Abstract
Objective
The neurobiological basis of inattentiveness, a core feature of attention-deficit/hyperactivity disorder (ADHD), is not yet well understood. Structural abnormalities in thalamus, especially the pulvinar nuclei, have recently been reported in ADHD. Pulvinar nuclei maintain reciprocal connections with cortical/subcortical areas, and play a central coordinating role during visual attention processing. The objective of this study was to test the hypothesis that children and young adolescents with ADHD would show atypical pulvinar–cortical functional pathways during sustained attention performance, and that these functional abnormalities would be associated with the inattentive symptoms of the disorder.
Method
Visual attention task-based functional magnetic resonance imaging (fMRI) data from 22 children and young adolescents with ADHD and 22 demographically matched, normal control subjects were analyzed. Cortical activation maps and temporal correlations of activity patterns between pulvinar nuclei and the remainder of brain were constructed for each participant. Correlations between activation magnitude of pulvinar and diagnostic measures were calculated in subjects with ADHD.
Results
Compared to controls, subjects with ADHD showed significantly reduced pulvinar activations bilaterally, significantly decreased functional connectivity between bilateral pulvinar and right prefrontal regions, and significantly increased connectivity between the right pulvinar and bilateral occipital regions. In addition, the activation magnitude in the left pulvinar was negatively correlated with the DSM-IV inattentive index in ADHD group.
Conclusions
Allied with previous evidence of structural abnormalities in pulvinar, the current data suggest that inappropriate development of pulvinar may lead to disrupted functional circuits for visual attention processing, and that these disruptions contribute significantly to the pathophysiological mechanisms of the inattentiveness symptoms in ADHD.
Keywords: ADHD, functional MRI, pulvinar, thalamo-cortical connectivity, attention
Attention-deficit/hyperactivity disorder (ADHD), characterized by cognitive deficits in attention and inhibitory processing, is the most common psychiatric disorder of childhood with a prevalence rate of 5% to 8%.1 It is frequently associated with lifelong impairment affecting both quality of life and learning. The development of objective biomarkers for this syndrome is a major goal of the neuroimaging research community.2
A large number of neuroimaging studies in ADHD have focused on the impulsive tendencies, implicating dysfunction within fronto-striatal circuits and other brain regions.3 For example, reduced functional connectivity between right inferior fronto-frontal, fronto-striatal, and fronto-parietal networks has been associated with performance of motor inhibition and task-switching tasks in this population.4 Reduced activity in right prefrontal cortex has been correlated with reduced ability to inhibit motor responses during a think/no-think task.5 Decreased activations in regions associated with sensorimotor timing during finger tapping has also been observed, including prefrontal and precentral gyri, cerebellum, inferior parietal lobule, and superior temporal gyrus.6
Inattention is the other well-recognized component of ADHD; yet the neurobiological underpinnings of inattention have not been nearly as well studied as those associated with impulsivity.1 Nonetheless, neuroimaging studies have reported functional deficits in prefrontal, anterior cingulate, and parietal cortices, regions well known to play important roles in attentional deployment.7 Recent studies have also reported aberrant cerebellar activation in ADHD during attention tasks 8 and at rest.9 Increasingly, studies have also implicated cortico-striato-thalamo-cortical (CSTC) loops in attentional and cognitive processing, and evidence suggests that disturbances of CSTC loops may be associated with the inattention symptom of ADHD.10
The thalamus is an important component of the CSTC loops.11 Functional magnetic resonance imaging (fMRI) studies have shown abnormal blood-oxygen–level dependence (BOLD) activation in the thalamus in patients with ADHD during task switching12 and selective attention,13 as well as abnormal functional connectivity between thalamus and cortical regions during the resting state.14,15 However, findings of these two resting-state functional connectivity studies are not fully consistent, probably because of the location difference of the region-of-interest (ROI) selected from the entire thalamus.
One key nucleus of the thalamus consistently implicated in attentional function is the pulvinar.16,17 As the largest of the thalamic nuclei, and given its extensive connectivity with the superior colliculus and visual cortices, the pulvinar plays a central role in modulating visual processing and orienting of the eyes to salient visual events.18 Lesions of pulvinar can cause hemispatial neglect syndrome in humans19 and primates.20 Injections of γ-aminobutyric acid (GABA)–related drugs into the pulvinar cause inhibition and facilitation of attention switches respectively, in nonhuman primates performing a spatial-cueing task.21 The pulvinar has connections with the prefrontal and inferior parietal lobes 22 and the medial nucleus of the pulvinar sends projection fibers to the posterior cingulate gyrus, the retrosplenial area, and the posterior parahippocampal gyrus.23 Functional neuroimaging studies in both monkeys and humans showed that networks including pulvinar, posterior parietal cortex, superior temporal cortex, and dorsolateral prefrontal cortex were associated with attention-related functions.16,24,25 Positron emission tomography (PET) studies across species make it clear that the pulvinar plays a central role in spatial attention orienting and in the filtering of distracting information.16
Very recently, two vertex-based shape analysis studies, one by Ivanov et al.26 and the other from our research group,27 reported significant structural atrophy in bilateral pulvinar nuclei in youth with ADHD. Given these findings of anomalous anatomy and the clear role of pulvinar during attentional processing, we set out here to examine functional activation in the pulvinar nuclei and to assess pulvinar-cortical connectivity during a visual sustained attention task, acquiring functional imaging measures in a cohort of children with ADHD combined type, and comparing their responses to those of age-matched control participants. We recognized the possible neural heterogeneities associated with the sub-phenotypes of ADHD would affect the brain activation and connectivity patterns. In this study, we focused our research on children with ADHD combined-type, who had both inattention and hyperactivity symptoms that confirm the diagnosis of ADHD. We did not include those who were diagnosed with inattentive subtype, considering that children with ADHD inattentive subtype are often argued with their diagnoses. We predicted functional deficits within the pulvinar nuclei and the relevant extending connections to the cerebral cortices, deficits that are likely associated with the neurobiological underpinnings of inattention that is a hallmark symptom of ADHD in children.
