Abstract
Cisplatin (cis-diammine-dichloroplatinum; CDDP) is an anticancer drug that induces significant hearing loss and balance dysfunction as side effects. Cilostazol (CS, 6-[4-(1-cyclohexyl-1H-tetrazol-5-yl) butoxy]-3, 4-dihydro-2-(1H)-quinolinone) has neuroprotective and antioxidant effects, whereas Ginkgo biloba extract (GbE) has preventive effects on CDDP-induced hearing loss in rats, and GbE enhances the antiatherogenic effect of CS by inhibiting the generation of reactive oxygen species (ROS). The purpose of this study was to investigate the effects of renexin (RXN), which contains GbE and CS, against CDDP-induced cochleo-vestibular dysfunction in rats and to elucidate the mechanism underlying the protective effects of RXN on auditory cells. Rats intraperitoneally injected with CDDP exhibited an increase in hearing threshold and vestibular dysfunction, which agreed with hair cell damage in the Organ of Corti and otoliths. However, these impairments were significantly prevented in a dose-dependent manner by pre- and co-treatment with RXN, and these preventive effects in RXN-treated rats were more prominent than those in GbE-treated rats. In a CDDP pharmacokinetic study, platinum concentration was very similar between CDDP-only treated and RXN+CDDP cotreated rats. RXN markedly attenuated CDDP-induced intracellular ROS and significantly reduced CDDP-activated expression of p-extracellular regulated kinase (ERK), BAX, cytochrome c, cleaved caspase-3 and cleaved poly (ADP-ribose) polymerase, but increased BCL-XL expression. These results show that RXN may have a synergistic effect by strongly protecting hearing and vestibular dysfunction induced by CDDP by inhibiting ROS production, mitochondrial pathways and the ERK pathway, without interfering with CDDP pharmacokinetics. Therefore, RXN could potentially be used to reduce CDDP-related hearing loss and dizziness.
Keywords: cisplatin, hearing loss, cilostazol, ginkgo biloba, dizziness
Cisplatin (cis-diammine-dichloroplatinum; CDDP) is a platinum-based chemotherapeutic agent that is widely used to treat several neoplastic diseases, including head and neck cancers.1 Unfortunately, its clinical application is limited by a broad spectrum of severe side effects, such as nephrotoxicity, myelotoxicity, gastrointestinal toxicity, ototoxicity, and peripheral neuropathy.2 In particular, nephrotoxicity and ototoxicity are dose-limiting side effects. Although nephrotoxicity can be diminished or controlled with hydration therapy, ototoxicity still poses a limitation to effective CDDP chemotherapy.3 CDDP-induced ototoxicity causes hair cell loss by inducing apoptosis in the cochlea. CDDP primarily damages the outer and inner hair cells, induces degeneration of the stria vascularis, and remarkably reduces the number of spiral ganglion cells.4 In such cases, CDDP often causes irreversible sensorineural hearing loss and serious tinnitus in humans, mice, and other animals.5 Approximately 23–54% of adults and>50% of pediatric patients with head and neck cancer treated with CDDP develop ototoxicity.6 In addition to serving as the organ of hearing, the ears have a significant role in the control of balance. CDDP-induced vestibulotoxicity has been assessed by various authors, and considerable clinical variability has been reported.7 To date, no effective prosthetic devices such as hearing aids or cochlear implants are available for the loss of vestibular function.8 Therefore, precautions against vestibular hair cell loss are an important issue.
Ginkgo biloba extract (GbE) leaves and their extracts are among the most widely used herbal products and/or dietary supplements in the world. The extract of G. biloba has long been safely used to treat patients with neurodegenerative, vascular, and audiovestibular disorders.9, 10 The in vitro and in vivo protective effects of GbE against oxidative stress have been shown,11 and these effects are closely related to the ability of GbE to scavenge free radicals, such as superoxide anion, hydroxyl, and peroxyl radicals.12 The free radical scavenging activity of GbE is comparable with that of other known antioxidants such as vitamins A and C, as well as the tripeptide glutathione.13 In addition, GbE has anti-inflammatory and antiapoptotic properties.14 Furthermore, GbE protects against CDDP-induced ototoxicity.15
Cilostazol (CS), 6-[4-(1-cyclohexyl-1H-tetrazol-5-yl) butoxy]-3, 4-dihydro-2-(1H)-quinolinone, is a potent selective phosphodiesterase type III inhibitor. CS functions as a platelet aggregation inhibitor16 and vasodilator,17 and is mainly used for treating patients with peripheral arterial disease18 and intermittent claudication.19 Previous studies have demonstrated that CS inhibits apoptosis under several conditions.20, 21, 22 Kim et al.23 reported a protective effect of CS against lipopolysaccharide-induced apoptosis in human umbilical vein endothelial cells, by scavenging hydroxyl radicals and intracellular reactive oxygen species (ROS) with the reduction in tumor necrosis factor-α formation and by increasing Bcl-2 protein expression, and decreasing Bax protein and cytochrome c release. The combination of CS and probucol, another potent lipid-soluble antioxidant, displays a synergistic effect on the suppression of ROS and inflammatory markers in human coronary artery endothelial cells.24 Moreover, the antiplatelet effect of CS may be potentiated by GbE without prolonging bleeding or coagulation times.25 A recent study indicated that cotreatment of GbE and CS synergistically decreases ROS production in ApoE null mice fed a high-fat diet.26
Thus, the present study investigated the protective effects of renexin (RXN), which is a combination of GbE and CS, against CDDP-induced hearing loss and vestibular toxicity in rats, and explored the mechanism of the protective effect of RXN in the House Ear Institute-Organ of Corti 1 (HEI-OC1) auditory cell line.
Results
RXN dose-dependently protected against CDDP-induced hearing loss and vestibular dysfunction
We administered CDDP in a single high dose (16 mg/kg, intraperitoneally) to simulate the high therapeutic doses used to manage human cancers. Hearing thresholds at 16 and 32 kHz were normal in the control group during the experimental period, but increased in all CDDP-administrated animals after 5 days (Supplementary Figure 1a).
The CDDP-only group showed worse hearing thresholds of 47.5±7.6 dB at 16 kHz and 48.8±8.8 dB at 32 kHz compared with that (14.0±2.1 dB at 16 kHz and 16.0±3.9 dB at 32 kHz) of the control group. RXN showed a dose-dependent effect against CDDP-induced hearing loss. Hearing threshold was significantly preserved in the RXN (90 mg/kg)+CDDP group (30.0±10.7 dB at 16 kHz and 33.8±6.4 dB at 32 kHz) and the RXN (180 mg/kg)+CDDP group (19.5±8.9 dB at 16 kHz and 22.5±7.9 dB at 32 kHz) (P<0.01), but not in the RXN (45 mg/kg)+CDDP group. Furthermore, significant differences in hearing thresholds were identified in the RXN (180 mg/kg) group compared with those in the middle dose (90 mg/kg) and low dose (45 mg/kg) groups. Representative sections of middle (16 kHz) and basal (32 kHz) turns (Supplementary Figure 1c) showed that the number of hair cells with normal stereocilia was extremely reduced in the CDDP-only group. Only a few hair cells with damaged stereocilia were observed in the RXN (90 mg/kg)+CDDP and the RXN (180 mg/kg)+CDDP groups. These differences in the number of hair cells (Supplementary Figure 1b) agreed with the hearing function evaluation (Supplementary Figure 1a).
