Abstract
We developed Drosophila melanogaster as a model to study correlated behavioral, neuronal and genetic effects of the neurotoxin lead, known to affect cognitive and behavioral development in children. We showed that, as in vertebrates, lead affects both synaptic development and complex behaviors (courtship, fecundity, locomotor activity) in Drosophila. By assessing differential behavioral responses to developmental lead exposure among recombinant inbred Drosophila lines (RI), derived from parental lines Oregon R and Russian 2b, we have now identified a genotype by environment interaction (GEI) for a behavioral trait affected by lead. Drosophila Activity Monitors (TriKinetics, Waltham, MA), which measure activity by counting the number of times a single fly in a small glass tube walks through an infrared beam aimed at the middle of the tube, were used to measure activity of flies, reared from eggs to 4 days of adult age on either control or lead-contaminated medium, from each of 75 RI lines. We observed a significant statistical association between the effect of lead on average daytime activity across lines and one marker locus, 30AB, on chromosome 2; we define this as a Quantitative Trait Locus (QTL) associated with behavioral effects of developmental lead exposure. When 30AB was from Russian 2b, lead significantly increased locomotor activity, whereas, when 30AB was from Oregon R, lead decreased it. 30AB contains about 125 genes among which are likely “candidate genes” for the observed lead-dependent behavioral changes. Drosophila are thus a useful, underutilized model for studying behavioral, synaptic and genetic changes following chronic exposure to lead or other neurotoxins during development.
Keywords: developmental lead exposure, developmental plasticity, behavior, quantitative trait locus, locomotor activity, Drosophila, developmental neurotoxicology, neurotoxin, endocrine disruptor
Introduction
The phase-out of leaded paint and gasoline resulted in substantial reduction in mean blood lead levels in the United States, but lead exposure remains a significant public health problem (White et al. 2007). In areas where environmental lead contamination has been eliminated, individuals may still carry in their skeletons lead from prior exposure, and this lead can be mobilized during times of stress. Such times include pregnancy and lactation, during which the lead can be passed on to the fetus or infant (Gulson et al. 2003). Because lead alters mechanisms underlying developmental neuronal plasticity (Lasley et al. 2001; Toscano and Guilarte 2005; White et al. 2007; Ruden et al., in press), chronic exposure of children, even at blood lead levels below the current community action level (10 μg/dL), can result in reductions in cognitive ability (Needleman et al. 1990; Surkan et al. 2007; White et al. 2007), increased likelihood of delinquency (Canfield et al. 2003; Dietrich et al. 2001; Wright et al. 2008), development of behaviors associated with Attention Deficit Hyperactivity Disorder (Braun et al. 2006; Jones et al. 2008), changes in activity (Padich et al. 1985) and altered sensory function (Altmann et al. 1998). In addition, even at very low doses, lead is an endocrine disruptor, delaying the onset of sexual maturity in girls (Wolff et al. 2008) and in rats (Dearth et al. 2002; Dearth et al. 2004; Iavicoli et al. 2004, 2006). To complicate matters, such other environmental factors as socio-economic status and stress (Virgolini et al. 2008) can modulate the severity of the effects of exposure to lead (Bellinger, 2008a; Weiss and Bellinger 2006).
Behavioral effects of chronic lead exposure have been seen in both vertebrate and invertebrate animals; the pattern of lead-dependent behavioral effects is similar, despite variation among species in the relationship between blood levels and neuronal toxicity (Garavan et al. 2000). Chronic lead exposure impairs sensory function in chickens (Lurie et al. 2006), mice (Jones et al. 2008), rats (Fox et al. 1994,1982) and possibly monkeys (Lasky et al. 1995, 2001) as well as locomotor activity levels in monkeys (Lasky and Laughlin 2001), rats (Tang et al. 1994) and Drosophila (Hirsch et al. 2003). Furthermore, lead impairs learning and memory in tadpoles (Strickler-Shaw and Taylor 1991), herring gulls (Burger 1990; Burger and Gochfeld 2005), mice (Sun et al. 2005) and rats (Alber and Strupp 1996; Jett et al. 1997; Moreira et al. 2001; Morgan et al. 2000).
Long term potentiation in the hippocampus, a model system to study neuronal plasticity, is impaired by developmental lead exposure (e.g. Gilbert and Mack 1998; Gilbert et al. 1996, 1999a, b; Lasley et al. 1993). Among lead’s effects in the hippocampus are changes in muscarinic modulation (Cory-Slechta and Pokora 1995; Tang et al. 2008; Wang et al. 2007), N-methyl-D-aspartate receptor function (Gilbert and Lasley 2007; Guilarte 1997; White et al. 2007), voltage-gated sodium channels (Yan et al. 2008), learning-induced activation of calcium-dependent protein kinase C (Vazquez and Pena de Ortiz 2004; Xu et al. 2005) and neurogenesis in adults (Gilbert et al. 2005; Verina et al. 2007). Other delayed effects of developmental lead exposure include a reduced ability to recover from stroke (Schneider and Decamp 2007) and an increase in the likelihood of such degenerative diseases as Alzheimer’s (White et al. 2007; Wu et al. 2008; Zawia and Basha 2005) and Parkinsonism (Landrigan et al. 2005; Winkel et al. 1995).
Lead affects synaptic development and function (Cooper and Manalis 1983; Morley et al. 2003; Toscano and Guilarte 2005; White et al. 2007). Chronic lead exposure decreases the density of dendritic spines and synapses in rats (Kiraly and Jones 1982; Petit and Alfano 1979; Petit and LeBoutillier 1979) and the number of synaptic contacts formed by retinotectal projections in frogs (Cline et al. 1996) but increases dendritic spine density (presumably synaptic sites) on cortical pyramidal cells (Patrick and Anderson 1995) and cerebellar neurons (Patrick and Anderson 2000) in cats.
Calcium plays an important role at the presynaptic terminal, where it regulates transmitter release (Zucker 1996) and synaptic plasticity (Zucker and Regehr 2002), entering through voltage-dependent channels resulting in a brief, localized, and large increase in [Ca2+]inside which in turn triggers the release of neurotransmitter (e.g. Atlas 2001; Neher and Sakaba 2008). Lead exposure interferes with normal calcium signaling in neurons (Audesirk and Audesirk 1989; Zhang et al. 2002), affecting both the calcium-dependent enzyme neuronal nitric oxide synthase (Chetty et al. 2001; Reddy et al. 2002) and the plasma membrane calcium ATPase (PMCA) (Vazquez and Pena de Ortiz 2004). In Drosophila larvae, developmental lead exposure increases the Ca2+ transient produced by action potential trains at identified neuromuscular synapses and this intracellular Ca2+ signal decays more slowly than in controls. These effects are most likely due to slower calcium extrusion from lead-exposed terminals (He et al. unpublished). Calcium extrusion from synaptic terminals involves PMCA, and it appears that lead exposure inhibits this vital enzyme both in mammals (Bettaiya et al. 1996; Mas-Oliva 1989; Sandhir and Gill 1994) and in Drosophila (He et al. 2008 unpublished).