METHOD
Participants
A total of 48 children and young adolescents, ranging from 9 through 15 years of age, were involved in this study. Three were excluded from analyses because of heavy head motions, and one was excluded because of low (<80%) response accuracy during fMRI acquisition. Finally, 22 children with ADHD combined type and 22 control subjects were included in analyses. All of the subjects were strongly right-handed, evaluated using the Edinburgh Handedness Inventory,28 and had estimated full-scale IQ≥ 80, measured by Wechsler Abbreviated Scale of Intelligence (WASI),29 to minimize neurobiological heterogeneity.
The patient group included children who met current DSM-IV criteria for ADHD combined type, by combining the Conners Rating Scale—Revised-L for both parent and self reports.30 This was confirmed with a parent interview using the Schedule for Affective Disorders and Schizophrenia for Children—Present and Lifetime Version (K-SADS-PL).31 The normal control group included children who had T-scores <60 (<1 SD) on all Conners parent and self-reports.
For both groups, we included the K-SADS-PL screening questions and supplements to rule out pervasive developmental disorders, substance use and abuse, and posttraumatic stress disorder. Similarly, oppositional defiant disorder with physical aggression (using DSM-IV diagnostic criteria), and all other current Axis I disorders (except for fear of the dark) were exclusionary. Children with any specific learning disorders were also excluded. The basic reading, mathematical reasoning, reading comprehension, and numerical operations subtests of the Wechsler Individual Achievement Test 2nd ed. (WIAT-II)32 were administered to determine the presence of impairments in reading or math.
General exclusion criteria for both groups also included the following: chronic medical/neurological illness or was taking systemic medication; specific or focal neurological disorder including epilepsy; treatment with any nonstimulant psychotropic within the past month; contraindications to magnetic resonance imaging (MRI) scanning; and heavy head motions (any of the six translation and rotation parameters >1.5 mm) during the fMRI data acquisition.
We avoided including siblings, considering that genetic and family factors might influence brain activation patterns during the fMRI tasks. Nine of the subjects with ADHD were taking short-acting Ritalin, whereas the others were not medicated. To eliminate the medication effects on brain activations during the fMRI tasks, we requested a 48-hour wash-out period before the study.
The children and young adolescents with ADHD were recruited from the Children's Evaluation and Rehabilitation Center at the Albert Einstein College of Medicine, and the Max and Celia Parnes Family Psychological and Psychoeducational Services Clinic at the Ferkauf Graduate School of Psychology. The controls were recruited from local schools through newspaper advertisements. This study received Institutional Review Board approval for human subjects’ research at the Albert Einstein College of Medicine. Written informed consents were provided by all participants and their parents after the nature of the study and its procedures were carefully explained. All procedures were conducted in keeping with the tenets for the ethical conduct of research as outlined in the Declaration of Helsinki.
MRI Data Acquisition Protocol
Imaging data were obtained using a 3.0 Tesla 32 Channel FreewaveAchieva MRI Scanner (Philips Medical Systems, Best, the Netherlands). All data acquisition protocols exceed the Biomedical Informatics Research Network (BIRN) recommendations for functional and morphometric data acquisition (http://www.nbirn.net/). High-resolution T1-weighted whole-head structural MRI data for each subject was acquired and used for image registration and head motion correction (240-mm field of view [FOV] with 240 × 240 in-plane matrix and 1 mm isotropic resolution, time echo [TE] = 4.6 milliseconds, repetition time [TR] = 9.8 milliseconds, α = 8°, sensitivity encoding [SENSE] factor = 2. Total scan time is 3 minutes). fMRI acquisition used whole-brain gradient echo-planar imaging (EPI) acquired over a 230-mm FOV on a 230 ×128 acquisition matrix with 2-mm slice thickness, and 2×2 mm2 in-plane resolution, 41 slices, TE = 28 milliseconds, TR = 2000 milliseconds.
Experimental Task for fMRI Acquisition
Sustained attention is impaired in patients with ADHD combined type. The continuous performance task (CPT) is one of the most widely used measures of impaired sustained attention, allowing for a parametric design that can systematically challenge sustained attention abilities.33 Studies have reported acceptable reliability and validity of the CPT,34 and strong correlation between the omission error rates and teacher ratings of inattention.35 The CPT tasks have also shown behavioral and functional brain impairments associated with inattention in previous fMRI studies of patients with ADHD.36,37
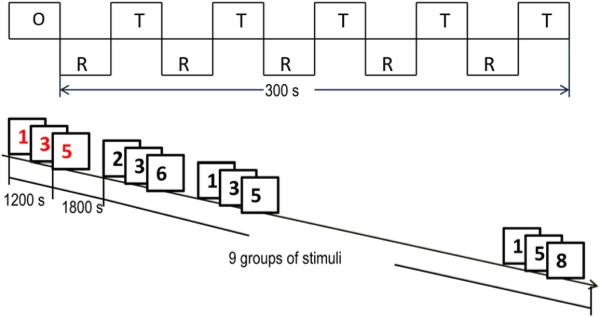
To achieve maximal measurement power in relatively brief periods, in the present study we designed a three-digit stimulus set and used a block design CPT task. Multi-digit stimuli evoke more attentional demands than single-digit stimuli. Block designs have superior measurement characteristics in a given time period relative to event-related designs (at a cost of experimental flexibility). Briefly, the block design is shown in Figure 1. In each of the five task blocks, a white box appeared in the center of the computer screen. A target sequence of three digits (1-3-5, 2-4-6, 3-5-7, 4-6-8, and 5-7-9 respectively) were shown in red at the rate of one digit per 400 milliseconds. Then after 1.8 seconds’ recalling time, nine sequences of three digits, from 1 to 9, appeared in black in a pseudo-random order, at the rate of 1 digit every 400 ms. A 1.8-seconds reaction period ensued after each sequence. In this period, participants were instructed to quickly use the right hand to press the left button when the sequence matched the target sequence, and to press the right button otherwise. During the rest blocks, participants were instructed to keep their eyes open, and to remain as relaxed and motionless as possible. Each participant was asked to perform two runs of the task, and the duration of each run was 5 minutes. Before the MRI scans, participants were provided a set of task training blocks to ensure that they understood the requirements of the task.
