Abstract
DHA (22:6n-3) supplementation during infancy has been associated with lower heart rate (HR) and improved neurobehavioral outcomes. We hypothesized that maternal DHA supplementation would improve fetal cardiac autonomic control and newborn neurobehavior. Pregnant women were randomized to 600 mg/day of DHA or placebo oil capsules at 14.4 (+/−4) weeks gestation. Fetal HRand HRV were calculated from magnetocardiograms (MCGs) at 24, 32 and 36 weeks gestational age (GA). Newborn neurobehavior was assessed using the Neonatal Behavioral Assessment Scale (NBAS). Postpartum maternal and infant red blood cell (RBC) DHA was significantly higher in the supplemented group as were metrics of fetal HRV and newborn neurobehavior in the autonomic and motor clusters. Higher HRV is associated with more responsive and flexible autonomic nervous system (ANS). Coupled with findings of improved autonomic and motor behavior, these data suggest that maternal DHA supplementation during pregnancy may impart an adaptive advantage to the fetus.
Keywords: Docosahexaenoic acid, Autonomic nervous system, Fetal, Magnetocardiology, Heart rate variability
1. Introduction
The rate, variability, and pattern of fetal cardiac responses are commonly used for the clinical evaluation of fetal well-being. Heart rate variability (HRV) is a measure of cardiac pacing that provides an indirect means for measuring the integrity of the developing autonomic nervous system (ANS). Metrics of HRV are used for the investigation of fetal neurobehaviors and for prediction to developmental outcomes in early childhood [1].
Studies in adults have demonstrated consistently that dietary intake of fish and/or long chain polyunsaturated fatty acid (LCPUFA) supplements reduce heart rate (HR) and improve measures of HRV, suggesting that LCPUFAs have an effect on cardiac autonomic function [2–4]. Term infants fed on milk or soy-based formulas with DHA had lower HR and higher HRV than infants fed on similar formulas without DHA [5]. We recently found that term infants fed on formulas with DHA as 0.32, 0.64, or 0.96% total fatty acids (with 0.64% arachidonic acid; ARA, 20:4n-6) had lower HR at 4, 6 and 9 months of age than infants in the control group fed on formula without DHA and ARA [6]. When these infants were administered a visual habituation protocol that yields both behavioral and psychophysical indices of attention, infants who received the DHA at 0.32 and 0.64% spent more time engaged in active stimulus processing (active phase of attention) than infants fed on unsupplemented formula.
We also observed that fetal HR was lower and HRV higher in women who reported prenatal intake of DHA alone or in combination with EPA (20:5n-3) in a pilot study [7]. This observation led to the conduct of the current clinical trial. These preliminary results and those from the previously cited studies suggest that pre- and postnatal supplementation of DHA (alone or in combination with EPA or ARA), may have an effect on fetal and infant cardiac autonomic function similar to what has been observed in adults. We tested the hypothesis that supplementing 600 mg/day of DHA during the 2nd and 3rd trimesters of pregnancy would result in lower fetal HR and higher HRV. At present, there is no USDA Dietary Reference Intake for DHA for any group; however, one expert group has recommended an average daily DHA intake of 200 mg per day during pregnancy and lactation [8].
Given the study's focus on the autonomic outcomes of DHA prenatal supplementation, we included the Neonatal Behavioral Assessment Scale (NBAS) as an outcome measure. The NBAS yields measures of state, arousal, physiological reactivity and fundamental forms of attention [9], and has been shown to be a modest predictor of developmental status across the short term [10,11]. More importantly, the scale has shown on numerous occasions to be a sensitive outcome variable for aspects of infant risk and status [12]. In addition, two studies found the NBAS to be sensitive to both prenatal [13] and postnatal [14] nutritional status. Here we present the primary outcome measures of the randomized clinical trial: fetal HR, HRV and the NBAS cluster scores.
2. Subjects and methods
2.1. Study design
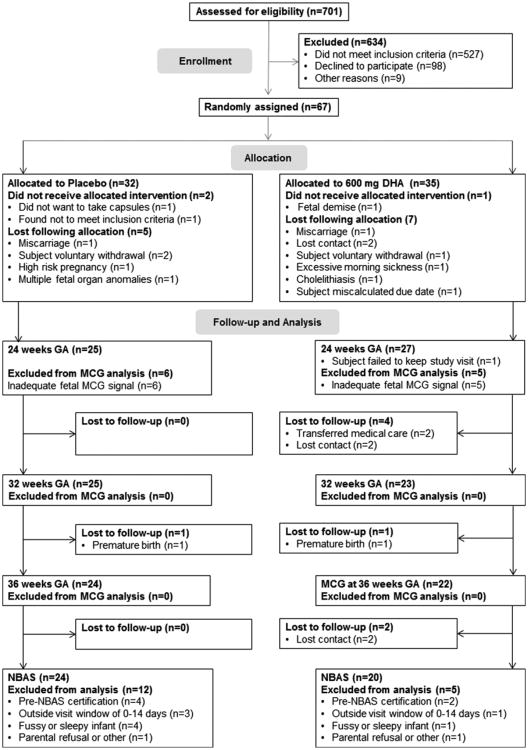
The clinical trial was a longitudinal, randomized, double-blind, placebo-controlled study conducted at the University of Kansas Medical Center in Kansas City, Kansas, USA, between May 2009 and July 2011. Study coordinators recruited subjects from the Obstetrics and Gynecology Clinic at the University of Kansas Medical Center by e-mail advertisement within the University, or by word of mouth. Women were included in the study if they were between 16–35.9 years of age and carrying a singleton pregnancy between the 12th and 20th week of gestation. Exclusion criteria were any serious health condition likely to affect the growth and development of the fetus or health of the mother including cancer, lupus, hepatitis, diabetes mellitus (Type1, Type 2 or gestational) or HIV/AIDS at baseline or fetal cardiac structural or conduction defects. Women who self-reported illicit drug use or alcohol use during pregnancy and those with hypertension or BMI ≥40 were excluded. Women who were taking more than 200 mg/day DHA in prenatal vitamins or over the counter supplements were excluded from participation. After screening, 67 eligible subjects were randomly assigned to the capsule allocation (Fig. 1). All subjects gave written, informed consent prior to enrolling in the study. The study was approved by the Human Subjects Committee at the University of Kansas in accordance with the Helsinki Declaration of 1975 as revised in 1983 and was overseen by a Data Safety and Monitoring Board.