The number of head rotations in the tail hanging test and swimming time duration in the swimming test were counted before and after CDDP administration for the vestibular function evaluation (Supplementary Figure 2a). Compared with the normal control group, all experimental groups showed a significantly increased number of head rotations (except RXN (180 mg/kg)+CDDP group) and prolonged swimming time after 5 days. A significant difference in the number of head rotations was observed between the RXN (180 mg/kg)+CDDP and the CDDP-only groups (P=0.000), which was supported by the quantitative analysis of the number of the hair cells on macula of the utricles and saccules (Supplementary Figure 2b). The saccules and utricles in the CDDP-only group showed an extremely reduced number of hair cells with an uneven distribution and abnormal appearance compared with those in the control group (Supplementary Figure 2c); however, hair cells in the RXN (180 mg/kg)+CDDP group were well-preserved with normal shaped stereocilia and a high density, which was consistent with the vestibular function evaluation in the tail hanging test.
Synergistic effects of GbE and CS in RXN against CDDP-induced hearing loss and vestibular dysfunction
The 180 mg/kg dose of RNX showed statistically significant protective effects against CDDP-induced hearing and vestibular impairment in the RXN (180 mg/kg)+CDDP group. Therefore, a RXN dose of 180 mg/kg was used to evaluate the synergistic effects of GbE and CS in the following study.
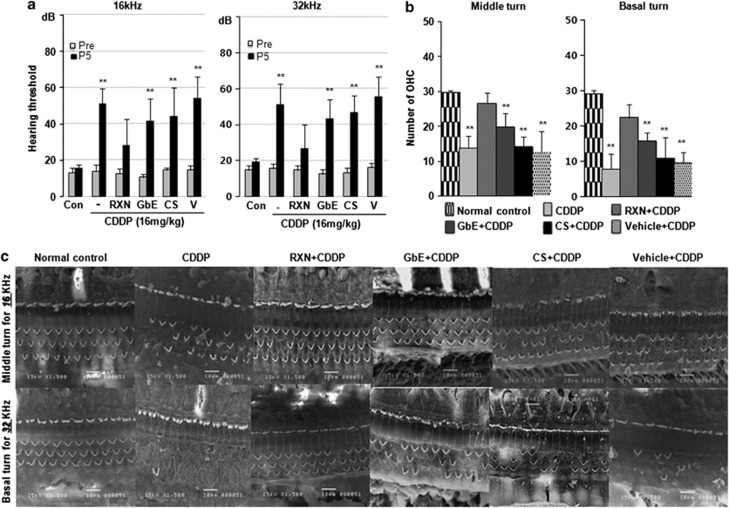
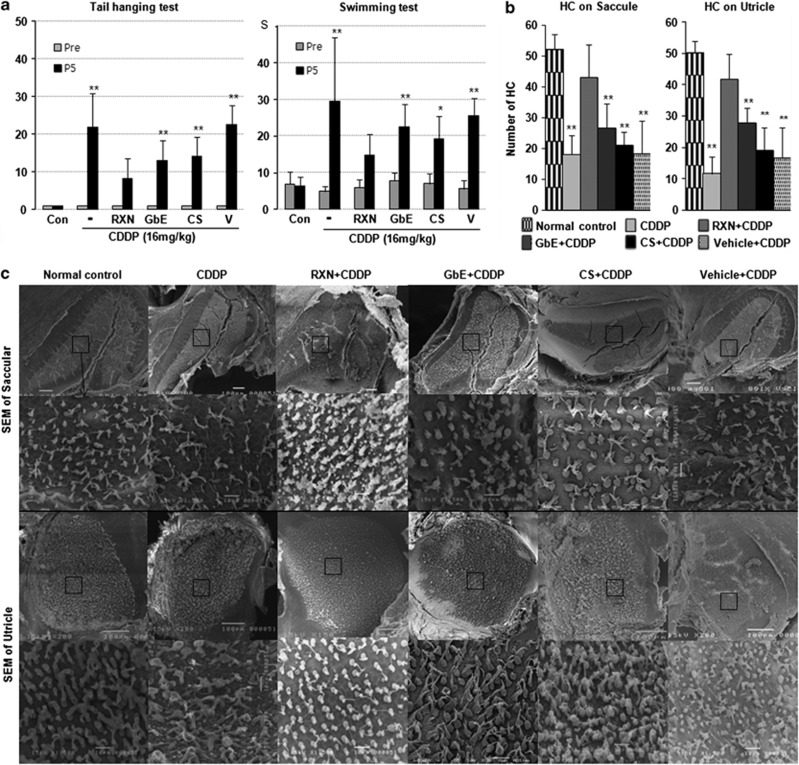
Compared with the normal control group, rats in the CDDP-only group showed marked hearing loss with average thresholds of 50.9±8.4 dB at 16 kHz and 51.3±9.4 dB at 32 kHz, as well as in the vehicle+CDDP, GbE+CDDP, and CS+CDDP groups (P<0.000); however, hearing preservation was observed in the RXN+CDDP group with average thresholds of 28.1±11.9 dB at 16 kHz and 28.3±11.7 dB at 32 kHz (Figure 1a). The hearing thresholds at 16 kHz and 32 kHz were 41.8±12.2 dB and 43.2±10.8 dB in the GbE+CDDP group and 44.2±15.9 dB and 46.7±17.0 dB in the CS+CDDP group, respectively. Both groups showed lower thresholds in favor of GbE and CS effects, and the difference between the GbE+CDDP group and CDDP-only group was significant (P<0.05). RXN showed a synergistic effect and significantly protected hearing thresholds, which differed from the GbE+CDDP group (P<0.05). The representative sections of middle and basal turns in Figure 1c showed a reduced number of intact stereocilia hair cells in CDDP-treated animals; however, preservation in the number and shape of hair cells was observed in the otoprotective agent-treated groups. The quantitative analysis of the number of hair cells is shown in Figure 1b. The RXN+CDDP and GbE+CDDP groups had significantly more preserved hair cells (P<0.001 and P<0.05) compared with those in the CDDP-only group, which agreed with the functional hearing evaluation. Hearing thresholds were not significantly different between the vehicle+CDDP group and the CDDP-only group (P>0.05), which agreed with the morphological evaluation results; therefore, carboxymethylcellulose as a solvent for otoprotective agents may have no effect on hearing function. The vestibular functional evaluation is shown in Figure 2a. The number of head rotations in the tail hanging test in the CDDP-only group was significantly greater than that in the RXN+CDDP (P<0.01). Average swimming time in the RXN+CDDP group was significantly shorter than that in the CDDP-only group (P<0.01). Fewer head rotations and shorter swimming times were found in the GbE+CDDP and CS+CDDP groups; however, the differences were not significant except the tail hanging test result in the GbE+CDDP group (P<0.05). The scanning electron microscopy findings (Figure 2c) showed damaged hair cell stereocilia in the otolith organs; however, preserved and well-shaped stereocilia were found in the RXN+CDDP group. This quantitative analysis result of hair cells (Figure 2b) agreed with the vestibular functional test results.