FIGURE 1.
Block design structure and the sequence of one task block of the visual attention task. Note: O = 8 seconds’ initial fixation period; R = 30 seconds’ rest block; s = seconds; T = 30 seconds task block.
Individual Imaging Data Preprocessing
The task-based fMRI and T1-weighted structural MRI data pre-processing was carried out using tools from the FMRIB Software Library (FSL)/fMRI Expert Analysis Tool (FEAT) and ART2.38 For the fMRI data from each participant, slice timing was corrected, spatial intensity was normalized, head motion was corrected, and each image was smoothed with an 8-mm full-width-at-half-maximum (FWHM) Gaussian spatial filter. Nonbrain structures in both the fMRI and T1-weighted data were extracted using the Brain Extraction Tool. The T1-weighted image was normalized to ICBM152 template in the Montreal Neurological Institute (MNI) space.39 The resulting transformation parameters were applied to the fMRI data to ensure accurate spatial normalization. Voxel-based functional activation maps responding to the fMRI task were generated using the FEAT tool from FSL. The Z statistic image was thresholded using clusters determined by Z>2.3 and a cluster corrected significance threshold of p < .05.
Pulvinar Seed Region Processing
An average image was generated by averaging the 44 high-resolution T1-weighted structural images, which had been registered to the T1-weighted standard MNI template during pre-processing. In the high-resolution (voxel size = 1 × 1 × 1 mm3) Automated Anatomical Labeling (AAL) template image provided by the Wake Forest University (WFU)–PickAtlas software40; the brain voxels were labeled by names of the anatomical structures in the standard NMI space. We thus investigated the locations and the number of the voxels (the approximate size) of the pulvinar nuclei, to determine the locations and size of the ROIs. Two spherical regions from both hemispheres (Origins [±14, –26, 8] and radius R = 4 mm) were identified accordingly, and were verified from the average image. For the task-based functional image from each individual, the average time series inside each seed region was calculated by using Featquery (FSL tool).
Functional Connectivity Analyses
Functional connectivity between each seed region and the remainder of the brain was examined by calculating the temporal correlation coefficient between the average time series of the seed region and the time series of the voxels from the whole brain using the FILM tool in FSL, where the average time series of the seed region was applied as the basic shape of the real event and was compared to the time series for the whole brain by a linear correlation model. Correlation coefficients were normalized by Z (Gaussianized T/F) transformation. The Z statistic images were thresholded using clusters determined by Z>2.3 and a corrected cluster significance threshold of p < .05.
Statistical Analyses
The group comparisons of the demographic and task performance data were analyzed using a general linear model (GLM-SPSS 17.0.2). Analyses were false discovery rate corrected for multiple comparisons (p < .05).
Group comparisons of the images were analyzed using the GML by adding sex as a fixed-effect covariate, task performance accurate rate, and omission error rate as random-effect covariates. The Z statistic images were thresholded using clusters determined by Z>2.3 and a cluster corrected significance threshold of p < .05.
Pearson partial correlations were carried out to measure the degree of associations among the task performance measure, the activation magnitudes within the pulvinar nuclei (Z value within each ROI), and the diagnostic features (DSM-IV Inattentive and Impulsive/Hyperactive Indices), while controlling for the possible effect of sex in the subjects with ADHD.
RESULTS
Analyses of the demographic and task performance data showed no significant between-group differences (Table 1), except the omission error rate for performing the fMRI task (p = .04).
TABLE 1.
Demographic Characteristics and Functional Magnetic Resonance Imaging (fMRI) Task Performance Measures in Both Groups
| NC (N = 22) | ADHD (N = 22) | Statistic | p | |
|---|---|---|---|---|
| Age (y) | 12.1 ± 2.23 | 11.6 ± 2.86 | t = 2.35 | .07 |
| Male/female | 10/12 | 12/10 | χ2 = 3.50 | .71 |
| Education (y) | 6.2 ± 2.21 | 5.8 ± 2.81 | t = 2.25 | .07 |
| Mother's education | 16.1 ± 3.46 | 14.7 ± 4.02 | t = 2.09 | .08 |
| Father's education | 16.5 ± 3.55 | 14.8 ± 4.37 | t = 1.77 | .06 |
| IQ | 114.7 ± 14.92 | 106.6 ± 16.21 | t = 0.06 | .73 |
| Race/ethnicity | χ2 = 4.33 | .11 | ||
| Caucasian | 11 | 9 | ||
| Black | 3 | 3 | ||
| Hispanic | 6 | 8 | ||
| Asian | 1 | 1 | ||
| Mixed | 1 | 1 | ||
| fMRI Task performance measures (normalized) | ||||
| Accuracy rate | 0.89 ± 0.07 | 0.86 ± 0.05 | t = 2.45 | .09 |
| Omission error | 0.08 ± 0.05 | 0.12 ± 0.08 | t = 4.18 | .04 |
| Commission error | 0.03 ± 0.01 | 0.01 ± 0.02 | t = 3.22 | .35 |
| Reaction time | 88.05 ± 9.4 | 76.39 ± 16.3 | t = 2.78 | .15 |
Note: Accuracy rate = number of correct responses/number of requested responses; ADHD = patients with attention-deficit/hyperactivity disorder; commission error = number of wrong responses/number of requested responses; NC = normal controls; omission error = number of missed responses/number of requested responses; y = years.