Fig. 1.
Flow diagram.
2.2. Intervention
The study biostatistician provided a randomization schedule to the investigational pharmacy that distributed the capsules. Only members of the investigational pharmacy knew the subject allocation. Participants and all members of the investigational team were blinded to the intervention assignment. Participants were allocated to either group based on the simple randomization procedure using random numbers generated by SAS version 9.1 (Cary, NC). Subjects were assigned to capsules (Martek Biosciences, now DSM Nutritional Products) and instructed to take 3 capsules a day. Each capsule contained 500 mg of oil from either: (a) algal oil as a source of DHA (200 mg of DHA per capsule for a total of 600 mg DHA/day) (n=35) or (b) three placebo capsules containing 50% soy and 50% corn oil (n=32). All capsules were the same color, size, weight and the oils were orange-flavored to prevent investigator or subject bias. One bottle containing 100 capsules was given to subjects at enrollment and then mailed every 30 days thereafter until the subject delivered her baby. Subjects were instructed to mail any remaining capsules back to the center in a self-addressed and stamped envelope provided with the capsule mailing. The sealed envelopes were returned to the Investigational Pharmacy who counted and recorded the number of remaining capsules and destroyed them. The subjects were instructed to stop taking study capsules when they delivered their baby.
2.3. Study outcomes
The primary outcome measures were fetal HR and metrics of time- and frequency-domain HRV at 24, 32 and 36 weeks gestational age (GA), and the NBAS was administered at 1–14 days postpartum. Secondary outcomes were maternal plasma and red blood cell (RBC) phospholipids at baseline and post-partum and newborn cord blood plasma and RBC phospholipids at delivery.
2.4. Fatty acid analyses
Maternal blood was collected by venipuncture at enrollment and on the morning following parturition. Cord blood was collected at delivery. All blood samples were placed on ice immediately after collection into 5 mL EDTA tubes (BD Vacutainer, Franklin Lakes, NJ). Plasma and red blood cells (RBC) were separated by centrifugation (3000g, 10 min; 4 °C), frozen, and stored under nitrogen at −80 °C until analysis. Lipids were isolated according to a modification of Folch et al. [15], and RBC phospholipids were isolated [16] by thin-layer chromatography, transmethylated with boron trifluoride-methanol [17], and the resulting fatty acid methyl esters (FAMEs) were separated and quantified using a Varian 3900 gas chromato-graph with an SP-2560 capillary column (100 m, Sigma Aldrich) and a Star 6.41 Chromatography Workstation for peak integration and analysis as previously reported [18]. Injector and detector temperatures were programmed at 260 °C. The temperature program for the 41-min column run was: 140 °C, 5 min; 4 °C increase/minute to 240 °C; 240 °C, 11 min. Individual peaks were identified by comparison with qualitative standards (PUFA no. 1 Marine Source 100 mg; PUFA no. 2 Animal Source 100 mg; Sigma Aldrich). A weighed standard fatty acid mixture (Supelco 37 component FAME mix, Sigma Aldrich) was employed to correct final DHA weight percent of total fatty acids (wt% TFA).
2.5. Magnetocardiogram
2.5.1. Data acquisition
Testing was conducted between 10:00 and 17:00 h. Women were instructed to eat a meal or snack 2 h prior to their visit. As much as possible, women were tested the same time of day at each visit. An ultrasound recording was obtained immediately prior to the MCG recording in order to document the position of the fetal body, obtain measures of fetal head circumference, abdominal circumference and femur length and to calculate estimated fetal weight. Participants were seated comfortably in the exam chair, slightly reclined and in contact with the surface of the biomagnetometer interface without applying pressure to the abdomen. Signals were recorded using an investigational 83 channel dedicated fetal biomagnetometer (CTF Systems, Inc.), housed in a magnetically shielded room. The axial gradiometer sensors are spatially distributed to cover the gravid maternal abdomen. Data collected during pregnancy were acquired in 2 blocks of a continuous 18 min recording (for a total of 36 min) using a 300 Hz sampling rate and recording filter of 0–75 Hz. Data were digitally filtered between 1 and 40 Hz offline (bidirectional fourth-order Butterworth filter). The raw data were divided into 6 consecutive 6 min sections for independent component analysis (ICA).
2.5.2. Data processing
The data in each sensor (channel) are a mixture of biomagnetic signals originating from various electrophysiological sources. The multivariate data were presented to an Infomax ICA algorithm implemented in EEGLAB toolbox (version 4.311) [19] in order to segregate the contributions from spatially distinct electrophysiolo-gical sources into individual components. Each MCG component was identified (maternal, fetal) and fiducial R-peaks were automatically detected using a template-matching algorithm described in detail in May et. al [20] in order to generate an inter-beat-interval (IBI) time series.
Fetal activity state has a significant influence on fetal HR and HRV; therefore all records undergo state classification by visual inspection of the fetal HR pattern [21] by two experienced study personnel. States 1F (calm, non-REM) and 3F (calm wakefulness) associated with absent, sporadic or short-lasting HR accelerations were classified as quiet states. States 2F (active REM) and 4F (active wakefulness) with frequent, long-lasting accelerations that return to the baseline were classified as active states [22]. If state determination differed between personnel, a third opinion was sought and consensus was reached. MCG data recorded during quiet fetal states were not considered for analysis.