We recently developed Drosophila melanogaster, as a model system to study behavioral, neuronal and also genetic effects of chronic lead exposure during development (Hirsch et al. 2003; Morley et al. 2003). We found that at low doses, lead affects development of such behaviors as courtship, which has been shown to be experience-dependent (Hirsch and Tompkins 1994; Hirsch and Ghiradella 2004; Hirsch et al. 2001), and fecundity (Hirsch et al. 2003); behaviors, such as locomotion, are affected at somewhat higher doses (Hirsch et al. 2003). In Drosophila larvae lead affects development of the neuromuscular junction (Morley et al. 2003) as well as inhibiting PMCA (He et al. unpublished).
Our objective in this study was to identify a genotype by environment interaction (GEI) for a behavioral response to lead among 75 recombinant inbred (RI) lines constructed from Oregon R and Russian 2b parental strains: that is, a variation among RI genotypes in the magnitude (and/or direction) of a lead-induced behavioral change (Sambandan et al. 2008). To do this we measured locomotor activity in control and lead-exposed individuals from each line. We identified a behavioral trait (Average Daytime Activity) which shows a significant GEI and used that to define an index that quantifies the effect of lead exposure on that trait. Using this index we performed quantitative trait locus (QTL) analysis and identified a chromosomal region for which differences in the alleles from the two parental strains impacted the magnitude (and/or direction) of the index (i.e. of the lead-induced variation in behavior). A brief report of these findings has been presented (Hirsch et al. unpublished).
Materials and Methods
Subjects
Drosophila melanogaster Recombinant Inbred (RI) Lines obtained from T. MacKay (Nuzhdin et al. 1997) were used in this study. Each RI line contains a unique sample of recombinant chromosomes marked with “roo” transposable element insertion sites (Nuzhdin et al. 1997). Eighty-one cytological insertion sites of the roo-transposable element were used as molecular markers to determine the genotype of the RI lines, with an average spacing between markers of 3.2 cM on the standard map (Leips and Mackay 2000).
Experimental Design
Two control and two lead-treated males from each of 75 roo lines were tested simultaneously in each of six replicates over a period of twelve weeks. Approximately 80% of the flies survived to the end of the experiment. The final sample size was not significantly different for the two treatment groups (n=685 for control flies and 663 for lead treated flies; chi-square p=0.55).
Lead and Rearing Conditions
RIs were raised at 25° C on Instant Drosophila Medium Formula 4–24 (Carolina Biological Supply Company, Burlington, NC) mixed with either distilled water (controls) or 250 μM Lead Acetate solution (lead-treated), under 12 hour light : 12 hour dark conditions with lights-on at 10 am and lights-off at 10 pm. The behavioral experiments described below were done at the same temperature and photoperiod conditions. Exposure to leaded or control food started at egg-laying by placing approximately 25 adult flies in each vial for two days to lay eggs. Newly eclosed adults were harvested within 24 hours of eclosion and placed on fresh medium, of the same type (leaded or control) as was present during pre-adult stages, for the first four days of adult life. All animals were transferred to control medium for day five and then transferred individually, in a randomized design and under carbon dioxide anesthesia into Drosophila Activity Monitors (DAMs) (TriKinetics, Waltham, MA) on day six.
Behavioral Assays
DAMs count the number of times a single fly trapped in a 5 mm diameter by 65 mm-long glass tube (with food, water and air) walks through an infrared beam aimed at the middle of the tube. Food in the DAMs was a gel made from apple juice (Mott’s, Rye Brook, NY) and grape agarose medium (Genessee Scientific, San Diego, CA). DAM activity data were collected from day 6 to day 12 of adult age. To allow the flies to recover from handling and anesthesia, our analysis is based data collected starting at “lights on” (10 am) of day 7. We ended the analysis at 10am on day 11, which insured that we used data from healthy flies. Data were analyzed using RhythmWatch software (Mini Mitter Company, Inc., Sunriver, OR).
Traits
DAM Activity Counts were collected and summed into 10 minute bins. We defined three traits: Average Total Activity (Day plus Night) (ATA); Average Daytime Activity (ADA), and Average Nighttime Activity (ANA).
Statistical Analysis of Treatment Effects
Lead burdens and behavioral data were subjected to analysis of variance using the General Linear Model (GLM) procedure from SPSS V14.0. The model investigated main effects for lead treatment, roo line, replicate and their interactions. The lead treatment by roo line interaction identifies genetic mediation of lead effects.
QTL Analysis
WindowsQTLCartographer, version 2.5 (Wang 2007) was used to assess the variance for each behavioral trait explained by each roo-marked chromosome segment to detect Quantitative Trait Loci which significantly influence the trait (the distribution of the markers on Chromosomes I, II and III are shown in Figure 1). Single Marker Analysis (SMA), Interval Mapping (IM) and Composite Interval Mapping (CIM) were used with default settings, except that 1000 permutations were run with IM and CIM to obtain p-values. The logarithmic ratio (−2 ln(H0/H1)) (LR) was computed for each measure, and r2 (the proportion of the variation explained by the QTL) was computed for IM and CIM. Only QTL that were statistically significant using all three measures (SMA, IM and CIM) were considered valid. QTL results from the CIM analysis are reported.
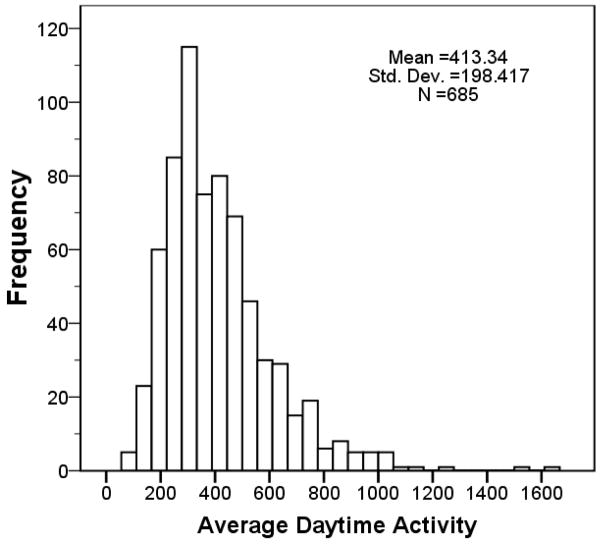
Figure 1.
Histogram of the distribution of ADA for control flies showing the variation in the behavioral trait (ADA) we used in our QTL analysis.
Lead Burden Assay
Males from the same vials of individual roo lines that were used for the behavioral testing described above were saved for the “lead burden” analysis. We recorded the number of surplus adults in each vial and transferred them into coded polypropylene centrifuge tubes so that the analysis could be run blind. These vials were then frozen for storage and transport. Lead burdens were obtained for 72 of the 75 strains.
