New methods for direct arylation, alkylation, and oxygenation of sp2 carbon-hydrogen bonds in directing-group containing benzenes have resulted in efficient synthetic routes to functionalized arenes.[1] In contrast, direct amination reactions that do not proceed via nitrenoid intermediates[2] are relatively rare. In most cases, palladium catalysis is used for aminations.[3a-p] Furthermore, majority of publications describe intramolecular C-N bond formation.[3a-i] Typically, protected amines or hydroxylamine derivatives are used to install nitrogen moiety. Simple amine coupling partners are employed only rarely. Several deprotonative, copper-catalyzed thiazole and oxazole aminations have been reported.[4b,c,e] Yu has reported a method for palladium-catalyzed benzamide amination by employing a removable auxiliary.[3k] We disclose here a method for an auxiliary-assisted amination of non-acidic benzamide β-C–H bonds and benzylamine derivative γ-C–H bonds that is catalyzed by copper(II) acetate.
Notably, the first copper-catalyzed directed amination of arene C-H bonds was reported by Yu in 2006 (Equation 1).[5a] Subsequently, several other groups have shown that 2-phenylpyridine derivatives can be ortho-aminated by employing copper salts.[5]
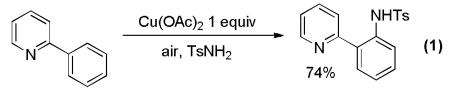 |
However, the scope of these reactions is limited by the presence of a non-removable pyridine moiety. We hypothesized that 8-aminoquinoline and picolinic acid auxiliaries[3g,6] would effect ortho-amination of sp2 C-H bonds based on the following considerations: (1) copper promotes amination and sulfenylation of 2-phenylpyridine derivatives,[5] and (2) sulfenylation can be directed by 8-aminoquinoline and picolinic acid moieties.[6c] The reaction 8-aminoquinoline p-methoxybenzamide and morpholine was investigated with respect to oxidant, additives, and amount o copper (II) acetate (Table 1). Use of 1 equiv Cu(OAc)2 gave 39% conversion to the product (entry 1). Higher conversion was obtained by employing 0.5 equiv of Cu(OAc)2 in the presence of oxygen (entry 2). If N-methylmorpholine oxide (NMO) oxidant was used in the presence of a catalytic amount of Ag2CO3 additive, 74% conversion to the product was observed (entry 4). It was possible to decrease the catalyst loading to 10 mol% (entry 5). Control experiment showed that Cu(OAc)2 is essential for the amination reaction (entry 7).
Table 1.
Optimization of Reaction Conditions
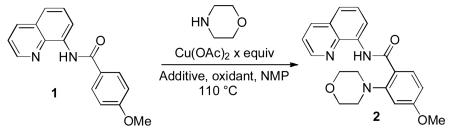
| entry | x equiv | oxidant | additive | yield, % |
|---|---|---|---|---|
| 1 | 1 | none | none | 39[a] |
| 2 | 0.5 | O2 | none | 51[a] |
| 3 | 0.25 | NMO | K2CO3 | 44[a] |
| 4 | 0.25 | NMO | Ag2CO3 0.25 equiv |
74[b] |
| 5 | 0.1 | NMO | Ag2CO3 0.13 equiv |
87[b] |
| 6 | 0.05 | NMO | Ag2CO3 0.075 equiv |
80[a] |
| 7 | 0 | NMO | Ag2CO3 0.125 equiv |
<2 |
Yield determined by 1H NMR of crude reaction mixtures.
Isolated yield.
The reaction of morpholine with 8-aminoquinoline benzamides is presented in Table 2. The amination is successful for both electron-rich (entries 1, 3, 5, 7, 8) and electron-poor amides (entries 2, 4, 6). In contrast with copper-promoted sulfenylation, amination selectively delivers monofunctionalization products at the less sterically demanding position (entries 5, 6, 8). Diamination products were not detected in crude reaction mixtures. The reaction shows good functional group tolerance. Ethers (entries 1 and 5), fluoride (entry 2), and ester substituents (entry 4) are tolerated. Moreover, the reaction is successful for five- and six-membered ring heterocycles. Pyridine (entry 9) and furan (entry 10) derivatives are aminated in good yields. Substrates possessing electron-withdrawing groups require higher catalyst loading. Reactions can be scaled up at least tenfold without significant loss of yield (entry 3).