2.5.3. Analysis of fetal HR and HRV
We used a template-matching algorithm developed by our team [23] in EEGLab 4.311 [19] to automatically detect R-peaks for time- and frequency-domain analysis. False positive and false negative detections and abnormal beats were manually corrected to create an interbeat interval (IBI) series from the MCG data. Metrics of HR and HRV were calculated from the IBI values in milliseconds (ms). The IBI series was then converted to an IBI time-series by linear interpolation at 10 Hz, resulting in a time-series that is appropriate for frequency analyses. We used the following metrics in our analysis:
Metrics of rate (mean IBI, mean HR) that are influenced by both parasympathetic and sympathetic activity. For time-domain HRV analysis we used metrics summarizing total HRV influenced by both parasympathetic and sympathetic activity. These include: (a) the standard deviation of normal R–R intervals (SDNN), a metric of overall HRV and (b) the root mean square of successive differences between consecutive IBIs (RMSSD), a metric of short-term HRV. For frequency-domain analysis we calculated the power integral of defined spectral frequency bands: (a) very low frequency (VLF) [0.02–0.08 Hz], (b) low frequency (LF) [0.08– 0.2 Hz], (c) high frequency (HF) [0.4–1.7 Hz] and the ratios of the frequency bands thought to reflect sympatho-vagal balance (VLF/LF, VLF/HF and LF/HF). It is generally accepted that both sympathetic and parasympathetic arms contribute to power in the VLF and LF bands while power in the HF band is largely from the parasympathetic branch [24]. Therefore, HF is considered a putative parasympathetic metric.
3. NBAS
The NBAS [9,25] was originally developed to demonstrate the behavioral abilities and capacities of the neonate to parents and health care practitioners; however, researchers adopted the scale to quantify individual differences in newborn behavior in studies of neonatal risk and behavior [26,27]. As a result, the psychometric properties of the scale [28–30] and various methods of data reduction [31,32] were established.
Suitable for use with newborns up to 2 months of age, the NBAS consists of 26 items scored on a 9-point scale plus the elicitation of 18 reflexes scored on a 4-point scale [9]. The dimensionality of the scales varies, optimality may be indicated by high, low, or midpoint scores. This issue is typically addressed by recoding scores for data reduction (see Table 1). The items have been reduced to seven item “clusters,” based on Lester et al. [32]. For neonatal behavior, the scale is generally assessed during the first 72 h after delivery, although some studies feature an assessment at 2-weeks, and some reports have used the scale out to 1 or 2 months of age. The NBAS must be administered by a tester trained to reliability. DJS was certified at The Brazelton Institute, Boston, MA and conducted all assessments. The scale was given when infants were one week of age (M=7.78 days, SD=3.99); groups did not vary at age of testing, t35=0.29, P=ns.
Table 1.
NBAS cluster scoring criteria (adapted from Lester et al., 1982).
| Cluster | Items | Cluster interpretation |
|---|---|---|
| Habituation1 | Light, rattle, bell, pinprick | Rapidity of behavioral shutdown to redundant input. More rapid shutdown thought to reflect a more efficient CNS |
| Orientation1 | Inanimate visual, inanimate auditory, animate visual, animate auditory, visual/auditory, alertness | Quality of responses and attention to salient environmental events (objects, faces, sounds). Higher scores reflect higher responsivity. |
| Motor2 | Tonus, maturity, pull-to-sit, defensive reaction, activity | Maturity of fundamental motor abilities. Higher scores reflect higher maturity. |
| Range of state3 | Peak of excitement, rapidity of buildup, irritability, lability of state | Extent and lability of arousal or reactivity to environmental input. Higher scores thought to reflect higher reactivity, and is regarded as more optimal |
| Regulation of state1 | Cuddliness, consolability, self-quieting, hand-to-mouth | Degree to which newborn can self-modulate or regulate state; includes ability and use of behavioral strategies to self-console. Higher scores reflect better self-regulation. |
| Autonomic stability4 | Tremors, startles, skin color | Measures physiologic indices of reactance to environmental stimuli, fragility of physiological response. Higher scores reflect more robust autonomic responses. |
| Reflexes | Abnormal score defined as 0, 1, or 3 for all reflexes except clonus, nystagmus, or TNR where 0, 1, and 2 are normal and 3 is abnormal. | Higher frequency of abnormal reflexes is regarded as nonoptimal. |
Total number of abnormal reflex scores.
Cluster score is total of all raw item scores.
Total of item scores, with tonus and activity recoded so high scores reflect optimal performance.
Total of item scores, with all items recoded so high scores reflect optimum performance.
Total of item scores. Tremors and skin color recoded so high scores reflect optimal performance; startles is inverted.
3.1. Statistical analyses
Power analyses established that 24 subjects per group would give 96% power to detect a group difference of 1 SD in fetal HR and 81% power to detect a difference of 0.75 SD in time-domain metrics of fetal HRV (α=0.05, β=0.20) according to 1-tailed paired t-test based on preliminary data [7]. The primary analysis was intention-to-treat and involved all subjects who were randomly assigned. MCG and fatty acid data were analyzed using SAS®, by SAS Institute, Inc., version 9.2. Statistical analysis of NBAS data were completed using PASW/IBM Statistics, SPSS version 20.0.
Variables with approximately normal distributions were summarized as means±SD. Outcome variables with skewed distributions were summarized by median and inter-quartile range and log-transformed to make their distributions approximately normal for comparisons. Attrition rates were assessed between groups and over GA periods by the generalized linear mixed models (GLMM) using the adaptive Gaussian-Quadrature method for estimation. The log-transformed maternal plasma and RBC DHA were modeled by random-intercept mixed-effects ANOVA models using factors of group, time and group-by-time interaction. Satterthwaite's method was used to calculate the degrees of freedom. Least-square (LS) means were computed for post hoc comparisons when the interaction was significant. Cord blood DHA was log-transformed and compared between groups by the 2-sample t-test. Similarly, repeated measures of HR and HRV at 24, 32 and 36 weeks GA from scans recorded during active fetal states were also analyzed by the random-intercept mixed-effects ANOVA models including factors of group and time and their interactions when they existed. The means of the NBAS cluster scores were compared across the two groups using t-tests. Maternal capsule intake was compared between groups by the Mann–Whitney U test.
