on average 74 artifact-free epochs per condition, the poor readers 66 (not significant, t-test, p > 0.1). The averaged epochs were low-pass filtered at 40 Hz; the baseline was adjusted according to a 100-ms pre-stimulus period.
T1-weighted magnetic resonance images (MRIs) were acquired on 3T Siemens scanners (TR = 2530 ms, TE = 3.25 ms, flip angle = 7°, 128 sagittal slices, voxel size = 1.3 × 1.0 × 1.3 mm3). A representation of each subject’s cortical surface was constructed using Freesurfer software; the surfaces were aligned across subjects using a spherical morphing method based on sulcal and gyral features14.
Magnetoencephalography source analysis
Cortical sources of the MEG signals were estimated using a distributed model, the weighted Minimum Norm Estimate (MNE)15 constrained to the cortical surface of each individual subject. Differences in the brain activation were quantified using regions of interest (ROI)16. Based on the omnibus (all subjects, both incongruent conditions combined) MNE solution in the 200–500 ms time range, an ROI in the anatomical vicinity of the auditory cortex in the STG was determined in each hemisphere. The regions thus obtained were then transformed into the cortical surface of each individual subject. For statistical comparisons we focused on three time windows of interest: 70–120 ms (acoustic), 200–300 ms (phonological), and 300–500 ms (semantic). For the earliest time window (acoustic analysis), MNEs for the two incongruent conditions (PS, PD) were used; for the others, differences between the incongruent conditions (PS–Congruent vs. PD–Congruent) were analyzed. The absolute values of the estimated current amplitude at each location were calculated and then averaged within each ROI and time window.
Results
Behavioral
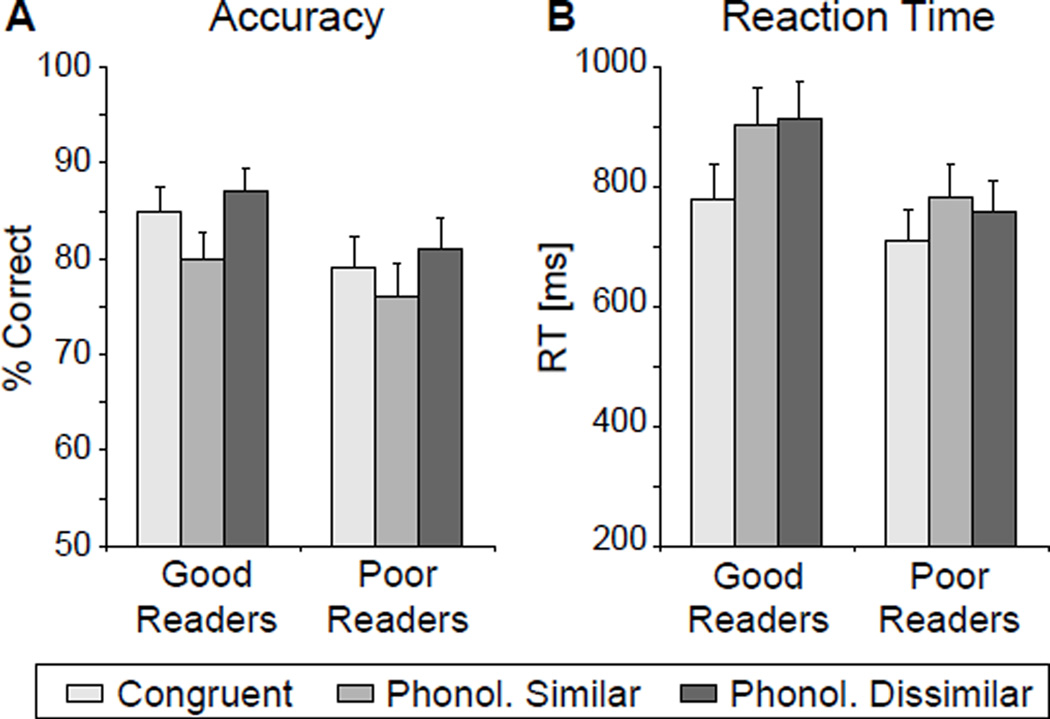
Repeated-measures group (good readers, poor readers) by condition (Congruent, PS, PD) ANOVAs did not yield any main effect of group or group×condition interaction for accuracy or RTs (Fig. 1). However, a main effect of condition was obtained for both accuracy (F(1,28) = 46.8, p < 0.001) and RT (F(2,56) = 15.1, p < 0.001). Post-hoc t-tests revealed that subjects were less accurate on the PS incongruent condition than the congruent condition (p < 0.03) and the PD incongruent condition (p < 0.0001). Additionally, subjects responded faster to the congruent condition than the PD (p < 0.001) and the PS (p < 0.0001) conditions.
