Abstract
Early detection is the key to effective treatment of prostate cancer, and to the prevention of deaths due to progression to untreatable advanced stage cancer. Because of mitigating factors, especially benign prostatic hyperplasia (BPH), that result in a low accuracy (about 60%) of prostate-specific antigen (PSA) testing, there is an urgent need for a more reliable biomarker for the identification of early stage through advanced stage prostate cancer and ‘at-risk’ individuals. To address this issue we propose that changes in prostatic fluid composition could provide accurate and reliable biomarkers for the screening of prostate cancer. Most notable is the consistent and significant decrease in citrate and zinc that is associated with the development and progression of prostate cancer. In this review we provide the clinical and physiological basis and the evidence in support of the utility of prostatic fluid analysis as an effective approach for screening/detection of prostate cancer, especially early stage and ‘at-risk’ subjects. The problem of BPH interference that plagues PSA testing is eliminated in the potential prostatic fluid biomarkers. The potential development of rapid, simple, direct, accurate clinical tests provides additional advantageous conditions. Further exploration and development of citrate, zinc and other electrolytes as prostatic fluid biomarkers are urgently needed to address this critical prostate cancer issue.
Keywords: prostatic fluid, zinc, citrate, electrolytes, screening and diagnosis
Introduction: the issue of reliable biomarkers for prostate cancer
A major problem that confronts the issue of prostate cancer is the absence of a reliable biomarker for the screening of subjects for prostate cancer, especially early stage prostate cancer and subjects at risk for prostate cancer. Currently, prostate-specific antigen (PSA) coupled with digital rectal examination (DRE) is the accepted initial diagnostic procedure for prostate cancer. Recent assessments have led to serious questions concerning the value and consequences of PSA for initial screening and detection of prostate cancer. Based predominantly on PSA results, urologists in the USA performed approximately 1.5 million needle biopsies in 2006, of which about 80% were found to be negative for adenocarcinoma. Despite this enormous testing, about 30 000 men died of prostate cancer due to the absence of early detection for effective treatment. PSA has an accuracy of about 60%, with the overriding problem that benign prostatic hyperplasia (BPH) causes an elevation in PSA as does cancer. The vast majority of men in the high prostate cancer >55 age will exhibit developing and progressing BPH. In addition PSA also results in ~25% false-negative results coupled with negative DRE for individuals who are pursuantly diagnosed with prostate cancer. These conditions have caused Carter et al.1 to conclude ‘…one key thrust of research(…) the identification of prostate cancer-specific markers in addressing the challenge of discriminating between benign disease and prostate cancer.’ Similarly, Stamey et al.2 concluded ‘the relationship of serum PSA in the last 5 years rests exclusively with benign enlargement of the prostate(…) We urgently need a serum marker that reflects prostate cancer in the current PSA range of 2 to 10 ng/ml(…) In the meantime what are we to do in the face of such massive, unwarranted PSA screening?’ To distinguish these events, a marker is needed that can provide a simple, reliable (≥80% accurate) and preferably noninvasive test that will identify individuals with early stage as well as advanced prostate cancer.
To address this issue, there has been a major thrust to identify and to develop biomarkers that can be employed in the identification and screening of individuals with prostate cancer or at risk for prostate cancer. The major direction of most investigations has been identification of blood plasma biomarkers and also genetic makers. We propose that a better approach would be the analysis of constituents of prostatic fluid, particularly the electrolyte composition. Prostatic fluid analysis can provide a more direct analysis of normal function versus pathological/ dysfunctional prostate than blood analysis. In addition, prostatic fluid provides a noninvasive procedure that can be obtained from a DRE routinely performed during a urology examination. The questions are whether or not prostatic fluid will develop and exhibit changes in composition associated specifically with prostate cancer, and can those changes be employed as an accurate clinical test for the detection of prostate cancer? To address these issues we will present a physiological and clinical review of prostatic fluid composition and dynamics and will provide evidence of the feasibility and plausibility of prostatic fluid changes as a potential ideal approach to identify and develop prostate cancer biomarkers.
