Abstract
In this study, we found an effective and novel therapeutic approach to atopic dermatitis (AD) therapy via treatment with a canine adipose tissue stem cell (cATSC) extract. We determined that the therapeutic application of cATSC-derived interleukin 10 (IL10) and transforming growth factor β1 (TGFβ1) effectively modulated the overloaded immune response after the induction of AD. In addition, we investigated the molecular role of the cATSC extract during AD treatment. Dogs with naturally occurring AD that was treated at Seoul National University Veterinary Teaching Hospital was enrolled in this study. Owner consent was obtained for privately owned dogs before enrollment. We prepared a primary fat-derived cATSC extract that contained various functional factors, including IL10 and TGFβ1, as a treatment for AD. We found that the cATSC extract significantly ameliorated the pathological symptoms of canine AD. The cATSC extract secreted the immunomodulatory cytokines IL10 and TGFβ1, which modulated the overloaded immune response after the induction of AD. Moreover, these immunomodulatory cytokines modulated AD-induced inflammation and inactivated the pathological signals IL6, INFγ, iNOS, eNOS and Nox4. Additionally, these cytokines protected against apoptotic keratinocyte degeneration. This study demonstrated the novel therapeutic efficacy of the cATSC extract during successive AD treatments, which suggests a potential therapeutic use for human AD patients.
Keywords: allergy, canine adipose tissue-derived stromal cells, immunomodulation, keratinocyte degeneration, protection
Atopic dermatitis (AD) is a common, chronically relapsing, inflammatory skin disease that is characterized by the disturbance of epidermal barrier function, recurrent inflammation and keratinocyte apoptosis.1, 2, 3 The T-helper (Th) type 2 cytokines interleukin 4 (IL4), IL5, IL6 and IL13 support humoral immunity and IgE generation, which is a general marker for atopic disease. A recent clinical study of allergies suggested a personal or familial tendency to produce IgE antibodies in response to low levels of allergens. Along with IgE, one of the potential diagnostic criteria for atopic disease is the presence of an extremely viscous mucoid effusion containing eosinophils.3 Wright et al.4 suggested that the increased expression of IL5 and an increased number of eosinophils along with significantly elevated levels of allergic mediators, such as IL4, IL6 and tumor necrosis factor α (TNFα), are characteristics of allergic individuals.5 Sobol et al.6 also suggested that atopic markers include a significantly higher percentage of T-lymphocytes, along with increased eosinophils, mast cells, basophils, IL4, IL5 and an eosinophilic cationic protein. A recent study also reported that the indoleamine 2, 3-dioxygenase (IDO) pathway contributes to the control of allergic inflammation. The increased expression of T-helper cell type, IL2, IFNγ and TNFα was observed in lesional atopic skin.7 Chronic human AD lesions also express IL4, IL13, IL5, IFNγ and IL12. Among these, IL12 is a Th1 differentiation factor.8, 9 The stimulation of the IDO pathway with distinct Toll-like receptors (TLRs) may complement other immunoregulatory pathways and be helpful in modulating the outcome of allergic inflammation and autoimmunity, among other diseases.10 However, the induction of regulatory T cells may be a major factor in controlling allergic inflammation. Signaling through the high-affinity IgE receptor, FcλR1 is a component of the IgE-mediated allergic reaction.7 Physical contact between the skin and specific agents (allergens) may provoke a hypersensitivity response in the skin, resulting in a polymorphic pattern of inflammation on the skin. Nickel-specific CD4+ T cells can produce IL17 in skin atopy.11, 12 IL17-deficient mice exhibited significantly reduced inflammatory cell infiltration and diminished chemokine and ICAM-1 levels, along with decreased neutrophil infiltration in hapten-treated ears. On the other hand, IFNγ, a characteristic TH1 cytokine, is dominant in the skin of patients with AD. The increased expression of IFNγ and other cytokines that accompany skin infection induces the disease-related apoptosis of keratinocytes in the lesions of patient with AD. The apoptotic death of keratinocytes is mediated through cell death receptors that are activated by ligands. Some of the apoptotic keratinocyte cell death signal ligands are TNFα and the TNFα receptor, TNFα-related apoptosis inducing ligand (TRAIL) receptors 1 and 2, fibroblast growth factor inducible 14 and TNF receptor superfamily member 6 (FAS). Recently, Rebane et al.13 reported that IFNγ influences the expression of cell death receptors and ligands in keratinocytes. In agreement with this finding, NOD2, DUSP1 and ADM were shown to be upregulated by IFNγ in human keratinocytes.13 In fact, IFNγ induced keratinocyte apoptosis and AD in patients, thus affecting the eczematous lesions that develop in AD skin.
In this study, we investigated the therapeutic efficacy of a canine adipose tissue stem cell (cATSC) extract for the treatment of canine AD through the effective modulation of the overloaded immune response and oxidative stress and the protection of keratinocytes against aggressive apoptotic cell death.
Results
The canine abdomen interscapula fat-derived cATSC extract was effectively ameliorated AD symptom
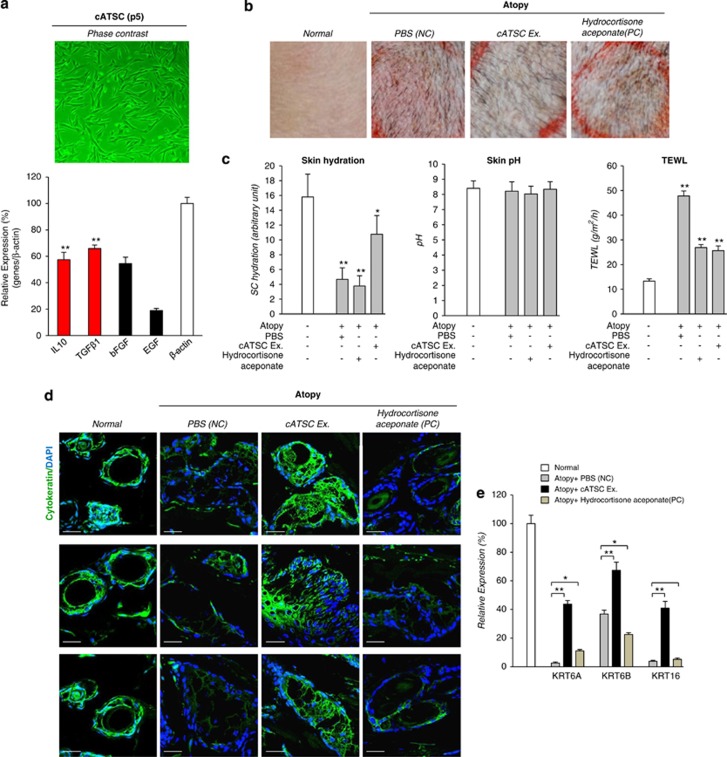
Fat-derived adipose tissue stromal cells express various components of the secretome, including both identified and unidentified immunomodulatory factors. Canine subdermal fat-derived cATSCs express the immunomodulatory factors IL10 and transforming growth factor β1 (TGFβ1), in addition to bFGF and EGF (Figure 1a). To evaluate the therapeutic efficacy of the cATSC extract for AD treatment, we also used PBS as a negative control, cATSC-derived whole extracts, and also hydrocortisone aceponate as a positive control on Dermatophagoides farinae induced inflamed atopic dog skin. At results, compared to negative control and also hydrocortisone aceponate application as a positive control in AD skin, cATSCs extracts treated canine skin showed prominently improved AD symptom with significantly decreased clinical evaluation score after cATSCs application. In contrast, there was no significant clinical improvement in negative control and also positive control. We are also measured skin hydration, pH change and TEWL for biophysical evaluation of AD skin before and after cATSCs extract application. As shown in Figure 1c, skin hydration and TEWL value of skin was significantly normalized after cATSCs extract application compared with PBS-treated AD group. Although, hydrocortisone aceponate application improved TEWL value, skin hydration was not significantly changed as cATSCs extract did (Figure 1c). Finally, treating AD lesions with IL10 or TGBβ1 alone significantly protected keratinocytes (KRT6A, KRT6B and KRT16). In contrast, the PBS and hydrocortisone aceponate treatments decreased the keratinocyte population (Figures 1d and e).
Figure 1.
