Abstract
A family history of dementia is associated with an increased risk of developing Alzheimer’s disease (AD) late in life (LOAD). This study marked the first attempt to assess the familial contribution to differences in cognitive performance in a large family-based group in the Chinese community. We enrolled 168 participants without dementia from a single pedigree with 9 probable AD patients diagnosed after age 65. These participants were evaluated with a comprehensive neuropsychological battery, the Chinese version of the Mini Mental State Examination, and the Alzheimer Disease Assessment Scale–Cognitive Subscale. Analyses found that extended family members of the LOAD pedigree showed similar performance on measures of global cognitive function and semantic memory compared to controls, but lower scores on episodic memory, attention, and executive function measures. These results indicate that the genetic influences on certain sub-cognitive domains are more detectable despite normal global cognitive function, and that family members with the LOAD pedigree are at risk for developing LOAD by virtue of their family history with an additive risk due to increased age. The findings in this study support the importance of documenting if there is a positive family history of AD in clinical evaluations.
Keywords: Alzheimer’s disease, Dementia, Genetics, Memory, Geriatric, Pedigree
INTRODUCTION
Alzheimer’s disease (AD) is a devastating neurodegenerative disorder that affects more than 13% of individuals aged 65 years and older, and between 40% and 50% aged 80 years and older (Alzheimer’s Association, 2012). Prior research suggested that many individuals with AD live in low- and middle-income countries (Sosa-Ortiz, Acosta-Castillo, & Prince, 2012). With the rapid aging of the Chinese population in the coming decades (Communiqué of the National Bureau of Statistics of People’s Republic of China on Major Figures of the 2010 Population Census), the prevalence of AD is projected to markedly increase and substantially impact public health. As there are few available efficacious treatment options, understanding genetic and environmental components that modulate AD risk and outcome may provide useful information to help manage this devastating disease.
While the etiology of AD is unknown, success in identifying genetic factors has been notable for the highly heritable early onset form that comprises a minority (1–5%) of AD cases (Goate et al., 1991; Levy-Lahad et al., 1995; Sherrington et al., 1995; Sweet et al., 2010). In contrast, the genetic factors or shared environmental factors are less clear in late onset Alzheimer’s disease (LOAD). Although some LOAD cases appear to be sporadic in nature, genetically mediated risk is evident from the familial aggregation of many LOAD cases (Gatz et al., 2006; Wilson et al., 2010). Several studies (Farrer et al., 1997; Gatz et al., 1997; Green et al., 2002; Johnson et al., 2006; Lee, Cheng, Graff-Radford, Foroud, & Mayeux, 2008; Reitz, Brayne, & Mayeux, 2011; Xu et al., 2009) have found that a family history of dementia is an important risk factor for AD, independent of apolipoprotein E (APOE). After advanced age, having a first-degree family history of LOAD, especially when a parent is positive, is the most significant risk factor for developing AD (Farrer et al., 1997; Gatz et al., 1997; Green et al., 2002; Xu et al., 2009). However, there is limited information regarding much of the genetic or family contribution to LOAD. To develop new preventative approaches for LOAD, it is important to identify persons who are cognitively intact and have high risk for developing LOAD.
Over the past 30 years, neuropsychological testing has identified the earliest, most definitive cognitive and behavioral symptoms of AD (Bennett, Wilson, Boyle, Buchman, & Schneider, 2012; Morris et al., 1989; Salmon & Bondi, 2009). As research has increasingly focused on earlier stages of the illness, it has become clear that biological markers of AD can precede cognitive and behavioral symptoms by many years. Amyloid and tau in the brain and cerebral spinal fluid (CSF) can be detected in vivo in asymptomatic individuals years before the onset of declining cognitive abilities (Jack et al., 2010; Perrin et al., 2009; Sperling et al., 2009). However, the recommended use of biomarkers to detect AD has been applicable mainly in clinical investigations and has yet to become standard practice. Thus, neuropsychological assessment continues to be the recommended method to provide a reliable phenotypic marker of AD that is critical for early detection.
Evidence indicates that AD symptoms begin with a deficit in memory for recent information and executive functions (Lindeboom & Weinstein, 2004; Storandt, 2008). When the process advances, impairment spreads to other cognitive domains including semantic memory, language function, and visuospatial ability (Lindeboom & Weinstein, 2004). Importantly, cognitive assessment is optimal for objectively documenting the degree of cognitive impairment for an individual. Hence, comprehensively examining cognitive performance in family members at high risk of AD may help them as well as their families better prepare for a later life with AD.
The primary aim of the present study was to estimate the family contribution to differences in performance on neurocognitive measures in a LOAD family-based group. We systematically examined the neuropsychological performance of non-demented members of a family with a history of LOAD that involved 9 patients across 4 generations. We hypothesized that at-risk participants from the LOAD cohort would show impaired performance in certain cognitive domains conveying the potential to prospectively develop global cognitive impairments.
METHODS
Study Overview
To accomplish the study aim, we compared performance on a comprehensive neurocognitive battery between LOAD family members and a demographically matched control group of individuals without a family background of AD. This study was approved by the Institutional Review Board of the Wuhan University of Science and Technology. Participants provided written, informed consent before completing study protocol procedures.
Cases Description
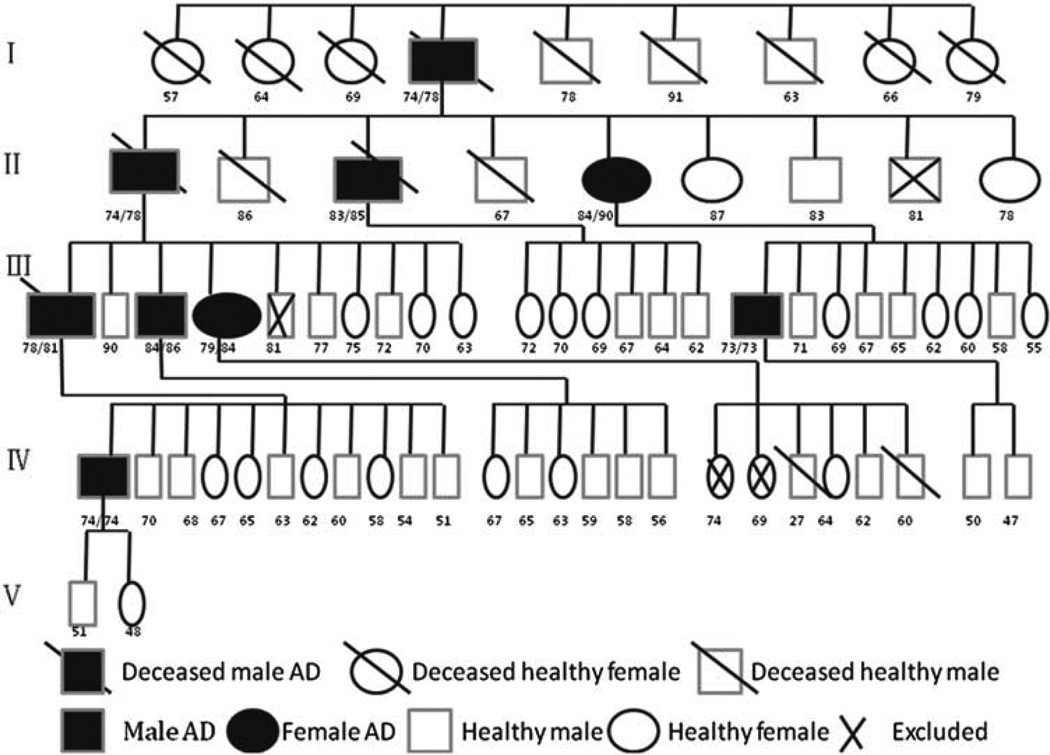
Nine patients (seven males, two females) in a single pedigree spanning four generations had been diagnosed with probable AD after age 65 by neurologists in the Wuzhi County People’s Hospital in the Henan province of northern China (see Figure 1). Seven patients were diagnosed based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (American Psychiatric Association, 1994). This diagnosis required the patients to have a history of cognitive decline and impairment in at least two cognitive domains, one of which must be memory (McKhann, 1984). The neurologists in the local hospital performed a detailed medical history through interviews with the patients and informants, and conducted physical, neurological, and neuropsychological examinations. The two other patients who passed away before 1994 were diagnosed based on a review of medical records. The neurologists examined their medical records and interviewed their family members to determine the diagnosis of AD.