Figure 1.
The synergistic effects of RXN (180 mg/kg), which contains GbE (80 mg/kg) and CS (100 mg/kg), against CDDP (16 mg/kg)-induced hearing loss. (a) Hearing threshold (dB) at 16 kHz and 32 kHz before (Pre) and after (post-5 day) CDDP administration. (b) OHC, number of hair cells counted in the middle and basal turns of the Organ of the Corti. *P<0.05, **P<0.01. Asterisk on the bar means compared with the normal control group. (c) Scanning electron micrographs of hair cells in different groups. Scale bars=10 μm
Figure 2.
The synergistic effects of RXN (180 mg/kg), which contains GbE (80 mg/kg) and CS (100 mg/kg), against CDDP (16 mg/kg)-induced vestibular dysfunction. (a) The number of head rotations in the tail hanging test and average swimming time on the swimming test before (Pre) and after (post-5 day) CDDP administration. (b) Quantitative analysis of hair cell (HC) number in the macula of saccules and utricles. *P<0.05, **P<0.01. Asterisk on the bar means compared with the normal control group. (c) Scanning electron micrographs of HCs in the different groups. Scale bars=100 μm and 10 μm
RXN protected HEI-OC1 cells from apoptotic death induced by CDDP
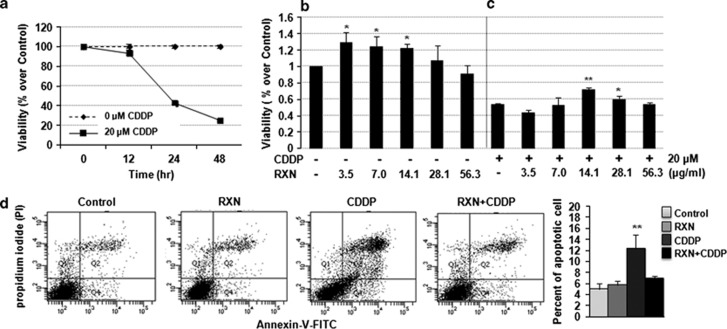
We examined the effect of RXN on CDDP-induced cytotoxicity in HEI-OC1 cells with the MTS assay. As shown in Figure 3a, CDDP decreased cell viability in a time-dependent manner. CDDP at a concentration of 20 μM for 24 h was chosen as an adequate condition for the following study. RXN alone significantly promoted cell viability at concentrations of 3.5, 7.0, and 14.1 μg/ml (Figure 3b). Pretreatment with RXN for 1 h significantly protected HEI-OC1 cells from CDDP toxicity in a dose-dependent manner (Figure 3c). The maximum protective effect was observed at 14.1 μg/ml; therefore, we chose 14.1 μg/ml RXN as an optimal concentration that resulted in significant protection (53.6% for CDDP versus 71.9% for the RXN-pretreated group) against the toxic effects of 20 μM CDDP over 24 h. A flow cytometry analysis (Annexin V/propidium iodide (PI) staining) was performed to quantify the effect of RXN on CDDP-induced apoptotic events, and the results are presented in Figure 3d. A significant increase in the number of apoptotic events occurred in the CDDP-only group (P<0.01) that was not observed in HEI-OC1 cells pretreated with RXN. Therefore, pretreatment with RXN markedly inhibited CDDP-induced apoptotic cell death.
Figure 3.
(a) CDDP-induced auditory cell toxicity. (b) Dose-dependent effect of RXN on HEI-OC1 cells. (c) Dose-dependent effect of RXN on CDDP-induced toxicity in HEI-OC1 cells. (d) Effect of RXN on CDDP-induced apoptosis: RXN significantly decreased CDDP-induced apoptosis in the RXN+CDDP group compared with that in the CDDP-only group. *P<0.05, **P<0.01. Asterisk on the bar means compared with the normal control group. All experiments were performed in triplicate
Pretreatment with RXN significantly attenuated the CDDP-induced increase in ROS
To investigate the effect of RXN on intracellular ROS generation induced by CDDP, HEI-OC1 cells were treated with 20 μM CDDP in the presence or absence of RXN for 24 h and stained with an oxidative stress detection reagent (green). Cells in the CDDP-only group showed higher green fluorescence induced by CDDP compared with that in the normal control group; however, the fluorescence density was lower in the RXN+CDDP group than that in the CDDP-only group (Figure 4a). A flow cytometry analysis was performed to quantify the effect of RXN on CDDP-induced intracellular ROS generation, and the results are presented in Figure 4b. The difference between the normal control and the CDDP-only group was significant (P<0.01); however, RXN attenuated the CDDP-induced increase in ROS significantly in the RXN+CDDP group (P<0.05), which was not significantly different from that in the normal control group.
Figure 4.
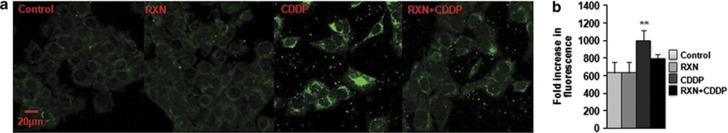
Measurement of intracellular ROS production. (a) A higher density of green florescence was observed in the CDDP-only group compared with that in RXN-treated cells. (b) Quantitative measurement by flow cytometry analysis showed significant difference between the RXN+CDDP and CDDP-only groups. **P<0.01. All experiments were performed in triplicate
Effect of RXN on CDDP-induced apoptosis via inhibition of the mitochondrial and ERK pathways
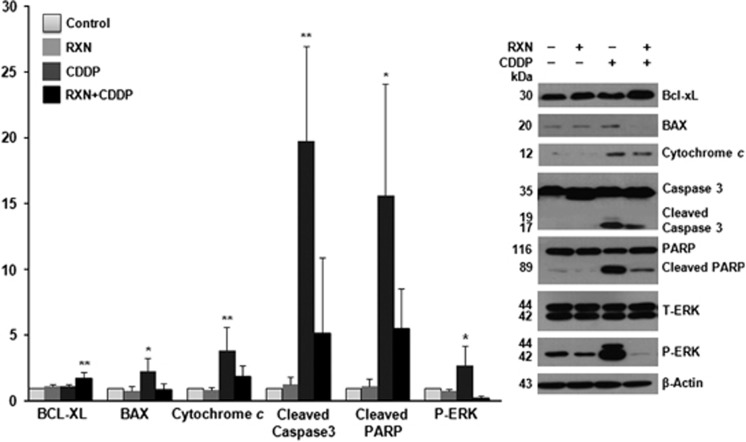
To elucidate the mechanism underlying the protective effects of RXN on HEI-OC1 cells, CDDP-mediated changes in apoptosis-related gene expression, including Bcl-xL, BAX, cytochrome c, caspase-3, poly-ADP-ribose polymerase (PARP), extracellular regulated kinase (ERK), and JNK were evaluated. A representative western blot is shown in Figure 5. The significantly increased expression of proteins encoded by the antiapoptotic gene Bcl-xL and decreased BAX, cytochrome c, cleaved caspase-3, and cleaved PARP expression in the RXN+CDDP group confirmed that the protective effects of RXN occur through the mitochondrial pathway. The significant downregulation of P-ERK in the RXN+CDDP group suggested that RXN blocked CDDP-mediated apoptosis via the ERK signaling pathway.
