Abstract
It is widely accepted that caloric restriction (CR) without malnutrition delays the onset of aging and extends lifespan in diverse animal models including yeast, worms, flies and laboratory rodents. The mechanism underlying this phenomenon is still unknown. We have hypothesized that a reprogramming of energy metabolism is a key event in the mechanism of CR (Anderson and Weindruch, Interdiscip. Topics in Gerontol. 2007). Data will be presented from studies of mice on CR, which lend support to this hypothesis. Effects of long-term CR (but not short-term CR) on gene expression in white adipose tissue (WAT) are overt. In mice and monkeys, a chronic 30% reduction in energy intake yields approximately a 70% decrease in adiposity. In mouse epididymal WAT, long-term CR causes overt shifts in the gene expression profile: CR increased the expression of genes involved in energy metabolism (Higami et al. FASEB J, 2004) while it down regulates the expression of more than 50 pro-inflammatory genes (Higami et al. J. Nutr., 2006). Whether aging retardation occurs in primates on CR is unknown. We have been investigating this issue in rhesus monkeys subjected to CR since 1989 and will discuss the current status of this project. A new finding from this project is that CR reduces the rate of age-associated muscle loss (sarcopenia) in monkeys (Colman et al., J. Gerontol., 2008).
Keywords: Caloric restriction, aging, mammals, primate, metabolism, PGC-1α
Introduction
CR is the only dietary intervention known to extend lifespan and delay the onset of age-associated phenotypes (Masoro 2002; Weindruch and Walford 1988). The retardation of aging by a simple reduction in calorie intake no doubt belies the complexity of the underlying mechanisms. Although the phenomenon was first described over seven decades ago, the mechanistic basis remains elusive. In this paper, we will present a brief overview of CR and describe some key early studies in rodents that reveal the potential of this regimen as a means to understand aging. Next, we will describe the studies in mice that led us to the hypothesis that reprogramming of energy metabolism is critical to the underlying mechanisms of CR and present PGC-1α (Peroxisome proliferator activated receptor-gamma coactivator 1 alpha) as a candidate regulator in this process. Finally, we will describe the relevance of CR studies to human health and longevity, including data from our two decade long study in rhesus monkeys and some of the findings from shorter-term human studies.
Aging retardation by CR
Throughout history, there has been considerable interest in identifying factors that confer increased longevity, from the elixir of life to the fountain of youth. It was not until about 75 years ago, however, that any credible evidence that longevity could be manipulated emerged (McCay et al. 1935). Reduction of caloric intake without malnutrition extended average and maximum lifespan in male and female rats and delayed the onset of age-associated pathologies, suggesting that the prospect of increased longevity was no longer a myth. Since then, scientific and public interest in CR as a means to extend lifespan has continued to grow. In rodents many of the diseases and disorders associated with age are opposed by CR; including cancer, obesity, diabetes, autoimmune diseases, sarcopenia and cardiovascular disease (Masoro 2002; Weindruch and Walford 1988). The impact of CR on these conditions has important implications for human health and has prompted further studies that can be divided into two groups. The first are “Early-onset CR” studies (e.g. start CR on 1 month old weanling rodents), which represent the best available model to study the biology of dietary-induced decelerated aging in mammals and the second is “Adult-onset CR” (e.g. start CR in middle age), which are most relevant for potential human application.