The canine abdominal fat-derived cATSC extract was effectively ameliorated AD symptoms and preserved skin cytokeratin. (a) Phase-contrast image of cultured canine ATSCs at passage 5 and the expression of various types of functional cytokines, such as IL10, TGFβ1, bFGF and EGF (**P<0.01). (b) The morphological characteristics of canine skin before and after treatment with the cATSC extract, PBS or hydrocortisone aceponate via phase-contrast microscopy analysis. (c) The diagnostic indicators for AD patients, such as moisture content, pH and transepidermal water loss (TEWL) values, were normalized after treatment with the cATSC extract. As a positive control, hydrocortisone aceponate treatment induces low moisture content and an increased TEWL value (*P<0.05, **P<0.01). (d) The immunohistochemical analysis and (e) optimized multiplex PCR analysis of keratinocytes revealed that the skin keratinocytes were almost completely protected by the cATSC treatment. In contrast, the PBS- or hydrocortisone aceponate-treated canine AD skin exhibited significantly degenerated, unprotected keratinocytes (representable keratinocyte markers; keratinocyte A (KRTA), keratinocyte 6B (KRT6B) and keratinocyte 16 (KRT16)) (*P<0.05, **P<0.01)
The effects of the cATSC extract on T-cell infiltration and propagation
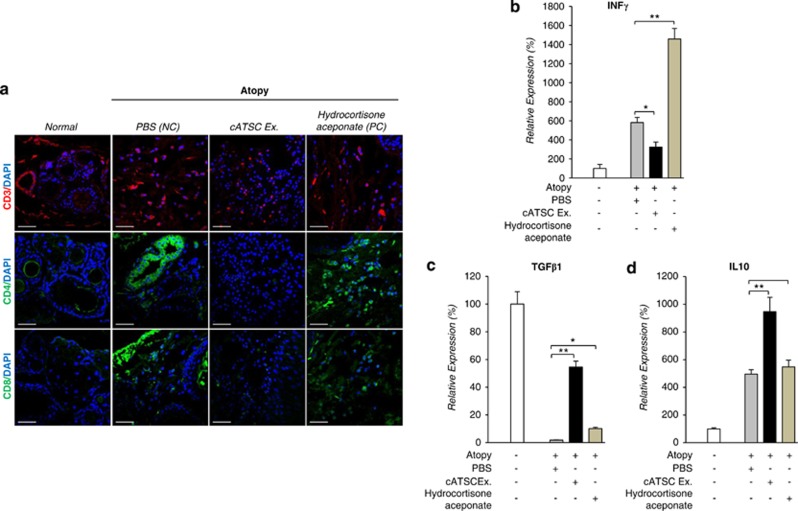
Next, we analyzed the protein expression of CD3, CD4 and CD8 in keratinocytes from canine AD (+PBS) and normal tissues, cATSC extract-treated canine AD tissues and hydrocortisone aceponate-treated AD canine skin. As shown in Figure 2a and Table1, compared with normal skin, AD effectively induced CD3, CD4 and CD8 propagation or mobilization into the skin epithelium (PBS NC in AD animals). As a positive control, hydrocortisone aceponate treatment also increased the CD3+, CD4+ and CD8+ immune cell population in the AD lesions. In contrast, the cATSC extract effectively modulated CD3, CD4 and CD8+ cell migration or propagation into the AD lesion in canines (Figure 2a). Compared with normal keratinocyte tissue, the PBS- and hydrocortisone aceponate-treated atopic animal skin showed significantly greater numbers of CD4 and CD8+ cells. As it were, the cATSC extract treatment effectively normalized the number of CD4 and CD8-positive immune cells (Figure 2a). We confirmed the statistically significant immunomodulatory behavior of cATSC extract treatment in canine AD lesions (Figure 2a). Additionally, the expression of the inflammatory factor INFγ in canine AD skin was significantly decreased after treatment with a cATSC extract. In contrast, hydrocortisone aceponate or PBS treatment significantly increased the INFγ expression (Figure 2b). On the other hand, the expression of the functional immunomodulatory factor TGFβ1 and IL10 was increased significantly after cATSC extract treatment in AD canine skin (Figures 2c and d).
Figure 2.
The effect of the cATSC extract on CD3, CD4 and CD8+ immune cell infiltration and INFγ, TGFβ and IL10 expression in canine AD skin. (a) The immunohistochemical analysis of CD3, CD4 and CD8 expression in canine skin before and after PBS, cATSC extract or hydrocortisone aceponate treatment. (b) Treatment with the cATSC extract effectively downregulated the INFγ expression in canine AD skin compared with the PBS- or hydrocortisone aceponate-treated AD animals. (c) The differential expression of immunomodulatory factors TGFβ1 and (d) IL10 in canine AD skin compared with PBS- or hydrocortisone aceponate-treated animals. The owners applied 20 ng of the cATSC extract, hydrocortisone aceponate or its negative control (PBS) to the lesional skin of the atopic dog once a day for 3 days. The cATSC extract treatment significantly increased the expression of TGFβ1 and IL10 (*P<0.05, **P<0.01)
Table 1. Population density of CD3, CD4 and CD8 cell before and after cATSCs treatment in AD skin (n=4).
|
Positive cell no. ( × 102 cells/mm2) |
|||||
|---|---|---|---|---|---|
| Normal | Atopy/PBS (NC) | Atopy/cATSC Ex. | Atopy/hydrocortisone aceponate (PC) | P-value | |
| CD3 | 1.02±0.08 | 3.66±0.29 | 1.89±0.15 | 3.87±0.31 | <0.01 |
| CD4 | 0.45±0.04 | 4.20±0.42 | 0.65±0.06 | 4.43±0.50 | <0.01 |
| CD8 | 0.61±0.06 | 4.07±0.39 | 0.74±0.06 | 3.79±0.33 | <0.01 |
Note: P-values are ANOVA.
Involvement of inflammatory factors and cytokine expression during cATSC extract-mediated AD amelioration
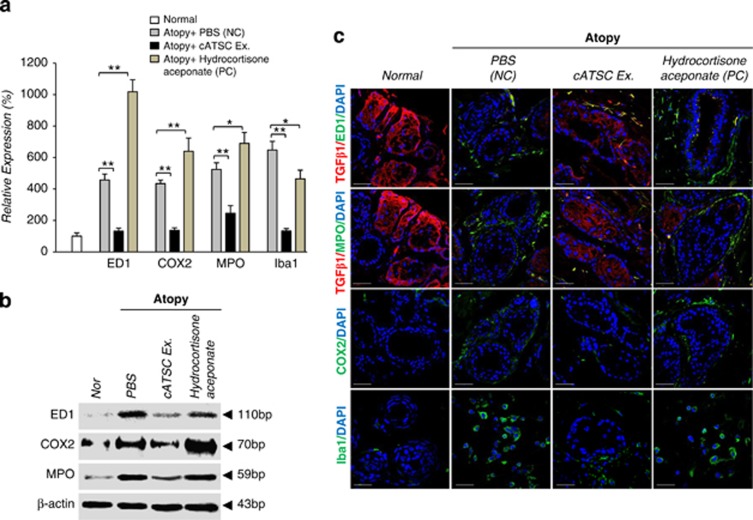
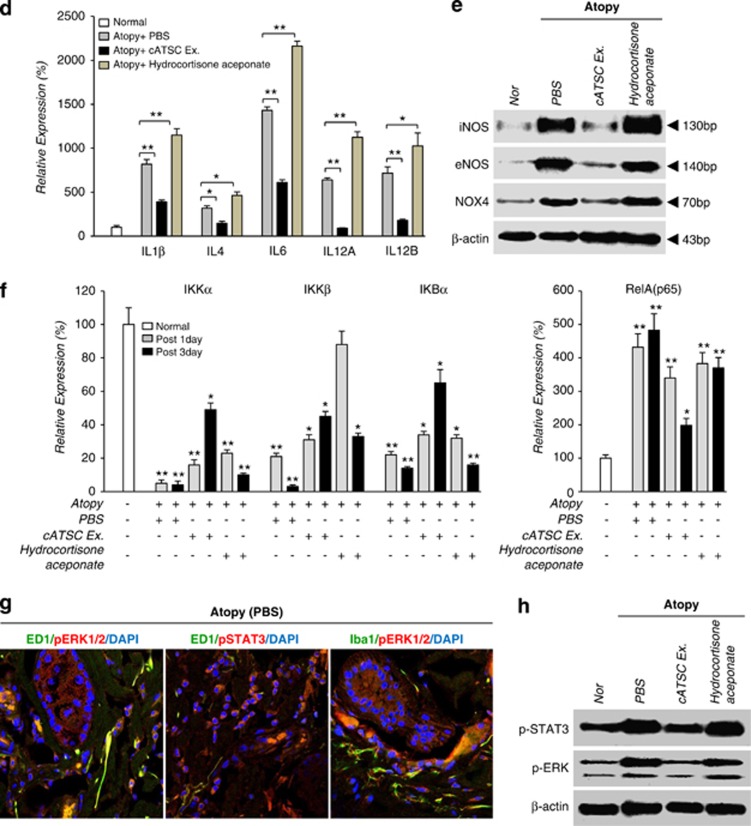
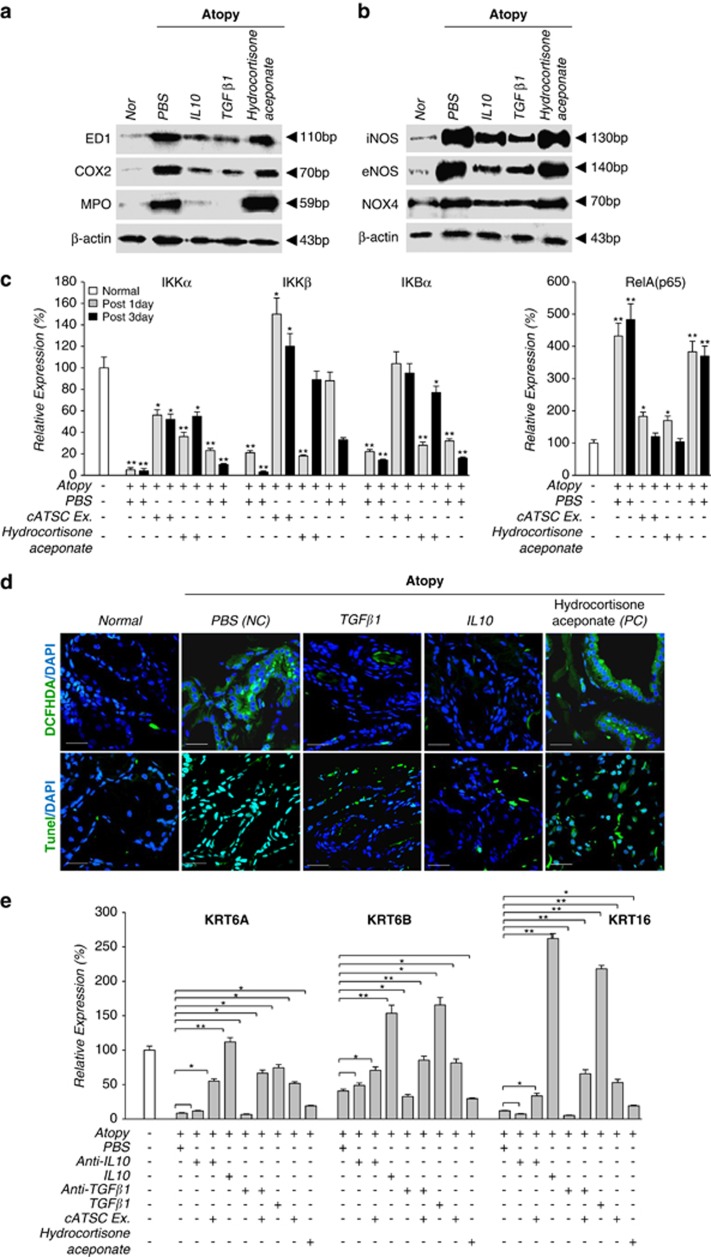