Fig. 1.
Family pedigree of patient cohort. Shows the family pedigree of 9 patients with Late Onset Alzheimer’s Disease (LOAD). I, II, III, IV, and V represents generations. The numbers below the boxes are the age at diagnosis (left) and the age of dying (right), the age at diagnosis (left) and the age of testing (right), or age at testing, respectively. The × in the boxes are individuals excluded from enrollment in the study. For purposes of simplicity, this pedigree does not include some healthy family members.
Four patients died before the study: three due to pneumonia, and one from fall complications. Five patients were seen at the time of this study. They were living in a positive family environment and were cared for by their adult children. The clinicians assessed these patients with the Chinese version of the Cognitive Abilities Screening Instrument (CASI) (Tsai, Lin, Wang, & Liu, 2007) and a structured neurological examination. All patients exhibited the same AD neurocognitive profile that included worsening of orientation, memory, language function, and executive function, which were more pronounced around the 73–79 age range (see detailed information in Table 1).
Table 1.
General information about nine cases
| Case no. | Age of onset (years) | Age of diagnosis (years) | Diagnosis | Age of dying (years) | Age at testing (years) | Year of dying |
|---|---|---|---|---|---|---|
| 1 | 73 | 74 | Possible AD | 78 | 1964 | |
| 2 | 73 | 74 | Probable AD | 78 | 1982 | |
| 3 | 82 | 83 | Probable AD | 85 | 2007 | |
| 4 | 84 | 84 | Probable AD | 90 | ||
| 5 | 74 | 78 | Probable AD | 81 | 2001 | |
| 6 | 79 | 84 | Probable AD | 86 | ||
| 7 | 76 | 79 | Probable AD | 84 | ||
| 8 | 73 | 73 | Probable AD | 73 | ||
| 9 | 74 | 74 | Probable AD | 74 | ||
| Average | 76.44 | 78.11 |
Participants
Participants were biologically related family members or relatives of the 9 LOAD patients from the single pedigree as mentioned above. These participants (LOAD group) were identified as family members or relatives of the LOAD patients based on the pedigree chart kept in their ancestral hall. This pedigree chart depicted all the descendants of this family’s ancestor. The demographically matched control participants (control group) were recruited from four pedigrees who resided in the same county and shared different surnames with the LOAD pedigree. The control participants were age-, sex-, and education-matched with the LOAD participants. We excluded from the control group those individuals with a family history of dementia. Participants with a prior history of neurological illness, stroke, severe or unstable physical illness, and prior or current substance/alcohol abuse and dependence were excluded for the relatives of the AD patients or for controls. We screened each participant with the Clinical Dementia Rating Scale (CDRS) (Hughes, Berg, Danziger, Coben, & Martin, 1982), and only those participants with a score of zero (indicating healthy) were included in the study.
Assessment of Global Cognitive Function
Trained and certified clinicians administered the neurocognitive battery to each participant. The neurocognitive battery included the Chinese version of the Mini Mental State Examination (CMMSE) (Chiu, Lee,Chung, & Kwong, 1994; Folstein, Folstein, & McHugh 1975) and the Chinese version of the Alzheimer Disease Assessment Scale–Cognitive Subscale (ADAS-Cog) (Chu et al., 2000). The CMMSE ranges from 0 to 30 points and assesses five cognitive domains including orientation (10 points), memory (3 points for registration and 3 points for recall), attention/calculation (5 points), language (8 points), and visuospatial abilities (1 point). The ADAS-Cog is a global cognitive measure that assesses domains of memory, language, and praxis. The error score ranges from 0 to 70 with higher scores reflective of greater cognitive impairment.
Measurement of Specific Cognitive Functions
Trained and certified psychometricians administered the neurocognitive battery to each participant two weeks after the completion of the global cognitive assessments. The battery included 8 brief subtests. The Wechsler Memory Scale-Revised (WMS-R, Wechsler, 1987) Logical Memory Test was used to assess episodic memory. Attention was assessed with Part A of the Trail Making Test (Bowie & Harvey, 2006) and Digit Span Forward (Wechsler, 1987). Executive function was assessed with Part B of the Trail Making Test and Digit Span Backward. Semantic fluency was assessed by asking participants to name exemplars of two semantic categories (animals, vegetables) in separate 1-min trials (Wilson & Bennett, 2005). The Clock Drawing Task (Quental, Brucki, & Bueno, 2009; Nair et al., 2010) was used to evaluate visuospatial ability. Specifically, the participants were instructed to draw a clock face displaying the time 2:45 (Quental, Brucki, & Bueno, 2009). Prior investigations (Gladsjo et al., 1999, Quental, Brucki, & Bueno, 2009; Wechsler, 1987; Werheid et al., 2002) found these neurocognitive measures to have adequate psychometric properties. Moreover, Wilson and Bennett (2005) found that change in performance on these measures has been associated with a genetic risk factor for AD. Both clinicians and psychometricians who assessed global cognitive function were masked to the nature of the study.
Statistical Analyses
The demographic data and neurocognitive scores between groups were analyzed using analysis of variance (ANOVA) models with adjustment for multiple comparisons using the Bonferroni correction technique. Chi-square statistics were used to compare frequencies of the categorical demographic data. For the neurocognitive data, we transformed raw scores to Z-scores based on reference to the mean scores of the control group. We also formed a composite measure of global cognitive function based on all 8 tests in the neurocognitive battery. Multiple regression analyses were conducted to test the family history on the relationship between age and individual neurocognitive test and composite scores. We computed correlation coefficients with corresponding 95% confidence intervals (CI) to determine the degree of relationship between measures. Analyses were conducted with the Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL) for Windows (version 17.0). An alpha level of 0.05 was used to determine significance in the interpretation of all results.