Figure 1.
Accuracy (A) and reaction times (RTs) (B) for the semantically congruent and the two semantically incongruent (Phonologically Similar and Phonologically Dissimilar) conditions.
Magnetoencephalography
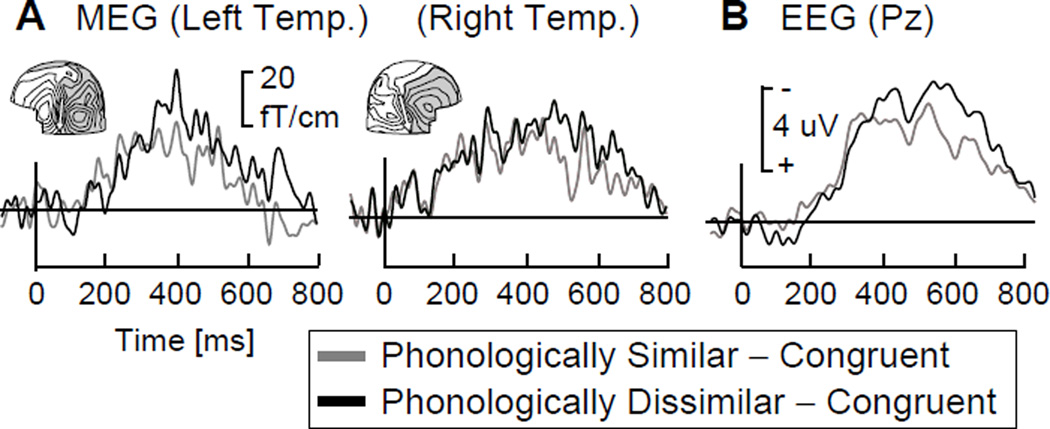
Figure 2 depicts MEG and EEG subtraction waveforms (Incongruent – Congruent), peaking in the latency range of the N400 event-related potential.
Figure 2.
Averaged event-related responses in the two subtraction conditions. A. MEG waveforms for one child in one left temporal and one right temporal gradiometer. The insets depict magnetic field patterns in the Phonologically Dissimilar – Congruent condition. The equivalent current dipoles (arrows) approximate the location of the underlying neural activity. B. EEG waveforms in a posterior midline (Pz) electrode, averaged across all subjects.
In the early (70–120 ms) time range, non-parametric Mann-Whitney tests revealed no significant differences for the MNE amplitude in the STG ROIs, suggesting that the groups did not differ in acoustic processing of the stimuli in the two incongruent conditions.
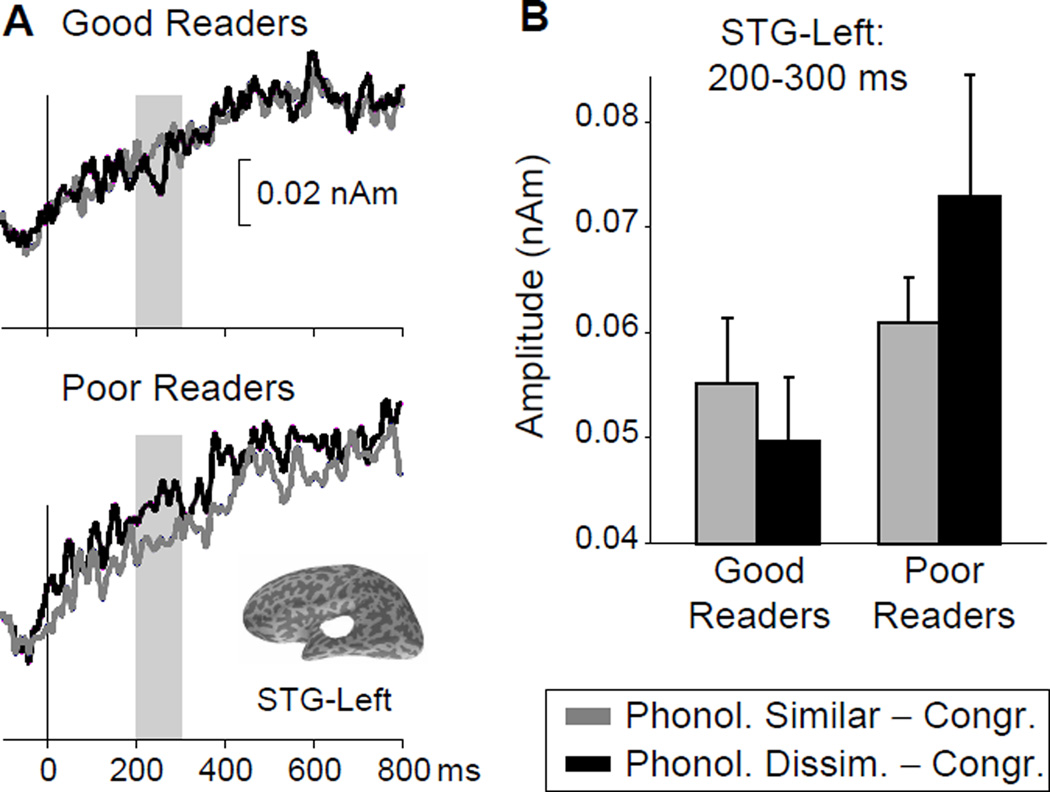
Group differences in MNE activation within the later time bins (200–300 and 300–500 ms) were examined using Mann-Whitney tests for the difference between the two incongruent conditions (PS–Congruent) – (PD–Congruent). A group difference (Mann-Whitney U = 66, p = 0.05, two-tailed, uncorrected), was observed in the left STG at 200–300 ms. The comparisons for the 200–300 ms time bin in the right hemisphere and for the 300–500 ms time bin in the left and right hemispheres were not significant. Figure 3 illustrates the group difference in the average waveforms for the two phonological contrasts (PS–Congruent, PD–Congruent) contributing to the effect in the left STG. On one hand, poor readers appeared to show more activation in the PD–Congruent contrast than in the PS–Congruent contrast. On the other hand, the poor readers appeared to show more activation in the PD–Congruent contrast than the good readers.
Figure 3.
Region of interest (ROI) analysis of the MEG data. A. MEG source waveforms for the left superior temporal gyrus (STG) ROI, obtained from the minimum-norm estimate (MNE) computed for the Incongruent (either Phonologically Similar or Phonologically Dissimilar) – Congruent conditions and averaged across all subjects in the good (top) and poor (bottom) reader groups. The shading indicates the 200–300 ms time range where a significant interaction effect was found. Time 0 ms corresponds to the onset of the critical final word in a sentence. The location of the left-STG ROI is shown on an inflated cortical surface reconstruction of one subject.