Table 2.
Copper-Catalyzed Reaction of Morpholine with Carboxylic Acid Derivatives[a]
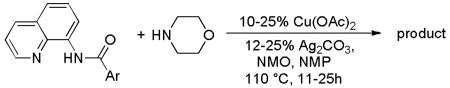
| entry | Ar | product | yield, % |
|---|---|---|---|
| 1 | 4-MeOC6H4 |
|
87 |
| 2 | 4-FC6H4 |
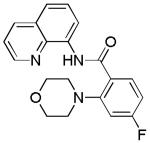
|
70 |
| 3 | 4-tBuC6H4 |
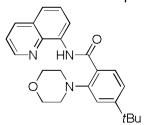
|
81 80[b] |
| 4 | 4-MeO2CC6H4 |
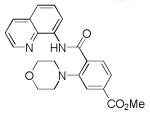
|
68 |
| 5 | 3-MeOC6H4 |
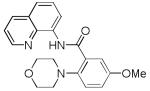
|
82 |
| 6 | 3-CF3C6H4 |
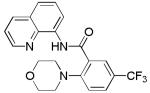
|
67 |
| 7 | 2-MeC6H4 |
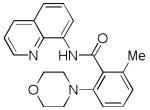
|
70 |
| 8 | 2-naphthyl |
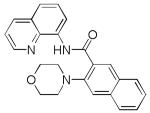
|
66 |
| 9 | 3-(2-Me- pyridyl) |
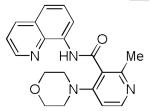
|
56 |
| 10 | 3-(2-Me-furyl) |
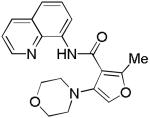
|
57 |
Scale: 0.5 mmol, 2 mL NMP, 2 equiv NMO, 2 equiv morpholine, 10-25 mol% Cu(OAc)2, 12-25 mol% Ag2CO3, 110 °C, 14-25 h. Yields are isolated yields. Please see Supporting Information for details.
Scale: 5 mmol.
α,α–Disubstituted benzylamine picolinamides can also be aminated, albeit in moderate yields (Scheme 1). Thus, α,α-dimethylbenzylamine picolinamide and its p-fluoroderivative were reacted with morpholine and the amination products were isolated in moderate yields. Benzylamine picolinamide amination reactions require relatively high temperatures to achieve reasonable conversions.
Scheme 1.
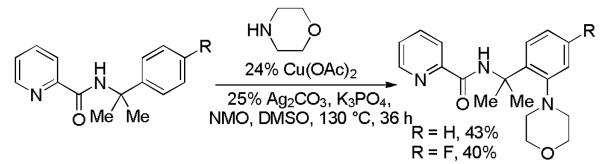
Benzylamine Picolinamide Amination
The reaction scope with respect to amines is presented in Table 3. Simple secondary amines such as methylbenzyl- and methylpropylamine are reactive and the products are obtained in good yields (entries 1 and 2). The reaction tolerates many functionalities on the amine coupling component. Ethyl isonipecotate (entry 3), 4-cyanopiperidine (entry 4), ethylene ketal of 4-ketopiperidine (entry 5), and Boc-protected 4-aminopiperidine (entry 6) afforded products in good yields. Selective coupling with amine NH in the presence of an amide NH was observed (entry 6). Reactions with primary amines were also successful, albeit with somewhat reduced yields. Thus, coupling with cyclohexyl (entry 7), cyclooctyl (entry 8), and neopentylamine (entry 9) afforded products in moderate yields. Straight-chain primary amines afford low yields (entry 10).
Table 3.