RESULTS
Characteristics of Participants
The demographic characteristics of the participants are shown in Table 2. This study recruited 168 at-risk participants from the LOAD pedigree and 187 demographically matched control participants from the other 4 pedigrees who resided in the same county. The response rate in the LOAD pedigree was 95%, while in the other four control pedigrees it was 67%. This suggested that the control families showed less interest in participating in this study. Eleven individuals in the LOAD pedigrees were excluded due to CDR score > 0 (n=1), stroke (n=2), diabetes (n=5 5), chronic obstructive pulmonary disease (n=1), liver cirrhosis (n=1), and lupus erythematosus (n=1). Thirteen individuals in the control pedigree were excluded due to stroke (n=5), diabetes (n=4), coronary heart disease (n=3), and lung cancer (n=1).
Table 2.
Demographic characteristics of the control and LOAD cohorts
| Characteristics | Control (N=187) | LOAD (N=168) | p value |
|---|---|---|---|
| Age | 65.52 (12.73) | 66.32 (13.27) | .99 |
| Education | 6.33 (3.05) | 6.39 (2.13) | 1.58 |
| Female sex | 75 (42%) | 66 (40%) | 1.38 |
| Relationship between participants and AD patients | 0 | 27 (16%) | |
| Siblings | 0 | 45 (27%) | |
| Offspring | 0 | 94 (56%) | |
| Grandchildren | 0 | 83 (50%) | |
| Great-grandchildren | 2 (1%) | ||
| Great-great-grandchildren |
Data are presented as mean and (standard deviation) or number (percentage).
LOA=Late Onset Alzheimer’s Disease; AD=Alzheimer’s disease.
There were no significant differences between groups with regard to age, gender distribution, or education. The LOAD participants were 60% male (n=102), ranged in age from 50 to 87 (mean (M)=66.32, standard deviation (SD)=13.27) years, and had 6.39 (SD=2.13) years of education on average. The control participants were 58% male (n=112), ranged in age from 50 to 87 (M = 65.52; SD=12.73) years, and had 6.33 (SD=3.05) years of education on average. The relationships between at-risk participants and nine patients included 45 offspring, 27 siblings, 94 grandchildren, 83 great-grandchildren, and 2 great-great-grandchildren.
Global Neuropsychological Characteristics
Comparisons of the CMMSE and ADAS-Cog scores of the LOAD and control groups are shown in Table 3. None of the participants scored above zero on the CDRS, thus all participants were eligible to perform the other neuropsychological measures. The LOAD and control groups showed similar performance on the CMMSE (p=.28). Although the ADAS-Cog error score was slightly higher in the LOAD relative to the control group, the difference was not significant (p=.10).
Table 3.
Performance of cohorts on measures of global neurocog-nitive performance
| Test | LOAD (n=168) |
Control (n=187) |
Z score | p value |
|---|---|---|---|---|
| CMMSE | 29.13 (3.21) | 29.25 (2.74) | −1.09 | .28 |
| ADAS-cog | 10.42 (2.06) | 13.23 (1.56) | −1.84 | .10 |
CMMSE=Chinese version of the Mini-Mental State Examination; ADAS-Cog=Alzheimer Disease Assessment Scale–Cognitive subscale; LOAD=Late Onset Alzheimer’s Disease. Data are presented as mean and (standard deviation).
Performance on Specific Neurocognitive Tests
In the control group, the distribution of scores on each test was approximately within the normal range, whereas the distributions of scores in the LOAD group were below the normal range (see Table 4). As reflected in the Z-scores, the LOAD group had significant impairment in the episodic memory, attention, and executive function domains relative to the other cognitive domains. Also, the LOAD group had significantly poorer performance compared to the control group in the episodic memory (p<.0001), attention (p<.0001), and executive function domains (p<.0001).
Table 4.
Comparison of performance between control and LOAD cohorts on neurocognitive measures and domains
| Cognitive domains | Control (n=187) | LOAD (n=168) | Z score | P value |
|---|---|---|---|---|
| Logical Memory Ia | 9.46 (4.25) | 6.45 (2.12) | −3.92 | <.0001 |
| Logical Memory IIa | 8.29 (3.12) | 5.34 (2.33) | −3.09 | .001 |
| Episodic Memory Composite | 3.57 (2.64) | 1.32 (1.36) | −3.15 | <.0001 |
| Digit Span Forward | 8. 98 (2.16) | 4.47 (1.24) | −3.15 | <.0001 |
| Trail Making Test part A | 26.72 (23.69) | 64.57 (26.74) | −3.64 | <.0001 |
| Attention Domain Composite | 11.11 (9.58) | 3.24 (7.23) | −3.03 | <.0001 |
| Trail Making Test part B | 79.87 (32.25) | 192.34 (53.12) | −2.93 | <.0001 |
| Digit Span Backward | 7.23 (2.12) | 4.82 (2.05) | −2.67 | <.0001 |
| Executive Function Composite | 3.56 (1.63) | 1.31 (1.28) | −2.66 | <.0001 |
| Fluency (animals) | 16.21 (5.23) | 16.34 (3.97) | −0.98 | .21 |
| Fluency (vegetables) | 15.57 (4.98) | 14.98 (3.28) | −0.29 | .12 |
| Semantic Memory Composite | 2.81 (1.78) | 1.39 (1.02) | −0.51 | .10 |
| Clocking drawing test | 7.58 (3.12) | 7.02 (2.89) | −1.09 | .29 |
Data are presented as mean and (standard deviation).
LOAD=Late Onset Alzheimer’s Disease.
Earlier studies found that some cognitive domains were more vulnerable due to the initially selective targeting of AD pathology to select cortical areas (Weintraub, Wicklund, & Salmon, 2012). As such, we examined for associations between the different cognitive domains. The correlations between the composite cognitive domains were low to moderate for the LOAD participants (r=0.39 for episodic memory–semantic memory, r=0.32 episodic memory–executive function, r=0.32 for semantic memory–executive function, r=0.31 for attention domain–semantic memory, all p <.0001). Conversely, the correlations between the composite cognitive domains were moderate to large for the control participants (r=0.81 for episodic memory–semantic memory, r=0.71 for episodic memory–executive function, r=0.68 for semantic memory–executive function, r=0.77 for attention–semantic memory, all p<.001).
Contribution of Age to Cognitive Performance
To examine the contributions of age to performance on neurocognitive measures, we used age as a subcategory within each group. Thus, the LOAD and control groups were categorized into distinct age-ranges (see Table 5). The results showed that lower cognitive scores in patients with AD relative to controls were observed only at older ages, especially in participants who were in the age range of ≥70 years. We then analyzed the difference in magnitude of the correlations between task composites in the two groups. In the LOAD group, better performance in cognitive domains was strongly associated with younger age (r=−0.67; p=.01 for episodic memory; r=−0.71; p=.01 for executive function; r=−0.61; p=.02 for attention). In the control group, there was no prominent correlation between global memory and age.
Table 5.