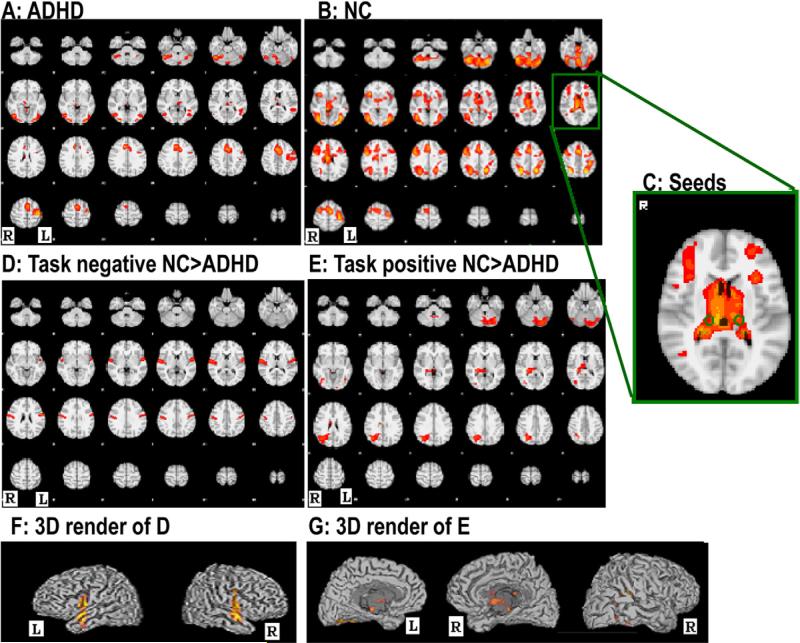
Group comparisons of the task-responsive activation maps are shown in Figure 2. As in Figure 2D, subjects with ADHD showed significantly reduced task-negative (the time series of those voxels were significantly negatively correlated with the design of the task) BOLD activations in bilateral superior temporal gyri (Heschl's gyri) (left side: Coordinates of Maximum [–44, –28, 18] Z = 3.61; right side: [54, –4, 4] Z = 3.94). From Figure 2E, children with ADHD also showed significantly reduced task-positive (the time series of those voxels were significantly positively correlated with the design of the task) BOLD activations in right thalamus ([12, –8, 24] Z = 3.53), right hippocampus ([26, –34, 12] Z = 4.66), right precuneus ([24, –59, 24] Z = 3.48), right angular gyrus ([30, –62, 44] Z = 4.59), left inferior occipital gyrus ([–44, –68, –12] Z = 3.92), and left cerebellum ([–38, –72, –28] Z = 4.17).
FIGURE 2.
Within-group averages and between-group differences of whole brain functional activations. Note: Images A and B show the group averages of the task-responsive brain activations in controls and patients, respectively; C shows the locations of the bilateral pulvinar seed regions; D and E showed the between-group differences of the task-negative and task-positive brain activations, respectively. ADHD = attention-deficit/hyperactivity disorder; L = left hemisphere; NC = normal controls; R = right hemisphere.
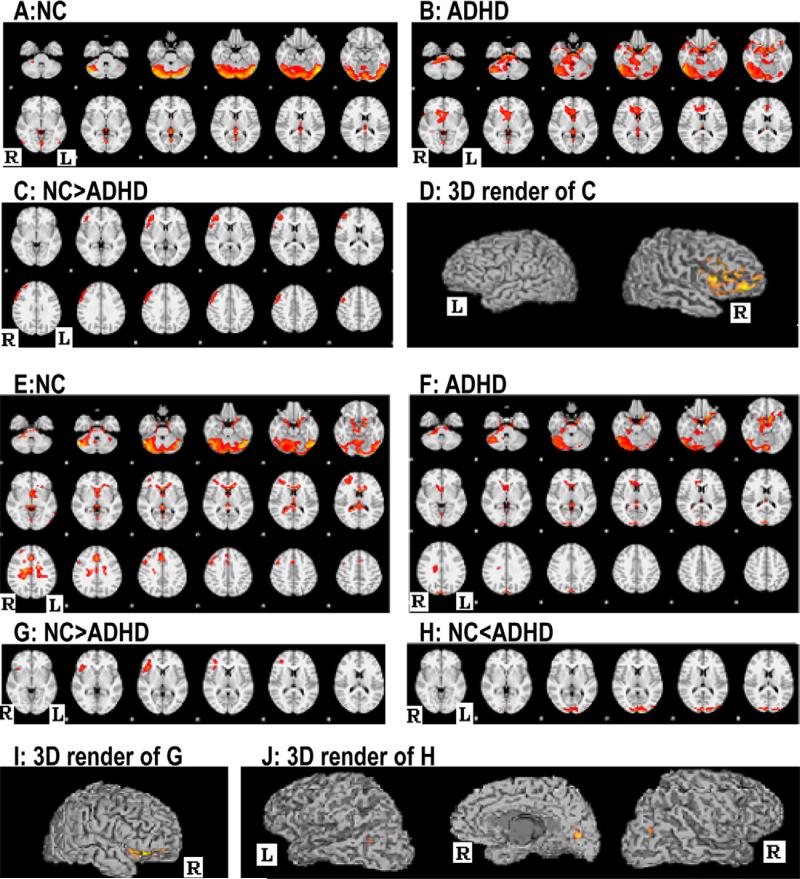
By calculating the functional connectivity between both pulvinar seed ROIs (Figure 2) and the remainder of the brain, we found that functional connectivities between the pulvinar nuclei of both hemispheres and right prefrontal lobe were significantly decreased relative to those in controls, whereas functional connectivities between the right pulvinar nuclei and bilateral occipital lobes were significantly increased in subjects with ADHD compared with controls (Figure 3 and Table 2).
FIGURE 3.
Images A–D show the within-group averages and between-group differences of functional connectivity between the left pulvinar nuclei and the remainder of whole brain, whereas images E–J show those of the right pulvinar nuclei and the reminder of the whole brain in controls and patients. Note: ADHD = Attention-Deficit/Hyperactivity Disorder; L = Left hemisphere; NC = Controls; R = Right hemisphere.
TABLE 2.