Figure 5.
The effect of RXN on CDDP-induced apoptotic pathways. Representative western blots are shown on the right, and each ratio indicates relative values of phosphor-protein normalized to β-actin compared with those in the control. *P<0.05, **P<0.01. Asterisk on the bar means compared with the normal control group. Western blots were performed in triplicate
Effect of RXN on CDDP pharmacokinetics in rats
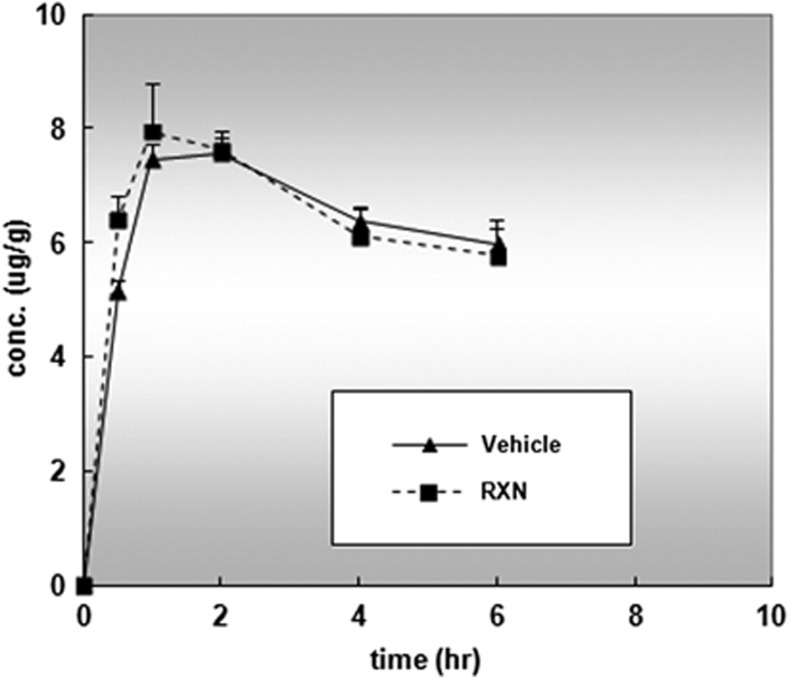
The effect of RXN on serum concentrations of platinum in rats was analyzed by ICP-MS to determine whether the chemoprotectant might bind to and eliminate circulating CDDP (Figure 6). The difference between the RXN+CDDP and vehicle+CDDP groups did not reach significance, indicating that RXN did not interfere with the anticancer effects of CDDP.
Figure 6.
CDDP pharmacokinetics measured as plasma platinum (Pt) concentrations by inductively coupled plasma mass spectrometry
Discussion
RXN's potential for chemoprotection against ototoxicity
Because severe side effects from CDDP-related ototoxicity limit its clinical use, many studies have been conducted to try to reduce CDDP ototoxicity. Yumusakhuylu et al.27 reported that significant otoprotection occurs in guinea pigs receiving resveratrol and high-dose CDDP. D-Methionine, an antioxidant amino acid and a sulfur-containing chemoprotectant, provides protection from CDDP-induced hearing loss by decreasing both the loss of cochlear hair cells28 and damage to the stria vascularis29 in animal studies. In another study by Choe et al.30, N-acetylcysteinewas administrated transtympanically in a guinea pig model, in which the inner ears were filled with a 2% N-acetylcysteine solution before CDDP treatment (20 mg/kg). As a result, the auditory brainstem response was significantly less reduced when compared with that in the control group. Different otoprotective medical interventions have been studied in an effort to prevent ototoxicity. Van As et al. performed the first systematic review evaluating all medical interventions to assess the efficacy of different otoprotective medical interventions for preventing hearing loss in children with cancer treated with platinum-based therapy, but no conclusions were made by the author about their efficacy. Thus, more high quality research is needed.31 Recent studies have shown that vestibular hair cells are damaged in guinea pigs even after low CDDP doses.32 Based on these findings and our data, we consider that the degree of vestibular functional impairment induced by CDDP may be correlated with the extent of morphological damage. Although neuroprotective agents (such as D-methionine, sodium thiosulfate, NAS) against CDDP-induced cochleotoxicity have been reported,33, 34 no study has investigated their protective effects against CDDP-induced vestibular toxicity.
Preventing platinum-induced hearing loss and vestibular dysfunction is very important. In the present study, we found that RXN at 180 mg/kg administered intragastrically protected against CDDP-mediated ototoxicity and vestibular toxicity. Morphological hair cells were clearly preserved in the RXN-treated group, as shown by the scanning electron microscopy evaluation, compared with marked hair cell loss observed in animals receiving CDDP. GbE and CS, components of RXN, have been involved in studies and clinical treatment for a long time. EGb 761 contains potent antioxidants capable of scavenging free radicals, inhibiting nitric oxide (NO) synthesis, reducing lipid peroxidation, and protecting against apoptosis and has been demonstrated to protect hearing loss induced by CDDP (13 mg/kg) in rats.35 CS prevents NO-induced apoptosis of chondrocytes via protein kinase CK2 in vitro and prevents cartilage destruction in a rat model of osteoarthritis.36 Jung et al. reported that GbE enhances the antiatherogenic effect of CS by inhibiting ROS generation. Consistent with these studies, we found that GbE-pretreated rats had significantly better hearing and vestibular function as well as hair cell morphology when compared to those in CDDP-treated rats; however, more significant protected hearing and vestibular functions were found in the RXN-pretreated rats, indicating that RXN has synergistic action.