Total fruit fly body lead burden was analyzed by Inductively Coupled Plasma Mass Spectrometry at the Geology Department, Union College, according to the methods described in Hirsch et al., 2003. Total number of vials tested was 522.
Results
Lead Burdens
To verify that lead exposure had been successful, we measured body lead burdens. Results of GLM analysis revealed a significant main effect (p < 0.001) for lead exposure, roo line and dose by roo interaction. Thus, lead treatment was effective, and there was significant genetic variability in the lead burdens of roo lines. The mean lead level in all the treated flies was 46.0 +/− 1.9 pg per fly, versus 0.1 +/−0.02 pg per control fly. Mean lead levels among roo lines treated with lead varied from 9.8 to 127.5 pg per fly.
Behavioral Effects
Treatment effects on behavior are summarized in Table 1. There were significant behavioral main effects for roo line and replicate for each of the three traits: Average Total Activity, Average Daytime Activity, and Average Nighttime Activity. There were no significant main effects for lead exposure for any of these traits. Differences in activity are thus most strongly affected both by genetic variations and by subtle differences between replicates (despite the standardization of the procedure). Consistent with the latter, there were significant interactions between lead treatment and replicate. In contrast, genetic variations produce stable behavioral differences; there were no significant interactions between roo line and replicate. One of the three traits, Average Daytime Activity (ADA), showed a significant Lead by roo line interaction (p<0.05). The third order interaction for this trait was also significant (p<0.013).
Table 1.
Behavioral Results
| Trait(n=1348) | Mean +/− SEM | ANOVAp-values for main effects and interactionsa | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| lead | line | lead*line | rep | lead*rep | line*rep | ||
| Average Daily Activity | 413.0 +/− 5.3 | 0.30 | 0.001 | 0.047 | 0.001 | 0.001 | 0.35 |
| Average Nightly Activity | 181.3 +/− 4.5 | 0.89 | 0.001 | 0.415 | 0.001 | 0.006 | 0.98 |
| Average Total Activity | 594.3 +/− 8.4 | 0.57 | 0.001 | 0.106 | 0.001 | 0.001 | 0.79 |
p< value shown
Even though the RI lines are derived from only two inbred parental strains, as Figure 1 shows, the control flies display considerable allelic variation in the behavioral trait (ADA) which we used in our QTL analysis.
QTL Analysis
For purposes of QTL analysis we used ADA to compute a “Lead Index” for each roo line from control and lead-treated strain means as a quantitative measure of the developmental effects of lead exposure: (ADA lead-treated − ADA control)/(ADA lead-treated + ADA control). Since ADA means varied significantly among roo lines we used a normalized measure. For purposes of the QTL analysis, we computed this ADA-based “Lead Index” for each roo line from control and lead-treated strain means.
Using the average Lead Index for each line, we detected one significant QTL on Chromosome 2 at 30AB (Composite Interval Mapping LR = 25.57, r2 = 0.26, p < 0.001; Figure 2).
Figure 2.
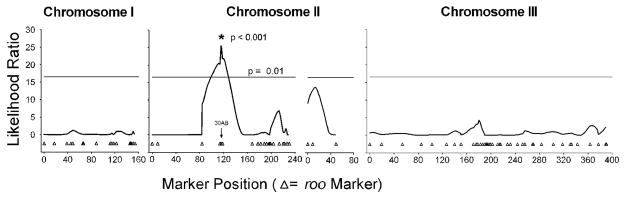
Composite Interval Mapping for the ADA Lead Index trait showing a significant QTL at cytological marker 30AB The The Y axis is in Likelihood Ratio (LR) Units; the horizontal line indicates p < 0.01 significance level. The X axis is in centiMorgans (cM), representing the genetic map for chromosomes 1, 2 and 3.
We used the QTL marker at 30AB to sort the RI lines into two groups, one in which the 30AB roo-marked chromosome segment was from the Russian 2b parental line (N = 502), and the other in which it was from Oregon R (N = 790). Figure 3 shows the distribution of the mean Lead Index for RI lines from each of the two groups, with values ranked from largest to smallest. Note that in each group there is a range from positive to negative, but the distribution of Lead Index scores differs between groups.
Figure 3.
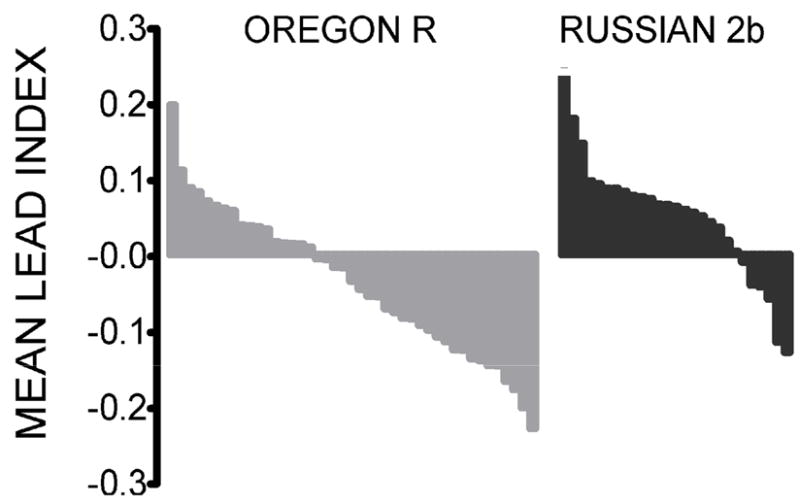
Distribution of roo lines as a function of ADA Lead Index (ranked from largest to smallest) for flies in which 30AB is from the Oregon R parental line (left) versus the Russian 2b line (right). Note there are many more lines in which the index is negative (and thus lead reduces activity) for Oregon R than for Russian 2b.
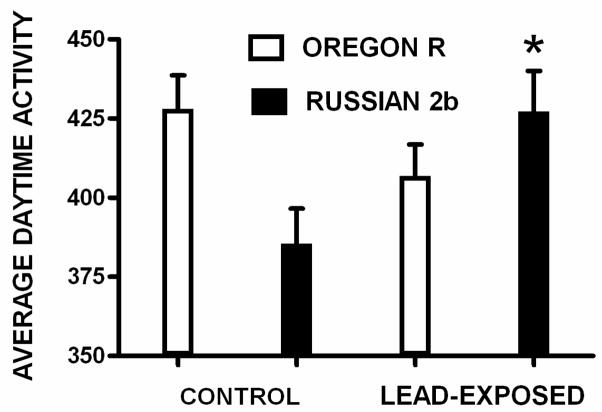
To verify that genetic variation linked to the roo line marker at 30AB influenced the magnitude (and/or direction) of lead-induced changes in ADA, we performed a GLM analysis for ADA using the same two groups. There was no significant main effect for lead exposure (F = 0.609; df = 1; p > 0.05), or parental genotype (F = 0.424; df = 1; p > 0.05). However, there was a significant lead exposure by parental genotype interaction (F = 8.173; df = 1; p < 0.005). As Figure 4 shows, when the parental line marker allele is from Russian 2b, lead exposure significantly increases ADA (F = 6.19; df= 1; p<0.015); in contrast, although ADA decreases with lead exposure when the parental line marker at 30AB is from Oregon R, there is no significant effect of lead exposure (F = 2.16; df= 1; p > 0.05). Thus, genetic variation at the QTL identified with the lead index affected the lead-induced changes in a complex behavioral trait: ADA.