We next analyzed the expression of inflammatory factors before and after treatment with the cATSC extract. At the transcript level, the AD control skin samples showed significantly increased expression of microglial and macrophage markers (ED1 and Iba1). Cox2 and MPO were upregulated in SC canine skin compared with normal canine skin (Figure 3a). We also investigated whether treatment with the cATSC extract affected AD-induced inflammation. The cATSC extract effectively downregulated ED1, Cox2, MPO and Iba1 expression at the transcriptional and protein level compared with the AD control animals (Figures 3a and b). On the other hand, hydrocortisone aceponate (positive control) treatment did not exert an immunomodulatory effect on canine AD (Figures 3a and b). We also completed an immunohistochemical analysis of the TGFβ1 and MPO or ED1, Cox2 (cyclooxygenase 2) and Iba1 expression patterns in PBS-treated, cATSC extract treated or hydrocortisone aceponate treated (positive control) animal skin. As shown in Figure 3c, the canine skin exhibiting AD showed significantly downregulated TGFβ1 expression compared with normal canine skin, but the TGFβ1 expression was rescued after treatment with the cATSC extract (Figure 3c). The expression levels of Cox2 and the microglial marker Iba1 were also significantly increased after AD induction compared with normal skin (Figure 3c). In contrast, treatment with the cATSC extract was effectively upregulated TGFβ1 expression while significantly decreasing the number of Iba1 and Cox2+ inflammatory cells (Figure 3c). Interestingly, hydrocortisone aceponate-treated canine skin also exhibited decreased TGFβ1 expression along with increased Iba1 and Cox2 expression (Figure 3c; Table 2). The proinflammatory factors IL1β, IL4, IL6 and IL12a and IL12b are also involved in AD. As shown in Figure 3d, the AD skin samples exhibited higher levels of IL1β, IL4, IL6 and IL12A and IL12B compared with normal canine skin. Treatment with the ADSC extract effectively modulated the excessive proinflammatory cytokine expression in the canine epidermis, but was not sufficient to normalize the secretion of proinflammatory factors in AD skin. In contrast to the cATSC treatment, the hydrocortisone aceponate treatment evoked the expression of proinflammatory factors in AD animals (Figure 3d). On the other hand, the AD-induced expression of inflammatory iNOS, eNOS and NOX4 was significantly attenuated after treatment with the ATSC extract (Figure 3e). Moreover, the AD-induced expression of IKKα, IKKβ and IKBα was negatively modulated but RelA expression was positively regulated by canine ATSC extract in the transcriptional level (Figure 3g). Interestingly, we also found that pERK1/2/ED1, pERK1/2/Iba1 and pSTAT3/ED1 colocalized in canine AD skin. Immunohistochemical and western blot analyses revealed that the induction of AD effectively enhances pERK1/2 and pSTAT3-mediated microglial and macrophage proliferation in canine skin (Figures 3g and h). On the other hand, our western blot showed that the cATSC extract treatment effectively inactivated pSTAT3 and pERK1/2, attenuating the inflammatory cell proliferation (Figure 3h).
Figure 3.
The molecular role of the cATSC extract in macrophage and microglial inactivation and the attenuation of inflammatory factor secretion. (a, b) The effect of the cATSC extract on macrophage and microglial cell infiltration or proliferation in comparison to PBS or hydrocortisone aceponate treatment. (c) An immunohistochemical analysis showed that, compared with hydrocortisone aceponate- or PBS-treated canine AD skin, cATSC-treated AD skin showed significantly increased TGFβ1 expression, but significantly attenuated COX2 and Iba1 expression. The table also shows the significantly increased expression of TGF β1 after cATSC extract treatment and the significant decrease in the number of inflammatory factor secreting cells. (d–f) The effect of the cATSC extract on the expression of inflammatory factors as following iNOS, eNOS, NOX4, IKKα, IKKβ, IKBα and RelA. In canine AD skin, the cATSC extract effectively downregulated AD-induced inflammatory factor and IKKα, IKKβ and IKBα expression. (g) An immunohistochemical analysis of canine AD skin revealed that the proliferation of macrophages and microglia was mediated by ERK1/2 and STAT3 phosphorylation. (h) A western blot analysis showed that the cATSC extract effectively downregulated AD-induced STAT3 and ERK1/2 phosphorylation in canine AD skin (*P<0.05, **P<0.01)
Table 2. Inflammatory factors and TGFβ1 expression before and after cATSCs extract treatment in AD skin (n=5).
|
Positive cell no. ( × 102 cells/mm2) |
|||||
|---|---|---|---|---|---|
| Normal | Atopy/PBS (NC) | Atopy/cATSC Ex. | Atopy/hydrocortisone aceponate (PC) | P-value | |
| TGFβ1 | 6.89±0.52 | 1.61±0.13 | 5.83±0.31 | 1.83±0.12 | <0.01 |
| ED1 | 0.32±0.04 | 2.99±0.18 | 1.72±0.12 | 3.33±0.31 | <0.01 |
| COX2 | 0.68±0.09 | 3.15±0.29 | 1.01±0.09 | 3.01±0.27 | <0.01 |
| MPO | 0.44±0.03 | 3.47±0.31 | 0.82±0.07 | 3.17±0.32 | <0.01 |
| Iba1 | 0.28±0.02 | 2.67±0.19 | 1.19±0.13 | 2.53±0.18 | <0.01 |
Note: P-values are ANOVA.