For all measures, the level of significance was set at 0.05. A one-tailed P-value was used to evaluate cardiac metrics of rate (HR, IBI) and time-domain metrics of HRV (RMSSD, SDNN) because our preliminary data showed that maternal LCPUFA supplement intake during the last two trimesters resulted in significantly lower fetal HR and higher HRV in time-domain metrics and the power calculation was based on the 1-tailed test. Since DHA supplementation was not expected to produce negative NBAS findings, one-tailed t-tests were also used to evaluate the effect of the intervention.
4. Results
4.1. Study population
Of the 67 women allocated to study capsules, 46 remained active in the study to full term delivery and gave birth to healthy singletons (22 female, 24 male), (Fig. 1). The study population characteristics at enrollment (baseline) and delivery are presented in Table 2. The GLMM indicated significant attrition rates changes over time (F1,132 = 3.42, P=0.036) but not between groups (F1,132=0.63, P=0.43). At enrollment, the randomized groups did not differ in maternal age, BMI, GA, plasma or RBC DHA. The study population was 37.3% African American, 46.3% Caucasian, 13.4% Hispanic and 3% Asian. Sixteen percent of the women enrolled smoked during pregnancy.
Table 2.
Maternal characteristics at enrollment; maternal and newborn characteristics at delivery. Mean ± SD or median (Q1 – Q3).
| Placebo | DHA | P-value | |||
|---|---|---|---|---|---|
|
|
|
||||
| Enrollment (n=32) | Delivery (n=24) | Enrollment (n=35) | Delivery (n=22) | ||
| Maternal characteristics | |||||
| Age (years) | 25.6±4.8 | 25.5±4.3 | |||
| Education (years) | 13.9±2.7 | 14.0±3.1 | |||
| Enrollment BMI (kg/m2) | 29.0±7.0 | 26.8±5.5 | |||
| GA (weeks) | 15.2±3.7 | 39.9±1.1 | 13.6±4.1 | 39.4±1.1 | |
| Plasma DHA (wt% TFA)1 | 3.91 (3.15–4.21) | 3.79 (3.28–4.26) | 3.94 (3.39–4.72) | 5.81 (3.87–6.95) | |
| RBC DHA(wt% TFA) 1 | 4.30 (3.99–5.03) | 4.99 (4.42–5.69) | 4.50 (3.73–5.44) | 7.09 (5.39–10.3) | |
| Capsule intake (per week) | 18.9±5.2 | 17.4±3.6 | 0.08 | ||
| Infant characteristics | |||||
| Birth weight (grams) | 3435.5±404.8 | 3416.8±552.9 | |||
| Birth length (cm) | 49.97±3.0 | 49.61±2.1 | |||
| Cord blood plasma DHA (wt% TFA) | 5.26 (4.58–6.19) | 5.64 (4.52–7.17) | 0.31 | ||
| Cord blood RBC DHA (wt% TFA) | 6.18 (5.81–6.87) | 7.75 (6.01–9.03) | 0.015 | ||
Log-transformed maternal plasma DHA, within group [Placebo; (F1, 58.7=0.00 P=0.99), DHA; (F1,63.5 =13.9 P=0.0004)] and between groups (F1, 61.1=7.11 P=0.010). Log-transformed maternal RBC DHA, within group [Placebo; (F1, 58.4 =−3.88, P=0.054), DHA; (F1, 62.9= −48.9, P<0.0001)] and between groups (F1, 60.7 = 13.0, P = 0.0006).
Post-hoc comparisons of enrollment-delivery changes within group and between groups.
4.2. Compliance analyses
Of the active study subjects at delivery, all but 2 (1 per group) complied with returning their completed capsule bottle every 30 days. There was no group difference in maternal compliance with recommended capsule intake (z=1.73, P=0.08). Women allocated to the DHA group consumed 17.4 (±3.6) capsules per week compared to 18.9 (±5.2) capsules per week in the placebo group, therefore, women in the supplemented group consumed on average, 497 mg (±103 mg) DHA per day from the supplement.
4.3. Fatty acid analysis
Maternal blood samples were collected from all women at enrollment and post-partum. Summary results and statistics are given in Table 2. Results of the random-intercept mixed-effects ANOVA show a significant main effect for group and time and a group-by-time interaction for maternal plasma and RBC DHA (Table 3). Post-hoc comparisons indicate a significant increase between enrollment and post-partum maternal log-transformed RBC DHA within the DHA group, (F1, 62.9= −48.9, P< 0.0001) and between DHA and Placebo groups (F1, 607 = 13.0, P=0.0006) but not within the Placebo group (F1, 58.4= −3.88, P=0.054). A similar pattern was observed in the log-transformed maternal plasma DHA, which increased between enrollment and post-partum within the DHA group (F1,635 = 13.9 P=0.0004) and between groups (F1, 61.1 = 7.11 P=0.010) but not within the Placebo group (F1, 58.7=0.00 P=0.99).
Table 3.
Maternal DHA and fetal cardiac metrics of rate and variability.
| Interaction-effects model | Main-effects model | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Group | Time | Group × time | Group | Time | |
| Maternal blood sample1 | |||||
| Log plasma DHA | 0.0007 | 0.011 | 0.010 | ||
| Log RBC DHA | 0.003 | <.0001 | 0.0006 | ||
| Fetal cardiac metrics2 | |||||
| HR3 | 0.08 | 0.003 | 0.34 | 0.095 | 0.003 |
| IBI3 | 0.075 | 0.001 | 0.29 | 0.074 | 0.001 |
| Log SDNN3 | 0.0195 | 0.023 | 0.19 | 0.017 | 0.022 |
| Log RMSSD3 | 0.008 | 0.004 | 0.27 | 0.007 | 0.004 |
| Log VLF | 0.015 | 0.021 | 0.41 | 0.013 | 0.021 |
| Log LF | 0.016 | <0.0001 | 0.61 | 0.014 | <.0001 |
| Log HF | 0.070 | 0.021 | 0.36 | 0.063 | 0.019 |
| Log (VLF/LF) | 0.56 | <0.0001 | 0.88 | 0.55 | <.0001 |
| Log (VLF/HF) | 0.31 | <0.0001 | 0.98 | 0.30 | <.0001 |
| Log (LF/HF) | 0.15 | <0.0001 | 0.96 | 0.15 | <.0001 |
Maternal measures: 2 time points (enrollment and post-partum).