Copper-Catalyzed Amination of 8-Amino-quinoline Amides[a]

| entry | amine | product | yield, % |
|---|---|---|---|
| 1 | HNMeBn |
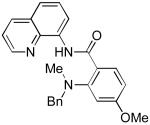
|
82 |
| 2 | HNMePr |
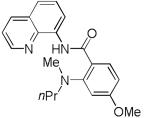
|
74 |
| 3 | 4-EtO2C- piperidine |
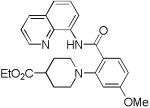
|
69 |
| 4 | 4-NC- piperidine |
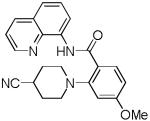
|
71 |
| 5 | Ethylene ketal of 4-keto- piperidine |
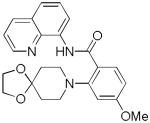
|
82 |
| 6 | 4-BocNH- piperidine |
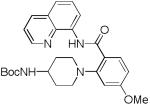
|
83 |
| 7 | Cyclohexyl- amine |
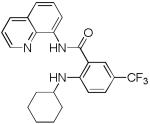
|
40 |
| 8 | Cyclooctyl- amine |
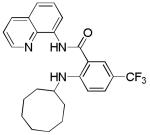
|
52 |
| 9 | Neopentyl- amine |
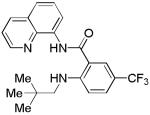
|
32 |
| 10[b] | n-C12H25NH2 |
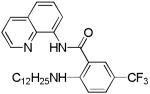
|
20 |
Scale: 0.5 mmol, 2 mL NMP, 2 equiv NMO, 2 equiv amine, 12-25 mol% Cu(OAc)2, 12-25 mol% Ag2CO3, 110 °C, 12 h. Yields are isolated yields.
Pyridine solvent. Please see Supporting Information for details.
The aminoquinoline directing group can be efficiently removed by base hydrolysis. Thus, 4 was heated with NaOH in ethanol for 72 h affording 4-t-butyl-2-morpholinobenzoic acid in high yield (Scheme 2).
Scheme 2.
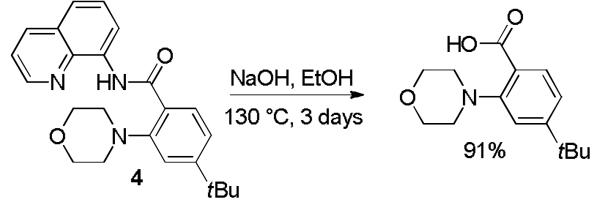
Directing Group Removal
The amination mechanism is unclear at this point. However, activation of an aryl C-H bond by a Cu(II) complex to form aryl-Cu(III) species has been reported in a highly geometrically constrained system.[7] Additionally, well-defined Cu(III) complexes react with N-nucleophiles forming carbon-nitrogen bonds.[8] Furthermore, an early report by Reinaud showed that copper(II) mediates aromatic hydroxylation by trimethylamine N-oxide.[9] The reaction presumably proceeds via a Cu(III) intermediate.
In conclusion, we have developed a method for direct, auxiliary-assisted amination of β-sp2 C–H bonds of benzoic acid derivatives and γ-sp2 C–H bonds of benzylamine derivatives. The reaction employs Cu(OAc)2 catalyst in conjunction with Ag2CO3 co-catalyst, amine coupling partner, NMP or DMSO solvent, and NMO oxidant. The utilization of inexpensive copper acetate catalyst and removable directing group are advantages that allow for a favorable comparison with the previous direct amination reactions. The reaction shows high generality and functional group tolerance. It provides a straightforward protocol for the preparation of ortho-aminobenzoic acid derivatives. Attempts to isolate and characterize reaction intermediates are in progress.