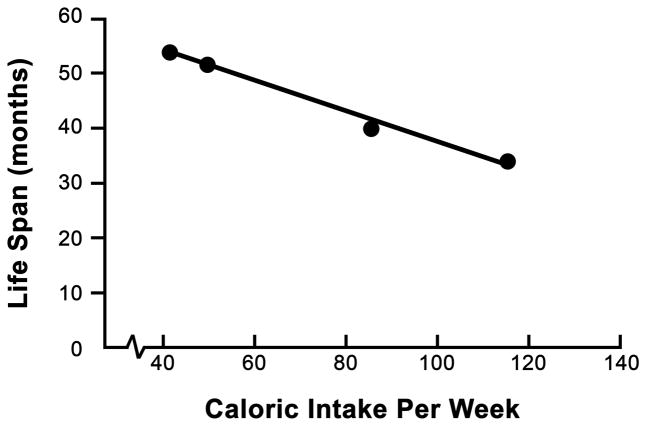
The relationship between caloric intake and lifespan was investigated in female mice from the long-lived C3B10F1 hybrid strain (Weindruch et al. 1986). With conventional maintenance, mice are group housed and fed ad libitum. For dietary studies, mice are individually housed and food intake is regulated (Pugh et al. 1999). This study led to the discovery that caloric intake is inversely proportional to lifespan, compared mice on ad libitum diet (~115kcal/mouse/wk) to mice on a variety of restricted diets (85, 50 and 40 kcal/mouse/wk). Nutrient enriched diets were fed to the two most severely restricted groups. The reduction in calorie intake was reflected the body weights of the four groups of mice. In addition, restricted mice lived longer than their ad libitum counterparts in a manner that was inversely proportional to their degree of restriction (Figure 1). While early-onset of the CR diet is acceptable in rodents, it would be unreasonable to apply the same study design in primates. Initiating a CR diet in young humans or non-human primates may negatively influence development and the attainment of maturity or may precipitate cognitive defects. Analysis of the effect of adult onset CR confirmed that reduced caloric intake increased average and maximal lifespan in both mice C3B10F1 and C57Bl/6 (Weindruch and Walford 1982), paving the way for a non-human primate study.
Figure 1. Inverse linear relationship between caloric intake and lifespan in mice.
C3B10F1 females were individually housed and placed on controlled diets (115kcal/wk, 85kcal/wk, 50 kcal/wk and 40kcal/wk) from weaning (n =49, 57, 71, 60 respectively). Animals with the highest caloric intake died earliest and survival improved with increased caloric restriction.
Exploration of the mechanisms of CR
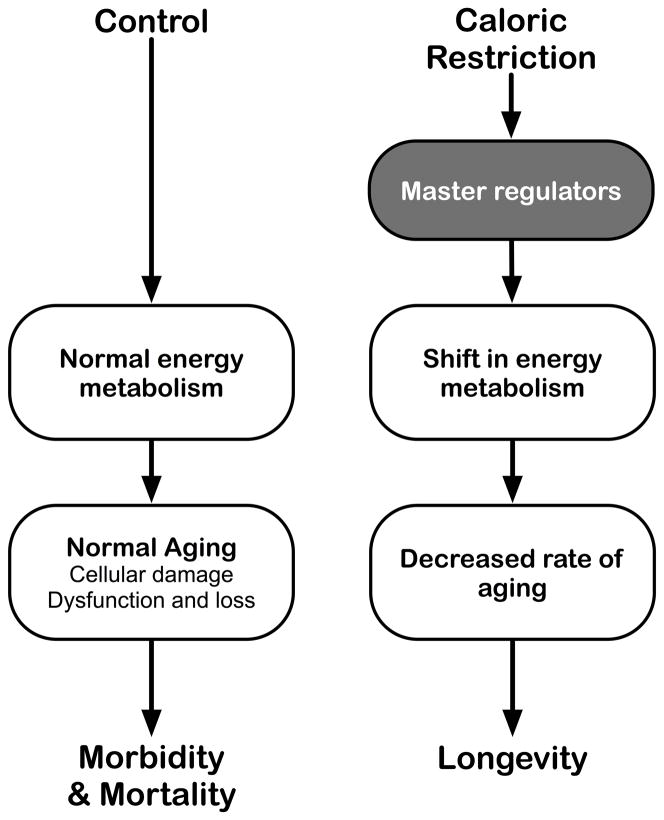
Numerous hypotheses have been presented to explain the effect of CR on longevity, including those involving alterations in membrane and lipid biology, insulin/IGF (insulin-like growth factor) axis, metabolic rate, systemic inflammation (Heilbronn and Ravussin 2003; Koubova and Guarente 2003; Masoro 2002; Smith et al. 2004; Weindruch et al. 2008). The inverse relationship between caloric intake and lifespan suggests that factors involved in regulation of energy metabolism may be key regulators of CR. A number of nutrient sensitive proteins that have been implicated in longevity, including the Sirtuins (Bordone and Guarente 2005), forkhead transcription factors (Greer and Brunet 2005) and the metabolic regulator mTOR (Schieke and Finkel 2006), may also be important in CR. These potential longevity pathways are most likely non-mutually exclusive. We have proposed a model where CR induces an active response to altered nutrient availability and propose that metabolic reprogramming is a primary event in the mechanism of CR (Anderson and Weindruch 2007) (Figure 2).