In the present era dominated by molecular biology and molecular technology focus, important physiological and pathophysiological relationships are outside the mainstream interests and are being discarded by the contemporary prostate clinical and research community. We hope that this presentation will provide an educational and informative background that will eliminate any unfortunate and unfounded ‘bias’, so that a rational, objective and scientifically credible assessment can be applied to the plausibility of the concept of prostatic fluid analysis for prostate cancer. For those who are or will be engaged in this area of investigation, we hope that this will serve as a basis in support of their presentations in pursuit of funding for this much needed research.
Citrate production: the normal function and metabolic characteristic of the human prostate gland
Before addressing the composition and dynamics of prostatic fluid, it is important that we briefly describe the functional relationships of the normal human prostate gland. However, we refer the reader to our recent reviews for expanded discussion and background.3–7 The major functional and anatomical region of the normal human prostate gland is the peripheral zone. This is also the major region where malignancy arises and develops. The major function of the prostate gland is the production and secretion of prostatic fluid and more specifically, the secretion of enormously high levels of citrate. As exemplified in Table 1, the normal peripheral zone contains uniquely high levels of citrate (20–70 times greater than other tissues); and prostatic fluid contains enormous levels of citrate (400–1500 times the level found in blood plasma). This is a unique function and capability that does not exist in any other tissues. To achieve this functional capability, the glandular epithelial cells have evolved for the function of citrate production. In normal mammalian cells, citrate is an indispensable intermediate of metabolism that is critical to the operation of the Krebs cycle for ATP production and for associated synthetic pathways, and as the essential source of cytosolic acetyl CoA for lipogenesis and cholesterogenesis. In prostate, as a secretory product the citrate is an end product of metabolism. We refer to this as ‘net citrate production’, and we characterize the prostate epithelial cells as ‘citrate-producing cells’. Thus the prostate epithelial cells sacrifice the typical metabolic utilization of citrate for bioenergetic and biosynthetic activities. Under such conditions, most mammalian cells would not survive. However, the successful evolution of the prostate epithelial cells for citrate production and secretion has been achieved through the incorporation of adaptive metabolic and cellular relationships that permit their survival and functional activities.
Table 1.
Typical citrate and zinc levels in prostate
| Citrate (nmols g−1) | Zinc (nmols g−1) | |
|---|---|---|
| Normal peripheral zone | 13 000 | 3000 |
| Malignant peripheral zone | 1000 | 500 |
| Benign hyperplasia | 14000 | 4000 |
| Other soft tissues | 300 | 200 |
| Normal PR fluid | 90000 | 9000 |
| Prostate cancer PR fluid | 8000 | 1000 |
| Plasma | 100 | 15 |
The next important consideration to address and to understand is the essential adaptive metabolic relationship that is associated with the specialized function and capability of net citrate production and secretion. The critical metabolic reaction that is responsible for the accumulation of citrate in the prostate cells is m-aconitase, which is the first reaction for the entry of citrate into the Krebs cycle. The peripheral zone glandular epithelial cells have the unique ability to accumulate high levels of zinc (Table 1), which inhibits m-aconitase activity. The accumulation of zinc results from the expression and activity of ZIP1 zinc uptake transporter, which is an important genetic characteristic of these specialized cells. The Krebs cycle in these cells is truncated, and synthesized citrate accumulates for secretion into prostatic fluid. Thus the specialized glandular epithelial cells have evolved for the capability of zinc accumulation, which is required for the metabolic production and accumulation of citrate for secretion into prostatic fluid. We further characterize these cells as ‘zinc-accumulating citrate-producing’ cells. With this brief background, we can now address the issue of normal prostatic fluid composition and dynamics.