Mechanism of RXN rescue from CDDP toxicity
Much progress has been made to define the mechanisms underlying hair cell degeneration in recent years. A toxic insult to a cell can activate a cascade of cell death genes, leading to demise. An effective otoprotective strategy might require intervention at several levels along pathways regulating cell death and survival; therefore, it is essential to further understand sensory hair cell death pathways.37 It is well established that ROS are generated in hair cells exposed to CDDP.38 Numerous studies have reported that enhancing antioxidant levels (through drug application or genetic manipulation) promotes hair cell survival while preserving function.39 When a cell is challenged, the balance between the proapoptotic and antiapoptotic Bcl-2 family members such as Bcl-xL serves as a primary control checkpoint regulating cell death and survival.40 Antiapoptotic Bcl-2 family members are able to form heterodimers with proapoptotic members, resulting in a neutral signal.41 When the balance tilts in favor of apoptosis, the proapoptotic Bcl-2 member Bax translocates from the cytoplasm to the mitochondria and promotes the formation of pores in the mitochondrial membrane.42 These cellular events lead to loss of mitochondrial transmembrane potential, the generation of ROS, and leakage of cytochrome c into the cytoplasm. So et al. reported that pretreatment with MEK1/ERK inhibitors, including PD98059 and U0126, markedly decreases CDDP cytotoxicity as measured by the MTT assay.43 Although JNK and c-jun become activated in response to noise, aminoglycosides, and CDDP, there is evidence indicating that inhibiting JNK effectively protects hair cells from aminoglycoside-induced and noise-induced hair cell death but not CDDP-induced hair cell death.44
The current in vitro study sought to explore the roles of RXN in signaling pathways regulating CDDP-induced apoptosis in an auditory cell line. Intracellular ROS generation and the JNK, ERK, and mitochondrial pathways were examined. RXN protected HEI-OC1 cells by intervening at several levels along the pathways. RXN improved HEI-OC1 cell viability against CDDP-induced cytotoxicityat a particular concentration and significantly attenuated CDDP-induced apoptosis and increased ROS production. RXN reduced cell apoptosis by increasing the antiapoptosis protein Bcl-xL and decreasing pro-apoptosis members such as BAX; consequently, the release of cytochrome c was inhibited and caspase-3 and PARP were down-regulated. Downregulation of P-ERK suggested that RXN blocked CDDP-induced apoptosis via the ERK pathway.
RXN interferes with CDDP therapy
Concern that the oncological effects of CDDP might be negatively impacted is the major reason that protective agents are rarely used clinically. Sodium thiosulfate, a thiol, is the most widely studied chemoprotectant against platinum-based chemotherapy. Although the exact mechanism of otological protection by sodium thiosulfate at the molecular level is unknown, it binds covalently to electrophilic platinum, rendering it inactive.45 Elferink et al. reported that sodium thiosulfate reacts irreversibly with CDDP to form Pt (S2O3)4, which is excreted by the kidney, reducing both its ototoxic activity and antineoplastic potency.46 In the current study, platinum serum concentration was evaluated, and the result indicated that RXN did not disturb the antineoplastic effect of CDDP.
Potential use of RXN in the clinical setting
Given the incidence of hearing loss and vestibular impairment associated with CDDP, a mechanism to decrease ototoxicity could be important for maximizing dosing and compliance and, therefore, chemotherapy efficacy. RXN, with its significant otoprotective effects, multi apoptotic pathway intervention, and non-interference with serum platinum concentration, might be a promising chemoprotective agent for rescue of CDDP-mediated ototoxicity. We suggest that a clinical trial to evaluate RXN may be warranted to extend the positive results to patients being treated with platinum chemotherapy. However, we also should consider possible complications from the combined use of RXN and CDDP. For example, RXN may show a rare complication of bleeding in patients treated with CDDP. Because bleeding risk may synergistically increase from the antiplatelet effect of RXN and CDDP-induced thrombocytopenia tendency. We cannot completely exclude the possibility even though we didn't find any RXN-related bleeding in the present study.
In conclusion, we demonstrated that administering RXN offered the synergistic action of GbE and CS and strongly protected against hearing and vestibular dysfunction induced by CDDP in vivo. The in vitro data suggest that the mechanism of the protective effects of RXN afforded in vivo were mainly dependent on its antiapoptotic role in CDDP-mediated toxicity in HEI-OC1 cells by inhibiting ROS production and mitochondrial and ERK pathways. Combined with the finding that RXN did not attenuate CDDP plasma platinum concentration, the results suggest that RXN can potentially be used to reduce CDDP-related vestibular dysfunction.
Materials and Methods
The Ajou University School of Medicine Institutional Animal Care and Use Committee approved the surgical procedures in accordance with the guidelines regarding the care and use of animals for experimental procedures. All efforts were made to minimize the number of animals used and their suffering. Sprague–Dawley rats (SD; male 7 weeks, 200–250 g) were purchased from Orient Bio Inc. (Seoul, Korea). CDDP was purchased from Sigma (St Louis, MO, USA), CS and GbE was kindly provided by SK Chemical Co. (Seoul, Korea).
Preparation of animal study groups
The in vivo study included two phases. The first phase consisted of a dosing study to identify the effect of RXN on CDDP-induced ototoxicity and vestibular toxicity. Twenty-six adult male SD rats were divided into five groups such as RXN (45 mg/kg)+CDDP (n=5), RXN (90 mg/kg)+CDDP (n=5), RXN (180 mg/kg)+CDDP (n=5), CDDP-only (n=6), and normal control (n=5). In the second phase, 69 rats were divided into six groups to investigate the synergistic action of GbE and CS on CDDP-induced hearing loss and vestibular toxicity. The groups were: normal control (n=10), CDDP-only group (n=13), RXN+CDDP (n=12), CS+CDDP (n=12), GbE+CDDP (n=12), and vehicle+CDDP (n=10). Following a functional hearing and vestibular evaluation, the rats were intragastrically treated with the experimental agents (water for the CDDP-only and normal control groups) from 7 days before CDDP administration until 5 days post-treatment. CDDP (16 mg/kg) (saline for the normal control groups) was injected intraperitoneally 2 h after the seventh treatment with the otoprotective agents. After hearing and vestibular functional tests were conducted for the postevaluation 5 days later, all rats were killed to harvest cochleae and utricles/saccules, and then a hair cell morphological evaluation was performed using scanning electron microscopy (SEM).
Chemicals
CDDP was dissolved in saline at a concentration of 0.15 mg/ml (low concentration for hydration therapy), and was administered intraperitoneally at a dose of 16 mg/kg. Carboxymethylcellulose sodium salt (Sigma-Aldrich, Kyunggi-Do, South Korea), as a RXN solvent, was dissolved in distillated water to 0.5%. GbE and CS were dissolved in carboxymethylcellulose solution by sonication to create 4.5, 9.0, and 18.0 mg/ml solutions at a ratio of GbE : CS=4 : 5. RXN was administrated intragastrically at doses of 45, 90, and 180 mg/kg.
Auditory and vestibular function assessment
The auditory brainstem response was tested with the Biosig 32 system (Tucker-Davis Technologies, Gainesville, FL, USA) as described previously.47 The tail hanging test was used to evaluate vestibular functioning; the rat was lifted by the tail and kept hanging at a height of 30 cm for a 15 s interval, and the number of head rotations was counted. For the swimming test, a stainless steel pool (length, 28 cm; width, 45 cm; and depth, 25 cm) was filled with body-temperature water at a depth of 19 cm. Rats were lifted by their tail and dropped into the center of the pool from a height of 20 cm. The time in seconds between contact with the water and separation from the water to the platform was noted by an independent observer.