B. Magnitude of the group-averaged MNE activation in the left STG ROI (200–300 ms).
Discussion
Perception of auditory words that varied in the degree of phonological contrast was examined in a sentence context in good vs. poor readers. The experimental manipulations were designed to stress the phonological processing abilities of the two groups. Not surprisingly, both groups were less accurate in the more demanding PS condition than the PD condition. However, poor readers did not do worse than the good readers in this comparison. Interestingly, despite similar task performance of the two groups, MEG source analysis suggested differences between the groups’ brain activation patterns as a function of phonological contrast.
When a sentence-terminal word is semantically incongruent with the preceding context, the brain typically elicits an N400 response12. Behaviorally, this takes the form of longer reaction times to the incongruent compared to the congruent sentences, as was seen in the present study. That both groups were less accurate in the PS condition, suggests that good and poor readers were more apt to confuse the PS than the PD stimuli as being semantically congruent with the preceding context. However, poor readers’ weaker phonological coding abilities may have led to their greater reliance, compared to good readers, on sentence context to perceptually disambiguate the PS, and hence confusable, stimuli5. This might account for the activation differences in the PS vs. PD conditions in the poor readers but not in the good readers, in the time range of the PMN, in the present study. Poor readers’ reduced activation in the PS condition compared to the PD condition in left STG between 200–300 ms suggests that the poor reader group may have misperceived the PS words as congruent with their phonological expectations from the semantic context; this account would be consistent with the reduced neural response to phonological repetition that has been found in superior temporal areas17. Conversely, good readers’ superior phonological coding abilities may have made them less vulnerable to the effects of context, regardless of the degree of phonological contrast between the congruent and incongruent sentence terminal words. Additionally, poor readers’ tendency to show more activation in the PD contrast, compared with good readers, may reflect their overall speech perception difficulties in reconciling the phonological mismatch between an expected vs. incoming phonetic percept.