Figure 4.
ADA is graphed for flies representing only the Oregon R and Russian 2b parental genotypes at 30AB under control (left) and leaded (right) conditions. When the 30AB marker is Russian 2b, lead significantly increases ADA. When the 30AB marker is Oregon R, lead does not have a significant effect on ADA. The genotype by lead-treatment was significant (p<0.005). (Error bars indicate standard error of the mean; * = p < 0.015).
To determine whether the variation in lead burden among roo lines could account for the variation in ADA or in Lead Index, we computed the correlation between RI strain means for lead burden and ADA, as well as for lead burden (for lead-treated flies) and Lead Index. The correlations were not significant (r = 0.024, p = 0.77, and r = 0.111, p = 0.36 respectively). Thus strain variation in lead burden could not account for strain variation in either ADA or Lead Index.
Discussion and Conclusions
Significant RI line differences in response to developmental lead exposure were found for average daytime activity. Approximately 25% of the lead-induced change in this behavior was explained by a significant QTL at 30AB on Chromosome 2. This effect is independent of strain differences in lead burden.
To the best of our knowledge, this is the first identification of a portion of the Drosophila genome that is involved in a behavioral response to lead. We demonstrated a significant Gene by Environment Interaction for a complex behavior (Lead Index) and by using the roo lines we were able to locate a genetic site on Chromosome 2, 30AB, involved in that interaction. We are thus able, in this species, to study behavioral (Hirsch et al. 2003), synaptic (He et al. unpublished; Morley et al. 2003) and now genetic changes resulting from chronic lead exposure during development.
Whole-genome microarrays are being used to measure changes in gene expression in response to various doses of a toxin or drug (reviewed in Foster et al. 2007 and Ruden 2007). However, since hundreds or even thousands of genes may undergo toxin-dependent changes in expression, it is difficult to take the next step and validate the results through follow-up genetic or molecular studies. RI analysis can provide an a priori method for identifying chromosomal regions where gene expression changes are most relevant to specific traits of interest. This study has identified a region of the Drosophila genome linked to the observed lead-dependent change in locomotor activity. There are approximately 125 genes closely linked to the roo marker at 30AB (Wilson et al. 2008). Some of the mechanisms these genes mediate include: nucleic acid binding (numb), transcription regulation (tai), signal transduction (Toll-4), defense responses (Toll-4 and CG3759), neurotransmitter secretion (Gdi), development of nervous system (numb), voltage-gated potassium channel activity (CG34366), ferroxidase activity (CG3759), iron ion transport (CG3759), and copper ion binding (CG3759).
Our results extend the growing list of traits shown to display significant allelic variation at identified QTL in these same roo lines. These include sternopleural bristle number (Gurganus et al. 1998,1999), reproductive performance (Fry, Nuzhdin et al. 1998), ovariole number, body size, early fecundity, competitive fitness and life span (Vieira et al. 2000; Wayne et al. 2001), olfactory behavior (Fanara et al. 2002), starvation resistance (Harbison et al. 2005), mating behavior (Moehring and Mackay 2004), longevity (Mackay 2002) and locomotor activity (Jordan et al. 2007). These studies demonstrate that the roo lines display significant allelic variation for many complex traits and represent a useful model for further analysis. They also suggest that much more genetic variation exists beyond these identified QTL since the roo lines capture allelic differences derived from only two inbred parent strains, neither of which was selected for particular behavioral responses other than reduced mating behavior in the Russian 2b parent strain (Kaidanov et al. 1991). Analysis of the same trait in independently derived Drosophila lines should reveal additional genetic variation for these traits, and might also provide independent confirmation for any QTL shared in common with the roo lines. Morgan and Mackay (2006) used the roo lines to identify seven QTL affecting thermal tolerance, and compared them to four QTL identified by Norry et al. (2004) in populations of different geographic origin that were artificially selected for response to thermal stress. Two QTL co-localized between the two studies, and the rest did not, suggesting that QTL analysis of complex quantitative traits in Drosophila in a single population can produce significant results that may generalize to other populations, but also may represent just a fraction of potential genetic variation for the trait. In the present study, our QTL associated with altered daytime activity in response to lead exposure did not match any of the four QTL identified by Jordan et al. (2006) for locomotor reactivity.
Using a different set of 41 inbred lines derived from a wild population, Sambandan et al. (2008) were able to identify and confirm, with quantitative complementation tests, genes involved in olfactory behavior of flies reared in different food sources. They used cluster analysis to identify groups of inbred strains with significant contributions to the heritability of GEI, and direct analysis of GEI effects on gene expression by employing replication of genomic expression assays within a subset of inbred strains representing the clusters associated with most of the relevant genetic variation.
Here we show that sets of RI strains can be used to identify QTL for developmental GEI effects similar to those described for olfactory behavior in non-recombinant inbred strains of flies raised on different food sources (Sambandan et al. 2008). Sambandan et al. (2008) also replicated gene expression assays within strains reared on different food sources and used analysis of variance to identify GEI at the level of transcription for individual loci. Future studies that include assays of global gene expression across RI lines will be able to directly correlate strain variation in expression with strain variation in complex trait phenotypes in different environments.
Developmental exposure to stressors (such as lead) can produce a range of behavioral changes in children, including a decrease in intelligence as measured by IQ tests (Bellinger 2008b; Jusko et al. 2008; White et al. 2007). We have suggested that lead is just one of many environmental factors that induces developmental changes (Hirsch and Ghiradella 2004) which may give rise to a slightly “modified” nervous system (Weiss and Bellinger 2006). This process of course involves changes in gene regulation (Bakheet et al. 2007; Zawia et al. 2000) and a first step is to identify some of these genes. Given the conservative nature of evolutionary changes (Gaziova and Bhat 2007; Keshishian et al. 1996) genes identified in Drosophila may help identify those involved in the response to lead in children.
Acknowledgments
RI lines were obtained from Trudy Mackay; we thank Kristi Montooth for help with the QTL and Helen Ghiradella as well as two anonymous reviewers for help with the manuscript. This work was supported by the Environmental Health Sciences Center in Molecular and Cellular Toxicology with Human Applications Grant P30 ES06639 at Wayne State University, NIH R01 grants (ES012933 and CA105349) to D.M.R.