The electrolyte composition and dynamics of normal prostatic fluid
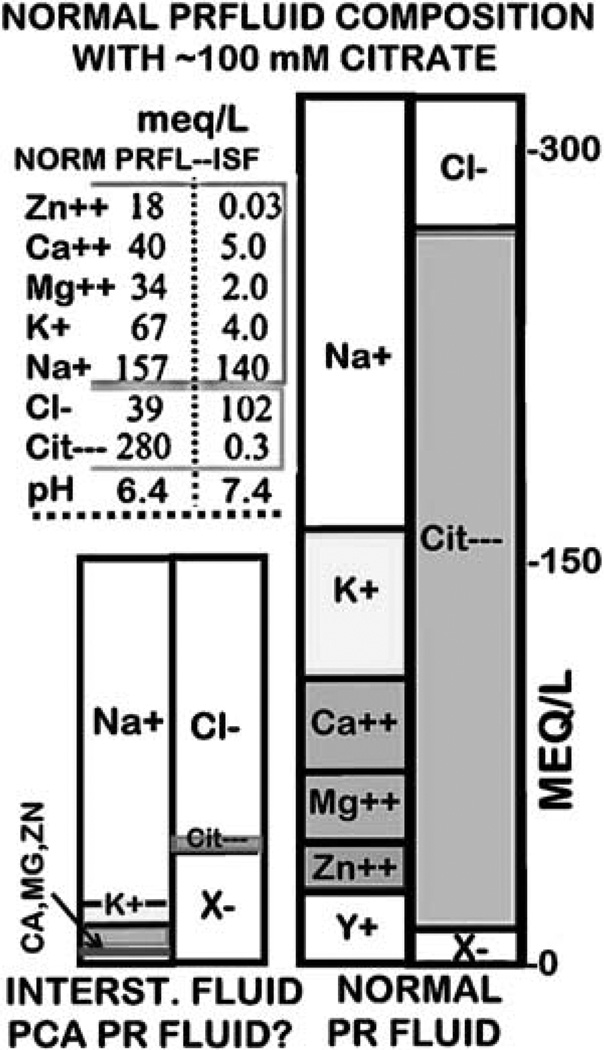
Scherstén8 first reported the high concentration of citrate in human prostatic fluid. Subsequent studies culminated in the characterization of the electrolyte composition of human prostatic fluid as described in the hallmark presentation of Nobel Laureate Dr Charles Huggins in the Harvey Lectures in 1946 entitled ‘The Prostatic Secretions’9. Kavanagh10 further advanced the information concerning the electrolyte composition and dynamics of prostatic fluid production. Figure 1 represents a Gamble plot derived from the reports of Huggins9 and Kavanagh10 of the composition of normal prostatic fluid. The first important principle to recognize is that all secretions are derived from the filtrate of blood plasma; that is, the interstitial fluid. Any changes in the composition of the secretory product from the composition of the plasma filtrate are due to cellular activities associated with the secretory process. In prostatic fluid, the major ‘driving force’ for the establishment of its electrolyte composition is the unique secretion of enormously high levels of citrate. In response to citrate as the major anion, accommodations of other electrolytes must occur to maintain the required fluid electrochemical balance. Thus, in addition to citrate, marked differences exist compared to interstitial fluid in the composition of other anions and cations. The important point for this discussion is that any prostatic condition that reduces or eliminates the concentration of citrate will result in corresponding adjustments in other anions and cations toward the direction of the composition of plasma.
Figure 1.
A Gamble plot of normal prostatic fluid electrolyte composition as compared to interstitial fluid.