Distributional characteristics of cognitive scores by age groups
| Cognitive domains | Control (n=187) | LOAD (n=168) | P value1 | Difference between means1 |
|---|---|---|---|---|
| Digit Span Forward | ||||
| 50–59 years | 8.41 (2.53) (n=54) | 8.32 (2.48) (n= 56) | .23 | 1.67 (0.11–3.30) |
| 60–69 years | 8.21 (2.16) (n=92) | 7.23 (2.45) (n= 78) | .05 | 0.13 (–0.14–0.47) |
| 70–79 years | 8.34 (2.83) (n=28) | 5.91 (2.73) (n= 25) | <.0001 | 2.40 (1.20–6.70) |
| ≥80 years | 7.87 (2.52) (n=13) | 5.73 (2.54) (n= 9) | <.0001 | 2.12 (1.14–5.11) |
| p value2 | .1 | <.0001 | ||
| Difference between means2 | 1.39 (0.31–2.92) | 2.65 (1.17–7.13) | ||
| Digit Span Backward | 2.34 (0.98–4.21) | |||
| 50–59 years | 7.36 (2.35) | 7.32 (2.56) | .23 | 1.09 (0.12–3.34) |
| 60–69 years | 7.21 (2.12) | 6.73 (2.71) | .33 | 3.14 (1.57–5.21) |
| 70–79 years | 7.84 (2.43) | 5.91 (2.54) | .007 | 2.29 (0.98–4.02) |
| ≥80 years | 7.07 (2.13) | 5.38 (2.91) | .009 | |
| p value2 | .1 | .0009 | ||
| Difference between means2 | 143 (0.33–2.42) | 1.87 (0.73–2.98) | ||
| Trail Making Test A | ||||
| 50–59 years | 31.82 (1.21) | 34.72 (1.09) | .48 | 11.35 (7.23–14.32) |
| 60–69 years | 34.23 (1.09) | 38.32 (0.58) | .10 | 10.96 (8.31–13.46) |
| 70–79 years | 38.19 (1.02) | 64.02 (0.97) | <.0001 | 9.93 (6.54–14.48) |
| ≥80 years | 42.97 (0.97) | 73.89 (0.78) | <.0001 | 12.18 (9.73–15.93) |
| p value2 | .02 | <.0001 | ||
| Difference between means2 | 7.07 (4.13–9.12) | 8.33 (3.53–9.96) | ||
| Trail Making Test B | ||||
| 50–59 years | 78.82 (1.21) | 79.72 (1.09) | .48 | 21.89 (13.73–37.12) |
| 60–69 years | 84.23 (1.09) | 89.32 (0.58) | .30 | 20.23 (10.75–37.21) |
| 70–79 years | 93.19 (1.02) | 194.02 (0.97) | <.0001 | 24.08 (12.73–36.58) 28.13 (10.68–44.76) |
| ≥80 years | 97.17 (0.97) | 243.89 (0.78) | <.0001 | |
| p value2 | .008 | <.0001 | ||
| Difference between means2 | 31.65 (19.73–45.42) | 43.33 (20.28–55.15) | ||
| Logical Memory Ia | ||||
| 50–59 years | 11.72 (3.24) | 11.68 (3.09) | .18 | 5.12 (2.34–7.87) |
| 60–69 years | 10.32 (4.12) | 9.89 (3.12) | .07 | 3.73 (1.73–5.18) |
| 70–79 years | 10.01 (3.98) | 5.45 (3.56) | .001 | 3.02 (1.26–6.67) |
| ≥80 years | 9.92 (3.78) | 4.43 (3.29) | .0002 | 2.96 (1.05–6.43) |
| p value2 | .1 | .002 | ||
| Difference between means2 | 3.11 (1.13–6.12) | 4.62 (2.13–6.12) | ||
| Logical Memory IIa | ||||
| 50–59 years | 10.32 (3.56) | 10.18 (3.37) | .28 | 5.02 (2.79–9.34) |
| 60–69 years | 10.02 (4.23) | 9.26 (3.66) | .06 | 4.37 (2.04–8.75) |
| 70–79 years | 9.18 (4.12) | 4.81 (3.59) | .0009 | 3.05 (1.04–5.92) |
| ≥80 years | 8.95 (3.98) | 3.74 (4.02) | <.0001 | 3.39 (1.27–6.37) |
| p value2 | .08 | .0009 | ||
| Difference between means2 | 4.15 (2.13–6.82) | 3.32 (1.24–6.71) | ||
| Fluency (animals) | ||||
| 50–59 years | 19.28 (5.23) | 18.83 (5.23) | .10 | 6.35 (3.14–11.57) |
| 60–69 years | 18.29 (5.09) | 17.34 (5.32) | .09 | 4.37 (1.95–7.84) |
| 70–79 years | 16.76 (5.12) | 16.23 (4.87) | .32 | 4.06 (2.11–8.33) |
| ≥80 years | 16.23 (4.98) | 15.98 (5.49) | .20 | 6.45 (2.27–12.91) |
| p value2 | .10 | .09 | ||
| Difference between means2 | 5.54 (2.74–9.65) | 7.32 (3.2–11.71) | ||
| Fluency (vegetables) | ||||
| 50–59 years | 17.31 (5.23) | 17.45 (5.23) | .32 | 8.68 (2.31–12.73) |
| 60–69 years | 16.14 (5.09) | 16.84 (5.32) | .23 | 7.45 (3.28–16.21) |
| 70–79 years | 15.45 (5.12) | 15.92 (4.87) | .21 | 5.78 (1.83–10.52) |
| ≥80 years | 15.27 (4.98) | 16.07 (5.49) | .10 | 5.73 (2.25–11.93) |
| p value2 | .07 | .10 | ||
| Difference between means2 | 6.05 (1.77–12.63) | 6.09 (1.28–12.92) | ||
| Clock Drawing Test | ||||
| 50–59 years | 8.38 (3.12) | 8.27(2.12) | .39 | 1.45 (0.07–5.91) |
| 60–69 years | 8.12 (2.77) | 7.66 (2.86) | .14 | 1.73 (0.33–6.24) |
| 70–79 years | 7.93 (3.09) | 7.43 (2.59) | .12 | 1.47 (0.07–4.36) |
| ≥80 years | 7.59 (2.72) | 7.27 (2.68) | .11 | 1.25 (0.02–4.98) |
| p value2 | .07 | .06 | ||
| Difference between means2 | 2.57 (0.27–4.94) | 2.46 (0.35–5.61) | ||
| Executive Function | ||||
| 50–59 years | 3.41 (3.32) | 3.44 (1.22) | .22 | 0.15 (−0.24–2.05) |
| 60–69 years | 3.39 (3.25) | 3.33 (1.35) | .10 | 0.16 (−0.2–2.93) |
| 70–79 years | 3.37 (2.87) | 1.34 (1.13) | .0002 | 0.17 (−0.17–2.07) |
| ≥80 years | 2.96 (2.66) | 1.27 (1.37) | <.0001 | 0.13 (−0.07–2.75) |
| p value2 | .06 | <.0001 | ||
| Difference between means2 | 0.05 (−0.01−2.97) | 0.11 (−0.31–2.28) | ||
| Episodic Memory | ||||
| 50–59 years | 3.53 (3.52) | 3.52 (3.35) | .16 | 0.12 (−0.29–2.93) |
| 60–69 years | 3.51 (3.45) | 3.51 (3.56) | .29 | 0.35 (−0.02–2.02) |
| 70–79 years | 3.49 (3.54) | 2.43 (2.13) | .001 | 0.29 (−0.34–3.13) |
| ≥80 years | 3.47 (3.48) | 1.36 (1.44) | <.0001 | 0.3 (−0.06–2.65) |
| p value2 | .07 | <.0001 | ||
| Difference between means2 | 0.05 (−0.27–2.55) | 0.04 (−0.11–3.31) | ||
| Attention | ||||
| 50–59 years | 11.55 (10.22) | 11.54 (10.42) | .30 | 0.18 (−0.02–2.81) |
| 60–69 years | 11.54 (10.24) | 11.52 (10.29) | .12 | 0.36 (−0.87–3.83) |
| 70–79 years | 1051 (10.46) | 5.52 (4.58) | .0001 | 0.07 (−0.01–2.55) |
| ≥80 years | 9.49 (9.45) | 3.49 (3.52) | .0001 | 0.12 (−0.04–2.63) |
| p value2 | .09 | .0001 | ||
| Difference between means2 | 0.25 (−0.07–1.96) | 0.04 (−0.27–2.87) | ||
| Semantic Memory | ||||
| 50–59 years | 0.55 (0.28) | 0.54 (0.42) | .30 | 0.15 (−0.07–2.01) |
| 60–69 years | 0.54 (0.98) | 0.52 (0.29) | .12 | 0.26 (−0.87–3.22) |
| 70–79 years | 0.51 (0.78) | 0.52 (0.58) | .20 | 0.25 (−0.03–2.55) |
| ≥80 years | 0.49 (0.32) | 0.49 (0.52) | .27 | 0.35 (−0.13–2.63) |
| p value2 | .09 | .10 | ||
| Difference between means2 | 0.25 (−0.07–1.96) | 0.14 (−0.57–2.88) |
Data are presented as mean and (standard deviation). The N for each age band is provided in the second row, and second and third column respectively. Difference in means is presented as means and (95% Confidence Intervals, CIs). Figures in parentheses are 95% CIs.