Footnotes
The authors declare there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alber SA, Strupp BJ. An in-depth analysis of lead effects in a delayed spatial alternation task: assessment of mnemonic effects, side bias, and proactive interference. Neurotoxicol Teratol. 1996;18:3–15. doi: 10.1016/0892-0362(95)02026-8. [DOI] [PubMed] [Google Scholar]
- Altmann L, Sveinsson K, Kramer U, Weishoff-Houben M, Turfeld M, Winneke G, Wiegand H. Visual functions in 6-year-old children in relation to lead and mercury levels. Neurotoxicol Teratol. 1998;20:9–17. doi: 10.1016/s0892-0362(97)00070-6. [DOI] [PubMed] [Google Scholar]
- Atlas D. Functional and physical coupling of voltage-sensitive calcium channels with exocytotic proteins: ramifications for the secretion mechanism. J Neurochem. 2001;77:972–85. doi: 10.1046/j.1471-4159.2001.00347.x. [DOI] [PubMed] [Google Scholar]
- Audesirk G, Audesirk T. Effects of in vitro lead exposure on voltage-sensitive calcium channels differ among cell types in central neurons of Lymnaea stagnalis. Neurotoxicology. 1989;10:659–69. [PubMed] [Google Scholar]
- Bakheet SA, Basha MR, Cai H, Zawia NH. Lead exposure: expression and activity levels of Oct-2 in the developing rat brain. Toxicol Sci. 2007;95:436–42. doi: 10.1093/toxsci/kfl163. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. Lead neurotoxicity and socioeconomic status: Conceptual and analytical issues. Neurotoxicology. 2008a;29:828–32. doi: 10.1016/j.neuro.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC. Very low lead exposures and children’s neurodevelopment. Curr Opin Pediatr. 2008b;20:172–7. doi: 10.1097/MOP.0b013e3282f4f97b. [DOI] [PubMed] [Google Scholar]
- Bettaiya R, Yallapragada PR, Hall E, Rajanna S. In vitro effect of lead on Ca(2+)-ATPase in synaptic plasma membranes and microsomes of rat cerebral cortex and cerebellum. Ecotoxicol Environ Saf. 1996;33:157–62. doi: 10.1006/eesa.1996.0020. [DOI] [PubMed] [Google Scholar]
- Braun JM, Kahn RS, Froehlich T, Auinger P, Lanphear BP. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U.S. children. Environ Health Perspect. 2006;114:1904–9. doi: 10.1289/ehp.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J. Behavioral effects of early postnatal lead exposure in herring gull (Larus argentatus) chicks. Pharmacol Biochem Behav. 1990;35:7–13. doi: 10.1016/0091-3057(90)90196-o. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Effects of lead on learning in herring gulls: an avian wildlife model for neurobehavioral deficits. Neurotoxicology. 2005;26:615–24. doi: 10.1016/j.neuro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Canfield RL, Henderson CR, Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med. 2003;348:1517–26. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetty CS, Reddy GR, Murthy KS, Johnson J, Sajwan K, Desaiah D. Perinatal lead exposure alters the expression of neuronal nitric oxide synthase in rat brain. Int J Toxicol. 2001;20:113–20. doi: 10.1080/109158101317097692. [DOI] [PubMed] [Google Scholar]
- Cline HT, Witte S, Jones KW. Low lead levels stunt neuronal growth in a reversible manner. Proc Natl Acad Sci U S A. 1996;93:9915–20. doi: 10.1073/pnas.93.18.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GP, Manalis RS. Influence of heavy metals on synaptic transmission: a review. Neurotoxicology. 1983;4:69–83. [PubMed] [Google Scholar]
- Cory-Slechta DA, Pokora MJ. Lead-induced changes in muscarinic cholinergic sensitivity. Neurotoxicology. 1995;16:337–47. [PubMed] [Google Scholar]
- Dearth RK, Hiney JK, Srivastava V, Burdick SB, Bratton GR, Dees WL. Effects of lead (Pb) exposure during gestation and lactation on female pubertal development in the rat. Reprod Toxicol. 2002;16:343–52. doi: 10.1016/s0890-6238(02)00037-0. [DOI] [PubMed] [Google Scholar]
- Dearth RK, Hiney JK, Srivastava V, Les Dees W, Bratton GR. Low level lead (Pb) exposure during gestation and lactation: assessment of effects on pubertal development in Fisher 344 and Sprague-Dawley female rats. Life Sci. 2004;74:1139–48. doi: 10.1016/j.lfs.2003.07.033. [DOI] [PubMed] [Google Scholar]
- Dietrich KN, Ris MD, Succop PA, Berger OG, Bornschein RL. Early exposure to lead and juvenile delinquency. Neurotoxicol Teratol. 2001;23:511–8. doi: 10.1016/s0892-0362(01)00184-2. [DOI] [PubMed] [Google Scholar]
- Fanara JJ, Robinson KO, Rollmann SM, Anholt RR, Mackay TF. Vanaso is a candidate quantitative trait gene for Drosophila olfactory behavior. Genetics. 2002;162:1321–8. doi: 10.1093/genetics/162.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster WR, Chen SJ, He A, Truong A, Bhaskaran V, Nelson DM, Dambach DM, Lehman-McKeeman LD, Car BD. A retrospective analysis of toxicogenomics in the safety assessment of drug candidates. Toxicol Pathol. 2007;35:621–35. doi: 10.1080/01926230701419063. [DOI] [PubMed] [Google Scholar]
- Fox DA, Srivastava D, Hurwitz RL. Lead-induced alterations in rod-mediated visual functions and cGMP metabolism: new insights. Neurotoxicology. 1994;15:503–12. [PubMed] [Google Scholar]
- Fox DA, Wright AA, Costa LG. Visual acuity deficits following neonatal lead exposure: cholinergic interactions. Neurobehav Toxicol Teratol. 1982;4:689–93. [PubMed] [Google Scholar]
- Fry JD, Heinsohn SL, Mackay TF. Heterosis for viability, fecundity, and male fertility in Drosophila melanogaster: comparison of mutational and standing variation. Genetics. 1998;148:1171–88. doi: 10.1093/genetics/148.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry JD, Nuzhdin SV, Pasyukova EG, Mackay TF. QTL mapping of genotype-environment interaction for fitness in Drosophila melanogaster. Genet Res. 1998;71:133–41. doi: 10.1017/s0016672398003176. [DOI] [PubMed] [Google Scholar]
- Garavan H, Morgan RE, Levitsky DA, Hermer-Vazquez L, Strupp BJ. Enduring effects of early lead exposure: evidence for a specific deficit in associative ability. Neurotoxicol Teratol. 2000;22:151–64. doi: 10.1016/s0892-0362(99)00057-4. [DOI] [PubMed] [Google Scholar]