Morphological evaluation of hair cells
Scanning electron microscopy was used for the hair cell morphological evaluation in the cochlea and saccules/utricles as described previously.46 Thirty outer hair cells in a given microscopic field were evaluated. Two to three microscopic fields were counted in each sample for the middle or basal turns, based on Viberg and Canlon's study48, and these were averaged to obtain a value for at least five samples. Five unit areas were chosen in the saccules and utricles, and hair cells were counted in each unit area for statistical analysis.
Cell culture and viability assays
The HEI-OC1 auditory cell line expresses several molecular markers characteristic of organ of corti sensory cells, and they are useful as an ototoxic study model for drugs such as CDDP. The cells were maintained in Dulbeco's Modified Eagle's Medium with 10% fetal bovine serum at 33 °C in a CO2 incubator. The HEI-OC1 cells (3000 cells/well in a 96-well plate) were incubated with 20 μM CDDP for 0, 12, 24, and 48 h, and the time-dependent effects of CDDP were measured using a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) assay (Promega, Madison, WI, USA). The cells (3000 cells/well in a 96-well plate) were treated with different concentrations of RXN (56.3, 28.1, 14.1, 7.0, and 3.5 μg/ml) for 24 h. To test the effects of RXN on CDDP-mediated cytotoxicity, the cells were pretreated with RXN for 1 h and exposed to CDDP (20 μM) for 24 h. A 20 μl aliquot of MTS solution was added to 100 μl medium, and the cells were incubated for 4 h at 33 °C in 10% CO2, and then the absorbance of the formazan at 490 nm was measured directly from the 96-well assay plates with a microplate reader (Model 680, Bio-Rad, Tokyo, Japan).
Annexin V-FITC/PI assay
Annexin V/PI staining was used to quantify the effect of RXN on CDDP-induced apoptosis with an Annexin V-FITC Apoptosis Detection kit (BD Biosciences, San Diego, CA, USA), following the manufacturer's protocol and quantified by flow cytometry. HEI-OC1 cells were cultured overnight in 6-well plates and then treated with 20 μM CDDP in the presence or absence of RXN. After washing with PBS, the cells were detached in trypsin and centrifuged (3 min, 4 °C, 2000 r.p.m.), followed by resuspending the cells gently in 200 μl PBS. The cells were centrifuged again, and then resuspended in 100 μl 1 × binding buffer. Then, the cells were incubated with Annexin V-FITC and PI (5 μl of each) for 15 min at room temperature. After adding 400 μl of 1 × binding buffer, the cells were analyzed by flow cytometry using a FACSAriaIII (BD Biosciences).
Western blot assay
Western blots were performed to evaluate involvement of the cell apoptosis pathway. The following primary antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA): β-Actin, PARP, BCL-xL, BAX, cytochrome c, cleaved caspase-3, and ERK. The treated HEI-OC1 cells were washed twice with PBS and then lysed in cold RIPA lysis buffer (Biosesang, Seoul, Korea) by adding Xpert Protease Inhibitor Cocktail Solution (100 × ) (GenDEPOT, Seoul, Korea), and the reaction was allowed to proceed for 30 min on ice. The homogenates were centrifuged at 13 000 r.p.m. for 30 min at 4 °C, and the supernatant fraction was collected for further analysis. Protein concentrations were determined by the DC Protein Assay (Bio-Rad,Hercules, CA, USA). Samples containing protein (25 μg sample) were immediately heated for 10 min at 100 °C and then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After electrophoresis, the proteins were transferred to a polyvinylidene fluoride membrane. The membranes were blocked with 5% skim milk in PBS containing 0.05% Tween (PBS-T) and subsequently incubated with primary polyclonal antibodies at a final dilution of 1 : 1000 at 4 °C overnight. After three washes in PBS-T, the membranes were incubated with peroxidase–conjugated secondary antibody (final dilution, 1 : 2000) in PBS-T at room temperature for 1 h. After washing the membranes, the protein bands were visualized using enhanced chemiluminescence on X-ray film according to the manufacturer's instructions (West-Q chemiluminescent Substrate kit, GenDEPOT). Band intensity was measured with NIH ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Measurement of intracellular ROS production
Intracellular ROS level was measured using a Total ROS Detection kit (Enzo Life Sciences, Plymouth Meeting, PA, USA) yielding a green fluorescence. After cell culture and treatment with CDDP in the presence or absence of RXN in 12-well plates on cover slips, the cells were loaded with ROS Detection solution and incubated for 1 h. After washing with 1 × Wash Buffer, the samples were immediately observed under a confocal microscope (LSM710, Carl Zeiss, Jena, Germany). The cells were cultured and treated with CDDP in the presence or absence of RXN in 6-well plates for the quantitative flow cytometry analysis. Media were removed, and the cells were washed twice with 1 × Wash Buffer. The cells were detached with trypsin, and centrifuged for 5 min at 400 × g and room temperature. The supernatant was discarded, and the cell pellet was resuspended in 500 μl of ROS Detection solution. The cells were stained for 30 min at 33 °C in the dark and analyzed by flow cytometry using a FACSAriaIII.
CDDP pharmacokinetics study
Ten rats were divided into a RXN+CDDP group (n=5) and a Vehicle+CDDP group (n=5), 2 h after the seventh RXN pretreatment, and CDDP was administered just before and after the CDDP administration. Blood samples were taken from the rats at 0, 0.5, 1.0, 2.0, 4.0, and 6.0 h, respectively. Plasma was prepared by centrifugation at 3000 × g for 10 min at 4 °C and was stored at −70 °C until analysis. Pharmacokinetics was assessed by platinum concentrations in plasma using inductively coupled plasma mass spectrometry (X-7 ICP-MS; Fisher Scientific, Waltham, MA, USA).
Statistical analysis
All data are presented as means±S.D.. The statistical analysis was performed using SPSS 12.0 for Windows (SPSS, Inc. Chicago, IL, USA). Differences were analyzed using a one-way analysis of variance, Tukey's HSD post hoc test, and t-tests. P-values≤0.05 were considered significant.
Acknowledgments
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education Science and Technology (2010-0010678).