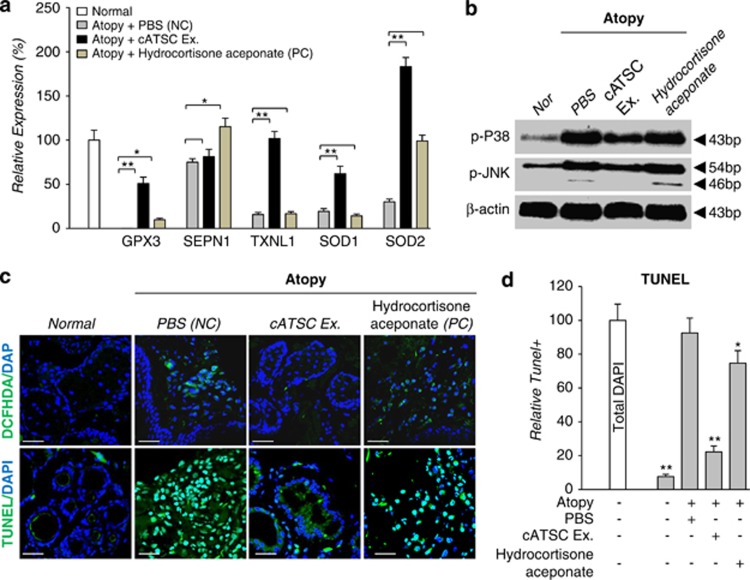
Involvement of Redox scavenging genes and p38/JNK inactivation for protection against AD-induced keratinocyte apoptosis
Next, we studied the relationship between redox regulatory gene expression and keratin preservation or degeneration in canine AD skin. As shown in Figure 4a, the AD skin tissue showed significantly lower redox scavenger gene (GPx3, SEPN1, TXNL1 and SOD1, 2) expression at the transcriptional level than normal skin (Figure 4a). In contrast, the cATSC extract treatment effectively rescued the expression of reactive oxygen species (ROS) scavenging genes, specifically TXNL1 and SOD2 (Figure 4a). Hydrocortisone aceponate treatment did not change the expression of GPx3, TXNL1 or SOD1, but did normalize SEPN1 and SOD2 expression (Figure 4a). The activation of p38/JNK was also significantly enhanced in canine AD skin and hydrocortisone aceponate-treated AD skin, but the cATSC extract significantly attenuated p38/JNK activation in AD skin (Figure 4b). In agreement with our immunohistochemical results, the hydrocortisone aceponate-treated and positive control AD-induced skin exhibited a significant accumulation of ROS (DCFDA+) along with increased TUNEL+ apoptotic cell death (Figure 4c). The level of TUNEL-positive apoptotic cell death was significantly decreased in the cATSC extract-treated skin samples compared with the PBS-treated samples (Figure 4d; Table 4)
Figure 4.
The involvement of ROS-mediated p38/JNK activation during canine AD-induced keratinocyte apoptosis. (a) Treatment with the cATSC extract effectively upregulated the AD-inducing decreased redox scavenger genes, GPx3, SEPN1, TXNL1, SOD1 and SOD2 at the transcriptional level. In contrast, the hydrocortisone aceponate treatment was not effective in normalizing the expression of redox scavenging genes other than SEPN1 and SOD2. (b) The involvement of p38/JNK phosphorylation in AD progression. The canine ATSC extract effectively reversed the AD-induced p38/JNK phosphorylation. (c) The involvement of ROS (1′,7′-Dichlodihydrofluorescein diacetate + DCFDA+) during AD-induced pathogenesis and keratinocyte apoptosis. (d) The cATSC extract effectively scavenged the AD-induced, accumulated ROS and protected against ROS-mediated keratinocyte apoptosis (TUNEL+) (*P<0.05, **P<0.01)
The effect of IL10 and immunomodulatory behavior on keratinocyte protection via NFκB inactivation in AD canine skin
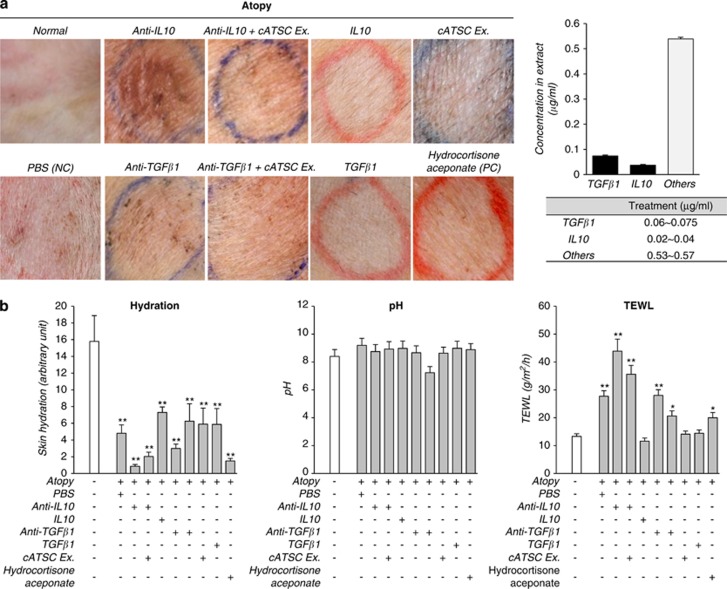
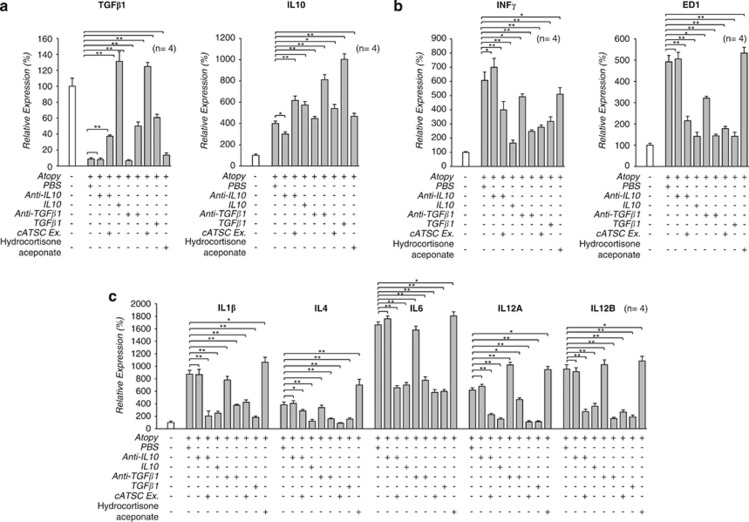
To confirm the immunomodulatory and therapeutic role of TGFβ1 in the cATSC extract, we compared the AD symptoms in PBS-treated AD control animals, TGFβ1-treated AD animals and anti-TGFβ1 and cATSC extract-treated AD animals. As shown in Figure 5a, the AD control animals developed a traditional AD phenotype, but the TGFβ1 and cATSC extract-treated animals exhibited significantly ameliorated AD symptoms (Figure 5a). As shown in Figure 5a, the PBS-treated AD skin was alleviated by IL10 or TGBβ1 treatments. In contrast, hydrocortisone aceponate treatment did not significantly alleviate the canine AD symptoms, which included scratching and the appearance of red spots (Figure 5a). The moisture content, pH and TEWL values were also more or less normalized by the canine ATSC extract (Figure 5b). We sought to identify the key immunomodulatory factor among the functional factors in the cATSC extract. As determined in the first step of this study, cATSCs express several functional cytokines, including IL10 and TGBβ1. Based on those result, we evaluated the immunomodulatory functions of IL10 and TGBβ1 as part of the cATSC extract. Additionally, the increased moisture content and altered TEWL conditions in the AD skin samples more or less normalized after cATSC treatment (Figure 5b). Treating AD lesioned skin with IL10 or TGBβ1 significantly upregulated TGFβ1 and IL10 expression (Figure 6a). In contrast, IL10 or TGBβ1 treatment significantly downregulated the INFγ expression and reduced the number of ED+ cells (Figure 6b). As expected, treating canine AD lesions with IL10 resulted in a strong immunomodulatory phenotype, with significantly decreased IL1β, IL4, 6, IL12α and IL12β expression and protected keratinocytes (Figure 6c). However, hydrocortisone aceponate treatment significantly enhanced the upregulation of inflammatory factors, similar to the AD control animals (Figure 6c). IL10 or TGBβ1 treatment significantly decreased the ED1, Cox2, MPO+ population present in AD skin samples and decreased the expression of iNOS, eNOS and Nox4 at the protein level (Figures 7a and b) or TGBβ1 treatment also significantly attenuated IKKα and IKBα expression along with increased IKKβ expression compared with the PBS control treatment (Figure 7c). The hydrocortisone aceponate treatment also attenuated the expression of Rel B in AD canine skin (Figure 7c). Generally, PBS- or hydrocortisone aceponate-treated AD induced skin cells usually overproduce DCFDA+ ROS. Treatment with the cATSC extract, IL10 or TGBβ1 significantly decreased ROS production or activated the ROS scavenging process, in addition to increasing protection against ROS-mediated apoptotic cell death (Figure 7d; Table 3).