The p value indicates difference in means between LOAD group and control group.
The p value indicates difference in means between contrast age groups (50–59 vs. 70–79 years).
LOAD=Late Onset Alzheimer’s Disease.
DISCUSSION
To our knowledge, this study marks the first attempt at documenting cognitive impairment in a single pedigree in this large sample from a Chinese community. The aim of the present study was to estimate the family contribution to the differences in neurocognitive function in a Chinese pedigree that had at least nine persons affected with LOAD across four generations. We evaluated global cognitive function as well as specific neurocognitive domains. Extended family members of the LOAD pedigree showed similar performance on measures of global cognitive function and semantic memory compared to controls, but lower scores on episodic memory, attention, and executive function measures. With recent genetic studies suggesting LOAD to be a genetically heterogeneous condition (Bertram & Tanzi, 2012; Johnson, Storandt, Morris, & Galvin, 2009; Wilson et al., 2010), the memory impairment we observed may be a strong predictor of AD in those with a positive family history. One individual from the LOAD pedigree with a CDR score greater than 0, but short of dementia was excluded from the study. This could explain the relatively comparable performance between the LOAD pedigree and control group on measures of global cognitive function. However, it could also be indicative of some cognitive effects associated with being in the LOAD pedigree.
The ε4 allele of the apolipoprotein E gene (APOE-4) and a family history of AD are both risk factors for the development of AD (Bloss, Delis, Salmon, & Bondi, 2008; Donix et al., 2012). Because APOE-4 accounts for only part of the genetic risk for AD, approximately 42% of persons with AD do not have an APOE-4 allele (Bird, 2005). Thus, a positive family history of dementia, regardless of whether an individual is an APOE-4 carrier, may increase the risk for developing AD (St. George-Hyslop, & Petit, 2005; Wilson et al., 2011).
Importantly, poor neurocognitive performance in the LOAD group was attributable to increased age, particularly age 70 and above. All measures in our neurocognitive battery were directly associated with age. These results suggested that members of the LOAD family were at risk for developing LOAD by virtue of their family history with additive risk due to increased age. Therefore, a family history of dementia is an age-specific risk factor for AD. Interestingly, this study found no significant differences in cognitive status between younger pedigree individuals and controls, despite prior studies that showed differences in brain metabolism and neurocognitive function in unaffected younger individuals with a positive family history of AD (Bloss et al., 2008; Donix et al., 2012), with and without APOE-4 (Bloss et al., 2008; Bookheimer et al., 2000; Johnson et al., 2006; Small et al., 2009; Trivedi et al., 2006).
The differences in neurocognitive performance in our sample became significant only when participants were in the elderly age range (e.g., 70 years old and above). In addition, there has been inconsistent information regarding whether family history of AD is independent from APOE-4 risk (Huang, Qiu, von Strauss, Winblad, & Fratiglioni, 2004), or whether APOE-4 is associated with the rate of progression of cognitive and functional decline in AD after its onset (Corder et al., 1995; Saunders, 2000). The risk for developing AD can be modulated by various and rarely studied factors including possible unidentified genes, gene–gene interactions, and environmental and lifestyle contributions (Bird, 2005). Based on the finding that the average age of onset and diagnosis of AD for 9 cases in this pedigree were at 76 and 78 years of age, it seems expected that some cognitive normal family members in this study would develop cognitive decline in their advanced age. This result may suggest that environmental, familial, and genetic factors impact cognitive functioning in the context of AD.
Our results suggest that family history influences on cognitive function are strong and grow stronger with increased age. We were unable to know if the LOAD sample would always have performed differently from the control cohort as the neurocognitive data were obtained only at one time point. Since the data were cross-sectional, we were unable to longitudinally observe change within each individual. However, from the nature of the inheritance and the relatively constant age (around 73–79 years of age) at disease onset and diagnosis within the LOAD family, participants around the mean age at onset for the family would be at high risk.
While the LOAD group was at risk for cognitive impairment, collectively, the data indicated that the LOAD group had normal performance on the majority of neuropsychological measures with the exception of episodic memory, attention, and executive function tests. The correlations between the composite cognitive domains were low to moderate in the LOAD participants, whereas the correlations were moderate to large in the control participants. These observations are consistent with prior evidence that some cognitive domains are more vulnerable to AD due to the initially selective cortical targeting of AD pathology (Weintraub, Wicklund, & Salmon, 2012). The earliest neurofibrillary changes that are part of the AD pathology usually occur in medial temporal lobe structures (Braak & Braak, 1991), which interrupts the neural network critical for episodic memory function. Over time, the pathology progresses to other cortical regions that produce additional cognitive symptoms that lead to the full dementia syndrome (Braak, & Braak, 1996a, 1996b; Braak, Arai, & Braak, 1999; Jack et al., 2000).