The metabolic characteristics in prostate cancer
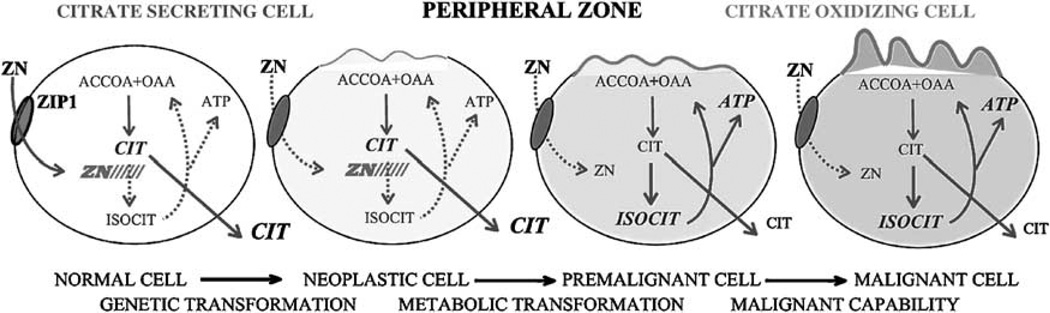
In contrast to the normal relationships described in the preceding sections, prostate cancer is characterized by marked changes in the citrate and zinc relationships (for extensive reviews see references 3–7). It is well established that citrate and zinc levels are dramatically and consistently decreased in prostate cancer. As shown in Table 1, malignant prostate tissue exhibits a 70–90% decrease in citrate and zinc levels. The development of malignancy involves a required metabolic transformation that results in the inability of the malignant cells to accumulate zinc, which is due to the down regulation of ZIP1 expression. In the absence of high zinc levels, m-aconitase activity is no longer inhibited and citrate is oxidized via a functional Krebs cycle. Therefore, in contrast to the ‘zinc-accumulating citrate-producing’ normal glandular epithelial cells, the malignant cells are ‘citrate-oxidizing cells that have lost the ability to accumulate zinc’. In addition, malignant cells are proliferating cells and require citrate for the lipogenic/cholesterogenic activities associated with membraneogenesis essential for cell growth and proliferation. Thus citrate accumulation and availability for secretion into prostatic fluid no longer exist in malignant cells. It is also important to note that this metabolic transformation occurs early in the malignant process as shown in Figure 2.
Figure 2.
The role of zinc and citrate metabolic changes in the development of prostate malignancy.
Altered prostatic fluid composition in prostate cancer
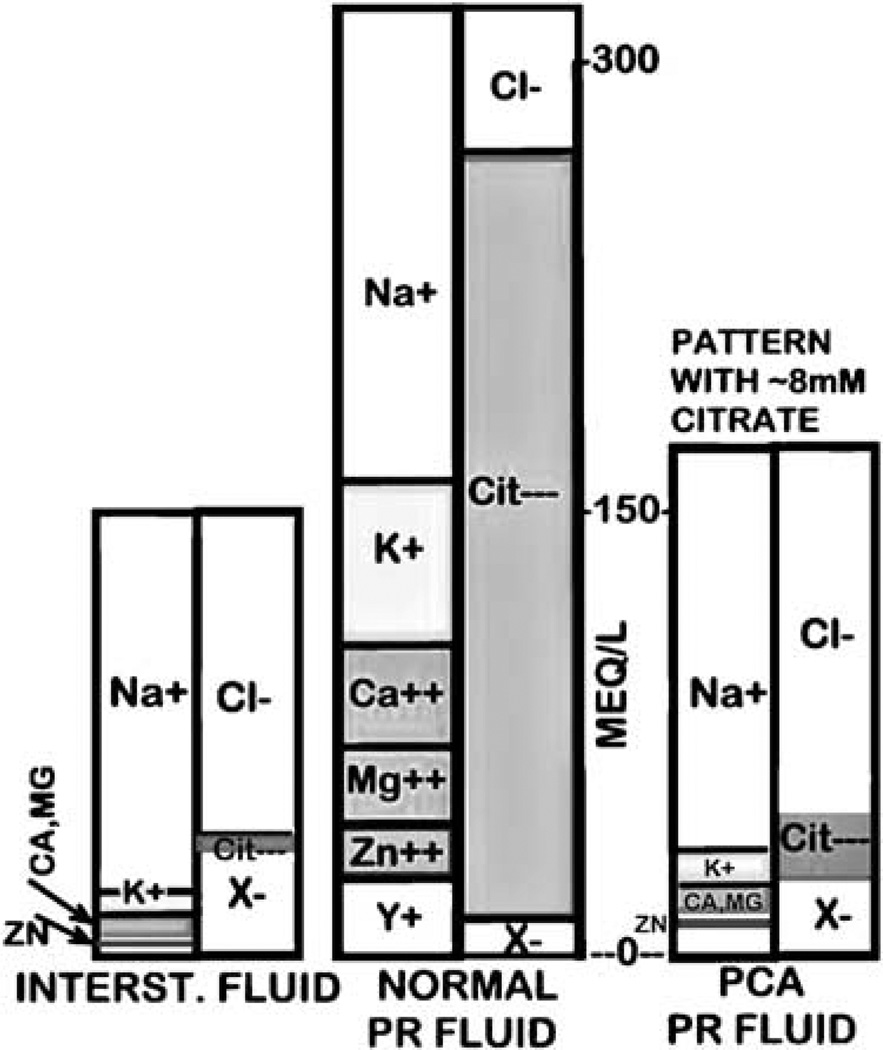
Since the adenocarcinomatous glands are no longer citrate producing glands, the concentration of citrate in prostatic fluid will be markedly diminished. As such, the attending electrolytes will also be adjusted in the direction of normal interstitial fluid composition. Figure 3 shows a representative electrolyte pattern under conditions that the citrate concentration of prostatic fluid in cancer is 8mM. In the case of zinc, the inability of the cells to accumulate high zinc levels decreases its concentration in prostatic fluid. While we expect and predict that these electrolyte relationships and changes will exist in prostate cancer, the key question is ‘What is the direct evidence in support of this concept?’ which we will address.