Figure 2. CR induced reprogramming of energy metabolism through activation of master regulators.
This metabolic reprogramming results in an altered metabolic state that positively influences tissue-specific effectors of longevity pathways, leading to a reduced rate of aging. Master regulators may include the transcriptional co-activator PGC-1α and members of the nuclear receptor family.
Through the application of gene expression profiling it has become possible to determine the signature of the biological age of a tissue at the molecular level. This technique also provides a means to uncover mechanisms of aging and its retardation by CR. To explore the effects of age and CR on gene regulation, we compared transcription profiles in tissues from young (5 month-old), old (30 month-old) and old CR mice (Lee et al. 2002; Lee et al. 1999; Lee et al. 2000). Our studies have focused on post-mitotic tissues, as these are most vulnerable to the effects of age. In these experiments, the control mice are mildly restricted (~90% ad libitum levels) to prevent obesity and control calorie intake; caloric intake for the restricted animals is reduced by a further 25% (~67% ad libitum). Our investigations of CR-dependent changes in transcription profiles in aged mice have revealed two types of transcriptional effects: first, the CR-induced prevention of age-associated changes and second, the changes associated with CR that are not age-dependent, i.e. those that are indicative of CR-induced transcriptional reprogramming. In heart, aging is associated with transcriptional changes that are consistent with increased structural protein turnover and neurodegeneration in addition to a shift from fatty acid to glucose metabolism (as illustrated in Lee et al. 2002). CR opposes the development of many of the age-associated changes and, importantly, was associated with increased expression of genes involved in energy metabolism and reduced expression of genes involved in inflammation (Lee et al. 2002). Regulation of metabolism emerged as an important theme from these studies and pointed to mitochondrial alterations as a critical feature of CR.
Our focus on the role of metabolism in aging led us to investigate the effect of CR on white adipose tissue (WAT). In recent times, there has been a considerable revision of the view of adipose tissue as a metabolically inert fat storage depot. It has become clear that adipose tissue is not only metabolically active, but it is also a source of hormones and inflammatory factors that influence metabolic homeostasis and systemic inflammation (Ahima 2006; Kershaw and Flier 2004; Mohamed-Ali et al. 1998). In order to determine the effect of CR independent of age-associated changes, we conducted a study in young adult, age-matched C57Bl6 male mice and compared the effect of fasting, short-term CR (23 days) and long-term CR (9 months) on WAT (Higami et al. 2006; Higami et al. 2004). Long-term CR had profound effects on the morphology, adiposity and transcriptional profile of WAT. These changes were not observed with fasting and short-term CR, regimens that are not associated with increased lifespan. Metabolic reprogramming is a prominent feature of the CR effect in WAT, with increased expression of genes involved in the glycolytic pathway, the lipolytic pathway, amino acid metabolism and mitochondrial metabolism (Higami et al. 2004). In addition to this striking activation of energy metabolism, there was a marked reduction in the expression of genes involved in inflammation in WAT (Higami et al. 2006). These alterations in WAT may provide an explanation for how CR delays the onset of age-associated diseases that have a basis in systemic inflammation, and points to a potential role for WAT derived factors in the aging process.
PGC-1α: a master regulator of energy metabolism
Based on the transcriptional data, we sought to identify factors that could be responsible for the metabolic reprogramming induced with CR. The transcriptional co-activator PGC-1α is a key regulator of genes involved in mitochondrial metabolism, including the nuclear encoded genes involved in the electron transport system (Finck and Kelly 2006; Scarpulla 2006). CR increases mRNA levels of PGC-1α in multiple tissues (Nisoli et al. 2005) and PGC-1α transcripts are elevated in cultured cells treated with serum from CR animals (Lopez-Lluch et al. 2006). In addition to regulating the genes involved in energy metabolism, PGC-1α also influences the balance between carbohydrate and fat metabolism through co-activation of the nuclear receptor family of transcription factors (Corton and Brown-Borg 2005; Puigserver and Spiegelman 2003). We recently identified a novel mechanism of PGC-1α regulation in the stress response in cultured cells (Anderson et al. 2008). Mitochondrial energy metabolism is regulated in the early response to oxidative stress in a PGC-1α dependent manner and there is an increase in mitochondrial membrane potential. Following stress, PGC-1α localization and transcriptional activity is regulated by SIRT1-dependent deacetylation. PGC-1α moves to the nucleus and activates transcription of ETS genes. The nutrient sensitive kinase GSK3β (Glycogen synthase kinase 3 beta) is activated upon stress and regulates PGC-1α protein stability by targeting it for proteasomal degradation in the nucleus. As a result, the nuclear pool of PGC-1α is degraded preventing continued activation of transcription, and the cytosolic pool of PGC-1α is replenished, re-establishing the pre-stress PGC-1α cellular distribution. This mechanism permits temporal regulation of mitochondrial function through alterations in PGC-1a stability, localization and activity (Figure 3). Analysis of tissues from mice under conditions of oxidative stress and from CR mice indicates that this pathway plays a role in the adaptive response in mice in vivo, and that regulation of mitochondria through manipulation of SIRT1 and GSK3β is a common feature of CR and the stress response (Anderson et al. 2008). These findings identify a potential link between longevity and stress resistance and may explain why regimens that extend lifespan often also confer increased stress resistance.