Glossary
- ABR
auditory brainstem response
- CDDP
Cisplatin
- CMC
Carboxymethylcellulose sodium salt
- CS
cilostazol
- GbE
Ginkgo biloba extract
- HEI-OC1
House Ear Institute-Organ of Corti 1
- ICP-MS
inductively coupled plasma mass spectrometry
- NAC
N-acetylcysteine
- NO
nitric oxide
- PARP
poly (ADP-ribose) polymerase
- p-ERK
phosphorylated extracellular regulated kinase
- ROS
intracellular reactive oxygen species
- RXN
renexin
- SEM
scanning electron microscopy
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by A Verkhratsky
Supplementary Material
References
- Sakamoto M, Kaga K, Kamio T. Extended high-frequency ototoxicity induced by the first administration of cisplatin. Otolaryngol Head Neck Surg. 2000;122:828–833. doi: 10.1016/S0194-59980070009-X. [DOI] [PubMed] [Google Scholar]
- Nader ME, Théorêt Y, Saliba I. The role of intratympanic lactate injection in the prevention of cisplatin-induced ototoxicity. Laryngoscope. 2010;120:1208–1213. doi: 10.1002/lary.20892. [DOI] [PubMed] [Google Scholar]
- Paksoy M, Ayduran E, Sanli A, Eken M, Aydın S, Oktay ZA. The protective effects of intratympanic dexamethasone and vitamin E on cisplatin-induced ototoxicity are demonstrated in rats. Med Oncol. 2011;28:615–621. doi: 10.1007/s12032-010-9477-4. [DOI] [PubMed] [Google Scholar]
- Peter SR, John AR.OtotoxicityIn: Michael AG, Brendan JSOtotoxicity of Platinum Compounds BC Decker Inc: Hamilton. London; 60–69.2004 [Google Scholar]
- Im GJ, Chang JW, Choi J, Chae SW, Ko EJ, Jung HH. Protective effect of Korean red ginseng extract on cisplatin ototoxicity in HEI-OC1 auditory cells. Phytother Res. 2010;24:614–621. doi: 10.1002/ptr.3082. [DOI] [PubMed] [Google Scholar]
- Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33:9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black FO, Myers EN, Schramm VL, Johnson J, Sigler B, Thearle PB, et al. Cisplatin vestibular ototoxicity: preliminary results. Laryngoscope. 1982;92:1363–1368. [PubMed] [Google Scholar]
- Wall C, Merfeld DM, Rauch SD, Black FO. Vestibular prostheses: the engineering and biomedical issues. J Vestib Res. 2002-2003;12:95–113. [PubMed] [Google Scholar]
- Burschka MA, Hassan HA, Reineke T, van Bebber L, Caird DM, Mösges R. Effect of treatment with Ginkgo biloba extract EGb 761 (oral) on unilateral idiopathic sudden hearing loss in a prospective randomized double-blind study of 106 outpatients. Eur Arch Otorhinolaryngol. 2001;258:213–219. doi: 10.1007/s004050100343. [DOI] [PubMed] [Google Scholar]
- Yang TH, Young YH, Liu SH. EGb 761 (Ginkgo biloba) protects cochlear hair cells against ototoxicity induced by gentamicin via reducing reactive oxygen species and nitric oxide-related apoptosis. J Nutr Biochem. 2011;22:886–894. doi: 10.1016/j.jnutbio.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Oyama Y, Chikahisa L, Ueha T, Kanemaru K, Noda K. Ginkgo biloba extract protects brain neurons against oxidative stress induced by hydrogen peroxide. Brain Res. 1996;712:349–352. doi: 10.1016/0006-8993(95)01440-3. [DOI] [PubMed] [Google Scholar]
- Yoshikawa I, Naito Y, Kondo M. Ginkgo biloba leaf extract: review of biological actions and clinical applications. Antioxid Redox Signal. 1999;1:469–480. doi: 10.1089/ars.1999.1.4-469. [DOI] [PubMed] [Google Scholar]
- Kose K, Dogan P. Lipoperoxidation induced by hydrogen peroxide in human erythrocyte membranes. 2. Comparison of the antioxidant effects of Ginkgo biloba extract (EGb 761) with those of water-soluble and lipid-soluble antioxidants. J Int Med Res. 1995;23:9–18. doi: 10.1177/030006059502300102. [DOI] [PubMed] [Google Scholar]
- Christen Y, Maixent J-M. What is Ginkgo biloba extract EGb 761? An overview-from molecular biology to clinical medicine. Cell Mol Biol. 2002;48:601–611. [PubMed] [Google Scholar]
- Huang X, Whitworth CA, Rybak LP. Ginkgo biloba extract (EGb 761) protects against cisplatin-induced ototoxicity in rats. Otol Neurotol. 2007;28:828–833. doi: 10.1097/mao.0b013e3180430163. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Tani T, Kanbe T, Watanabe K. Effect of cilostazol on platelet aggregation and experimental thrombosis. Arzneimittelforschung. 1985;35:1144–1149. [PubMed] [Google Scholar]
- Tanaka K, Gotoh F, Fukuuchi A, Amano T, Uematsu D, Kawamura J, et al. Effects of a selective inhibitor of cyclic AMP phosphodiesterase on the pial microcirculation in feline cerebral ischemia. Stroke. 1989;20:668–673. doi: 10.1161/01.str.20.5.668. [DOI] [PubMed] [Google Scholar]
- Jaff MR. Pharmacotherapy for peripheral arterial disease: emerging therapeutic options. Angiology. 2002;53:627–633. doi: 10.1177/000331970205300602. [DOI] [PubMed] [Google Scholar]
- Dawson DL, Cutler BS, Meissner MH, Strandness DE. Cilostazol as beneficial effects in treatment of intermittent claudication: result from a multicenter, randomized, prospective, double-blind trial. Circulation. 1998;98:678–686. doi: 10.1161/01.cir.98.7.678. [DOI] [PubMed] [Google Scholar]
- Kim KY, Shin HK, Choi JM, Hong KW. Inhibition of lipopolysaccharide-induced apoptosis by cilostazol in human umbilical vein endothelial cells. J Pharmacol Exp Ther. 2002;300:709–715. doi: 10.1124/jpet.300.2.709. [DOI] [PubMed] [Google Scholar]
- Shin HK, Kim YK, Kim KY, Lee JH, Hong KW. Remnant lipoprotein particles induce apoptosis in endothelial cells by NAD(P)H oxidase-mediated production of superoxide and cytokines via lectin-like oxidized low-density lipoprotein receptor-1 activation: prevention by cilostazol. Circulation. 2004;109:1022–1028. doi: 10.1161/01.CIR.0000117403.64398.53. [DOI] [PubMed] [Google Scholar]
- Choi JM, Shin HK, Kim KY, Lee JH, Hong KW. Neuroprotective effect of cilostazol against focal cerebral ischemia via antiapoptotic action in rats. J Pharmacol Exp Ther. 2000;300:787–793. doi: 10.1124/jpet.300.3.787. [DOI] [PubMed] [Google Scholar]
- Kim KY, Shin HK, Choi JM, Hong KW. Inhibition of LPS induced apoptosis by cilostazol in human umbilical vein endothelial cells. J Pharmacol Exp Ther. 2002;300:709–715. doi: 10.1124/jpet.300.2.709. [DOI] [PubMed] [Google Scholar]
