Abstract
Using brain surgery, specific areas in the brain can be stimulated with electrical impulses to reversibly change their activity and alleviate symptoms related to mental illnesses. This so-called deep brain stimulation and other methodological advances that even more selectively activate specific group of neurons can give us clues as to what neural circuitry is involved in a particular mental disorder and whether therapeutic activation of these brain areas and neurons may be effective. In ‘Bedside to Bench’, Eric Nestler discusses two trials of individuals with anorexia nervosa in which deep brain stimulation of different brain areas resulted in improvement of behavioral domains associated with the syndrome. The results and potential of this technique in animals and humans may bring us closer to understanding the neurobiology of anorexia nervosa, which still remains a mystery and poses a challenge for treatment. In ‘Bench to Bedside’, Jennifer Warner-Schmidt peruses recent findings that uncover the functional connectivity of brain regions involved in depression and how activation of cortical regions can result in antidepressant effects that can compensate for the malfunction of other brain circuits that results in depression.
Anorexia nervosa is a potentially fatal eating disorder characterized by self-starvation, where an individual’s weight is, by arbitrary definition, <85% of expected levels. The syndrome is approximately tenfold more common in females than in males, with a typical age of onset during adolescence. Today, roughly 0.3% of females in the United States develop anorexia nervosa.
Although its causes are virtually completely unknown, there is evidence of an important genetic component, despite there having been no disease-causing genes identified so far. Several environmental and psychological factors have also been implicated, but they have provided little insight into causative mechanisms. Treatments remain inadequate for most patients; only 30% make a complete recovery in their life-time with different types of behavioral therapies. Several classes of psychotropic medications are used, but none are clearly effective. Roughly 10% of all patients diagnosed with anorexia nervosa die from starvation-related complications or suicide—the highest mortality rate seen for any mental illness.
Two recent studies report the potential utility of deep brain stimulation (DBS) in treating severe cases of anorexia nervosa1,2. In DBS, electrodes are implanted—typically bilaterally—within a targeted region of the brain; patients have power supplies implanted in their chests, which drive continuous, high-frequency stimulation of the targeted brain area. In one study, four female patients with anorexia received DBS targeted to the nucleus accumbens, a region of ventral striatum that controls reward and motivation1. In the second study, six female patients received DBS targeted to Brodmann area 25, the subgenual region of the anterior cingulate cortex (part of the prefrontal cortex) thought to be important for mood and decision making2. After several months of continuous DBS, all four patients in the first study, and four of six in the second one, showed significant improvement in weight along with improvements in several other behavioral domains. Although the studies were exploratory phase 1 trials and were not adequately powered to determine efficacy, the provocative findings are cause for cautious optimism and provide a possible path toward an improved understanding of this syndrome, for which no truly effective treatment exists.
What causes someone to starve herself (or himself) to the point of death? Psychological explanations, such as having a distorted body image (thinking one is fat despite being cachectic) or being overly controlling or perfectionistic, seem like obvious descriptions of the core symptoms of the syndrome as opposed to mechanistic causes. Functional and structural brain imaging studies have implicated several regions of forebrain that are known to be crucial in reward, mood, motivation and decision making in anorexia nervosa3,4. These include several regions of the prefrontal cortex, nucleus accumbens and dorsal striatum, amygdala (important for learned associations for both rewarding and aversive stimuli) and insula (a cortical region suggested to serve as a neural substrate of disgust). Previous evidence has shown that patients with anorexia nervosa have abnormal brain responses—for example, in the prefrontal cortex and nucleus accumbens—to food stimuli that are rewarding in normal individuals, leading to the suggestion of ‘abnormal reward processing’ in anorexia nervosa. But these findings also seem derivative of the behavioral symptoms of the illness—where self-starvation is rewarding, in contrast to the extreme aversion felt by people without the disorder—and are therefore of limited utility.
A further limitation in understanding the neurobiological basis of anorexia nervosa is the lack of bona fide animal models5. Most models involve forced calorie restriction of rodents and the resulting hyperactivity; other models disrupt gonadal steroids, based on the female preponderance of the syndrome in humans. However, in the absence of known anorexia-causing genes of strong effect and high penetrance in humans, all current animal models are of limited etiological and face validity.