Experimental Section
((N-(2-Morpholino-4-t-butylbenzoyl)-8-aminoquinoline (Table 2, entry 3)
To a 1-dram vial equipped with stir bar was added N-4-t-butylbenzoyl-8-aminoquinoline (152 mg, 0.5 mmol), Cu(OAc)2 (11 mg, 0.06 mmol), and Ag2CO3 (17 mg, 0.06 mmol). Inside the glove box, NMO (117 mg, 1.0 mmol) was added to the vial. Outside the glove box NMP (2 mL) and morpholine (0.09 mL, 1.03 mmol) were added to the resulting mixture. The vial was wrapped with aluminum foil and stirred at 110 °C for 11 h 15 min. After completion, the reaction mixture was cooled down to room temperature and dry absorbed on silica gel. Purification by column chromatography in hexanes/ethyl acetate (4:1) gave 158 mg (81%) of product as a tan solid. Rf = 0.40 (SiO2, hexanes/EtOAc, 3:1), mp 142-144 °C (from hexanes/EtOAc). 1H NMR (400 MHz, CDCl3, ppm) δ 12.75–12.67 (s, 1H) 9.13 (dd, J = 7.8 Hz, J = 1.4 Hz, 1H) 8.87 (dd, J = 4.1 Hz, J = 1.8 Hz, 1H) 8.19 (dd, J = 8.2 Hz, J = 1.4 Hz, 1H) 8.13 (d, J = 8.2 Hz, 1H) 7.63–7.51 (m, 2H) 7.48 (dd, J = 8.2 Hz, J = 4.1 Hz, 1H) 7.31–7.26 (m, 2H) 4.03–3.93 (m, 4H) 3.21–3.15 (m, 4H) 1.37 (s, 9H) . 13C NMR (100 MHz, CDCl3, ppm) δ 166.0, 156.3, 151.2, 148.4, 139.2, 136.7, 136.1, 132.3, 128.7, 127.9, 126.3, 121.9, 121.8, 118.0, 116.4, 66.5, 54.3, 35.6, 31.3. Signal for one carbon could not be located. FT-IR (neat, cm−1) U 2961, 1660, 1526, 1486, 1112. HRMS (ESI+): Calculated for C24H27N3O2 [M+H]+ 390.2182, Found 390.2184.))
Supplementary Material
Footnotes
We thank the Welch Foundation (Grant No. E-1571), NIGMS (Grant No. R01GM077635), Camille and Henry Dreyfus Foundation, and Norman Hackerman Advanced Research Program for supporting this research.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- [1].Reviews: Colby DA, Bergman RG, Ellman JA. Chem. Rev. 2010;110:624. doi: 10.1021/cr900005n. Ackermann L, Vicente R, Kapdi A. Angew. Chem. 2009;121:9976. doi: 10.1002/anie.200902996. Ackermann L, Vicente R, Kapdi AR. Angew. Chem., Int. Ed. 2009;48:9792. doi: 10.1002/anie.200902996. Chen X, Engle KM, Wang D-H, Yu J-Q. Angew. Chem. 2009;121:5196. doi: 10.1002/anie.200806273. Chen X, Engle KM, Wang D-H, Yu J-Q. Angew. Chem., Int. Ed. 2009;48:5094. doi: 10.1002/anie.200806273. Seregin IV, Gevorgyan V. Chem. Soc. Rev. 2007;36:1173. doi: 10.1039/b606984n. Lyons TW, Sanford MS. Chem. Rev. 2010;110:1147. doi: 10.1021/cr900184e. Daugulis O, Do H-Q, Shabashov D. Acc. Chem. Res. 2009;42:1074. doi: 10.1021/ar9000058. Alberico D, Scott ME, Lautens M. Chem. Rev. 2007;107:174. doi: 10.1021/cr0509760. Yeung CS, Dong VM. Chem. Rev. 2011;111:1215. doi: 10.1021/cr100280d. Sun C-L, Li B-J, Shi Z-J. Chem. Commun. 2010:677. doi: 10.1039/b908581e. Li C-J. Acc. Chem. Res. 2009;42:335. doi: 10.1021/ar800164n.