Figure 5.
The function of IL10 and TGFβ1 in canine AD treatment. (a) The phenotypic improvement of canine AD skin before and after IL10 or TGFβ1 and anti-IL10/cATSC extract or anti-TGFβ1/cATSC extract treatment. (b) The change in biophysical indicators before and after IL10 or TGFβ1 and anti-IL10/cATSC extract or anti-TGFβ1/cATSC extract treatment. The moisture content, pH and TEWL were determined in each skin tissue sample as the biophysical factor evaluation (*P<0.05, **P<0.01)
Figure 6.
The molecular function and regulation of IL10 and TGFβ1 expression by the cATSC extract during canine AD treatment. (a) The effect of IL10 and TGFβ1 in the cATSC extract on IL10 and TGFβ1 expression (n=4). Interleukin 10 or the cATSC extract significantly increased the expression of TGFβ1 in AD animal skin. In contrast, the anti-IL10 antibody effectively downregulated the TGFβ1 expression in AD animal skin. On the other hand, TGFβ1 or the cATSC extract significantly increased the IL10 expression in AD animal skin. In contrast, the anti-TGFβ1 antibody effectively downregulated the IL10 expression in AD animal skin. (b) The expression of interferon γ and ED1 was negatively regulated by both IL10 and TGFβ1. However, the expression of the same genes was significantly upregulated by anti-IL10 or anti-TGFβ1 treatment. (c) Additionally, the inflammatory factors IL1β, IL4, IL6, IL12A and IL12B were negatively regulated by IL10 or TGFβ1 treatment in canine AD animal skin. The cATSC extract also significantly downregulated the expression of the same inflammatory factors (*P<0.05, **P<0.01)
Figure 7.
The effect of IL10 and TGFβ1 on inflammatory factor expression in canine AD skin in the protein level. (a) Treatment with IL10 or TGFβ1 significantly downregulated the expression of ED1, Cox2, MPO, (b) iNOS, eNOS, NOX4, (c) and IKKα, IKKβ, IKBα and RelA at the protein or transcript level compared with PBS- or hydrocortisone aceponate-treated AD skin. (d) A histological analysis of IL10- or TGFβ1-treated canine AD skin was used to determine the effects of both factors during ROS accumulation and keratinocyte cell death. (e) The effect of the cATSC extract, IL10, TGFβ1, anti-IL10 or anti-TGFβ1 on the expression of the keratinocyte genes KRT6A, KRT6B and KRT16 at the transcript level (*P<0.05, **P<0.01)
Table 3. Reactive oxygen species (ROS) generation and keratinocyte apoptosis before and after cATSCs Ex, TGFβ and IL10 treatment in AD skin (n=4).
|
Positive cell no. ( × 102 cells/mm2) |
|||||||
|---|---|---|---|---|---|---|---|
| Normal | Atopy/PBS (NC) | Atopy/cATSC Ex. | Atopy/TGFβ1 | Atopy/IL10 | Atopy/hydrocortisone aceponate (PC) | P-value | |
| DCFHDA | 0.32±0.05 | 1.74±0.12 | 0.59±0.03 | 0.91±0.06 | 1.02±0.11 | 1.57±0.18 | <0.05 |
| TUNEL | 0.13±0.02 | 2.21±0.26 | 0.82±0.09 | 1.23±0.18 | 1.04±0.16 | 1.96±0.21 | <0.05 |
Note: P-values are ANOVA.
Next, to confirm the clinical potential of cATSCs extract on atopic dogs, we conduct double-blind, placebo-controlled trial of cATSCs on five atopic dogs. After application of cATSCs extract by the owner for 3 days, clinical symptom of AD was ameliolated in their clinical evaluation score and skin hydration was improved than PBS placebo (Table 4).
Table 4. Double-blind, placebo-controlled trial of cATSCs on five atopic dogs.
|
Atopy |
||||
|---|---|---|---|---|
| Normal (n=5) | PBS (n=5) | cATSC Ex. (n=5) | P-value | |
| Clinical evaluation score | — | 5.2±1.64 | 4.2±0.84 | <0.05 |
| Skin hydration | 16.2±3.03 | 6.27±1.08 | 12.35±3.62 | <0.05 |
| TEWL | 13.38±0.98 | 42.16±12.72 | 27.48±6.34 | NS |
| Skin pH | 8.44±0.52 | 8.37±0.47 | 8.34±0.48 | NS |
Note: P-values are ANOVA.
After application of cATSCs extract, clinical symptom of AD was ameliorated in their clinical evaluation score and skin hydration was improved than PBS placebo.
Discussion
One of the tissue-specific stem cells, adipose tissue-derived stem cells (ATSCs), differentiates into multiple mesenchymal lineages and various other cells or tissues.14, 15 Thus, ATSCs have emerged as a promising tool for tissue engineering, in addition to cell and gene therapy.16 On the other hand, it has been suggested that MSCs may exert beneficial effects through cell-to-cell contact and paracrine factor secretion, possibly mediating cell proliferation, anti-inflammatory/immunoregulatory and anti-apoptotic properties through the secretion of bioactive molecules. Of these properties, one of the most attractive and clinically important features of MSCs is their immunosuppressive potential. Mesenchymal stem cells have been found to inhibit T lymphocyte proliferation and activation in response to alloantigens and nonspecific mitogens. Moreover, MSCs have been found to decrease IFNγ production by T-helper (Th)-1 cell while increasing IL4 secretion, suggesting a shift from a proinflammatory state to an anti-inflammatory state. The MSC-derived soluble factor includes TGFβ1, indoleamine 2,3-dioxygenase (IDO), nitric oxide (NO) and prostaglandin E2 (PGE2), among others.
AD is an inflammatory allergic skin disease with characteristic clinical symptoms. During AD development, inflammation was also evokes oxidative stress, which plays an important role in the AD pathogenesis process in mammals.17, 18 The induction of oxidative stress has been related to a surplus of ROS and is also dependent on deficiencies in the antioxidant system, including the ROS scavenging machinery.19 Increased lymphocyte, monocyte and eosinophil infiltration follows. When overloaded, those immune cells not only secrete bioactive substances, and proinflammatory cytokines such as IL6, IL7 and TNFα, but also increase their ROS production. The accumulation of ROS in keratinocytes is a significant factor in the inflammatory process and induces p38/JNK-mediated apoptotic cell death.18, 20 The inflammatory pathways active in human AD cause oxidative stress that damages lipids, proteins and DNA. In canine AD patients, ROS production and lipid peroxidation are increased during the inflammatory process.21, 22, 23, 24, 25, 26, 27, 28 Finally, the activation of inflammatory pathways in AD patients provokes tissue damage, including keratinocyte damage.
The proinflammatory cytokines TNFα, IL1β, INFγ, IL1α and IL6 are involved in AD pathology in the skin.29, 30, 31 Moreover, these inflammatory factors are activated by the inflammatory transcription factor NFκB, which activates a number of genes, including IL1β, TNF and IL6. The expression of inflammatory cytokines is associated with decreased selenoprotein expression and increased plasma levels of the proinflammatory cytokines IL1β, TNFα and IL6.32 Somatic cell death, or apoptosis, is the general process of cell renewal. However, epithelial keratinocytes have an important role in AD pathogenesis during pathological conditions.31, 32, 33, 34, 35 We recently showed that a pathogenic signal induces the upregulation of IFNκ-inducible genes in chronic AD skin lesions. In line with the findings of Akkoc et al.36 INFγ expression was significantly upregulated in chronically lesioned skin. In addition, several apoptosis-related genes are differentially expressed in primary keratinocytes from the skin of AD patients. They also suggested that γ-inducible genes (RAB) and the apoptosis-related genes DUSP1 and ADM were overexpressed by IFNγ and that those genes were responsible for the IFNγ-induced apoptosis of keratinocytes in AD patients. On the other hand, activated T cells are also involved in keratinocyte death through IFNγ secretion and final activation.36, 37
To confirm the therapeutic efficacy of IL10 or TGFβ1 in canine AD skin, we first treated the animals with IL10 or TGFβ1 individually to evaluate the therapeutic efficacy of those factors. Treating canine AD skin with IL10 or TGFβ1 significantly improved and normalized AD symptoms (Figures 5a and b). In contrast, treating normal skin with anti-IL10 or anti-TGFβ1 antibodies induced AD, in addition to abnormal moisture, pH and TEWL conditions. Interestingly, the treatment of anti-IL10- or TGF β1-treated animal skin with the cATSC extract returned the AD lesions to a normal skin phenotype (Figure 5). In this study, we have finally identified a canine AD ameliorating factor and conclude that IL10 or TGFβ1 deficiency induces AD symptoms in normal skin. These immunomodulatory factors are also crucial to maintaining normal keratinocytes in vivo. Moreover, the interruption of IL10 or TGFβ1 action with anti IL10 or TGFβ1 antibodies caused traditional AD-like symptoms, with the overexpression of inflammatory factors and significantly decreased levels of KRT6A, KRT6B and KRT16. As shown in Figure 7, both IL10 and TGFβ1 directly or indirectly protected keratinocytes against ROS-mediated apoptotic cell death through microglia and macrophages, in addition to downregulating NOX4 and NFκB.