Figure 3.
A Gamble plot representing electrolytes changes in prostate cancer prostatic fluid that contains low (~ 8mM) citrate.
Citrate and zinc in the central zone and the issue of BPH
Because of the problem associated with PSA, we need to describe the relationship that exists with BPH and changes in the prostate gland and in prostatic fluid. Whereas the peripheral zone is the major region of malignancy, the central zone is the major region of BPH that arises from the transition zone and periurethral region. The normal central zone constitutes about 25% of the prostate compared to about 70% peripheral zone. The normal glands of the central zone are neither zincaccumulating nor citrate-producing glands. However in BPH, the central zone is ‘invaded’ by hyperplastic glands that are zinc-accumulating citrate-producing glands. It is well established that the zinc and citrate levels are low in normal central zone and elevated in BPH. The zinc and citrate concentrations of glandular BPH tissue approximate the levels found in normal peripheral zone. Consequently, the low concentration of zinc and citrate that differentiates prostate cancer from normal prostate is not interfered by BPH levels. This is a major advantage of the prostatic fluid changes over the PSA assay.
Citrate concentration in prostate cancer prostatic fluid
Huggins9 first noted that the citrate level in prostate cancer prostatic fluid was significantly lower than levels found in prostatic fluid from normal subjects or from BPH subjects. His reported values calculate as normal ~20–130mM; BPH ~10–70mM; cancer ~0.5–5mM; which reflects an approximate 95% decrease in prostate cancer levels compared to normal or BPH values. As this potentially important observation was not emphasized by Huggins at that time, it remained essentially obscured for the following 60 years. A recent important advancement was provided by Sillerud’s group reports11,12 of the results of magnetic resonance spectroscopy (MRS) determination of citrate levels of expressed prostatic fluid. They corroborated Huggins’ observation of a significant decrease in the citrate content of the cancer prostatic fluid samples. Similar results were obtained by Serkova et al.13 However, MRS calculations of citrate concentration are > 2.5-fold higher than the expected ‘true’ citrate concentration. The reported MRS normal prostatic fluid citrate values range from ~100 to 800mM with a mean of ~250mM, and MRS cancer citrate values range from ~14 to 450mM with a mean of ~140mM. The ‘true’ normal prostatic fluid citrate concentration is ~40–150mM with a mean ~90mM (Kavanagh10; Averna et al.11; our studies). Such discrepancies are apparently due to MRS signal properties; and need to be resolved by investigators who employ MRS measurements. Nevertheless, the results of the MRS studies demonstrate the significant relative decrease in citrate levels of cancer samples and corroborate the plausibility of decreased prostatic fluid citrate as a biomarker for prostate cancer detection.