Several previous studies have suggested that the episodic memory deficit has important clinical utility for the early detection of AD (Backman, Small, & Fratiglioni, 2001; Kawas et al., 2003; Small, Fratiglioni, Viitanen, Winblad, & Backman, 2000). Evidence suggests that a subtle impairment in episodic memory often occurs before the emergence of the obvious cognitive and behavioral changes required for a clinical diagnosis of AD (Welsh, Butters, Hughes, Mohs, & Heyman, 1991, 1992; Welsh et al., 1994). Also, deficits in executive function occur early in the course of AD and are often evident in the mild cognitive impairment (MCI) stage (Backman, Jones, Berger, Laukka, & Small, 2005; Baudic et al., 2006; Bisiacchi, Borella, Bergamaschi, Carretti, & Mondini, 2008; Dickerson, Sperling, Hyman, Albert, & Blacker, 2007). Executive function deficits, in addition to difficulties with delayed memory recall, predict subsequent progression to AD dementia (Albert, 1996; Marshall et al., 2011). Consistent with this possibility, many studies have found that poor performance on episodic memory and executive function measures in nondemented elderly adults predict cognitive decline and progression to AD over a time period of 1 to 6 years (Albert, Moss, Tanzi, & Jones, 2001; Backman et al., 2005; Blacker et al., 2007; Bondi et al., 1995; Bondi, Salmon, Galasko, Thomas, & Thal, 1999; Bondi et al., 2008; Chen et al., 2001; Fine et al., 2008; Lange et al., 2002). Thus, the decline in episodic memory and executive function in members of the LOAD family may be a cognitive marker of future decline in global cognitive abilities. Given the relationship between these specific cognitive deficits and prodromal AD, clearer insight into the specific cognitive changes that occur in our sample may lead to earlier and more accurate identification of elderly adults at higher risk of developing AD. These findings indicate that the genetic influences on individual cognitive domains are different, which is important to consider when exploring the roles of genetic and environmental risk factors.
This study was strengthened by the large sample size of the extended LOAD family and by comparing their neurocognitive performance to a demographically matched control group. The neurocognitive measures included in the study were selected to comprehensively assess those neurocognitive domains found to be sensitive to and detect early cognitive changes related to AD, before the functional difficulties required for a diagnosis (Gladsjo et al., 1999; Quental, Brucki, & Bueno, 2009; Wechsler, 1987; Weintraub et al., 2012; Werheid et al., 2002). Thus, this study provided a unique opportunity for future studies to longitudinally trace the progression across familial pedigrees. As the participants in this study were functioning at a high level in daily life without significant memory difficulties, our findings reflect very early cognitive changes in this at-risk family. The importance of the findings hinges upon the issue of the efficacy of early screening for the detection of early signs of dementia. Indeed, our results provide evidence for clinical practice to implement systematic neurocognitive evaluations at critical time points for those persons with a family history of AD.
In conclusion, we used a psychometrically sound neurocognitive battery to comprehensively assess the cognitive effects of advancing age and AD. The findings suggested that family members with the LOAD pedigree are at increased risk for developing LOAD. Thus, it is critical to develop comprehensive strategies to prevent, or at minimum minimize the decline in cognitive abilities in individuals at risk for AD. Further research is warranted to continue this important line of investigation to both determine the risk factors of LOAD, and develop cognitive remediation strategies for those extended family members who are in the young adult stage. Follow-up will be necessary to determine which individuals will ultimately develop AD. Our findings underline the importance of documenting if there is a positive family history of AD in clinical evaluations, which could serve as a significant risk factor for the prospective development of AD.
ACKNOWLEDGMENTS
Our deepest gratitude is extended to the families who provided their invaluable time to participate in this study. This work was supported by the grant (31271199, PI: Y. Zeng) from the National Natural Science Foundation of China, as well as the Key Technology Research Programs (2013060602010278, PI: Y. Zeng) from Wuhan city, China, and in part by a grant (K23 MH087739, PI: S. McClintock) from the National Institute of Mental Health. The information in this manuscript and the manuscript itself has never been published either electronically or in print.
Footnotes
Disclosure: No conflicts of interest exist.
REFERENCES
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2012;8(2):131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Albert MS. Cognitive and neurobiologic markers of early Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(24):13547–13551. doi: 10.1073/pnas.93.24.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. Journal of the International Neuropsychological Society. 2001;7(5):631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (Pub.) Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition—Text Revision (DSMIV-TR) Washington, DC: American Psychiatric Press, Inc; 1994. [Google Scholar]
- Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer’s disease: A meta-analysis. Neuropsychology. 2005;19(4):520–531. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- Backman L, Small BJ, Fratiglioni L. Stability of the preclinical episodic memory deficit in Alzheimer’s disease. Brain. 2001;124(Pt 1):96–102. doi: 10.1093/brain/124.1.96. [DOI] [PubMed] [Google Scholar]
- Baudic S, Dalla Barba G, Thibaudet MC, Smagghe A, Remy P, Traykov L. Executive function deficits in early Alzheimer’s disease and their relations with episodic memory. Archives of Clinical Neuropsychology. 2006;21(1):15–21. doi: 10.1016/j.acn.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Boyle PA, Buchman AS, Schneider JA. Relation of neuropathology to cognition in persons without cognitive impairment. Annals of Neurology. 2012;72(4):599–609. doi: 10.1002/ana.23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, Tanzi RE. The genetics of Alzheimer’s disease. Progress in Molecular Biology and Transitional Science. 2012;107:79–100. doi: 10.1016/B978-0-12-385883-2.00008-4. [DOI] [PubMed] [Google Scholar]
- Bloss CS, Delis DC, Salmon DP, Bondi MW. Decreased cognition in children with risk factors for Alzheimer’s disease. Biological Psychiatry. 2008;64(10):904–906. doi: 10.1016/j.biopsych.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird TD. Genetic factors in Alzheimer’s disease. The New England Journal of Medicine. 2005;352:862–864. doi: 10.1056/NEJMp058027. [DOI] [PubMed] [Google Scholar]
- Bisiacchi PS, Borella E, Bergamaschi S, Carretti B, Mondini S. Interplay between memory and executive functions in normal and pathological aging. Journal of Clinical and Experimental Neuropsychology. 2008;30(6):723–733. doi: 10.1080/13803390701689587. [DOI] [PubMed] [Google Scholar]