In this study, we found that an autologous adipose tissue-derived mesenchymal stem cell extract significantly ameliorated the pathological symptoms of canine AD by secreting the immunomodulatory cytokines, IL10 and TGFβ1, which modulated the overloaded immune response. Moreover, these immunomodulatory cytokines modulated AD-induced inflammation and inactivated ROS by inducing pathological signals that protected against apoptotic keratinocyte degeneration.
Materials and Methods
Animals
Dogs with naturally occurring AD were recruited by the Seoul National University Veterinary Teaching Hospital. The owners provided consent for privately owned dogs prior to enrollment. The dogs were diagnosed with a combination of history, clinical signs and a positive result on an allergen-specific IgE test and/or the intradermal skin test. All other causes of pruritus were excluded. Glucocorticoids and antihistamines were withdrawn at least 3 weeks prior to the intradermal skin test, allergen-specific IgE test and study enrollment. All of the dogs were healthy except for skin problems. The dogs with superficial pyoderma or Malassezia pachydermatis associated dermatitis were treated appropriately until the clinical signs resolved. The dogs were stabilized on their usual diet for at least 2 weeks prior to study enrollment, and the owners were instructed not to change the diet offered during the study. In this study, five healthy beagles were used as controls. None of the beagles had a history or clinical signs of pruritus nor had conditions likely to alter immune function. All of the experiments were approved by the Institute of Laboratory Animal resources of Seoul National University (Grant number: SNU-120316-1).
Isolation of canine adipose-derived stromal cells (cATSCs) and cATSC cytoplasmic extract
Raw canine fat tissue was obtained from subcutaneous tissue from the interscapular region using standard surgical procedures and mild sedation with medetomidine (0.1 ml/kg body weight). The raw canine fat tissue was processed according to established methods to obtain stem cells from the isolated fat tissue. To isolate the stem cells, the samples were digested with 0.075% collagenase IV (Sigma, St Luis, MO, USA) and centrifuged at 1200 × g for 10 min to acquire a high-density cell pellet. The pellet was then suspended in red blood cell (RBC) lysis buffer (Biowhittaker, Walkersville, MD, USA) and incubated for 10 min at room temperature to lyse the contaminating RBCs. The stem cell pellet was then collected and incubated overnight at 37 °C/5% CO2 in α-MEM medium (Gibco BRL, Grand Island, NY, USA) containing 10% FBS. This work was approved by the Seoul National University Institutional Review Board (IRB No. 0603/001-002), and the ethics committee specifically approved the procedure.
Application of cATSCs extract on allergen challenges D. farinae derived inflamed skin
The atopic dogs were epicutaneously sensitized to D. farinae to provoke an acute flare-up of AD. The flank hair was trimmed using a clipper (Oster pro 76; Oster Co., McMinnville, TN, USA) and painted with D. farinae solution. The D. farinae solution was prepared using a traditional method. Specifically, a pure culture of D. farinae (Greer Laboratories, Lenoir, NC, USA, >99% pure, whole bodied, natural milled D. farinae) was mixed with sterile saline to obtain a final concentration of 400 mg/ml. Each dog received 0.3 ml of the HDM mixture once daily for 3 consecutive days. Successful sensitization was demonstrated by dermatitis flare-ups that were clinically compatible with AD. After an environmental challenge, 5, 10 or 30 ng of the cATSC extract was applied to the inflamed skin for 24 h using the Finn chamber (Finn chambers on Scanpor; Epitest Ltd. Oy, Finland). PBS and 0.0584% hydrocortisone aceponate (1.3 μl/1.13 cm2) (Cortavance; Virvac, Carros Cedex, France) were used as negative and positive controls, respectively, and were also applied using the same method. At days 0 and 3, the clinical severity was assessed using a modified Canine Atopic Dermatitis Extent and Severity Index (CADESI) survey, and the biophysical state of the skin was measured. Skin samples were obtained by a skin biopsy (5 mm punch biopsy; Miltex Instrument Company, Maroubra, NSW, Australia) after the assessment of clinical severity on day 3. Half of the skin samples were fixed with 10% formalin solution for cryosectioning, and the remainders of the samples were immediately frozen at −70 °C for cytokine analysis.
The treatment of atopic dogs with cATSC extract
To evaluate the clinical potential of the cATSC extract on atopic dogs, randomized, placebo-controlled trial of the cATSC extract was conducted. The owners applied 20 ng of the cATSC extract, hydrocortisone aceponate or its negative control (PBS) to the lesional skin of the atopic dog once a day for 3 days. At days 0 and 3, the treatment response was evaluated by modified the CADESI (Canine Atopic Dermatitis Extent and Severity Index) score, prorates severity scale and a measurement of the biophysical state of skin. In the CADESI, the same investigator examined a particular dog throughout the study. The owners were asked to assess the severity of pruritus and to make an assessment of the overall response (0∼10; 0=normal, 10=severe). The biophysical state of the skin was evaluated by measuring the TEWL (transepidermal water loss), capacitance and pH as previously mentioned.
Clinical severity assessment
The Modified Canine AD Extent and Severity Index (CADESI) were used to evaluate the clinical severity of the atopic skin. Briefly, the severity of erythema, lichenification, excoriation and self-induced alopecia of the skin were scored from 0–5 (normal=0, severe=5) to give a total score of 20. The biophysical state of the skin was evaluated by measuring the TEWL, capacitance and pH. The transepidermal water loss was measured using an evaporimeter (VapoMeter; Delfin Technologies Ltd, Kuopio, Finland), and the results were expressed as g/m2/h. The electrical capacitance of the stratum corneum was measured with a capacitance meter (corneometer CM 825; Courage & Khazaka, Cologne, Germany) and expressed in arbitrary units. The skin pH was recorded using a skin surface pH probe and meter (skin pH meter PH 905; Courage & Khazaka).
Clinical evaluation
Clinical signs were evaluated using a clinical evaluation score prior to and 72 h after the Finn chamber application. Briefly, severity of erythema, lichenification, excoriation and self-induced alopecia of the applied lesion were scored from 0 to 5 (0–5, normal=0, severe=5)
Biophysical state of skin
Biophysical state of skin was evaluated by measuring trans epidermal water loss (TEWL), electrical capacitance of the stratum corneum and skin pH. TEWL was measured using an evaporimeter (VapoMeter; Delfin Technologies Ltd) and the results were expressed as g/m2/h. Electrical capacitance of the stratum corneum was measured with a capacitance meter (corneometer CM 825; Courage & Khazaka) and expressed in arbitrary units. Skin pH was recorded using a skin surface pH probe and meter (Skin pH meter PH 905; Courage & Khazaka).
Skin sampling
After clinical evaluation and measurement of biophysical state of skin, skin samples were obtained by skin biopsy (5 mm punch biopsy; Miltex Instrument Company). Half of skin samples were fixated on 10% formalin solution for cryosection and the rest were immediately frozen at −70 °C for cytokine analysis.