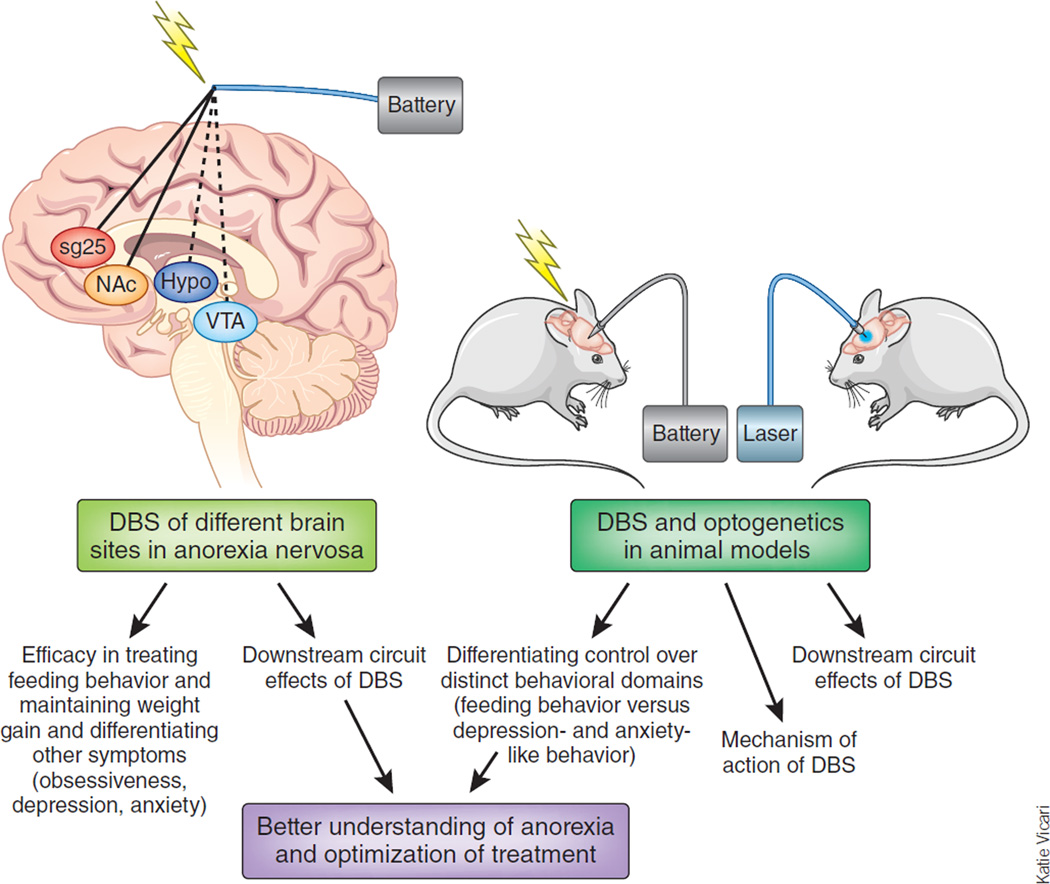
It is in this context that experimental trials of DBS in anorexia nervosa might break new ground. DBS enables the direct modification of the electrical activity within a given region of brain, and various brain imaging modalities allow for the elaboration of the circuit consequences downstream of such chronic DBS, approaches possible in both humans and animals (Figure 1). It is thus possible that use of DBS, targeting distinct regions of brain with different frequencies and time of stimulation, will provide fundamental insight into the brain circuits that underlie anorexia nervosa. Given that most individuals with anorexia nervosa show symptoms of other mental disorders, including obsessive-compulsive traits, anxiety and depression, such exploratory DBS studies could help parse the neural substrates of anorexia and its psychological concomitants from these other symptom domains.
Figure 1.
Potential DBS treatment of anorexia nervosa. The scheme illustrates two sites of DBS recently reported in humans1,2 (solid lines) and possible additional sites (dashed line) and the ways in which coordinated studies in animals might explain the mechanism of action of DBS both at the site of stimulation and affected downstream circuitry.
What, then, is the right DBS target site for anorexia nervosa? Most studies to date that examine new applications of DBS to neuropsychiatric disorders take the approach of using targets already established for other conditions. This makes sense, as the relative safety of these targets is known even after many years of continuous stimulation. Additionally, DBS of a site used for an established condition (for example, motor abnormalities of Parkinson’s disease) can provide information as to whether other symptoms that accompany the syndrome, such as depression seen in Parkinson’s disease, are also alleviated. Thus, DBS of the nucleus accumbens (and nearby regions involving the anterior limb of the internal capsule) has shown some early efficacy for the treatment of severe obsessive-compulsive disorder6, whereas DBS of Brodmann area 25 is a leading target for the experimental treatment of severe depression7,8. The ability of DBS of these regions to induce some improvement in anorexia nervosa1,2 raises the question of whether such improvements occur primarily through the alleviation of obsessive-compulsive or depressive symptoms rather than the direct alleviation of abnormal feeding behavior. DBS of the sub-thalamic nucleus, a mainstay in the treatment of Parkinson’s disease, has been shown to cause weight gain in a subset of patients8, raising the possibility that this region could be targeted for anorexia nervosa as well.