- Gaziova I, Bhat KM. Generating asymmetry: with and without self-renewal. Prog Mol Subcell Biol. 2007;45:143–78. doi: 10.1007/978-3-540-69161-7_7. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Kelly ME, Samsam TE, Goodman JH. Chronic developmental lead exposure reduces neurogenesis in adult rat hippocampus but does not impair spatial learning. Toxicol Sci. 2005;86:365–74. doi: 10.1093/toxsci/kfi156. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Lasley SM. Developmental lead (Pb) exposure reduces the ability of the NMDA antagonist MK-801 to suppress long-term potentiation (LTP) in the rat dentate gyrus, in vivo. Neurotoxicol Teratol. 2007;29:385–93. doi: 10.1016/j.ntt.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Mack CM. Chronic lead exposure accelerates decay of long-term potentiation in rat dentate gyrus in vivo. Brain Res. 1998;789:139–49. doi: 10.1016/s0006-8993(97)01517-5. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Mack CM, Lasley SM. Chronic developmental lead exposure increases the threshold for long-term potentiation in rat dentate gyrus in vivo. Brain Res. 1996;736:118–24. doi: 10.1016/0006-8993(96)00665-8. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Mack CM, Lasley SM. Chronic developmental lead exposure and hippocampal long-term potentiation: biphasic dose-response relationship. Neurotoxicology. 1999a;20:71–82. [PubMed] [Google Scholar]
- Gilbert ME, Mack CM, Lasley SM. The influence of developmental period of lead exposure on long-term potentiation in the adult rat dentate gyrus in vivo. Neurotoxicology. 1999b;20:57–69. [PubMed] [Google Scholar]
- Guilarte TR. Pb2+ inhibits NMDA receptor function at high and low affinity sites: developmental and regional brain expression. Neurotoxicology. 1997;18:43–51. [PubMed] [Google Scholar]
- Gulson BL, Mizon KJ, Korsch MJ, Palmer JM, Donnelly JB. Mobilization of lead from human bone tissue during pregnancy and lactation--a summary of long-term research. Sci Total Environ. 2003;303:79–104. doi: 10.1016/s0048-9697(02)00355-8. [DOI] [PubMed] [Google Scholar]
- Gurganus MC, Fry JD, Nuzhdin SV, Pasyukova EG, Lyman RF, Mackay TF. Genotype-environment interaction at quantitative trait loci affecting sensory bristle number in Drosophila melanogaster. Genetics. 1998;149:1883–98. doi: 10.1093/genetics/149.4.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurganus MC, Nuzhdin SV, Leips JW, Mackay TF. High-resolution mapping of quantitative trait loci for sternopleural bristle number in Drosophila melanogaster. Genetics. 1999;152:1585–604. doi: 10.1093/genetics/152.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison ST, Chang S, Kamdar KP, Mackay TF. Quantitative genomics of starvation stress resistance in Drosophila. Genome Biol. 2005;6:R36. doi: 10.1186/gb-2005-6-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch HV, Mercer J, Sambaziotis H, Huber M, Stark DT, Torno-Morley T, Hollocher K, Ghiradella H, Ruden DM. Behavioral effects of chronic exposure to low levels of lead in Drosophila melanogaster. Neurotoxicology. 2003;24:435–42. doi: 10.1016/S0161-813X(03)00021-4. [DOI] [PubMed] [Google Scholar]
- Hirsch HVB, Tompkins L. The flexible fly: experience-dependent development of complex behaviors in Drosophila melanogaster. J Exp Biol. 1994;195:1–18. doi: 10.1242/jeb.195.1.1. [DOI] [PubMed] [Google Scholar]
- Hirsch HVB, Ghiradella H. Environmental Influences on Behavioral Development in Insects. In: Capinera J, editor. Encyclopedia of Entomology. Dordrecht, Netherlands; Kluwer Press: 2004. [Google Scholar]
- Hirsch HVB, Tieman S, Barth M, Ghiradella H. Tunable Seers: Activity-Dependent Development of Vision in Cat and Fly. In: Blass E, editor. Developmental Psychobiology, Handbook of Behavioral Neurobiology. New York: Kluwer Academic/Plenum Publishers; 2001. pp. 81–142. [Google Scholar]
- Iavicoli I, Carelli G, Stanek EJ, 3rd, Castellino N, Calabrese EJ. Effects of low doses of dietary lead on puberty onset in female mice. Reprod Toxicol. 2004;19:35–41. doi: 10.1016/j.reprotox.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Iavicoli I, Carelli G, Stanek EJ, Castellino N, Li Z, Calabrese EJ. Low doses of dietary lead are associated with a profound reduction in the time to the onset of puberty in female mice. Reprod Toxicol. 2006;22:586–90. doi: 10.1016/j.reprotox.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Jett DA, Kuhlmann AC, Guilarte TR. Intrahippocampal administration of lead (Pb) impairs performance of rats in the Morris water maze. Pharmacol Biochem Behav. 1997;57:263–9. doi: 10.1016/s0091-3057(96)00349-8. [DOI] [PubMed] [Google Scholar]
- Jones LG, Prins J, Park S, Walton JP, Luebke AE, Lurie DI. Lead exposure during development results in increased neurofilament phosphorylation, neuritic beading, and temporal processing deficits within the murine auditory brainstem. J Comp Neurol. 2008;506:1003–17. doi: 10.1002/cne.21563. [DOI] [PubMed] [Google Scholar]
- Jordan KW, Carbone MA, Yamamoto A, Morgan TJ, Mackay TF. Quantitative genomics of locomotor behavior in Drosophila melanogaster. Genome Biol. 2007;8:R172. doi: 10.1186/gb-2007-8-8-r172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan KW, Morgan TJ, Mackay TF. Quantitative trait loci for locomotor behavior in Drosophila melanogaster. Genetics. 2006;174:271–84. doi: 10.1534/genetics.106.058099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusko TA, Henderson CR, Lanphear BP, Cory-Slechta DA, Parsons PJ, Canfield RL. Blood lead concentrations < 10 microg/dL and child intelligence at 6 years of age. Environ Health Perspect. 2008;116:243–8. doi: 10.1289/ehp.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidanov LZ, Bolshakov VN, Tzygvintzev PN, Gvozdev VA. The sources of genetic variability in highly inbred long-term selected strains of Drosophila melanogaster. Genetica. 1991;85:73–8. doi: 10.1007/BF00056108. [DOI] [PubMed] [Google Scholar]
- Keshishian H, Broadie K, Chiba A, Bate M. The drosophila neuromuscular junction: a model system for studying synaptic development and function. Annu Rev Neurosci. 1996;19:545–75. doi: 10.1146/annurev.ne.19.030196.002553. [DOI] [PubMed] [Google Scholar]
- Kiraly E, Jones DG. Dendritic spine changes in rat hippocampal pyramidal cells after postnatal lead treatment: a Golgi study. Exp Neurol. 1982;77:236–9. doi: 10.1016/0014-4886(82)90158-3. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Sonawane B, Butler RN, Trasande L, Callan R, Droller D. Early environmental origins of neurodegenerative disease in later life. Environ Health Perspect. 2005;113:1230–3. doi: 10.1289/ehp.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky RE, Laughlin NK. Exploring a partially enclosed space by lead-exposed female rhesus monkeys. Neurotoxicol Teratol. 2001;23:177–83. doi: 10.1016/s0892-0362(01)00120-9. [DOI] [PubMed] [Google Scholar]