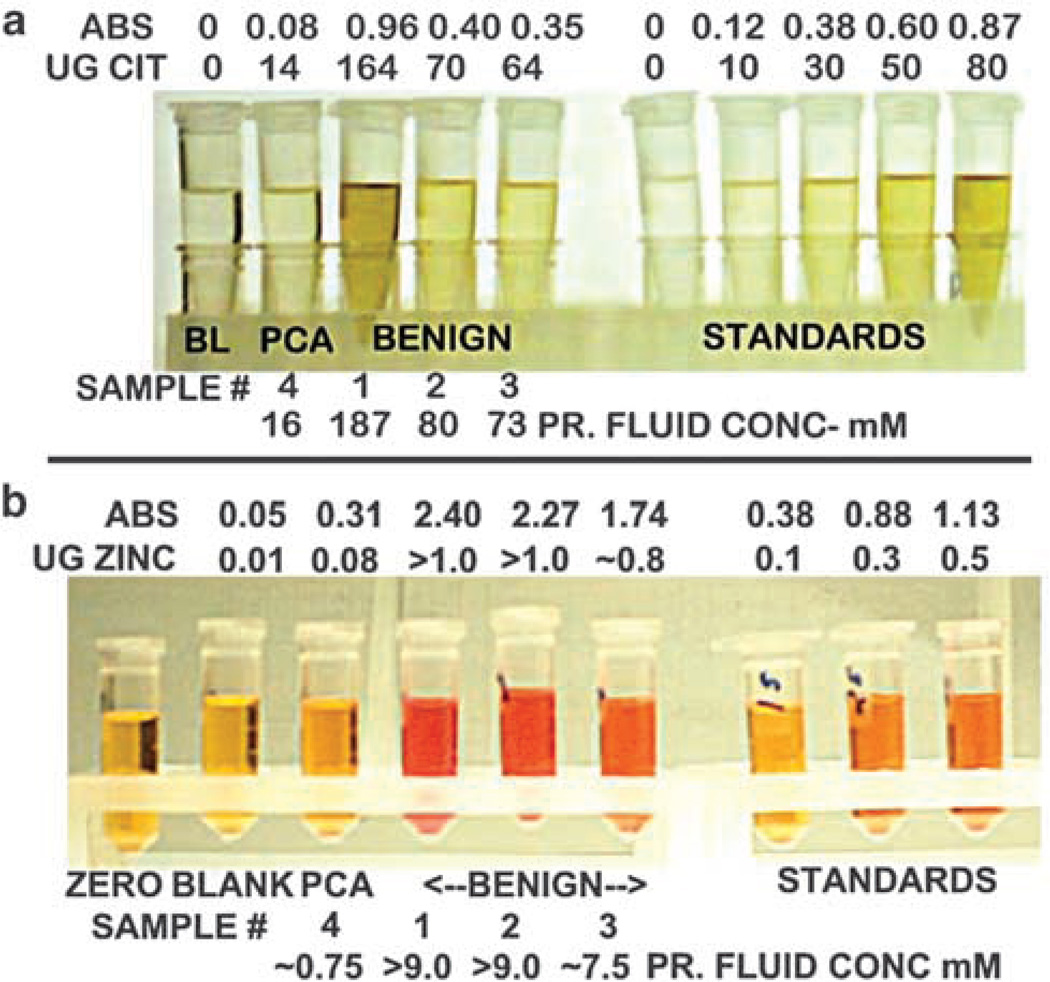
In a recent pilot study we obtained five blinded prostatic fluid samples and attempted to determine if we could identify the cancer prostatic fluid sample(s) from the BPH sample(s) with the use of a simplified colorimetric citrate assay. We anticipated that BPH samples would contain ~40–150mM citrate, and cancer samples would contain ~5–20mM citrate. The prostatic fluid samples were diluted and an aliquot of each sample was assayed against a set of standards. As shown in Figure 4, one of the samples (sample no. 4) exhibited a very low citrate level (~16mM) compared to the other samples that ranged from 73 to 187mM. The latter values are consistent with the normal/BPH range of ~40–150mM. We correctly identified that sample no. 4 was from a subject with prostate cancer. Even with the somewhat ‘crude’ colorimetric assay, the results fit the pattern that we anticipate.
Figure 4.
Simplified colorimetric assays of expressed prostatic fluid showing (a) the decreased citrate level of cancer versus BPH samples (note: the standard volume in the assay was twice the volume of prostatic fluid samples.) and (b) the decreased zinc level of cancer versus BPH samples.