Figure 3. Alterations in PGC-1α activity and stability through SIRT1 and GSK3β.
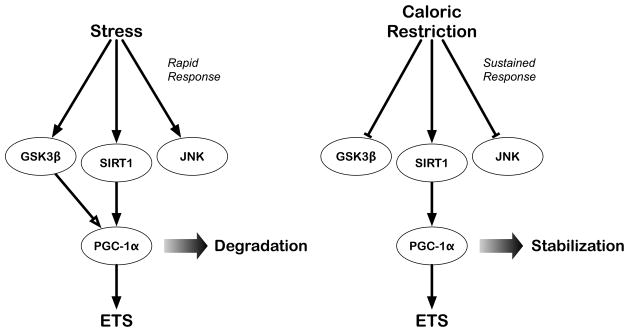
In response to acute stress, activation of SIRT1 and GSK3β causes activation and subsequent degradation of PGC-1α, resulting in a rapid and transient effect on mitochondrial energy metabolism. In response to CR, a chronic stress, SIRT1 is activated and GSK3β is inhibited, causing increased PGC-1a activity and stability, resulting in a prolonged effect on mitochondrial energy metabolism.
Monkey CR studies and implications for human health
The positive effect of CR on lifespan has been documented in multiple species from yeast to mammals but has yet to be demonstrated in a primate species. To determine whether CR could attenuate rates of change of most biological indicators of aging, including increased “health span” and increased longevity, a rhesus monkey study was initiated in 1989 at the Wisconsin National Primate Research Center at the University of Wisconsin (Kemnitz et al. 1993). Applying a model of adult-onset CR (~30% restricted), subjects were monitored for food intake before being randomized into control and CR groups. The overall goals of this study were to develop the rhesus monkey as a model for the study of aging and to determine the influence of CR on disease patterns and longevity. In addition to the expected reduction in body weight, a number of phenotypes have emerged in the restricted cohort that indicate that CR is also be efficacious in primates. Similar to the rodent studies, CR animals show a reduction in adiposity (Colman et al. 1998), increased insulin sensitivity (Gresl et al. 2003), and reduced levels of oxidative damage (Zainal et al. 2000). In addition, we have recently observed attenuation in the development of sarcopenia in the restricted animals (Colman et al. 2008). Preservation of skeletal muscle function may play a role in preventing age-associated increases in insulin resistance. To date, the CR monkeys are protected from gluco-regulatory disorders and the symptoms associated with Type 2 diabetes. In addition, the restricted animals have improved cardiovascular profiles, including reduced levels of CRP (C-reactive protein) and decreased levels of triglyceride and phospholipids associated with low density lipoproteins (Edwards et al. 1998).
Long-term studies exploring the effect of CR on human lifespan may be beyond reasonable grasp, however there is limited information on the effect of CR on humans from population studies and from short-term studies. Although reduced caloric intake is prevalent in numerous global populations, the effects of malnutrition often overshadow any benefits of CR. An exception is the relatively large proportion of centagenarians in the Okinawan population. Caloric intake is reduced in the island population compared to that of Japan as a whole (Chan et al. 1997; Kagawa 1978). Increased longevity among these people has been taken as evidence that reduced caloric intake increases average lifespan. In contrast, there is increasing evidence that overindulgence and the resulting obesity accelerate the onset of numerous disorders previously associated with aging, including diabetes, hypertension, atherosclerosis and cancer (Das et al. 2004; Wisse 2004). Short-term studies of CR have confirmed a positive health effect in people (Heilbronn et al. 2006), although feasibility and compliance issues arise in studies of longer duration (Racette et al. 2006). In individuals who voluntarily restrict themselves, CR proved highly effective in protecting against risk factors associated with atherosclerosis (Fontana et al. 2004).