- [2].a) Ng K-H, Chan ASC, Yu W-Y. J. Am. Chem. Soc. 2010;132:12862. doi: 10.1021/ja106364r. [DOI] [PubMed] [Google Scholar]; b) Zalatan DN, Du Bois J. In: Topics in Current Chemistry. Yu J-Q, Shi Z, editors. Vol. 292. Springer-Verlag; Berlin, Germany: 2010. pp. 347–378. [DOI] [PubMed] [Google Scholar]; c) Hamilton CW, Laitar DS, Sadighi JP. Chem. Commun. 2004:1628. doi: 10.1039/b404515g. [DOI] [PubMed] [Google Scholar]; d) Badiei YM, Dinescu A, Dai X, Palomino RM, Heinemann FW, Cundari TR, Warren TH. Angew. Chem. 2008;120:10109. doi: 10.1002/anie.200804304. [DOI] [PubMed] [Google Scholar]; Badiei YM, Dinescu A, Dai X, Palomino RM, Heinemann FW, Cundari TR, Warren TH. Angew. Chem., Int. Ed. 2008;47:9961. doi: 10.1002/anie.200804304. [DOI] [PubMed] [Google Scholar]; e) Thu H-Y, Yu W-Y, Che C-M. J. Am. Chem. Soc. 2006;128:9048. doi: 10.1021/ja062856v. [DOI] [PubMed] [Google Scholar]; f) Lu H, Subbarayan V, Tao J, Zhang XP. Organometallics. 2010;29:389. [Google Scholar]; g) Ichinose M, Suematsu H, Yasutomi Y, Nishioka Y, Uchida T, Katsuki T. Angew. Chem. 2011;123:10058. doi: 10.1002/anie.201101801. [DOI] [PubMed] [Google Scholar]; Ichinose M, Suematsu H, Yasutomi Y, Nishioka Y, Uchida T, Katsuki T. Angew. Chem., Int. Ed. 2011;50:9884. doi: 10.1002/anie.201101801. [DOI] [PubMed] [Google Scholar]; h) Vedernikov AN, Caulton KG. Chem. Commun. 2004:162. doi: 10.1039/b309519c. [DOI] [PubMed] [Google Scholar]; i) Kim HJ, Kim J, Cho SH, Chang S. J. Am. Chem. Soc. 2011;133:16382. doi: 10.1021/ja207296y. [DOI] [PubMed] [Google Scholar]; j) Kantak AA, Potavathri S, Barham RA, Romano KM, DeBoef B. J. Am. Chem. Soc. 2011;133:19960. doi: 10.1021/ja2087085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Intramolecular: Tsang WCP, Zheng N, Buchwald SL. J. Am. Chem. Soc. 2005;127:14560. doi: 10.1021/ja055353i. Inamoto K, Saito T, Katsuno M, Sakamoto T, Hiroya K. Org. Lett. 2007;9:2931. doi: 10.1021/ol0711117. Wasa M, Yu J-Q. J. Am. Chem. Soc. 2008;130:14058. doi: 10.1021/ja807129e. Jordan-Hore JA, Johansson CCC, Gulias M, Beck EM, Gaunt MJ. J. Am. Chem. Soc. 2008;130:16184. doi: 10.1021/ja806543s. Mei T-S, Wang X, Yu J-Q. J. Am. Chem. Soc. 2009;131:10806. doi: 10.1021/ja904709b. Tan Y, Hartwig JF. J. Am. Chem. Soc. 2010;132:3676. doi: 10.1021/ja100676r. Nadres ET, Daugulis O. J. Am. Chem. Soc. 2012;134:7. doi: 10.1021/ja210959p. He G, Lu C, Zhao Y, Nack WA, Chen G. Org. Lett. 2012;14:2944. doi: 10.1021/ol301352v. He G, Zhao Y, Zhang S, Lu C, Chen G. J. Am. Chem. Soc. 2012:134. doi: 10.1021/ja3023972. 3. Intermolecular: Sun K, Li Y, Xiong T, Zhang J, Zhang Q. J. Am. Chem. Soc. 2011;133:1694. doi: 10.1021/ja1101695. Yoo EJ, Ma S, Mei T-S, Chan KSL, Yu J-Q. 2011;133:7652. doi: 10.1021/ja202563w. Xiao B, Gong T-J, Xu J, Liu Z-J, Liu L. J. Am. Chem. Soc. 2011;133:1466. doi: 10.1021/ja108450m. Yin G, Wu Y, Liu G. J. Am. Chem. Soc. 2010;132:11978. doi: 10.1021/ja1030936. Rice GT, White MC. J. Am. Chem. Soc. 2009;131:11707. doi: 10.1021/ja9054959. Du H, Zhao B, Shi Y. J. Am. Chem. Soc. 2008;130:8590. doi: 10.1021/ja8027394. Pan J, Su M, Buchwald SL. Angew. Chem. 2011;123:8806. doi: 10.1002/anie.201102880. Pan J, Su M, Buchwald SL. Angew. Chem., Int. Ed. 2011;50:8647. doi: 10.1002/anie.201102880. Pt catalysis: Dangel BD, Johnson JA, Sames D. J. Am. Chem. Soc. 2001;123:8149. doi: 10.1021/ja016280f.