Regions That Had Significant Functional Connectivity with the Pulvinar Nuclei, and Had Significant Between-Group Differences During the Functional Magnetic Resonance Imaging (fMRI) Tasks
| Coordinate |
||||||
|---|---|---|---|---|---|---|
| Z | X | Y | Z | BA | Anatomical Location | |
| Left pulvinar nuclei | ||||||
| NC | 5.96 | ±38 | –35 | –30 | Bilateral cerebellum | |
| 3.11 | ±25 | 15 | –25 | 38 | Bilateral inferior frontal gyrus | |
| 4.23 | ±52 | –55 | –25 | 37 | Bilateral temporal lobe | |
| 3.37 | ±47 | –76 | –16 | 19 | Bilateral occipital lobe | |
| 3.32 | ±12 | –35 | 7 | 0 | Bilateral hippocampus | |
| 3.11 | 0 | –55 | 16 | 19 | Left precuneus | |
| ADHD | 6.29 | ±50 | –54 | –22 | Bilateral cerebellum | |
| 5.65 | 48 | –50 | –22 | 20 | Right inferior temporal lobe | |
| 4.45 | ±4 | –40 | 10 | 29 | Bilateral posterior cingulum | |
| 3.09 | ±3 | –50 | 15 | 30 | Bilateral precuneus | |
| NC>ADHD | 3.91 | 39 | 44 | –14 | 45/47 | Right inferior frontal gyrus |
| 3.79 | 47 | 19 | 45 | 9 | Right middle frontal lobe | |
| Right pulvinar nuclei | ||||||
| NC | 5.49 | ±52 | 59 | –34 | Bilateral cerebellum | |
| 3.81 | 30 | 46 | 26 | 46 | Right middle frontal lobe | |
| 3.81 | 38 | 32 | 10 | 45 | Right inferior frontal gyrus | |
| 3.61 | 52 | 12 | 36 | 34 | Left inferior orbital frontal gyrus | |
| 3.31 | 15 | –34 | 7 | 27 | Right hippocampus | |
| ADHD | 5.36 | –22 | 18 | –22 | 11 | Left orbital frontal gyrus |
| 4.55 | 12 | –6 | –12 | 34 | Right hippocampus | |
| 4.49 | ±2 | –94 | 20 | 19/19 | Bilateral cuneus | |
| 3.1 | 14 | –30 | 24 | Right precuneus | ||
| 3.77 | –52 | –66 | –22 | 29 | Right cerebellum | |
| NC>ADHD | 3.97 | 40 | 22 | 4 | 47 | Right prefrontal lobe |
| NC<ADHD | 3.96 | ±4 | –94 | 14 | 19/19 | Bilateral occipital lobe |
Note: ADHD = patients with attention-deficit/hyperactivity disorder; BA = Brodmann area; NC = normal controls.
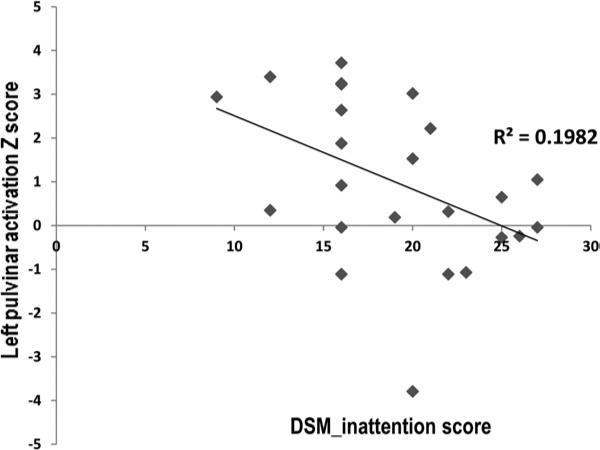
In the patient group, a significant negative correlation was shown between the activation magnitude of the left pulvinar nuclei and the DSM-IV inattentive index (r =–0.87; p = 0.009). This result is shown in Figure 4. The fMRI performance measures did not show significant correlations with the DSM-IV diagnostic indices or with the pulvinar activation measures.
FIGURE 4.
Significant negative correlation between left pulvinar activation and inattentive index in the patient group.
DISCUSSION
During performance of a sustained visual attention task, children with ADHD showed significantly decreased functional activations in a large area of the thalamus in the right hemisphere, significantly decreased functional connectivity between the right pulvinar and right prefrontal cortex, and significantly increased functional connectivity between the right pulvinar and bilateral occipital regions. Reduced activation in an ROI within the left pulvinar was also observed in the patient group, although between-group differences did not reach predetermined significance levels. Nonetheless, children with ADHD showed significantly decreased functional connectivity between the left pulvinar and the right prefrontal lobe. This study also found significant negative correlation between activation magnitude of the left pulvinar and the DSM-IV inattentive index in the 22 children with ADHD.
The decreased functional activations of bilateral pulvinar, their under-communication with the right prefrontal cortex, and the over-communication between right pulvinar and bilateral occipital lobes suggests that functional abnormalities within the occipital-pulvinar-prefrontal circuit may play an important role in the inattentiveness that is seen in the children with ADHD. These findings are in agreement with existing fMRI studies that have reported reduced thalamic activations during various tasks in ADHD.4,7 Furthermore, decreased BOLD activation and blood flow perfusion in the prefrontal lobe and increased blood flow perfusion in bilateral occipital lobes during various visual tasks has been consistently reported in patients with ADHD.9,10 Structural imaging studies have consistently shown decreased frontal volume,41,42 reduced prefrontal cortical convolution complexity,43 dramatic delay of cortical maturation in terms of cortical topography and thickness in ADHD,44 and severe structural atrophy in bilateral pulvinar in youth with ADHD.26,27 Delayed or inadequate development of these crucial structures may well contribute to significant disturbances across the functional circuits for visual attention processing, and may play an important role in the pathophysiology of inattentiveness in ADHD.
Consistent with the results of a recent study in ADHD,45 we found significantly reduced brain activations in the right hippocampus and precuneus during sustained visual attention. However, findings of structural abnormalities in these regions are not always consistent in ADHD. As reviewed by Shaw and Rabin,46 many MRI studies have reported no significant structural differences in the precuneus of ADHD children. A recent study reported enlarged subregions of bilateral hippocampi in subjects with ADHD compared with controls.47 Shaw and Rabin suggested that ADHD in childhood may be characterized by a delay in subcortical maturation, and that different clinical outcomes may be associated with different developmental trajectories in adolescence and beyond.46
The current study also demonstrated significant activation reductions in cerebellum in children with ADHD. An increasing number of studies have now reported functional and structural deficits in the cerebellum.48-50 Together with these existing findings, the current study suggests that cerebellum plays an important role in attention processing, and that functional and structural anomalies in cerebellum may contribute to the cognitive and behavioral impairments seen in ADHD.