- Lasky RE, Luck ML, Torre P, 3rd, Laughlin N. The effects of early lead exposure on auditory function in rhesus monkeys. Neurotoxicol Teratol. 2001;23:639–49. doi: 10.1016/s0892-0362(01)00175-1. [DOI] [PubMed] [Google Scholar]
- Lasky RE, Maier MM, Snodgrass EB, Hecox KE, Laughlin NK. The effects of lead on otoacoustic emissions and auditory evoked potentials in monkeys. Neurotoxicol Teratol. 1995;17:633–44. doi: 10.1016/0892-0362(95)02006-3. [DOI] [PubMed] [Google Scholar]
- Lasley SM, Green MC, Gilbert ME. Rat hippocampal NMDA receptor binding as a function of chronic lead exposure level. Neurotoxicol Teratol. 2001;23:185–9. doi: 10.1016/s0892-0362(01)00116-7. [DOI] [PubMed] [Google Scholar]
- Lasley SM, Polan-Curtain J, Armstrong DL. Chronic exposure to environmental levels of lead impairs in vivo induction of long-term potentiation in rat hippocampal dentate. Brain Res. 1993;614:347–51. doi: 10.1016/0006-8993(93)91054-v. [DOI] [PubMed] [Google Scholar]
- Leips J, Mackay TF. Quantitative trait loci for life span in Drosophila melanogaster: interactions with genetic background and larval density. Genetics. 2000;155:1773–88. doi: 10.1093/genetics/155.4.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie DI, Brooks DM, Gray LC. The effect of lead on the avian auditory brainstem. Neurotoxicology. 2006;27:108–17. doi: 10.1016/j.neuro.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Mackay TF. The nature of quantitative genetic variation for Drosophila longevity. Mech Ageing Dev. 2002;123:95–104. doi: 10.1016/s0047-6374(01)00330-x. [DOI] [PubMed] [Google Scholar]
- Mas-Oliva J. Effect of lead on the erythrocyte (Ca2+, Mg2+)−ATPase activity. Calmodulin involvement. Mol Cell Biochem. 1989;89:87–93. doi: 10.1007/BF00228283. [DOI] [PubMed] [Google Scholar]
- Moehring AJ, Mackay TF. The quantitative genetic basis of male mating behavior in Drosophila melanogaster. Genetics. 2004;167:1249–63. doi: 10.1534/genetics.103.024372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira EG, Vassilieff I, Vassilieff VS. Developmental lead exposure: behavioral alterations in the short and long term. Neurotoxicol Teratol. 2001;23:489–95. doi: 10.1016/s0892-0362(01)00159-3. [DOI] [PubMed] [Google Scholar]
- Morgan RE, Levitsky DA, Strupp BJ. Effects of chronic lead exposure on learning and reaction time in a visual discrimination task. Neurotoxicol Teratol. 2000;22:337–45. doi: 10.1016/s0892-0362(00)00065-9. [DOI] [PubMed] [Google Scholar]
- Morgan TJ, Mackay TF. Quantitative trait loci for thermotolerance phenotypes in Drosophila melanogaster. Heredity. 2006;96:232–42. doi: 10.1038/sj.hdy.6800786. [DOI] [PubMed] [Google Scholar]
- Morley EJ, Hirsch HV, Hollocher K, Lnenicka GA. Effects of chronic lead exposure on the neuromuscular junction in Drosophila larvae. Neurotoxicology. 2003;24:35–41. doi: 10.1016/s0161-813x(02)00095-5. [DOI] [PubMed] [Google Scholar]
- Needleman HL, Schell A, Bellinger D, Leviton A, Allred EN. The long-term effects of exposure to low doses of lead in childhood. An 11-year follow-up report. N Engl J Med. 1990;322:83–8. doi: 10.1056/NEJM199001113220203. [DOI] [PubMed] [Google Scholar]
- Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–72. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Norry FM, Dahlgaard J, Loeschcke V. Quantitative trait loci affecting knockdown resistance to high temperature in Drosophila melanogaster. Mol Ecol. 2004;13:3585–94. doi: 10.1111/j.1365-294X.2004.02323.x. [DOI] [PubMed] [Google Scholar]
- Nuzhdin SV, Pasyukova EG, Dilda CL, Zeng ZB, Mackay TF. Sex-specific quantitative trait loci affecting longevity in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1997;94:9734–9. doi: 10.1073/pnas.94.18.9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padich RA, Dietrich KN, Pearson DT. Attention, activity level, and lead exposure at 18 months. Environ Res. 1985;38:137–43. doi: 10.1016/0013-9351(85)90079-9. [DOI] [PubMed] [Google Scholar]
- Patrick GW, Anderson WJ. Dendritic alterations of cortical pyramidal neurons in postnatally lead-exposed kittens: a Golgi-Cox study. Dev Neurosci. 1995;17:219–29. doi: 10.1159/000111290. [DOI] [PubMed] [Google Scholar]
- Patrick GW, Anderson WJ. Dendritic alterations of cerebellar Purkinje neurons in postnatally lead-exposed kittens. Dev Neurosci. 2000;22:320–8. doi: 10.1159/000017456. [DOI] [PubMed] [Google Scholar]
- Petit TL, Alfano DP. Differential experience following developmental lead exposure: effects on brain and behavior. Pharmacol Biochem Behav. 1979;11:165–71. doi: 10.1016/0091-3057(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Petit TL, LeBoutillier JC. Effects of lead exposure during development on neocortical dendritic and synaptic structure. Exp Neurol. 1979;64:482–92. doi: 10.1016/0014-4886(79)90226-7. [DOI] [PubMed] [Google Scholar]
- Reddy GR, Suresh A, Murthy KS, Chetty CS. Lead neurotoxicity: heme oxygenase and nitric oxide synthase activities in developing rat brain. Neurotox Res. 2002;4:33–9. doi: 10.1080/10298420290007600. [DOI] [PubMed] [Google Scholar]
- Ruden DM. Personalized medicine and quantitative trait transcripts. Nat Genet. 2007;39:144–5. doi: 10.1038/ng0207-144. [DOI] [PubMed] [Google Scholar]
- Ruden DM, Possidente D, Lnenicka G, Hirsch HVB. Gene Environment Interactions: Toxicogenomics of Heavy Metals Using Drosophila Model. In: Nriagu Jerome., editor. Encyclopedia of Environmental Health. Elsevier; Amsterdam, Netherlands: in press. [Google Scholar]
- Sambandan D, Carbone MA, Anholt RR, Mackay TF. Phenotypic Plasticity and Genotype by Environment Interaction for Olfactory Behavior in Drosophila melanogaster. Genetics. 2008;179:1079–88. doi: 10.1534/genetics.108.086769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhir R, Gill KD. Alterations in calcium homeostasis on lead exposure in rat synaptosomes. Mol Cell Biochem. 1994;131:25–33. doi: 10.1007/BF01075721. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Decamp E. Postnatal lead poisoning impairs behavioral recovery following brain damage. Neurotoxicology. 2007;28:1153–7. doi: 10.1016/j.neuro.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Strickler-Shaw S, Taylor DH. Lead inhibits acquisition and retention learning in bullfrog tadpoles. Neurotoxicol Teratol. 1991;13:167–73. doi: 10.1016/0892-0362(91)90007-j. [DOI] [PubMed] [Google Scholar]