The promise of retardation of human aging brings renewed focus to the biology of aging in mammals and the elucidation of the mechanistic basis of aging retardation by CR. While improved health and increased longevity are enormously attractive to most people, it seems unlikely that people would find the idea of reduced caloric intake sufficiently attractive to actually undertake the regimen. As a result, the development of nutracueticals that mimic the effects of CR without requiring a severe limitation of calorie intake is an area of active investigation (Barger et al. 2008a; Barger et al. 2008b).
Acknowledgments
This work was supported by NIH funding P01 AG011915.
Abbreviations
- CR
Caloric restriction
- WAT
White adipose tissue
References
- Ahima RS. Adipose tissue as an endocrine organ. Obesity (Silver Spring) 2006;14(Suppl 5):242S–249S. doi: 10.1038/oby.2006.317. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Barger JL, Edwards MG, Braun KH, O’Connor CE, Prolla TA, Weindruch R. Dynamic regulation of PGC-1alpha localization and turnover implicates mitochondrial adaptation in calorie restriction and the stress response. Aging Cell. 2008;7:101–11. doi: 10.1111/j.1474-9726.2007.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Weindruch R. Metabolic reprogramming in dietary restriction. Interdiscip Top Gerontol. 2007;35:18–38. doi: 10.1159/000096554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger JL, Kayo T, Pugh TD, Prolla TA, Weindruch R. Short-term consumption of a resveratrol-containing nutracuetical mixture mimics gene expression of long-term caloric restriction in mouse heart. Experimental Gerontology. 2008a doi: 10.1016/j.exger.2008.06.013. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS ONE. 2008b;3:e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- Chan YC, Suzuki M, Yamamoto S. Dietary, anthropometric, hematological and biochemical assessment of the nutritional status of centenarians and elderly people in Okinawa, Japan. J Am Coll Nutr. 1997;16:229–35. doi: 10.1080/07315724.1997.10718679. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Allison DB, Weindruch R. Attenuation of sarcopenia by dietary restriction in rhesus monkeys. J Gerontol A Biol Sci Med Sci. 2008;63:556–9. doi: 10.1093/gerona/63.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Roecker EB, Ramsey JJ, Kemnitz JW. The effect of dietary restriction on body composition in adult male and female rhesus macaques. Aging (Milano) 1998;10:83–92. doi: 10.1007/BF03339642. [DOI] [PubMed] [Google Scholar]
- Corton JC, Brown-Borg HM. Peroxisome proliferator-activated receptor gamma coactivator 1 in caloric restriction and other models of longevity. J Gerontol A Biol Sci Med Sci. 2005;60:1494–509. doi: 10.1093/gerona/60.12.1494. [DOI] [PubMed] [Google Scholar]
- Das M, Gabriely I, Barzilai N. Caloric restriction, body fat and ageing in experimental models. Obes Rev. 2004;5:13–9. doi: 10.1111/j.1467-789x.2004.00115.x. [DOI] [PubMed] [Google Scholar]
- Edwards IJ, Rudel LL, Terry JG, Kemnitz JW, Weindruch R, Cefalu WT. Caloric restriction in rhesus monkeys reduces low density lipoprotein interaction with arterial proteoglycans. J Gerontol A Biol Sci Med Sci. 1998;53:B443–8. doi: 10.1093/gerona/53a.6.b443. [DOI] [PubMed] [Google Scholar]
- Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–22. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101:6659–63. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–25. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- Gresl TA, Colman RJ, Havighurst TC, Byerley LO, Allison DB, Schoeller DA, Kemnitz JW. Insulin sensitivity and glucose effectiveness from three minimal models: effects of energy restriction and body fat in adult male rhesus monkeys. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1340–54. doi: 10.1152/ajpregu.00651.2002. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. Jama. 2006;295:1539–48. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–9. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- Higami Y, Barger JL, Page GP, Allison DB, Smith SR, Prolla TA, Weindruch R. Energy restriction lowers the expression of genes linked to inflammation, the cytoskeleton, the extracellular matrix, and angiogenesis in mouse adipose tissue. J Nutr. 2006;136:343–52. doi: 10.1093/jn/136.2.343. [DOI] [PubMed] [Google Scholar]