Fetal measures: 3 time points (24, 32, 36 weeks GA).
One-tailed P-value, all others two-tailed.
Forty-one newborn cord blood samples were collected (DHA n=20, Placebo n=21, Table 1). Five samples were lost due to failure to collect (n=2), blood sample frozen (n=1), subject delivered at home (n = 1) and mislabeling (n=1). Newborn RBC DHA was significantly higher in the supplemented group (t30.8=2.57, P=0.015) whereas plasma DHA was not significantly different between groups (t39=1.03, 0=0.31)
4.4. Metrics of fetal HR and HRV
Summary statistics for fetal HR, IBI and metrics of fetal HRV for the 3 MCG time points are shown in Table 4. At 24 weeks GA, we were able to record fetal MCG in 76% of the subjects randomized to Placebo and 81% of the subjects randomized to DHA. One subject in the DHA group failed to keep the 24 week MCG appointment. Loss of fetal MCG signal at this GA is most often the result of the fetus being too far from the recording sensors (maternal overweight), fetal orientation, fetal movements or a combination thereof. After 24 weeks GA, we recorded fetal MCGs in 100% of the study participants. We were also successful in capturing the fetal MCG during active fetal states in 100% of subjects at 32 and 36 weeks GA. Therefore, no fetal MCG data were lost to state differences at 32 and 36 weeks.
Table 4.
Fetal cardiac metrics of rate and variability. Mean 7 SD; median (Q1 – Q3).
| Placebo | DHA | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| 24 weeks (n=19) | 32 weeks (n=25) | 36 weeks (n=24) | 24 weeks (n=21) | 32 weeks (n=23) | 36 weeks (n=22) | |
| Metrics of rate | ||||||
| HR (bpm) | 146.0±6.5 | 142.5±7.7 | 145.0±8.6 | 145.2±6.8 | 140.8±7.6 | 141.7±9.3 |
| IBI (ms) | 410.8±17.8 | 421.3±722.6 | 414.6±23.6 | 413.3±19.4 | 426.9±22.7 | 425.5±27.1 |
| Time-domain metrics | ||||||
| SDNN (ms) | 16.0 (14.0–23.0) | 17.8 (16.3–21.8) | 20.5 (16.0–31.5) | 18.0 (16.0–20.5) | 24.0 (19.5–29.5) | 26.5 (22.0–30.0) |
| RMSSD (ms) | 7.0 (4.5–11.0) | 5.0 (4.0–6.0) | 4.8 (4.0–8.0) | 7.5 (5.0–10.5) | 6.5 (5.5–9.0) | 6.5 (5.5–8.0) |
| Frequency-domain metrics | ||||||
| VLF (ms2) | 64.6 (30.0–162.9) | 37.9 (28.8–62.7 | 40.4 (19.6–93.8) | 69.4 (40.7–123.5) | 76.4 (47.0–116.4) | 89.0 (50.1–111.9) |
| LF (ms2) | 38.2 (16.2–123.3) | 15.6 (7.0–22.9) | 14.3 (4.4–38.6) | 49.4 (19.4–91.4) | 30.7 (17.3–63.1) | 30.3 (16.6–42.7) |
| HF (ms2) | 8.2 (4.9–11.2) | 6.2 (4.3–10.0) | 7.0 (4.5–15.1) | 8.3 (6.13–10.1) | 10.4 (7.1–18.3 | 10.7 (7.4–15.4) |
| Ratios attributed to sympatho-vagal balance | ||||||
| VLF/LF | 1.7 (1.3–2.5) | 2.9 (2.1–3.7) | 3.0 (2.0–4.7) | 1.8 (1.2–2.7) | 2.4 (1.9–3.6) | 2.7 (2.3–3.7) |
| VLF/HF | 9.8 (6.4–16.1) | 6.8 (4.7–7.9) | 6.8 (4.7–7.9) | 9.0 (6.9–21.1) | 8.2 (4.2–11.5) | 6.5 (3.7–9.7) |
| LF/HF | 5.8 (2.9–11.6) | 2.3 (1.3–3.4) | 1.8 (1.2–3.2) | 5.9 (4.2–15.9) | 3.3 (1.6–5.3) | 2.6 (1.3–3.6) |
Note: MCGs missing at 24 weeks were due to inadequate fetal MCG signal (DHA, n=6, Placebo, n=5). One subject in the DHA group did not keep the 24 week appointment. Assessments of rate are HR=mean heart rate, IBI=mean interbeat interval. Time domain measures of total heart rate variability in msec are SDNN=standard deviation of normal R–R intervals and RMSSD=root mean square of differences between IBIs. The frequency domain measures of band specific power (integrals in msec2) include very low frequency (VLF) [0.02–0.08 Hz], low frequency (LF) [0.08–0.2 Hz], and high frequency (HF) [0.4–1.7 Hz] and their ratios.
There was a significant effect of advancing GA on all metrics of fetal HR and HRV (Table 3); this was expected, given that fetal HR decreases and HRV increases with advancing GA. Trends towards lower fetal HR (P=0.095) and longer IBIs (P=0.074) were observed in the DHA supplemented group (effect sizes estimated from the differences in LS means were 0.40 and 0.44, respectively). For the time-domain metrics representing overall HRV (SDNN) and short-term HRV (RMSSD), maternal DHA supplementation resulted in significantly higher values on both metrics of fetal HRV (SDNN; P=0.017, RMSSD; P=0.007). Similarly, the frequency-domain metrics that reflect a combination of sympathetic and parasympathetic input (VLF, LF power) are significantly higher in the supplemented group (VLF; P=0.013, LF; P=0.014). The putative parasympathetic metric, HF power, was higher in the group randomized to DHA intervention, though falling just short of conventional levels of significance (P=0.06). DHA supplementation had no effect on cardiac autonomic balance as reflected by group comparisons of the frequency band ratios. There were no significant group-by-time interactions seen for any metric of fetal HR or HRV.