Western blot
To analyze and confirm the proteins expressed in AD animal skin, the tissues were lysed in 500 μl of lysis buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM EGTA, 1 mM glycerophosphate, 1 mM Na3VO4 and 1 mM PMSF). The lysates were clarified by 10 min of centrifugation at 15 000 × g, and the total protein content was determined using a Bio-Rad (Millano, Italy) protein quantification kit. For western blotting, equal amounts (40 μg) of protein extract were subjected to 10% SDS-PAGE analysis and transferred to a nitrocellulose membrane. The anti ED1 (1 : 100, AbD Serotec, Oxford, UK), COX2 (1 : 200, Santa Cruz, Dallas, Texas, USA), MPO (1 : 500, DAKO, Glostrup, Denmark), RelA (1 : 1000, Cell Signaling, Danvers, MA, USA), RelB (1 : 200, Santa Cruz), iNOS (1 : 2000, BD Bioscience, San Jose, CA, USA), eNOS (1 : 2500, BD Bioscience), NOX4 (1 : 200, Santa Cruz), p-STAT3 (1 : 1000, Cell Signaling), p-Erk (1 : 1000, Cell Signaling), p-p38 (1 : 1000, Cell Signaling), p-JNK (1 : 1000, Cell Signaling) and β-actin (1 : 500, Millipore, Billerica, MA, USA) antibodies were incubated with the membranes. After western blotting, the relative band intensities were determined and compared using Quality-one 1-D analysis software (Bio-Rad, Hercules, CA, USA).
Measurement of intracellular ROS
The level of intracellular ROS in the skin tissue was evaluated using the fluorescent probe DCFDA [5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate]. For visualization by fluorescent microscopy, cells were plated in glass bottom dishes and treated with 0.3 mM H2O for 6 h. The cells were then washed and loaded with 10 μM DCFDA for 30 min at 37 °C and imaged by fluorescent microscopy (ex/em=495/525 nm). For the quantitative assessment of ROS production, UCB-MSCs in 96-well plates were washed with HBSS and loaded with 10 μM DCFDA for 30 min at 37 °C. The cells were washed three times with HBSS and exposed to 100 μM tBHP alone or in combination with SS-31. The oxidation of DCF was monitored in real-time using a microplate spectrofluorometer (Molecular Devices, Sunnyvale, CA, USA) using excitation/emission wavelengths of 485/530 nm.
RT-PCR for gene expression analysis
One week after the initiation of culture, the total cellular RNA was extracted from cultured ATSCs with Trizol (Life Technologies, Frederick, MA, USA). The total RNA was reverse-transcribed into first-strand cDNA using an oligo-dT primer, then amplified by PCR using the indicated gene-specific primers (20 pM). The PCR reactions were conducted using an ABI 7700 Prism Sequence Detection System and SYBER green detection kit (Applied Biosystems, Foster, CA, USA). The primer sequences were designed with Primer Express software (PE-Applied Biosystems, Warrington, UK) using gene sequences obtained from the GenBank database.
Tissue immunohistochemical analysis
For the immunohistochemical analysis of the frozen tissues, the tissue sections were fixed for 30 min in 4% paraformaldehyde. The sections were then washed three times in PBS, and the nonspecific binding was blocked with 10% normal horse serum. The sections were then incubated overnight at 4 °C with the following antibodies: anti-TGFβ1 (1 : 200, Santa Cruz), anti-ED1 (1 : 100, AbD Serotec), anti-COX2 (1 : 200, Santa Cruz), anti-MPO (1 : 500, DAKO), anti-Iba1 (1 : 100, Wako, Osaka, Japan), anti-CD3 (1 : 500, MACS, Gladbach, Germany), anti-CD4 (1 : 500, MACS), anti-CD8 (1 : 500, MACS), anti-Cytokeratin14 (1 : 500, Millipore). After rinsing with the primary antibodies, the sections were incubated for 1 h. After extensive washing in PBS, the cells were incubated for 30 min with FITC or Texas Red-conjugated secondary antibodies (1 : 250, Invitrogen, Grand Island, NY, USA, respectively). The controls in which the primary antibodies were omitted or replaced with irrelevant IgG did not show any detectable staining. The specimens were evaluated using a Leica fluorescence microscope (Leica Microsystems, Exon, PA, USA). The immunocytochemical studies were repeated at least three times and detected with confocal microscopy using a Leica TCS sp2 laser scanning microscope (Leica Microsystems) equipped with three lasers. The double- or single-labeled cells were verified by the imaging of 1-μm sections through the slices. For the relative quantification of the positive cells, area counts of the tissue were conducted. In each section, five adjacent fields were sampled, beginning where the upper and lower blades were joined. The average number of positive cells in five adjacent fields per section was counted, along with the percentage of antibody positive cells per section. The error bars represent standard deviations.
TUNEL assay
The death of apoptotic skin tissue was estimated in terms of the skin's ability to reduce the MTT dye (3, 4, 5-dimethyl thiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (Sigma) to blue purple formazan crystal. The effect of H2O2 on the induction of cell death was determined with the TdT in situ apoptosis detector kit (Roche, Indianapolis, IN, USA), which was used according to the manufacturer's specifications. After fixation with 4% paraformaldehyde, the cells were incubated in a TUNEL reaction mixture containing deoxynucleotidyl transferase (TdT) buffer with TdT and biotinylated dUTP, incubated in a humidified atmosphere at 37 °C for 90 min, and then washed with PBS. The cells were incubated at room temperature for 30 min with anti-horseradish peroxidase-conjugated antibody, and the signals were visualized with diaminobenzidine. The results were analyzed using a fluorescent microscope (Leica Microsystems). The TUNEL-positive apoptotic cells were quantified by counting the positively stained cells. Three digital microscopic images at a magnification of 100 were randomly captured in the areas with positive cells. The number of positively stained cells in each of the three images was averaged.
Double-blind, placebo-controlled trial of cATSCs extract on atopic dogs
To evaluate the clinical potential of cATSCs extract on atopic dogs, double-blind, and placebo-controlled trial of cATSCs extract were conducted on five privately owned atopic dogs. In all, 200 ng of cATSCs extract or its negative control PBS was applied on one lesional skin of atopic dog by the owner once a day for 3 consecutive days. Application site was within 4 × 4 cm2 in width. At day 0 and 3, treatment response was evaluated by clinical evaluation score, and measuring of biophysical state of skin. In the clinical evaluation score, the same investigator examined a particular dog throughout the study. Biophysical state of the skin was evaluated by measuring TEWL, skin hydration and skin pH as previously mentioned.
Statistical analysis
The significance of the results was tested by completing a t-test or ANOVA using GraphPad InStat 3.0 software (La Jolla, CA, USA). For the repeated-measures ANOVA and t-test in vitro studies where Kaplan–Meier curves and a log-rank analysis were performed, MedCalc software (Ostend, Belgium) was used.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST, 2010-0020265).
Glossary
- TGFβ1
transforming growth factor β1
- IL
interleukin
- ATSC
adipose tissue stem cell
- AD
atopic dermatitis
- TEWL
transepidermal water loss
- INFγ
interferon gamma
- COX2
Cyclooxygenase2
- MPO
Myeloperoxidase
- NOX4
NADPH oxidase 4
- NOS
nitric oxide synthase
- DCFHDA
2′,7′-dichlorfluorescein-diacetate
- Tunel
terminal deoxynucleotidyl transferase dUTP nick end labeling
- GPX3
Glutathione peroxidase 3
- SEPN1
Selenoprotein N1
- TXNL1
Thioredoxin-like protein 1
The authors declare no conflict of interest.