- Blacker D, Lee H, Muzikansky A, Martin EC, Tanzi R, McArdle JJ, Albert M. Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Archives of Neurology. 2007;64(6):862–871. doi: 10.1001/archneur.64.6.862. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer’s disease. The New England Journal of Medicine. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Jak AJ, Delano-Wood L, Jacobson MW, Delis DC, Salmon DP. Neuropsychological contributions to the early identification of Alzheimer’s disease. Neuropsychology Review. 2008;18(1):73–90. doi: 10.1007/s11065-008-9054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Salmon DP, Galasko D, Thomas RG, Thal LJ. Neuropsychological function and Apolipopro-tein E genotype in the preclinical detection of Alzheimer’s disease. Psychology and Aging. 1999;14(2):295–303. doi: 10.1037//0882-7974.14.2.295. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Salmon DP, Monsch AU, Galasko D, Butters N, Klauber MR, Saitoh T. Episodic memory changes are associated with the APOE-epsilon 4 allele in nondemented older adults. Neurology. 1995;45(12):2203–2206. doi: 10.1212/wnl.45.12.2203. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Harvey PD. Administration and interpretation of the trail making test. Nature Protocols. 2006;1(5):2277–2281. doi: 10.1038/nprot.2006.390. [DOI] [PubMed] [Google Scholar]
- Braak E, Arai K, Braak H. Cerebellar involvement in Pick’s disease: Affliction of mossy fibers, monodendritic brush cells, and dentate projection neurons. Experimental Neurology. 1999:153–163. doi: 10.1006/exnr.1999.7131. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathologica (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Development of Alzheimer-related neurofibrillary changes in the neocortex inversely recapitulates cortical myelogenesis. Acta. Neuropathologica (Berl) 1996a;92:197–201. doi: 10.1007/s004010050508. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurologica Scandinavica Supplementary. 1996b;165:3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- Chen PJ, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M. Patterns of cognitive decline in presymptomatic Alzheimer disease: A prospective community study. Archives of General Psychiatry. 2001;58(9):853–858. doi: 10.1001/archpsyc.58.9.853. [DOI] [PubMed] [Google Scholar]
- Chiu HF, Lee HC, Chung WS, Kwong PK. Reliability and validity of the Cantonese version of the Mini-Mental State Examination — a preliminary study. Journal of Hong Kong College of Psychiatrists. 1994;2(5):25–28. [Google Scholar]
- Chu LW, Chiu KC, Hui SL, Yu GK, Tsui WJ, Lee PW. The reliability and validity of the Alzheimer’s Disease Assessment Scale Cognitive Subscale (ADAS-Cog) among the elderly Chinese in Hong Kong. Annals Academy of Medicine Singapore. 2000;29(4):474–485. [PubMed] [Google Scholar]
- Communique´ of the National Bureau of Statistics of People’s Republic of China on Major Figures of the 2010 Population Census (No. 1) (2011, April 28) National Bureau of Statistics of China; Retrieved from http://www.stats.gov.cn/english/newsandcomingevents/t20110428_402722244.htm. [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr, Rimmler JB. Apolipoprotein E, survival in Alzheimer’s disease patients, and the competing risks of death and Alzheimer’s disease. Neurology. 1995;45:1323–1328. doi: 10.1212/wnl.45.7.1323. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Sperling RA, Hyman BT, Albert MS, Blacker D. Clinical prediction of Alzheimer disease dementia across the spectrum of mild cognitive impairment. Archives of General Psychiatry. 2007;64(12):1443–1450. doi: 10.1001/archpsyc.64.12.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donix M, Ercoli LM, Siddarth P, Brown JA, Martin-Harris L, Burggren AC, Bookheimer SY. Influence of Alzheimer disease family history and genetic risk on cognitive performance in healthy middle-aged and older people. The American Journal of Geriatric Psychiatry. 2012;20(7):565–573. doi: 10.1097/JGP.0b013e3182107e6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Journal of the American Medical Association. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- Fine EM, Delis DC, Wetter SR, Jacobson MW, Jak AJ, McDonald CR, Bondi MW. Cognitive discrepancies versus APOE genotype as predictors of cognitive decline in normal-functioning elderly individuals: A longitudinal study. American Journal of Geriatric Psychiatry. 2008;16(5):366–374. doi: 10.1097/JGP.0b013e3181629957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. “Mini-mental state”. [DOI] [PubMed] [Google Scholar]
- Gatz M, Pedersen NL, Berg S, Johansson B, Johansson K, Mortimer JA, Ahlbom A. Heritability for Alzheimer’s disease: The study of dementia in Swedish twins. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1997;52(2):M117–M125. doi: 10.1093/gerona/52a.2.m117. [DOI] [PubMed] [Google Scholar]
- Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, Berg S, Pedersen NL. Role of genes and environments for explaining Alzheimer disease. Archives of General Psychiatry. 2006;63(2):168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- Gladsjo JA, Schuman CC, Evans JD, Peary GM, Miller SW, Heaton RK. Norms for letter and category fluency: Demographic corrections for age, education, and ethnicity. Assessment. 1999;6(2):147–178. doi: 10.1177/107319119900600204. [DOI] [PubMed] [Google Scholar]
- Green RC, Cupples LA, Go R, Benke KS, Edeki T, Griffith PA. Risk of dementia among white and African American relatives of patients with Alzheimer disease. Journal of the American Medical Association. 2002;287(3):329–336. doi: 10.1001/jama.287.3.329. MIRAGE Study Group. [DOI] [PubMed] [Google Scholar]
- Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, James L. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Huang W, Qiu C, von Strauss E, Winblad B, Fratiglioni L. APOE genotype, family history of dementia, and Alzheimer disease risk: A 6-year follow-up study. Archives of Neurology. 2004;61(12):1930–1934. doi: 10.1001/archneur.61.12.1930. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. British Journal of Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu Y, O’Brien PC, Smith GE, Ivnik RJ, Kokmen E. Rates of hip-pocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55(4):484–89. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DK, Storandt M, Morris JC, Galvin JE. Longitudinal study of the transition from healthy aging to Alzheimer disease. Archives of Neurology. 2009;66(10):1254–1259. doi: 10.1001/archneurol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Trivedi MA, Ries ML, Torgerson BM, Carlsson CM, Sager MA. The influence of Alzheimer disease family history and apolipoprotein E epsilon4 on mesial temporal lobe activation. Journal of Neuroscience. 2006;26(22):6069–6076. doi: 10.1523/JNEUROSCI.0959-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawas CH, Corrada MM, Brookmeyer R, Morrison A, Resnick SM, Zonderman AB, Arenberg D. Visual memory predicts Alzheimer’s disease more than a decade before diagnosis. Neurology. 2003;60(7):1089–1093. doi: 10.1212/01.wnl.0000055813.36504.bf. [DOI] [PubMed] [Google Scholar]