5. NBAS
Forty-four of the active study participants returned for newborn NBAS testing. Six infants were born prior to tester certification and those data were not included in the analysis. Other reasons for excluding the data are detailed in Fig. 1. The NBAS was successfully completed and scored in 27 infants (12 Placebo, 15 DHA). The analysis of the NBAS clusters as a function of randomized group is shown in Table 5. Infants from women randomized to the DHA intervention during pregnancy showed significantly higher (i.e., more optimal) scores on the Motor (t25=1.87, P=0.038) and Autonomic clusters (t25=1.99, P=0.029), and differences approached significance on the Orienting cluster (t25=0.55, P=0.092).
Table 5.
Means±SD for NBAS clusters.
| Cluster | Placebo (n=12) | DHA (n=15) | t(25) | P |
|---|---|---|---|---|
| Habituation | 9.92±9.28 | 8.47±9.26 | 0.404 | ns |
| Orienting | 19.75±15.45 | 23.40±18.32 | 0.550 | .092 |
| Motor | 23.08±11.40± | 26.07±18.13 | 1.866 | .038 |
| State organization | 13.50±13.89 | 15.13±8.02 | 1.370 | ns |
| State regulation | 16.42±20.02 | 16.93±20.06 | 0.245 | ns |
| Autonomic | 14.83±16.90 | 18.13±14.48 | 1.992 | .029 |
| Reflexes | 21.92±14.45 | 22.60±14.33 | 0.550 | ns |
6. Discussion and conclusions
This study represents the first longitudinal assessment of fetal cardiac autonomic control, as indexed by HR and HRV, in women randomized to 600 mg/day DHA. The results largely supported our hypothesis that DHA supplementation during pregnancy would increase fetal HRV. We find that the effect extends to both branches of the developing ANS as evidenced by greater overall HRV (SDNN) and greater power in frequency bands that contain both sympathetic and parasympathetic input (VLF, LF). Furthermore, the frequency band ratios, thought to represent autonomic balance, were not different between groups. Thus, the effect of DHA on fetal cardiac autonomic control was global, rather than constrained or specific to one arm of the ANS.
Findings of improved HRV in the DHA-supplemented group were strengthened by positive findings from the NBAS, a perinatal outcome assessment. Two NBAS clusters (motor and autonomic) were significantly higher in infants whose mothers received DHA during pregnancy; higher scores on these clusters reflect improved function in specific neurobehavioral domains. In particular, the autonomic cluster is thought to reflect the quality of the newborn's physiological reactivity to the environment; more mature autonomic function is an indication of flexibility and integrity of the ANS and is ultimately thought to be related to cognitive functions. Our findings are consistent with the hypothesis that the effects of DHA on development may be mediated through the ANS [7].
The period where we observed the greatest group difference in metrics of fetal HRV occurred in the third trimester. This was expected since variability in fetal HR begins to emerge between 32–34 weeks GA largely due to increased vagal inhibition [33]. Another fundamental event in the development of brainstem mediated systems during the third trimester is the emergence of fetal sleep states associated with activity or quiescence [34]. The maturation of these two endogenously regulated, brain-stem mediated systems coincides with the period in which there is greatest DHA accrual into the developing fetal brain and nervous system [35]. Newborn sleep organization has been associated with maternal DHA status during pregnancy [36] and both sleep and HRV have been linked to improved developmental and cognitive outcomes [37,38].
It is important to consider metrics of HRV as more than a measure of heart function but rather, as a proxy for the degree of integration between central and peripheral nervous systems in the developing fetus. Porges et al. [39] propose that the maturation and myelination of the ANS during fetal development and the first year of life provides the neural foundation to adapt to a changing and often challenging environment and that the response needs to be flexible for the development of social engagement and cognitive development. As such, the ANS may be a target for developmental programming effects.
The central theme of the developmental origins hypothesis is that prenatal exposures such as under or over-nutrition, exposure to maternal smoking, alcohol or significant maternal stressors influence postnatal function and future health of the offspring [40]. The majority of human studies use GA or birth weight as a proxy for the effects of in utero exposure; however, these measures are limited with regard to understanding neural mechanisms behind group differences or observed associations. In contrast, fetal HRV has been shown to be significantly associated with later developmental outcomes including mental, psychomotor and language development in the 3rd year of life [41]. In addition to fetal HRV, synchronous periods of fetal HR and motor activity are thought to reflect the maturation and development of the central nervous system during gestation [42]. Our results of higher fetal HRV and positive autonomic and motor neurobehaviors from the newborn assessment are in keeping with these constructs. Exploratory analyses to determine what effect maternal DHA intake may have on other fetal motor activities (breathing movements, non-nutritive suck) and HR-movement coupling are currently underway.
We hypothesized that maternal DHA intake would result in significantly lower fetal HR and higher HF power based on our earlier findings [7]. The current randomized trial resulted only in trends towards significance for both metrics. This could be attributed to differences in supplement composition (lack of EPA in the current trial) or other differences in the two populations studied, e.g., other lifestyle differences between women in a convenience sample compared to a randomized sample. That said, the current findings from these measures are fairly ambiguous, as they trend strongly toward a positive effect yet do not attain conventional levels of statistical significance. Nonetheless, the current data do not definitively answer the research question, and so the observed trend is interesting and worthy of further study.
There were limitations to the study. We estimated attrition at 25% based our experience in previous clinical trials, however, attrition was higher than anticipated, reaching 35% at the neonatal assessment. Finally, NBAS data was excluded from the analysis in 39% of the neonates which could have limited our ability to detect group differences in other behavioral clusters. In spite of these limitations, the results support our research hypothesis and warrant continued investigation of the effects of DHA on fetal autonomic development and neurobehaviors.