- Park SY, Lee JH, Shin HK, Kim CD, Lee WS, Rhim BY, et al. Synergistic efficacy of concurrent treatment with cilostazol and probucol on the suppression of reactive oxygen species and inflammatory markers in cultured human coronary artery endothelial cells. Korean J Physiol Pharmacol. 2008;12:165–170. doi: 10.4196/kjpp.2008.12.4.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu KH, Han HY, Lee SY, Jeon SD, Im GJ, Lee BY, et al. Ginkgo biloba extract enhances antiplatelet and antithrombotic effects of cilostazol without prolongation of bleeding time. Thromb Res. 2009;124:328–334. doi: 10.1016/j.thromres.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Jung IH, Lee YH, Yoo JY, Jeong SJ, Sonn SK, Park JG, et al. Ginkgo biloba extract(GbE) enhances the anti-atherogenic effect of cilostazol by inhibiting ROS generation. Exp Mol Med. 2012;44:311–318. doi: 10.3858/emm.2012.44.5.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumusakhuylu AC, Yazici M, Sari M, Binnetoglu A, Kosemihal E, Akdas F, et al. Protective role of resveratrol against cisplatin induced ototoxicity in guinea pigs. Int J Pediatr Otorhinolaryngol. 2012;76:404–408. doi: 10.1016/j.ijporl.2011.12.021. [DOI] [PubMed] [Google Scholar]
- Korver KD, Rybak LP, Whitworth C, Campbell KM. Round window application of D-methionine provides complete cisplatin otoprotection. Otolaryngol Head Neck Surg. 2002;126:683–689. doi: 10.1067/mhn.2002.125299. [DOI] [PubMed] [Google Scholar]
- Cheng PW, Liu SH, Hsu CJ, Lin-Shiau SY. Correlation of increased activities of Na+, K+-ATPase and Ca2+-ATPase with the reversal of cisplatin ototoxicity induced by D-methionine in guinea pigs. Hear Res. 2005;205:102–109. doi: 10.1016/j.heares.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Choe WT, Chinosornvatana N, Chang KW. Prevention of cisplatin ototoxicity using transtympanic N-acetylcysteine and lactate. Otol Neurotol. 2004;25:910–915. doi: 10.1097/00129492-200411000-00009. [DOI] [PubMed] [Google Scholar]
- van As JW, van den Berg H, van Dalen EC. Medical interventions for the prevention of platinum-induced hearing loss in children with cancer. Cochrane Database Syst Revl. 2012;5:CD009219. doi: 10.1002/14651858.CD009219.pub2. [DOI] [PubMed] [Google Scholar]
- Sergi B, Ferraresi A, Troiani D, Paludetti G, Fetoni AR. Cisplatin ototoxicity in the guinea pig: vestibular and cochlear damage. Hear Res. 2003;182:56–64. doi: 10.1016/s0378-5955(03)00142-4. [DOI] [PubMed] [Google Scholar]
- Muldoon LL, Pagel MA, Kroll RA, Brummett RE, Doolittle ND, Zuhowski EG, et al. Delayed administration of sodium thiosulfate in animal models reduces platinum ototoxicity without reduction of antitumor activity. Clin Cancer Res. 2000;6:309–315. [PubMed] [Google Scholar]
- Thomas DD, Muldoon LL, Kraemer DF, Neuwelt EA. Protection against cisplatin-induced ototoxicity by N-acetylcysteine in a rat model. Hear Res. 2004;193:25–30. doi: 10.1016/j.heares.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Huang X, Whitworth CA, Rybak LP. Ginkgo biloba extract (EGb 761) protects against cisplatin-induced ototoxicity in rats. OtolNeurotol. 2007;28:828–833. doi: 10.1097/mao.0b013e3180430163. [DOI] [PubMed] [Google Scholar]
- Lee SW, Song YS, Shin SH, Kim KT, Park YC, Park BS, et al. Cilostazol protects rat chondrocytes against nitric oxide-induced apoptosis in vitro and prevents cartilage destruction in a rat model of osteoarthritis. Arthritis Rheum. 2008;58:790–800. doi: 10.1002/art.23220. [DOI] [PubMed] [Google Scholar]
- Cheng AG, Cunningham LL, Rubel EW. Mechanisms of hair cell death and protection. Curr Opin Otolaryngol Head Neck Surg. 2005;13:343–348. doi: 10.1097/01.moo.0000186799.45377.63. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Whitworth C, Somani S. Application of antioxidants and other agents to prevent cisplatin ototoxicity. Laryngoscope. 1999;109:1740–1744. doi: 10.1097/00005537-199911000-00003. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Sha SH, Minoda R, Izumikawa M, Kuriyama H, Schacht J, et al. Antioxidant gene therapy can protect hearing and hair cells from ototoxicity. Mol Ther. 2004;9:173–181. doi: 10.1016/j.ymthe.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Xiang H, Kinoshita Y, Knudson CM, Korsmeyer SJ, Schwartzkroin PA, Morrison RS. Bax involvement in p53-mediated neuronal cell death. J Neurosci. 1998;18:1363–1373. doi: 10.1523/JNEUROSCI.18-04-01363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner C. The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol Immunol. 2003;39:615–647. doi: 10.1016/s0161-5890(02)00252-3. [DOI] [PubMed] [Google Scholar]
- So H, Kim H, Lee JH, Park C, Kim Y, Kim E, et al. Cisplatin cytotoxicity of auditory cells requires secretions of proinflammatory cytokines via activation of ERK and NF-kappaB. J Assoc Res Otolaryngol. 2007;8:338–355. doi: 10.1007/s10162-007-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ladrech S, Pujol R, Brabet P, Van De Water TR, Puel JL. Caspase inhibitors, but not c-Jun NH2-terminal kinase inhibitor treatment, prevent cisplatin-induced hearing loss. Cancer Res. 2004;64:9217–9224. doi: 10.1158/0008-5472.CAN-04-1581. [DOI] [PubMed] [Google Scholar]
- Blakley BW, Cohen JI, Doolittle ND, Muldoon LL, CampbeII KC, Dickey DT, et al. Strategies for prevention of toxicity caused by platinum-based chemotherapy: review and summary of the annual meeting of the Blood-Brain Barrier Disruption Program, Gleneden Beach, Oregon, March 10, 2001. Laryngoscope. 2002;112:1997–2001. doi: 10.1097/00005537-200211000-00016. [DOI] [PubMed] [Google Scholar]
- Whitworth CA, Ramkumar V, Jones B, Tsukasaki N, Rybak LP. Protection against cisplatin ototoxicity by adenosine agonists. Biochem Pharmacol. 2004;67:1801–1807. doi: 10.1016/j.bcp.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Choung YH, Kim SW, Tian CJ, Min JY, Lee HK, Park SN, et al. Korean red ginseng prevents gentamicin-induced hearing loss in rats. Laryngoscope. 2011;121:1294–1302. doi: 10.1002/lary.21756. [DOI] [PubMed] [Google Scholar]
- Viberg A, Canlon B. The guide to plotting a cochleogram. Hear Res. 2004;197:1–10. doi: 10.1016/j.heares.2004.04.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.