The latency range as well as the cortical location of the activation difference under phonologically similar vs. dissimilar stimulus contrast is consistent with the PMN component8,18 and may thus reflect poor readers’ difficulties with phonological coding. In fact, this area has been implicated bilaterally in speech discrimination19 and linked with several aspects of reading20. No group differences were found in the N400 latency range (300–500 ms) in keeping with previous findings of semantic activation in dyslexic readers10; and from similar tasks that did not involve explicit phonological processing21,22. To our knowledge, there have been no previous reports of PMN effects in children, although some studies have reported a childhood N250 with a fronto-central distribution23, which occurs in the same time range as the PMN and for which sources have been localized in the superior temporal plane24. Interestingly, increased N250 latencies have been found in reading disordered children in some studies, suggestive of slower refractory periods for the N250 generators in this population25.
Developmental factors related to the age range of the subjects (viz., 7–13 yrs), combined with the subtle nature of speech perception deficits in poor readers3, may have played a role in the findings not being as robust as expected. Notwithstanding, the MEG results agree with those obtained with these same subjects in a previous study involving speech perception of isolated words under phonologically demanding conditions13. Overall, our findings are consistent with a phonological deficit in reading disability.
Conclusion
Both good and poor readers made more errors on the phonologically similar than dissimilar contrasts, reflecting perhaps the use of similar phonological coding strategies in speech perception by the two groups. However, MEG source analysis suggested differences between the groups’ brain activation patterns as a function of the degree of phonological contrast. Overall, the findings point to subtle differences in phonological processing abilities of the two groups that are consistent with a phonological account of reading disability.
Acknowledgments
We thank S. Basho, S. Mosher, C. Sahyoun, D. Von Pechmann, and M. Hämäläinen for various aspects of data collection and analysis, and S. Shattuck-Hufnagel, K. Stevens, and C. Perfetti for helpful comments. Supported by NIH: DC00159 and HD046171, and in part by NCRR P41RR14075 and the MIND Institute. DTW was also supported by an NIH Training Grants DC00038, and 5T32EB001680 (PI: Bruce Rosen). We thank all the children and parents for their willingness to participate in the study.
Footnotes
There was no conflict of interest.
References
- 1.Shaywitz SE, Fletcher JM, Holahan JM, Shneider AE, Marchione KE, Stuebing KK, et al. Persistence of dyslexia: the Connecticut Longitudinal Study at adolescence. Pediatrics. 1999;104:1351–1359. doi: 10.1542/peds.104.6.1351. [DOI] [PubMed] [Google Scholar]
- 2.Liberman IY, Shankweiler D, Liberman AM, Fowler C, Fischer FW. Phonetic segmentation and recoding in the beginning reader. In: Reber AS, Scarborough D, editors. Toward a psychology of reading: The proceedings of the CUNY Conference; Erlbaum; Hillsdale, N.J.. 1977. [Google Scholar]
- 3.Mody M, Studdert-Kennedy M, Brady S. Speech perception deficits in poor readers: auditory processing or phonological coding? Journal of Experimental Child Psychology. 1997;64:199–231. doi: 10.1006/jecp.1996.2343. [DOI] [PubMed] [Google Scholar]
- 4.Nation K, Snowling MJ. Individual differences in contextual facilitation: evidence from dyslexia and poor reading comprehension. Child Development. 1998;69:996–1011. [PubMed] [Google Scholar]
- 5.Stanovich KE, West RF. The effect of sentence context on on-going word recognition: Tests of a two-process theory. Journal of Experimental Psychology: Human Perception and Performance. 1981;7:658–672. [Google Scholar]
- 6.Perfetti CA, Goldman SR, Hogaboam TW. Reading skill and the identification of words in discourse context. Memory and Cognition. 1979;7:273–282. doi: 10.3758/bf03197600. [DOI] [PubMed] [Google Scholar]
- 7.Chiappe P, Chiappe DL, Gottardo A. Vocabulary, Context, and Speech Perception Among Good and Poor Readers. Educational Psychology. 2004;24:825–843. [Google Scholar]