However, based on our increasing knowledge of normal feeding pathways in animals9, it would be interesting to carry out exploratory DBS studies of numerous other brain regions in individuals with anorexia nervosa, with an eye on treatment of selected domains of behavioral abnormalities. For example, it would be important to target more specific components of the reward circuitry, such as the ventral tegmental area and subregions of nucleus accumbens, given that DBS of different nucleus accumbens subregions has been shown to differentially modify food intake versus motivation for food in rats10. It would also be interesting to target distinct hypothalamic nuclei that are known to have very different roles in the control of feeding behavior9. As efficacy is established, it will be important to determine how long DBS must be continued to maintain a treatment response. As well, determining the long-term safety of DBS of various sites will be crucial, although few long-term side effects have been reported to date in other conditions.
Once DBS targets of robust and perhaps selective efficacy in anorexia nervosa are established, it would be possible to use this information to explore underlying mechanisms in animal models (see Figure 1). As one example, would the opposite type of manipulation of the homologous region in a rodent or nonhuman primate result in a syndrome of self-starvation, which has heretofore been very difficult to induce in animals? In parallel, it would be important to use animals to understand how DBS of a given region can be therapeutic in humans. Thus, it is not currently understood, for any clinical application of DBS, whether DBS works by affecting cell bodies or nerve terminals within, or axons that traverse, the area of stimulation8. Effects on several types of glial cells are also possible.
Moreover, DBS, as currently applied, involves very-high-frequency sg25, subgenual anterior cingulate cortex; NAc, nucleus accumbens; Hypo, hypothalamus; VTA, ventral tegmental area. Stimulation of a targeted brain region, which might just as readily inactivate these neural elements through depolarization blockade. Work in animals, where it is now possible to selectively stimulate particular types of cell bodies or nerve terminals in a given brain region—including even nerve terminals arising from one particular afferent region—through the use of optogenetic and related tools11,12, will make it possible to delineate the mechanism of action of DBS and its likely complex circuit-level consequences8. It might even be possible to one day apply these more specific tools back to the clinic. A recent study in mice showing opposite effects of optogenetic stimulation of either of two major subtypes of nucleus accumbens neurons on behavioral responses to cocaine indicates the importance of this specificity13.
These are clearly very early days in DBS treatments for anorexia nervosa. Nevertheless, for a syndrome that has defied any substantial neurobiological explanation, or robust biological treatment, despite decades of research, preliminary neurosurgical data in humans provide a potential lead toward eventually conquering this debilitating illness.
Footnotes
COMPETING FINANCIAL INTERESTS
The author declares no competing financial interests.
References
- 1.Lipsman N, et al. Lancet. 2013;381:1361–1370. doi: 10.1016/S0140-6736(12)62188-6. [DOI] [PubMed] [Google Scholar]
- 2.Wu H, et al. World Neurosurg. (in the press) [Google Scholar]
- 3.Kaye WH, Wierenga CE, Bailer UF, Simmons AN, Bischoff-Grethe A. Trends Neurosci. 2013;36:110–120. doi: 10.1016/j.tins.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keating C, Tilbrook AJ, Rossell SL, Enticott PG, Fitzgerald PB. Neuropsychologia. 2012;50:567–575. doi: 10.1016/j.neuropsychologia.2012.01.036. [DOI] [PubMed] [Google Scholar]
- 5.Avena NM, Bocarsly ME. Neuropharmacology. 2012;63:87–96. doi: 10.1016/j.neuropharm.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodman WK, Alterman RL. Annu. Rev. Med. 2012;63:511–524. doi: 10.1146/annurev-med-052209-100401. [DOI] [PubMed] [Google Scholar]
- 7.Mayberg HS. J. Clin. Invest. 2009;119:717–725. doi: 10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lozano AM, Lipsman N. Neuron. 2013;77:406–424. doi: 10.1016/j.neuron.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 10.van der Plasse G, Regina Schrama R, van Seters SP, Vanderschuren LJMJ, Westenberg HGM. PLoS ONE. 2012;7:e33455. doi: 10.1371/journal.pone.0033455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tye KM, Deisseroth K. Nat. Rev. Neurosci. 2012;13:251–266. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogan SC, Roth BL. Pharmacol. Rev. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lobo MK, et al. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]