- Higami Y, Pugh TD, Page GP, Allison DB, Prolla TA, Weindruch R. Adipose tissue energy metabolism: altered gene expression profile of mice subjected to long-term caloric restriction. Faseb J. 2004;18:415–7. doi: 10.1096/fj.03-0678fje. [DOI] [PubMed] [Google Scholar]
- Kagawa Y. Impact of Westernization on the nutrition of Japanese: changes in physique, cancer, longevity and centenarians. Prev Med. 1978;7:205–17. doi: 10.1016/0091-7435(78)90246-3. [DOI] [PubMed] [Google Scholar]
- Kemnitz JW, Weindruch R, Roecker EB, Crawford K, Kaufman PL, Ershler WB. Dietary restriction of adult male rhesus monkeys: design, methodology, and preliminary findings from the first year of study. J Gerontol. 1993;48:B17–26. doi: 10.1093/geronj/48.1.b17. [DOI] [PubMed] [Google Scholar]
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- Koubova J, Guarente L. How does calorie restriction work? Genes Dev. 2003;17:313–21. doi: 10.1101/gad.1052903. [DOI] [PubMed] [Google Scholar]
- Lee CK, Allison DB, Brand J, Weindruch R, Prolla TA. Transcriptional profiles associated with aging and middle age-onset caloric restriction in mouse hearts. Proc Natl Acad Sci U S A. 2002;99:14988–93. doi: 10.1073/pnas.232308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–3. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000;25:294–7. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- Lopez-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci U S A. 2006;103:1768–73. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Caloric restriction: a key to understanding and modulating aging. Elsevier; Amsterdam: 2002. [Google Scholar]
- McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Nutrition. 1935;5:155–71. discussion 172. [PubMed] [Google Scholar]
- Mohamed-Ali V, Pinkney JH, Coppack SW. Adipose tissue as an endocrine and paracrine organ. Int J Obes Relat Metab Disord. 1998;22:1145–58. doi: 10.1038/sj.ijo.0800770. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–7. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- Pugh TD, Klopp RG, Weindruch R. Controlling caloric consumption: protocols for rodents and rhesus monkeys. Neurobiol Aging. 1999;20:157–65. doi: 10.1016/s0197-4580(99)00043-3. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, Fontana L, Klein S, Holloszy JO. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci. 2006;61:943–50. doi: 10.1093/gerona/61.9.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla RC. Nuclear control of respiratory gene expression in mammalian cells. J Cell Biochem. 2006;97:673–83. doi: 10.1002/jcb.20743. [DOI] [PubMed] [Google Scholar]
- Schieke SM, Finkel T. Mitochondrial signaling, TOR, and life span. Biol Chem. 2006;387:1357–61. doi: 10.1515/BC.2006.170. [DOI] [PubMed] [Google Scholar]
- Smith JV, Heilbronn LK, Ravussin E. Energy restriction and aging. Curr Opin Clin Nutr Metab Care. 2004;7:615–22. doi: 10.1097/00075197-200411000-00005. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Colman RJ, Perez V, Richardson AG. How does caloric restriction increase the longevity of mammals? In: Guarente L, Partridge L, Wallace D, editors. Molecular Biology of Aging. Cold Spring Harbor Laboratory Press; New York: 2008. [Google Scholar]
- Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982;215:1415–8. doi: 10.1126/science.7063854. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116:641–54. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- Weindruch RH, Walford RL. The retardation of aging and disease by dietary restriction. Charles C Thomas; Springfield, Illinois, USA: 1988. [Google Scholar]
- Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004;15:2792–800. doi: 10.1097/01.ASN.0000141966.69934.21. [DOI] [PubMed] [Google Scholar]
- Zainal TA, Oberley TD, Allison DB, Szweda LI, Weindruch R. Caloric restriction of rhesus monkeys lowers oxidative damage in skeletal muscle. Faseb J. 2000;14:1825–36. doi: 10.1096/fj.99-0881com. [DOI] [PubMed] [Google Scholar]