Some of the earliest functional neuroimaging studies noted that performing a focused visual attention task caused a decrease in blood flow in the auditory cortex and its associated areas, even though no explicit auditory stimulation was presented.51 Clearly, in the magnet environment, the ongoing noise of the scanner is a potential source of distracting input, and it is perhaps not surprising that a relative suppression of processing within auditory cortex has often been observed, although similar blood flow suppressions have been reported in visual cortices during attention-demanding somatosensory52 and auditory tasks.53 An interesting study, during which participants performed a sustained auditory attention (vigilance) task, also showed initial suppression of activity in the visual cortex, but this suppression declined as a function of the time participants spent engaged in the task.54 The fall-off in suppression was not, however, accompanied by a decrement in ongoing performance, and the authors interpreted this pattern as reflecting a withdrawal of resources as the auditory task became more automated. Electrophysiological data also suggest that auditory cortex activity is actively suppressed during performance of a visual demanding task.55
In the current study, the finding of significant functional connectivity between the pulvinar and bilateral auditory cortices in controls also suggests that an important aspect of focusing visual attention involves attentional modulation of ongoing auditory processing. In addition, it was evident from the voxel-based whole-brain activation maps (Figure 2D) that subjects with ADHD showed significantly reduced deactivation in bilateral temporal lobes. To verify this primary result, an ROI-based supplementary analysis was carried out (Supplement 1 and Figure S1, available online). It revealed that the hemodynamic signals from bilateral Heschl's gyri, during the visual attention task, showed significantly less negative correlations in the ADHD cohort compared with controls. Thus, these data point to an apparent attenuation of constitutively activated cross-sensory attentional suppression mechanisms in ADHD. Although there is a tendency to focus on the mechanisms by which attention is focused on “to-be-attended” inputs, clearly the mechanisms by which “to-be-ignored” stimulation streams are selected against deserve equal emphasis, and the current data point to potentially important deficits in these suppressive mechanisms that will require further study. Altered attentional suppression mechanisms may play an important role in the distractibility that is often seen in this phenotype.
There are some issues of this study that need to be considered. First, this study included both male and female subjects. Clinical studies have reported differences in the symptoms and profiles of comorbidities between male and female individuals with ADHD. It is unclear yet whether the neuropathological mechanisms of ADHD have gender differences. This study matched the number of male and female participants within and between groups, and included sex as a fixed-effect covariate for between-group analyses. We further tested the data by comparing the imaging results between the 12 male and 10 female patients with ADHD, and did not find significant differences. However, considering the limited sample size in each subgroup, the statistical power of this test was limited. Future studies can focus on investigations in the trajectories of the neurocognitive function, symptom profiles, neural development, and sex effect in ADHD, by recruiting a very large study sample and using longitudinal follow-ups. Second, several subjects with ADHD had been taking short-acting medications, whereas the others were medication naive. We requested a minimum of a 48-hour wash-out period to eliminate the medication effects on the brain activations. Whole-brain activations did not differ significantly between the medicated and medication-naive patients. Third, the patient group showed significantly higher omission error rate and a trend of lower accuracy rate for the fMRI task performance. These items had been controlled during group comparisons to remove potential confounders. For any task-based fMRI data, response errors contribute to the signal noise. Altered brain activations and connectivity could be the result of high-response errors. We thus requested >80% response accurate rate as an inclusion criterion. In this study, temporal correlations (functional connectivities) between the pulvinar nuclei and the remainder of the whole brain were investigated during the visual sustained attention task. In Supplement 2 and Figure S2 (available online), we also carried out a Psychophysiological Interactions (PPI) analysis to investigate the group differences of the effective connectivities between the pulvinar nuclei and other brain regions. In addition, head motion can significantly affect the BOLD signals during fMRI acquisition. Framewise head motion analyses and comparisons between the head motion measures and the BOLD signals are detailed in Supplement 3 and Figure S3, available online).
Supplementary Material
Clinical Guidance.
Inappropriate development of the pulvinar nuclei in subjects with ADHD may contribute significantly to disruption of cortical–pulvinar–cortical circuits for attention processing, and further contributes to the pathophysiological mechanisms of inattention of this disorder.
Future studies may assess the impact of treatment strategies on the connectivity and efficiency of the pulvinar–cortical circuits for attention processing, which will help in the formulation of more effective intervention strategies.
Acknowledgments
This work was partially supported by the Rose F. Kennedy Intellectual and Developmental Disabilities Research Center (RFK-IDDRC) through a program grant (HD071593) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). We would also like to acknowledge the contributions of the staff at the Human Clinical Phenotyping Core (HCP) of the RFK-IDDRC during the recruitment and clinical classification of a portion of the participants.
Footnotes
Disclosure: Drs. Li, Sroubek, Kelly, Lesser, Sussman, He, Branch, and Foxe report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Xiaobo Li, Gruss Magnetic Resonance Research Center, Albert Einstein College of Medicine of Yeshiva University.
Ariane Sroubek, Ferkauf Graduate School of Psychology of Yeshiva University.
Mary S. Kelly, Albert Einstein College of Medicine of Yeshiva University.
Iris Lesser, Albert Einstein College of Medicine of Yeshiva University.
Elyse Sussman, Albert Einstein College of Medicine of Yeshiva University.
Yong He, State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University.
Craig Branch, Gruss Magnetic Resonance Research Center, Albert Einstein College of Medicine of Yeshiva University.
John J. Foxe, Nathan S. Kline Institute for Psychiatric Research.