In summary, we have shown that the fetal ANS and newborn neurobehavior are responsive to maternal DHA intake. As such, the fetal ANS is a likely target for nutritional programming effects. We propose that maternal DHA intake during the last two trimesters of pregnancy results in more responsive autonomic function in the offspring, giving the fetus and newborn an adaptive advantage.
Acknowledgments
The authors thank JoAnn Lierman, RN, PhD and Lori L. Blanck, R. EEG/EP T. of the Hoglund Brain Imaging Center, University of Kansas Medical Center for their expertise in recording the fetal ultrasound and data acquisition. We thank E. Anda Popescu, PhD for helpful discussions and designing the programs used for MCG analysis.
Footnotes
This study was supported by The Eunice Kennedy Shriver National Institute of Child Health and Development (R21 HD059019) (KMG) and in part received core support from by the Kansas Intellectual Development and Disabilities Research Center (P30 NICHD HD 002528). The investigational product was generously donated by DSM Nutritional Products. This trial was registered at clinicaltrials.gov, Identifier: NCT01007110.
Presented as a poster at the 10th Congress of the International Society for the Study of Fatty Acids and Lipids, Vancouver, Canada, 28 May 2012.
References
- 1.DiPietro JA, Bornstein MH, Hahn CS, Costigan K, Achy-Brou A. Fetal heart rate and variability: stability and prediction to developmental outcomes in early childhood. Child Dev. 2007;78:1788–1798. doi: 10.1111/j.1467-8624.2007.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christensen JH. n-3 fatty acids and the risk of sudden cardiac death. Emphasis on heart rate variability. Dan Med Bull. 2003;50:347–367. [PubMed] [Google Scholar]
- 3.Christensen JH, Schmidt EB. Autonomic nervous system, heart rate variability and n-3 fatty acids. J Cardiovasc Med (Hagerstown) 2007;8(1):S19–22. doi: 10.2459/01.JCM.0000289276.10675.a1. [DOI] [PubMed] [Google Scholar]
- 4.Holguin F, Tellez-Rojo MM, Lazo M, Mannino D, Schwartz J, Hernandez M, Romieu I. Cardiac autonomic changes associated with fish oil vs soy oil supplementation in the elderly. Chest. 2005;127:1102–1107. doi: 10.1378/chest.127.4.1102. [DOI] [PubMed] [Google Scholar]
- 5.Pivik RT, Dykman RA, Jing H, Gilchrist JM, Badger TM. Early infant diet and the omega 3 fatty acid DHA: effects on resting cardiovascular activity and behavioral development during the first half-year of life. Dev Neuropsychol. 2009;34:139–158. doi: 10.1080/87565640802646726. [DOI] [PubMed] [Google Scholar]
- 6.Colombo J, Carlson SE, Cheatham CL, Fitzgerald-Gustafson KM, Kepler A, Doty T. Long-chain polyunsaturated fatty acid supplementation in infancy reduces heart rate and positively affects distribution of attention. Pediatr Res. 2011;70:406–410. doi: 10.1203/PDR.0b013e31822a59f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gustafson KM, Colombo J, Carlson SE. Docosahexaenoic acid and cognitive function: is the link mediated by the autonomic nervous system? Prostaglandins Leukot Essent Fatty Acids. 2008;79:135–140. doi: 10.1016/j.plefa.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koletzko B, Lien E, Agostoni C, Bohles H, Campoy C, Cetin I, Decsi T, Dudenhausen JW, Dupont C, Forsyth S, Hoesli I, Holzgreve W, Lapillonne A, Putet G, Secher NJ, Symonds M, Szajewska H, Willatts P, Uauy R. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. J Perinat Med. 2008;36:5–14. doi: 10.1515/JPM.2008.001. [DOI] [PubMed] [Google Scholar]
- 9.Brazelton TB, Nugent JK. The neonatal behavioral assessment scale. 3rd. Mac Keith Press; Cambridge: 1995. [Google Scholar]
- 10.Moss M, Colombo J, Mitchell DW, Horowitz FD. Neonatal behavioral organization and visual processing at 3 months. Child Dev. 1988;59:1211–1220. doi: 10.1111/j.1467-8624.1988.tb01490.x. [DOI] [PubMed] [Google Scholar]
- 11.Vaughn BE, Taraldson B, Crichton L, Egeland B. Relationships between neonatal behavioral organization and infant behavior during the 1st year of life. Infant Behav Dev. 1980;3:47–66. [Google Scholar]
- 12.Colombo J, Mitchell DW, Coldren JT, Atwater JD. Discrimination learning during the first year: stimulus and positional cues. J Exp Psychol Learn Mem Cogn. 1990;16:98–109. doi: 10.1037//0278-7393.16.1.98. [DOI] [PubMed] [Google Scholar]
- 13.Lester BM. Synergistic process approach to the study of prenatal malnutrition. Int J Behav Dev. 1979;2:377–393. [Google Scholar]
- 14.DiPietro JA, Larson SK, Porges SW. Behavioral and heart rate pattern differences between breast-fed and bottle-fed neonates. Dev Psychol. 1987;23:467–474. [Google Scholar]
- 15.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 16.Zail SS, Pickering A. Fatty acid composition of erythrocytes in hereditary spherocytosis. Br J Haematol. 1979;42:399–402. doi: 10.1111/j.1365-2141.1979.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 17.Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride—methanol. J Lipid Res. 1964;5:600–608. [PubMed] [Google Scholar]
- 18.Smuts CM, Huang M, Mundy D, Plasse T, Major S, Carlson SE. A randomized trial of docosahexaenoic acid supplementation during the third trimester of pregnancy. Obstet Gynecol. 2003;101:469–479. doi: 10.1016/s0029-7844(02)02585-1. [DOI] [PubMed] [Google Scholar]