- Sun L, Zhao ZY, Hu J, Zhou XL. Potential association of lead exposure during early development of mice with alteration of hippocampus nitric oxide levels and learning memory. Biomed Environ Sci. 2005;18:375–8. [PubMed] [Google Scholar]
- Surkan PJ, Zhang A, Trachtenberg F, Daniel DB, McKinlay S, Bellinger DC. Neuropsychological function in children with blood lead levels <10 microg/dL. Neurotoxicology. 2007;28:1170–7. doi: 10.1016/j.neuro.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang HW, Liang YX, Hu XH. Effects of low level lead exposure on behavior of young rats. Zhongguo Yao Li Xue Bao. 1994;15:316–9. [PubMed] [Google Scholar]
- Tang M, Luo L, Zhu D, Wang M, Luo Y, Wang H, Ruan DY. Muscarinic cholinergic modulation of synaptic transmission and plasticity in rat hippocampus following chronic lead exposure. Naunyn Schmiedebergs Arch Pharmacol. 2008 doi: 10.1007/s00210-008-0344-1. [DOI] [PubMed] [Google Scholar]
- Toscano CD, Guilarte TR. Lead neurotoxicity: from exposure to molecular effects. Brain Res Brain Res Rev. 2005;49:529–54. doi: 10.1016/j.brainresrev.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Vazquez A, Pena de Ortiz S. Lead (Pb(+2)) impairs long-term memory and blocks learning-induced increases in hippocampal protein kinase C activity. Toxicol Appl Pharmacol. 2004;200:27–39. doi: 10.1016/j.taap.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Verina T, Rohde CA, Guilarte TR. Environmental lead exposure during early life alters granule cell neurogenesis and morphology in the hippocampus of young adult rats. Neuroscience. 2007;145:1037–47. doi: 10.1016/j.neuroscience.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira C, Pasyukova EG, Zeng ZB, Hackett JB, Lyman RF, Mackay TF. Genotype-environment interaction for quantitative trait loci affecting life span in Drosophila melanogaster. Genetics. 2000;154:213–27. doi: 10.1093/genetics/154.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgolini MB, Rossi-George A, Lisek R, Weston DD, Thiruchelvam M, Cory-Slechta DA. CNS effects of developmental Pb exposure are enhanced by combined maternal and offspring stress. Neurotoxicology. 2008;29:812–27. doi: 10.1016/j.neuro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Luo L, Luo YY, Gu Y, Ruan DY. Effects of Pb2+ on muscarinic modulation of glutamatergic synaptic transmission in rat hippocampal CA1 area. Neurotoxicology. 2007;28:499–507. doi: 10.1016/j.neuro.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Wang S, Basten CJ, Zeng ZB. Windows QTL Cartographer 2.5. 2007. Department of Statistics, North Carolina State University; Raleigh, NC: [Google Scholar]
- Wayne ML, Hackett JB, Dilda CL, Nuzhdin SV, Pasyukova EG, Mackay TF. Quantitative trait locus mapping of fitness-related traits in Drosophila melanogaster. Genet Res. 2001;77:107–16. doi: 10.1017/s0016672300004894. [DOI] [PubMed] [Google Scholar]
- Wayne ML, Mackay TF. Quantitative genetics of ovariole number in Drosophila melanogaster. II. Mutational variation and genotype-environment interaction. Genetics. 1998;148:201–10. doi: 10.1093/genetics/148.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B, Bellinger DC. Social ecology of children’s vulnerability to environmental pollutants. Environ Health Perspect. 2006;114:1479–85. doi: 10.1289/ehp.9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LD, Cory-Slechta DA, Gilbert ME, Tiffany-Castiglioni E, Zawia NH, Virgolini M, Rossi-George A, Lasley SM, Qian YC, Basha MR. New and evolving concepts in the neurotoxicology of lead. Toxicol Appl Pharmacol. 2007;225:1–27. doi: 10.1016/j.taap.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Wilson RJ, Goodman JL, Strelets VB. FlyBase: integration and improvements to query tools. Nucleic Acids Res. 2008;36:D588–93. doi: 10.1093/nar/gkm930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel R, Kuhn W, Przuntek H. Chronic intoxication with lead- and sulfur compounds may produce Parkinson’s disease. J Neural Transm Suppl. 1995;46:183–7. [PubMed] [Google Scholar]
- Wolff MS, Britton JA, Boguski L, Hochman S, Maloney N, Serra N, Liu Z, Berkowitz G, Larson S, Forman J. Environmental exposures and puberty in inner-city girls. Environ Res. 2008;107:393–400. doi: 10.1016/j.envres.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JP, Dietrich KN, Ris MD, Hornung RW, Wessel SD, Lanphear BP, Ho M, Rae MN. Association of prenatal and childhood blood lead concentrations with criminal arrests in early adulthood. PLoS Med. 2008;5:e101. doi: 10.1371/journal.pmed.0050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Basha MR, Zawia NH. The environment, epigenetics and amyloidogenesis. J Mol Neurosci. 2008;34:1–7. doi: 10.1007/s12031-007-0009-4. [DOI] [PubMed] [Google Scholar]
- Xu SZ, Bullock L, Shan CJ, Cornelius K, Rajanna B. PKC isoforms were reduced by lead in the developing rat brain. Int J Dev Neurosci. 2005;23:53–64. doi: 10.1016/j.ijdevneu.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Yan D, Wang L, Ma FL, Deng H, Liu J, Li C, Wang H, Chen J, Tang JL, Ruan DY. Developmental exposure to lead causes inherent changes on voltage-gated sodium channels in rat hippocampal CA1 neurons. Neuroscience. 2008;153:436–45. doi: 10.1016/j.neuroscience.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Zawia NH, Basha MR. Environmental risk factors and the developmental basis for Alzheimer’s disease. Rev Neurosci. 2005;16:325–37. doi: 10.1515/revneuro.2005.16.4.325. [DOI] [PubMed] [Google Scholar]
- Zawia NH, Crumpton T, Brydie M, Reddy GR, Razmiafshari M. Disruption of the zinc finger domain: a common target that underlies many of the effects of lead. Neurotoxicology. 2000;21:1069–80. [PubMed] [Google Scholar]
- Zhang HS, Song LH, Wang L, Qin YH. Lead can inhibit NMDA-, K(+)-, QA/KA-induced increases in intracellular free Ca2+ in cultured rat hippocampal neurons. Biomed Environ Sci. 2002;15:330–40. [PubMed] [Google Scholar]
- Zucker RS. Exocytosis: a molecular and physiological perspective. Neuron. 1996;17:1049–55. doi: 10.1016/s0896-6273(00)80238-x. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]