- Lange KL, Bondi MW, Galasko DG, Delis DC, Salmon DP, Thal LJ. Decline in verbal memory during preclinical Alzheimer’s disease: Examination of the effect of Apolipoprotein E genotype. Journal of the International Neuropsychological Society. 2002;8(7):943–955. doi: 10.1017/s1355617702870096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Cheng R, Graff-Radford N, Foroud T, Mayeux R. Analyses of the National Institute on Aging Late-Onset Alzheimer’s Disease Family Study: Implication of additional loci. Archives of Neurology. 2008;65(11):1518–1526. doi: 10.1001/archneur.65.11.1518. National Institute on Aging Late-Onszheimer’s Disease Family Study Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Wang K. Candidate gene for the chromosome 1 familial Alzheimer’s; disease locus. Science. 1995;269(5266):973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- Lindeboom J, Weinstein H. Neuropsychology of cognitive ageing, minimal cognitive impairment, Alzheimer’s disease, a vascular cognitive impairment. European Journal of Pharmacology. 2004;490(1–3):83–86. doi: 10.1016/j.ejphar.2004.02.046. [DOI] [PubMed] [Google Scholar]
- Marshall GA, Rentz DM, Frey MT, Locascio JJ, Johnson KA, Sperling RA. Executive function and instrumental activities of daily living in mild cognitive impairment and Alzheimer’s disease. Alzheimer’s Disease Neuroimaging Initiative. Alzheimers & Dementia. 2011;7(3):300–308. doi: 10.1016/j.jalz.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(1):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Clark C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Nair AK, Gavett BE, Damman M, Dekker W, Green RC, Mandel A, Stern RA. Clock drawing test ratings by dementia specialists: Interrater reliability and diagnostic accuracy. The Journal of Neuropsychiatry and Clinical Neuroscience. 2010;22(1):85–92. doi: 10.1176/appi.neuropsych.22.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature. 2009;461(1266):916–922. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quental NBM, Brucki SMD, Bueno OFA. Visuospatial function in early Alzheimer’s disease. Dementia & Neuropsychologia. 2009;3(3):234–240. doi: 10.1590/S1980-57642009DN30300010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nature Review Neurology. 2011;7(3):137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon DP, Bondi MW. Neuropsychological assessment of dementia. Annual Review of Psychology. 2009;60:257–282. doi: 10.1146/annurev.psych.57.102904.190024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders AM. Apolipoprotein E and Alzheimer disease: An update on genetic and functional analyses. Journal of Neuropathology Experimental Neurology. 2000;59(9):751–758. doi: 10.1093/jnen/59.9.751. [DOI] [PubMed] [Google Scholar]
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, St George-Hyslop PH. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375(6534):754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- Small BJ, Fratiglioni L, Viitanen M, Winblad B, Backman L. The course of cognitive impairment in preclinical Alzheimer disease: Three- and 6-year follow-up of a population-based sample. Archives of Neurology. 2000;57(6):839–844. doi: 10.1001/archneur.57.6.839. [DOI] [PubMed] [Google Scholar]
- Small GW, Siddarth P, Burggren AC, Kepe V, Ercoli LM, Miller KJ, Barrio JR. Influence of cognitive status, age, and APOE-4 genetic risk on brain FDDNP positron-emission tomography imaging in persons without dementia. Archives of General Psychiatry. 2009;66(1):81–87. doi: 10.1001/archgenpsychiatry.2008.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa-Ortiz AL, Acosta-Castillo I, Prince MJ. Epidemiology of dementias and Alzheimer’s disease. Archives of Medical Research. 2012;43(8):600–608. doi: 10.1016/j.arcmed.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, Johnson KA. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. George-Hyslop PH, Petit A. Molecular biology and genetics of Alzheimer’s disease. Comptes Rendus Biologies. 2005;328(2):119–130. doi: 10.1016/j.crvi.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Storandt M. Cognitive deficits in the early stages of Alzheimer’s disease. Current Directions in Psychological Science. 2008;17(3):198–202. [Google Scholar]
- Sweet RA, Bennett DA, Graff-Radford NR, Mayeux R. Assessment and familial aggregation of psychosis in Alzheimer’s disease from the National Institute on Aging Late Onset Alzheimer’s Disease Family Study. Brain. 2010;133(Pt4):1155–1162. doi: 10.1093/brain/awq001. National Institute on Aging Late-Onszheimer’s Disease Family Study Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MA, Schmitz TW, Ries ML, Torgerson BM, Sager MA, Hermann BP, Johnson SC. Reduced hippocampal activation during episodic encoding in middle-aged individuals at genetic risk of Alzheimer’s disease: A cross-sectional study. BMC Medicine. 2006;4:1–14. doi: 10.1186/1741-7015-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai RC, Lin KN, Wang HJ, Liu HC. Evaluating the uses of the total score and the domain scores in the cognitive abilities screening instalment, Chinese version (CASI C-2.0): Results of confirmatory factor analysis. International Psychogeriatrics. 2007;19(6):1051–1063. doi: 10.1017/S1041610207005327. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler memory scale-revised manual. San Antonio: Psychological Corporation; 1987. [Google Scholar]
- Weintraub S, Wicklund HA, Salmon DP. The Neuropsychological profile of Alzheimer disease. Cold Spring Harbor Perspectives in Medicine. 2012;2(a006171):1–18. doi: 10.1101/cshperspect.a006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh KA, Butters N, Hughes J, Mohs RC, Heyman A. Detection of abnormal memory decline in mild cases of Alzheimer’s disease using CERAD neuropsychological measures. Archives of Neurology. 1991;48(3):278–281. doi: 10.1001/archneur.1991.00530150046016. [DOI] [PubMed] [Google Scholar]
- Welsh KA, Butters N, Hughes JP, Mohs RC, Heyman A. Detection and staging of dementia in Alzheimer’s disease. Use of the neuropsychological measures developed for the Consortium to Establish a Registry for Alzheimer’s Disease. Archives of Neurology. 1992;49(5):448–52. doi: 10.1001/archneur.1992.00530290030008. [DOI] [PubMed] [Google Scholar]
- Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G, Heyman A. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD).V. A normative study of the neuropsychological battery. Neurology. 1994;44(4):609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- Werheid K, Hoppe C, Thone A, Muller U, Mungersdorf M, von Cramon DY. The Adaptive Digit Ordering Test: Clinical application, reliability, and validity of a verbal working memory test. Archives of Clinical Neuropsychology. 2002;17(6):547–565. [PubMed] [Google Scholar]
- Wilson RS, Barral S, Lee JH, Leurgans SE, Foroud TM, Sweet RA, Bennett DA. Heritability of different forms of memory in the Late Onset Alzheimer’s Disease Family Study. Journal of Alzheimer’s Disease. 2011;23(2):249–255. doi: 10.3233/JAD-2010-101515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Bennett DA. Assessment of cognitive decline in old age with brief tests amenable to telephone administration. Neuroepidemiology. 2005;25(1):19–25. doi: 10.1159/000085309. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Leurgans SE, Foroud TM, Sweet RA, Graff-Radford N, Mayeux R, Bennett DA. Assessment of cognitive function in the Late Onset Alzheimer’s Disease Family Study. Archives of Neurology. 2010;67(1):855–861. doi: 10.1001/archneurol.2010.129. National Institute on Aging Late-Onszheimer’s Disease Family Study Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, McLaren DG, Ries ML, Fitzgerald ME, Bendlin BB, Rowley HA, Johnson SC. The influence of parental history of Alzheimer’s disease and apolipoprotein E epsilon4 on the BOLD signal during recognition memory. Brain. 2009;132(Pt 2):383–391. doi: 10.1093/brain/awn254. [DOI] [PMC free article] [PubMed] [Google Scholar]