REFERENCES
- 1.Shafritz KM, Marchione KE, Gore JC, Shaywitz BA, Shaywitz SE. The effects of methylphenidate on neural systems of attention in attention-deficit/hyperactivity disorder. Am J Psychiatry. 2004;161:1990–1997. doi: 10.1176/appi.ajp.161.11.1990. [DOI] [PubMed] [Google Scholar]
- 2.Martin Fernandez-Mayoralas D, Fernandez-Jaen A, Garcia-Segura JM, Quinones-Tapia D. [Neuroimaging in attention deficit hyperactivity disorder]. Rev Neurol. 2010;50(Suppl 3):S125–S133. [PubMed] [Google Scholar]
- 3.Bush G. Attention-deficit/hyperactivity disorder and attention networks. Neuropsychopharmacology. 2010;35:278–300. doi: 10.1038/npp.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cubillo A, Halari R, Ecker C, Giampietro V, Taylor E, Rubia K. Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood attention-deficit hyperactivity disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. J Psychiatr Res. 2010;44:629–39. doi: 10.1016/j.jpsychires.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Depue BE, Burgess GC, Willcutt EG, Ruzic L, Banich MT. Inhibitory control of memory retrieval and motor processing associated with the right lateral prefrontal cortex: evidence from deficits in individuals with ADHD. Neuropsychologia. 2010;48:3909–3917. doi: 10.1016/j.neuropsychologia.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valera EM, Spencer RM, Zeffiro TA, et al. Neural substrates of impaired sensorimotor timing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;68:359–367. doi: 10.1016/j.biopsych.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamm L, Menon V, Reiss AL. Parietal attentional system aberrations during target detection in adolescents with attention deficit hyperactivity disorder: event-related fMRI evidence. Am J Psychiatry. 2006;163:1033–1043. doi: 10.1176/ajp.2006.163.6.1033. [DOI] [PubMed] [Google Scholar]
- 8.Durston S, Davidson MC, Mulder MJ, et al. Neural and behavioral correlates of expectancy violations in attention-deficit hyperactivity disorder. J Child Psychol Psychiatry. 2007;48:881–889. doi: 10.1111/j.1469-7610.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim BN, Lee JS, Shin MS, Cho SC, Lee DS. Regional cerebral perfusion abnormalities in attention deficit/hyperactivity disorder. Statistical parametric mapping analysis. Eur Arch Psychiatry Clin Neurosci. 2002;252:219–225. doi: 10.1007/s00406-002-0384-3. [DOI] [PubMed] [Google Scholar]
- 10.Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 11.McFarland NR, Haber SN. Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. J Neurosci. 2002;22:8117–8132. doi: 10.1523/JNEUROSCI.22-18-08117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dibbets P, Evers ATE, Hurks PMP, Bakker K. Differential brain activation patterns in adult attention-deficit hyperactivity disorder (ADHD) associated with task switching. Neuropsychology. 2011;24:413–423. doi: 10.1037/a0018997. [DOI] [PubMed] [Google Scholar]
- 13.Beauregard M, Levesque J. Functional magnetic resonance imaging investigation of the effects of neurofeedback training on the neural bases of selective attention and response inhibition in children with attention-deficit/hyperactivity disorder. Appl Psychophysiol Biofeedback. 2006;31:3–20. doi: 10.1007/s10484-006-9001-y. [DOI] [PubMed] [Google Scholar]
- 14.Qiu M, Ye z, Li Q, Liu G, Xie B, Wang J. Changes of brain structure and function in ADHD children. Brain Topogr. 2011;24:243–252. doi: 10.1007/s10548-010-0168-4. [DOI] [PubMed] [Google Scholar]
- 15.Cao X, Cao Q, Long X, et al. Abnormal resting-state functional connectivity patterns of the putamen in medication-naïve children with attention deficit hyperactivity disorder. Brain Res. 2009;1303:195–206. doi: 10.1016/j.brainres.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 16.Robinson DL, McClurkin JW, Kertzman C, Petersen SE. Visual responses of pulvinar and collicular neurons during eye movements of awake, trained macaques. J Neurophysiol. 1991;66:485–96. doi: 10.1152/jn.1991.66.2.485. [DOI] [PubMed] [Google Scholar]
- 17.Kastner S, De Weerd P, Desimone R, Ungerleider LG. Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science. 1998;282:108–111. doi: 10.1126/science.282.5386.108. [DOI] [PubMed] [Google Scholar]
- 18.Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 19.Annoni JM, Khateb A, Gramigna S, et al. Chronic cognitive impairment following laterothalamic infarcts: a study of 9 cases. Arch Neurol. 2003;60:1439–43. doi: 10.1001/archneur.60.10.1439. [DOI] [PubMed] [Google Scholar]
- 20.Ungerleider LG, Christensen CA. Pulvinar lesions in monkeys produce abnormal scanning of a complex visual array. Neuropsychologia. 1979;17:493–501. doi: 10.1016/0028-3932(79)90056-3. [DOI] [PubMed] [Google Scholar]
- 21.Petersen SE, Robinson DL, Morris JD. Contributions of the pulvinar to visual spatial attention. Neuropsychologia. 1987;25:97–105. doi: 10.1016/0028-3932(87)90046-7. [DOI] [PubMed] [Google Scholar]
- 22.Van Essen DC. Corticocortical and thalamocortical information flow in the primate visual system. Prog Brain Res. 2005;149:173–85. doi: 10.1016/S0079-6123(05)49013-5. [DOI] [PubMed] [Google Scholar]
- 23.Baleydier C, Mauguiere F. Anatomical evidence for medial pulvinar connections with the posterior cingulate cortex, the retrosplenial area, and the posterior parahippocampal gyrus in monkeys. J Comp Neurol. 1985;232:219–28. doi: 10.1002/cne.902320207. [DOI] [PubMed] [Google Scholar]
- 24.Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- 25.Culham JC, Brandt SA, Cavanagh P, Kanwisher NG, Dale AM, Tootell RB. Cortical fMRI activation produced by attentive tracking of moving targets. J Neurophysiol. 1998;80:2657–2670. doi: 10.1152/jn.1998.80.5.2657. [DOI] [PubMed] [Google Scholar]
- 26.Ivanov I, Bansal R, Hao X, et al. Morphological abnormalities of the thalamus in youths with attention deficit hyperactivity disorder. Am J Psychiatry. 2010;167:397–408. doi: 10.1176/appi.ajp.2009.09030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia S, Li X, Kimball A, Kelly M, Lesser I, Branch C. Thalamic shape and connectivity abnormalities in children with attention deficit/hyperactivity disorder. Psychiatry Research: Neuroimaging. doi: 10.1016/j.pscychresns.2012.04.011. http://dx.doi.org/10.1016/j.pscychresns.2012.04.011. [DOI] [PMC free article] [PubMed]