Footnotes
Edited by E Candi
References
- Rothe MJ, Grant-Kels JM. Atopic dermatitis: an update. J Am Acad Dermatol. 1996;35:1–13. doi: 10.1016/S0190-9622(96)90486-7. [DOI] [PubMed] [Google Scholar]
- Scott DW, Miller WH, Griffin C.Skin immune system and allergic skin diseaseIn: Scott DW, Miller WH, Griffin C, (eds)Muller and Kirk's Small Animal Dermatology6th edSaunders: Philadelphia; 2001543–666. [Google Scholar]
- Shambaugh G.Pathology and clinical course of inflammatory diseases of the middle earIn: Shambaugh G Jr, (eds)Surgery of the Ear Saunders: Philadelphia; 1967271–272. [Google Scholar]
- Wright ED, Hurst D, Miotto D, Giguere C, Hamid Q. Increased expression of major basic protein (MBP) and interleukin-5(IL-5) in middle ear biopsy specimens from atopic patients with persistent otitis media with effusion. Otolaryngol Head Neck Surg. 2000;123:533–538. doi: 10.1067/mhn.2000.109472. [DOI] [PubMed] [Google Scholar]
- Fireman P. Therapeutic approaches to allergic rhinitis: treating the child. J Allergy Clin Immunol. 2000;105:616–621. doi: 10.1067/mai.2000.106152. [DOI] [PubMed] [Google Scholar]
- Sobol SE, Taha R, Schloss MD, Mazer BD, Manoukian JJ, Tewfik TL, et al. T(H)2 cytokine expression in atopic children with otitis media with effusion. J Allergy Clin Immunol. 2002;110:125–130. doi: 10.1067/mai.2002.125697. [DOI] [PubMed] [Google Scholar]
- Bieber T. The pro- and anti-inflammatory properties of human antigen-presenting cells expressing the high affinity receptor for IgE (Fc epsilon RI) Immunobiology. 2007;212:499–503. doi: 10.1016/j.imbio.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Nuttall TJ, Knight PA, McAleese SM, Lamb JR, Hill PB. T-helper 1, t-helper 2 and immunosuppressive cytokines in canine atopic dermatitis. Vet Immunol Immunopathol. 2002;87:379–384. doi: 10.1016/s0165-2427(02)00076-4. [DOI] [PubMed] [Google Scholar]
- Muraille E, Leo O. Revisiting the Th1/Th2 paradigm. Scand J Immunol. 1998;47:1–9. doi: 10.1111/j.1365-3083.1998-47-1.00383.x. [DOI] [PubMed] [Google Scholar]
- Maneechotesuwan K, Supawita S, Kasetsinsombat K, Wongkajornsilp A, Barnes PJ. Sputum indoleamine-2, 3-dioxygenase activity is increased in asthmatic airways by using inhaled corticosteroids. J Allergy Clin Immunol. 2008;121:43–50. doi: 10.1016/j.jaci.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645–649. doi: 10.1046/j.1523-1747.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- Albanesi C, Cavani A, Girolomoni G. IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: synergistic or antagonist effects with IFN-gamma and TNF-alpha. J Immunol. 1999;162:494–502. [PubMed] [Google Scholar]
- Rebane A, Zimmermann M, Aab A, Baurecht H, Koreck A, Karelson M, et al. Mechanisms of IFN-gamma-induced apoptosis of human skin keratinocytes in patients with atopic dermatitis. J Allergy Clin Immunol. 2012;129:1297–1306. doi: 10.1016/j.jaci.2012.02.020. [DOI] [PubMed] [Google Scholar]
- Kim BS, Jung JS, Jang JH, Kang KS, Kang SK. Nuclear Argonaute 2 regulates adipose tissue-derived stem cell survival through direct control of miR10b and selenoprotein N1 expression. Aging Cell. 2011;10:277–291. doi: 10.1111/j.1474-9726.2011.00670.x. [DOI] [PubMed] [Google Scholar]
- Kang SK, Yeo JE, Kang KS, Phinney DG. Cytoplasmic extracts from adipose tissue stromal cells alleviates secondary damage by modulating apoptosis and promotes functional recovery following spinal cord injury. Brain Pathol. 2007;17:263–275. doi: 10.1111/j.1750-3639.2007.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SK, Putnam L, Dufour J, Ylostalo J, Jung JS, Bunnell BA. Expression of telomerase extends the lifespan and enhances osteogenic differentiation of adipose tissue-derived stromal cells. Stem Cells. 2004;22:1356–1372. doi: 10.1634/stemcells.2004-0023. [DOI] [PubMed] [Google Scholar]
- Tsuboi H, Kouda K, Takeuchi H, Takigawa M, Masamoto Y, Takeuchi M, et al. 8-hydroxydeoxyguanosine in urine as an index of oxidative damage to DNA in the evaluation of atopic dermatitis. Br J Dermatol. 1998;138:1033–1035. doi: 10.1046/j.1365-2133.1998.02273.x. [DOI] [PubMed] [Google Scholar]
- Niwa Y, Sumi H, Kawahira K, Terashima T, Nakamura T, Akamatsu H. Protein oxidative damage in the stratum corneum: evidence for a link between environmental oxidants and the changing prevalence and nature of atopic dermatitis in Japan. Br J Dermatol. 2003;149:248–254. doi: 10.1046/j.1365-2133.2003.05417.x. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Nakai K, Yoneda K, Maeda R, Munehiro A, Fujita N, Yokoi I, et al. Urinary biomarker of oxidative stress in patients with psoriasis vulgaris and atopic dermatitis. J Eur Acad Dermatol Venereol. 2009;23:1405–1408. doi: 10.1111/j.1468-3083.2009.03327.x. [DOI] [PubMed] [Google Scholar]
- Kapun AP, Salobir J, Levart A, Kotnik T, Svete AN. Oxidative stress markers in canine atopic dermatitis. Res Vet Sci. 2012;92:469–470. doi: 10.1016/j.rvsc.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Briganti S, Picardo M. Antioxidant activity, lipid peroxidation and skin diseases. What's new. J Eur Acad Dermatol Venereol. 2003;17:663–669. doi: 10.1046/j.1468-3083.2003.00751.x. [DOI] [PubMed] [Google Scholar]
- Dotan Y, Lichtenberg D, Pinchuk I. Lipid peroxidation cannot be used as a universal criterion of oxidative stress. Prog Lipid Res. 2004;43:200–227. doi: 10.1016/j.plipres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Fuchs J, Zollner TM, Kaufmann R, Podda M. Redox-modulated pathways in inflammatory skin diseases. Free Radic Biol Med. 2001;30:337–353. doi: 10.1016/s0891-5849(00)00482-2. [DOI] [PubMed] [Google Scholar]
- Grewe M, Walther S, Gyufko K, Czech W, Schopf E, Krutmann J. Analysis of the cytokine pattern expressed in situ in inhalant allergen patch test reactions of atopic dermatitis patients. J Invest Dermatol. 1995;105:407–410. doi: 10.1111/1523-1747.ep12321078. [DOI] [PubMed] [Google Scholar]
- Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis--part I: clinical and pathologic concepts. J Allergy Clin Immunol. 2011;127:1110–1118. doi: 10.1016/j.jaci.2011.01.053. [DOI] [PubMed] [Google Scholar]
- Contassot E, Gaide O, French LE. Death receptors and apoptosis. Dermatol Clin. 2007;25:487–501. doi: 10.1016/j.det.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Meyer N, Zimmermann M, Burgler S, Bassin C, Woehrl S, Moritz K, et al. IL-32 is expressed by human primary keratinocytes and modulates keratinocyte apoptosis in atopic dermatitis. J Allergy Clin Immunol. 2010;125:e810. doi: 10.1016/j.jaci.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Matsue H, Kobayashi H, Hosokawa T, Akitaya T, Ohkawara A. Keratinocytes constitutively express the Fas antigen that mediates apoptosis in IFN gamma-treated cultured keratinocytes. Arch Dermatol Res. 1995;287:315–320. doi: 10.1007/BF01105085. [DOI] [PubMed] [Google Scholar]
- Klunker S, Trautmann A, Akdis M, Verhagen J, Schmid-Grendelmeier P, Blaser K, et al. A second step of chemotaxis after transendothelial migration: keratinocytes undergoing apoptosis release IFN-gamma-inducible protein 10, monokine induced by IFN-gamma, and IFN-gamma-inducible alpha-chemoattractant for T cell chemotaxis toward epidermis in atopic dermatitis. J Immunol. 2003;171:1078–1084. doi: 10.4049/jimmunol.171.2.1078. [DOI] [PubMed] [Google Scholar]
- Zimmermann M, Koreck A, Meyer N, Basinski T, Meiler F, Simone B, et al. TNF-like weak inducer of apoptosis (TWEAK) and TNF-alpha cooperate in the induction of keratinocyte apoptosis J Allergy Clin Immunol 2011127207e201–e210. [DOI] [PubMed] [Google Scholar]
- Curran JE, Jowett JB, Elliott KS, Gao Y, Gluschenko K, Wang J, et al. Genetic variation in selenoprotein S influences inflammatory response. Nat Genet. 2005;37:1234–1241. doi: 10.1038/ng1655. [DOI] [PubMed] [Google Scholar]
- Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233–246. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22:125–137. doi: 10.5021/ad.2010.22.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann A, Akdis M, Kleemann D, Altznauer F, Simon HU, Graeve T, et al. T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J Clin Invest. 2000;106:25–35. doi: 10.1172/JCI9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkoc T, de Koning PJ, Ruckert B, Barlan I, Akdis M, Akdis CA. Increased activation-induced cell death of high IFN-gamma-producing T(H)1 cells as a mechanism of T(H)2 predominance in atopic diseases. J Allergy Clin Immunol. 2008;121:e651. doi: 10.1016/j.jaci.2007.12.1171. [DOI] [PubMed] [Google Scholar]
- Jang CH, Kim YH. Characterization of cytokines present in pediatric otitis media with effusion: comparison of allergy positive and negative. Int J Pediatr Otorhinolaryngol. 2002;66:37–40. doi: 10.1016/s0165-5876(02)00185-4. [DOI] [PubMed] [Google Scholar]