- 8.Connolly JF, Phillips NA, Stewart SH, Brake WG. Event-related potential sensitivity to acoustic and semantic properties of terminal words in sentences. Brain and Language. 1992;43:1–18. doi: 10.1016/0093-934x(92)90018-a. [DOI] [PubMed] [Google Scholar]
- 9.van den Brink D, Brown CM, Hagoort P. Electrophysiological evidence for early contextual influences during spoken-word recognition: N200 versus N400 effects. Journal of Cognitive Neuroscience. 2001;13:967–985. doi: 10.1162/089892901753165872. [DOI] [PubMed] [Google Scholar]
- 10.Helenius P, Salmelin R, Service E, Connolly JF. Semantic cortical activation in dyslexic readers. Journal of Cognitive Neuroscience. 1999;11:535–550. doi: 10.1162/089892999563599. [DOI] [PubMed] [Google Scholar]
- 11.Halgren E, Dhond RP, Christensen N, Van Petten C, Marinkovic K, Lewine JD, et al. N400-like magnetoencephalography responses modulated by semantic context, word frequency, and lexical class in sentences. Neuroimage. 2002;17:1101–1116. doi: 10.1006/nimg.2002.1268. [DOI] [PubMed] [Google Scholar]
- 12.Kutas M, Neville HJ, Holcomb PJ. A preliminary comparison of the N400 response to semantic anomalies during reading, listening and signing. Electroencephalography and Clinical Neurophysiology Supplement. 1987;39:325–330. [PubMed] [Google Scholar]
- 13.Wehner DT, Ahlfors SP, Mody M. Effects of phonological contrast on auditory word discrimination in children with and without reading disability: A magnetoencephalography (MEG) study. Neuropsychologia. 2007;45:3251–3262. doi: 10.1016/j.neuropsychologia.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin FH, Witzel T, Ahlfors SP, Stufflebeam SM, Belliveau JW, Hämäläinen MS. Assessing and improving the spatial accuracy in MEG source localization by depth-weighted minimum-norm estimates. Neuroimage. 2006;15:160–171. doi: 10.1016/j.neuroimage.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 16.Wehner DT, Ahlfors SP, Mody M. The influence of semantic processing on phonological decisions in children and adults: a magnetoencephalography (MEG) study. J Speech Lang Hear Res. 2007;50:716–731. doi: 10.1044/1092-4388(2007/050). [DOI] [PubMed] [Google Scholar]
- 17.Wei Q, Ihara A, Hayakawa T, Murata T, Matsumoto E, Fujimaki N. Phonological influences on lexicosemantic processing of kanji words. Neuroreport. 2007;18:1775–1780. doi: 10.1097/WNR.0b013e3282f16ddf. [DOI] [PubMed] [Google Scholar]
- 18.Kujala A, Alho K, Service E, Ilmoniemi RJ, Connolly JF. Activation in the anterior left auditory cortex associated with phonological analysis of speech input: localization of the phonological mismatch negativity response with MEG. Brain Research: Cognitive Brain Research. 2004;21:106–113. doi: 10.1016/j.cogbrainres.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Zaehle T, Geiser E, Alter K, Jancke L, Meyer M. Segmental processing in the human auditory dorsal stream. Brain Res. 2007 doi: 10.1016/j.brainres.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Kevan A, Pammer K. Making the link between dorsal stream sensitivity and reading. Neuroreport. 2008;19:467–470. doi: 10.1097/WNR.0b013e3282f5f7ad. [DOI] [PubMed] [Google Scholar]
- 21.Bonte ML, Blomert L. Developmental dyslexia: ERP correlates of anomalous phonological processing during spoken word recognition. Brain Research: Cognitive Brain Research. 2004;21:360–376. doi: 10.1016/j.cogbrainres.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Sabisch B, Hahne A, Glass E, von Suchodoletz W, Friederici AD. Auditory language comprehension in children with developmental dyslexia: evidence from event-related brain potentials. Journal of Cognitive Neuroscience. 2006;18:1676–1695. doi: 10.1162/jocn.2006.18.10.1676. [DOI] [PubMed] [Google Scholar]
- 23.Ceponiene R, Rinne T, Näätänen R. Maturation of cortical sound processing as indexed by event-related potentials. Clinical Neurophysiology. 2002;113:870–882. doi: 10.1016/s1388-2457(02)00078-0. [DOI] [PubMed] [Google Scholar]
- 24.Takeshita K, Nagamine T, Thuy DH, Satow T, Matsuhashi M, Yamamoto J, et al. Maturational change of parallel auditory processing in school-aged children revealed by simultaneous recording of magnetic and electric cortical responses. Clinical Neurophysiology. 2002;113:1470–1484. doi: 10.1016/s1388-2457(02)00202-x. [DOI] [PubMed] [Google Scholar]
- 25.Sharma M, Purdy SC, Newall P, Wheldall K, Beaman R. Refractory effects on auditory-evoked responses in children with reading disorders. Neuroreport. 2007;18:133–136. doi: 10.1097/WNR.0b013e32800fef71. [DOI] [PubMed] [Google Scholar]