- 19.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Meth. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 20.May LE, Glaros A, Yeh HW, Clapp JF, 3rd, Gustafson KM. Aerobic exercise during pregnancy influences fetal cardiac autonomic control of heart rate and heart rate variability. Early Hum Dev. 2010;86:213–217. doi: 10.1016/j.earlhumdev.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Nijhuis IJ, ten Hof J. Development of fetal heart rate and behavior: indirect measures to assess the fetal nervous system. Eur J Obstet Gynecol Reprod Biol. 1999;87:1–2. doi: 10.1016/s0301-2115(99)00143-8. [DOI] [PubMed] [Google Scholar]
- 22.Ten Hof J, Nijhuis IJ, Mulder EJ, Nijhuis JG, Narayan H, Taylor DJ, Westers P, Visser GH. Longitudinal study of fetal body movements: nomograms, intrafetal consistency, and relationship with episodes of heart rate patterns a and B. Pediatr Res. 2002;52:568–575. doi: 10.1203/00006450-200210000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Popescu EA, Popescu M, Bennett TL, Lewine JD, Drake WB, Gustafson KM. Magnetographic assessment of fetal hiccups and their effect on fetal heart rhythm. Physiol Meas. 2007;28:665–676. doi: 10.1088/0967-3334/28/6/005. [DOI] [PubMed] [Google Scholar]
- 24.Acharya UR, Joseph KP, Kannathal N, Lim CM, Suri JS. Heart rate variability: a review. Med Biol Eng Comp. 2006;44:1031–1051. doi: 10.1007/s11517-006-0119-0. http://dx.doi.org/10.1007/s11517-006-0119-0. [DOI] [PubMed] [Google Scholar]
- 25.Brazelton TB. Spastics Int Med. Publications; London: 1973. Neonatal behavioral assessment scale. [Google Scholar]
- 26.Als H, Tronick E, Lester BM, Brazelton TB. The Brazelton neonatal behavioral assessment scale (BNBAS) J Abnorm Child Psychol. 1977;5:215–231. doi: 10.1007/BF00913693. [DOI] [PubMed] [Google Scholar]
- 27.Osofsky JD, Danzger B. Relationships between neonatal characteristics and mother–infant interaction. Dev Psychol. 1974;10:124–130. [Google Scholar]
- 28.Lancioni GE. Infant operant—conditioning and its implications for early intervention. Psychol Bull. 1980;88:516–534. [PubMed] [Google Scholar]
- 29.Lester BM, Emory EK, Hoffman SL, Eitzman DV. Multivariate study of effects of high-risk factors on performance on brazelton neonatal assessment scale. Child Dev. 1976;47:515–517. [PubMed] [Google Scholar]
- 30.Yang RK, Federman EJ, Douthitt TC. Characterization of neonatal behavior—dimensional analysis. Dev Psychol. 1976;12:204–210. [Google Scholar]
- 31.Jacobson JL, Jacobson SW, Fein GG, Schwartz PM. Factors and clusters for the Brazelton scale—an investigation of the dimensions of neonatal behavior. Dev Psychol. 1984;20:339–353. [Google Scholar]
- 32.Lester BM, Als H, Brazelton TB. Regional obstetric anesthesia and newborn behavior—a reanalysis toward synergistic effects. Child Dev. 1982;53:687–692. [PubMed] [Google Scholar]
- 33.Groome LJ, Loizou PC, Holland SB, Smith LA, Hoff C. High vagal tone is associated with more efficient regulation of homeostasis in low-risk human fetuses. Dev Psychobiol. 1999;35:25–34. doi: 10.1002/(sici)1098-2302(199907)35:1<25::aid-dev4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 34.Mirmiran M, Maas YG, Ariagno RL. Development of fetal and neonatal sleep and circadian rhythms. Sleep Med Rev. 2003;7:321–334. doi: 10.1053/smrv.2002.0243. [DOI] [PubMed] [Google Scholar]
- 35.Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Intrauterine fatty acid accretion rates in human brain: implications for fatty acid requirements. Early Hum Dev. 1980;4:121–129. doi: 10.1016/0378-3782(80)90015-8. [DOI] [PubMed] [Google Scholar]
- 36.Cheruku SR, Montgomery-Downs HE, Farkas SL, Thoman EB, Lammi-Keefe CJ. Higher maternal plasma docosahexaenoic acid during pregnancy is associated with more mature neonatal sleep-state patterning. Am J Clin Nutr. 2002;76:608–613. doi: 10.1093/ajcn/76.3.608. [DOI] [PubMed] [Google Scholar]
- 37.Feldman R. The development of regulatory functions from birth to 5 years: insights from premature infants. Child Dev. 2009;80:544–561. doi: 10.1111/j.1467-8624.2009.01278.x. [DOI] [PubMed] [Google Scholar]
- 38.Weisman O, Magori-Cohen R, Louzoun Y, Eidelman AI, Feldman R. Sleep– wake transitions in premature neonates predict early development. Pediatrics. 2011;128:706–714. doi: 10.1542/peds.2011-0047. [DOI] [PubMed] [Google Scholar]
- 39.Porges SW, Furman SA. The early development of the autonomic nervous system provides a neural platform for social behavior: a polyvagal perspective. Infant Child Dev. 2011;20:106–118. doi: 10.1002/icd.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langley-Evans SC. Nutritional programming of disease: unravelling the mechanism. J Anat. 2009;215:36–51. doi: 10.1111/j.1469-7580.2008.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DiPietro JA, Kivlighan KT, Costigan KA, Rubin SE, Shiffler DE, Henderson JL, Pillion JP. Prenatal antecedents of newborn neurological maturation. Child Dev. 2010;81:115–130. doi: 10.1111/j.1467-8624.2009.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DiPietro JA, Hodgson DM, Costigan KA, Hilton SC, Johnson TR. Development of fetal movement—fetal heart rate coupling from 20 weeks through term. Early Hum Dev. 1996;44:139–151. doi: 10.1016/0378-3782(95)01704-6. [DOI] [PubMed] [Google Scholar]