- 28.Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 29.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- 30.Conners CK. Conners’ Rating Scales—Revised User's Manual. Multi-Health Systems; North Tonawanda, NY: 1997. [Google Scholar]
- 31.Kaufman J, Birmaher B, Brent D, et al. The Schedule for Affective Disorders and Schizophrenia for School Aged Children: Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Wechsler D. Wechsler Individual Achievement Test, 2nd ed. (WIAT-II) Psychological Corporation; New York: 2001. [Google Scholar]
- 33.Barkley RA, DuPaul GJ, McMurray MB. Comprehensive evaluation of attention deficit disorder with and without hyperactivity as defined by research criteria. J Consult Clin Psychol. 1990;58:775–789. doi: 10.1037//0022-006x.58.6.775. [DOI] [PubMed] [Google Scholar]
- 34.Halperin JM, O'Brien JD, Newcorn JH, et al. Validation of hyper-active, aggressive, and mixed hyperactive/aggressive childhood disorders: a research note. J Child Psychol Psychiatry. 1990;31:455–460. doi: 10.1111/j.1469-7610.1990.tb01582.x. [DOI] [PubMed] [Google Scholar]
- 35.Tana MG, Montin E, Cerutti S, Bianchi AM. Exploring cortical attentional system by using fMRI during a Continuous Perfomance Test. Comput Intell Neurosci. 2010:329213. doi: 10.1155/2010/329213. http://dx.doi.org/10.1155/2010/329213. [DOI] [PMC free article] [PubMed]
- 36.Rubia K, Halari R, Cubillo A, Mohammad AM, Brammer M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naive children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57:640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Schneider MF, Krick CM, Retz W, et al. Impairment of frontostriatal and parietal cerebral networks correlates with attention deficit hyperactivity disorder (ADHD) psychopathology in adults—a functional magnetic resonance imaging (fMRI) study. Psychiatry Res. 2010;183:75–84. doi: 10.1016/j.pscychresns.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Ardekani BA, Guckemus S, Bachman A, Hoptman MJ, Wojtaszek M, Nierenberg J. Quantitative comparison of algorithms for inter-subject registration of 3D volumetric brain MRI scans. J Neurosci Methods. 2005;142:67–76. doi: 10.1016/j.jneumeth.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 40.Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 41.Seidman LJ, Biederman J, Monuteaux MC, Valera E, Doyle AE, Faraone SV. Impact of gender and age on executive functioning: do girls and boys with and without attention deficit hyperactivity disorder differ neuropsychologically in preteen and teenage years? Dev Neuropsychol. 2005;27:79–105. doi: 10.1207/s15326942dn2701_4. [DOI] [PubMed] [Google Scholar]
- 42.Mostofsky SH, Cooper KL, Kates WR, Denckla MB, Kaufmann WE. Smaller prefrontal and premotor volumes in boys with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2002;52:785–794. doi: 10.1016/s0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- 43.Li X, Jiang J, Zhu W, et al. Asymmetry of prefrontal cortical convolution complexity in males with attention-deficit/hyperactivity disorder using fractal information dimension. Brain Dev. 2007;29:649–655. doi: 10.1016/j.braindev.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Shaw P, Eckstrand K, Sharp W, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheridan MA, Hinshaw S, D'Esposito M. Efficiency of the prefrontal cortex during working memory in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:1357–1366. doi: 10.1097/chi.0b013e31812eecf7. [DOI] [PubMed] [Google Scholar]
- 46.Shaw P, Rabin C. New insights into attention-deficit/hyperactivity disorder using structural neuroimaging. Curr Psychiatry Rep. 2009;11:393–8. doi: 10.1007/s11920-009-0059-0. [DOI] [PubMed] [Google Scholar]
- 47.Plessen KJ, Bansal R, Zhu H, et al. Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63:795–807. doi: 10.1001/archpsyc.63.7.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castellanos FX, Acosta MT. [The neuroanatomy of attention deficit/hyperactivity disorder]. Rev Neurol. 2004;38(Suppl 1):131–136. [PubMed] [Google Scholar]
- 49.Emond V, Joyal C, Poissant H. Structural and functional neuro-anatomy of attention-deficit hyperactivity disorder (ADHD) [abstract]. Encephale. 2009;35:107–114. doi: 10.1016/j.encep.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Kobel M, Bechtel N, Specht K, et al. Structural and functional imaging approaches in attention deficit/hyperactivity disorder: does the temporal lobe play a key role? Psychiatry Res. 2010;183:230–236. doi: 10.1016/j.pscychresns.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 51.Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL. The functional organization of human extrastriate cortex: a PET-rCBF study of selective attention to faces and locations. J Neurosci. 1994;14(11 Pt 1):6336–6353. doi: 10.1523/JNEUROSCI.14-11-06336.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawashima R, O'Sullivan BT, Roland PE. Positron-emission tomography studies of cross-modality inhibition in selective attentional tasks: closing the “mind's eye”. Proc Natl Acad Sci U S A. 1995;92:5969–5972. doi: 10.1073/pnas.92.13.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mozolic JL, Joyner D, Hugenschmidt CE, et al. Cross-modal deactivations during modality-specific selective attention. BMC Neurol. 2008;8:35. doi: 10.1186/1471-2377-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paus T, Zatorre R, Hofle N, et al. Time-related changes in neural systems underlying attention and arousal during the performance of an auditory vigilance task. J Cogn Neurosci. 1997;9:392–408. doi: 10.1162/jocn.1997.9.3.392. [DOI] [PubMed] [Google Scholar]
- 55.Gomez-Ramirez M, Kelly SP, Molholm S, Sehatpour P, Schwartz TH, Foxe JJ. Oscillatory sensory selection mechanisms during intersensory attention to rhythmic auditory and visual inputs: a human electrocorticographic investigation. J Neurosci. 2011;31:18556–67. doi: 10.1523/JNEUROSCI.2164-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.