- [4].a) Brasche G, Buchwald SL. Angew. Chem. 2008;120:1958. doi: 10.1002/anie.200705420. [DOI] [PubMed] [Google Scholar]; Brasche G, Buchwald SL. Angew. Chem., Int. Ed. 2008;47:1932. doi: 10.1002/anie.200705420. [DOI] [PubMed] [Google Scholar]; b) Wang Q, Schreiber SL. Org. Lett. 2009;11:5178. doi: 10.1021/ol902079g. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Kawano T, Hirano K, Satoh T, Miura M. J. Am. Chem. Soc. 2010;132:6900. doi: 10.1021/ja101939r. [DOI] [PubMed] [Google Scholar]; d) Cho SH, Yoon J, Chang S. J. Am. Chem. Soc. 2011;133:5996. doi: 10.1021/ja111652v. [DOI] [PubMed] [Google Scholar]; e) Monguchi D, Fujiwara T, Furukawa F, Mori A. Org. Lett. 2009;11:1607. doi: 10.1021/ol900298e. [DOI] [PubMed] [Google Scholar]; f) Michaudel Q, Thevenet D, Baran PS. J. Am. Chem. Soc. 2012;134:2547. doi: 10.1021/ja212020b. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Tang C, Jiao N. J. Am. Chem. Soc. 2012;134:18294. doi: 10.1021/ja3089907. [DOI] [PubMed] [Google Scholar]
- [5].a) Chen X, Hao X-S, Goodhue CE, Yu J-Q. J. Am. Chem. Soc. 2006;128:6790. doi: 10.1021/ja061715q. [DOI] [PubMed] [Google Scholar]; b) Uemura T, Imoto S, Chatani N. Chem. Lett. 2006;35:842. [Google Scholar]; c) John A, Nicholas KM. J. Org. Chem. 2011;76:4158. doi: 10.1021/jo200409h. [DOI] [PubMed] [Google Scholar]; d) Shuai Q, Deng G, Chua Z, Bohle DS, Li C-J. Adv. Synth. Catal. 2010;352:632. [Google Scholar]
- [6].a) Zaitsev VG, Shabashov D, Daugulis O. J. Am. Chem. Soc. 2005;127:13154. doi: 10.1021/ja054549f. [DOI] [PubMed] [Google Scholar]; b) Shabashov D, Daugulis O. J. Am. Chem. Soc. 2010;132:3965. doi: 10.1021/ja910900p. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Tran LD, Popov I, Daugulis O. J. Am. Chem. Soc. 2012;134:18237. doi: 10.1021/ja3092278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ribas X, Jackson DA, Donnadieu B, Mahía J, Parella T, Xifra R, Hedman B, Hodgson KO, Llobet A, Stack TDP. Angew. Chem. 2002;114:2991. doi: 10.1002/1521-3773(20020816)41:16<2991::AID-ANIE2991>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]; Ribas X, Jackson DA, Donnadieu B, Mahía J, Parella T, Xifra R, Hedman B, Hodgson KO, Llobet A, Stack TDP. Angew. Chem. Int. Ed. 2002;41:2991. doi: 10.1002/1521-3773(20020816)41:16<2991::AID-ANIE2991>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- [8].a) Huffman LM, Stahl SS. J. Am. Chem. Soc. 2008;130:9196. doi: 10.1021/ja802123p. [DOI] [PubMed] [Google Scholar]; b) King AE, Huffman LM, Casitas A, Costas M, Ribas X, Stahl SS. J. Am. Chem. Soc. 2010;132:12068. doi: 10.1021/ja1045378. [DOI] [PubMed] [Google Scholar]
- [9].Reinaud O, Capdevielle P, Maumy M. J. Chem. Soc., Chem. Commun. 1990:566. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