Zinc concentration of prostate cancer prostatic fluid
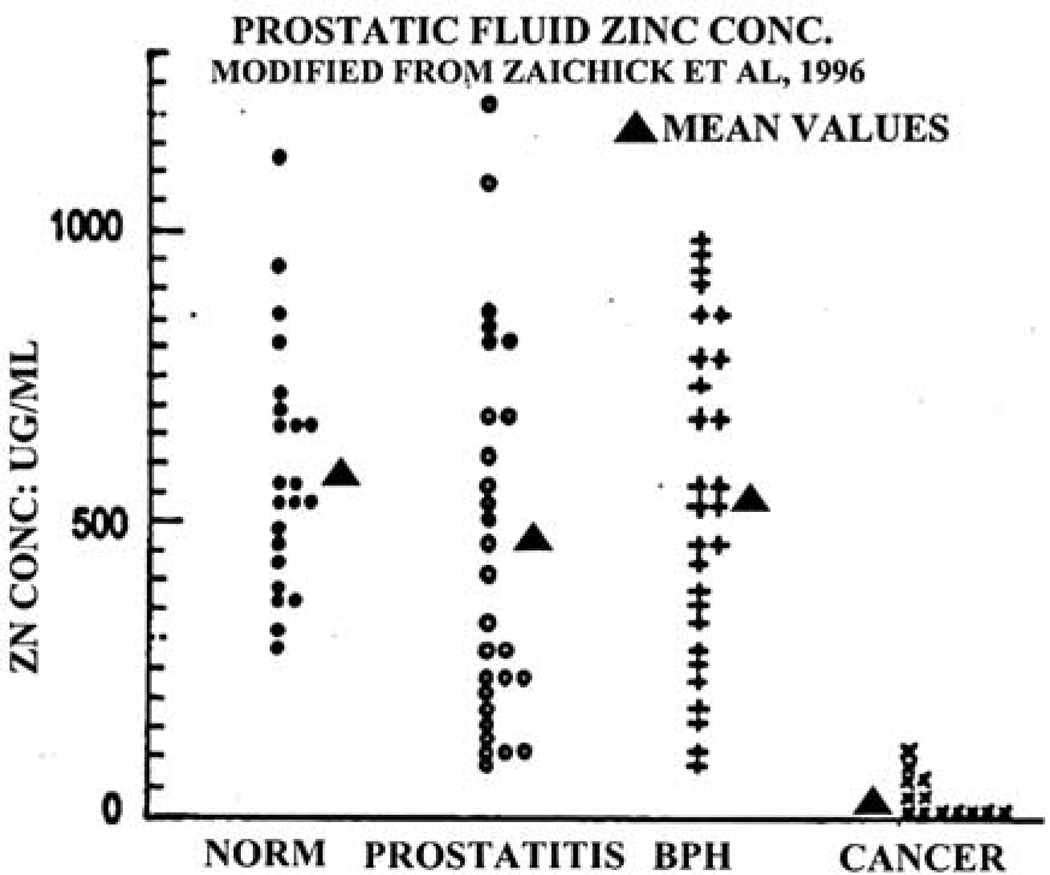
Zaichick et al.14 compared the prostatic fluid zinc levels from normal, BPH, prostatitis and cancer subjects (Figure 5). The results show that the zinc levels of normal and BPH prostatic fluid, as well as prostatitis are essentially the same. However, zinc levels of prostate cancer prostatic fluid are significantly lower, and the mean values represent a >90% decrease. Most notably, there exists no case in which the prostate cancer zinc levels exhibit the high zinc levels that characterize normal, BPH, or prostatitis prostatic fluid. This is consistent with the relationship that malignant cells do not exist as zinc-accumulating citrate-producing cells as we have discussed. It is also notable that the Zaichick et al. study results in an AUROC ≅ 0.95, which compares to PSA AUROC≅0.60.
Figure 5.
Comparison of zinc levels of cancer versus noncancer prostatic fluid samples.
We determined the zinc levels in the same prostatic fluid samples that we described above for the citrate analysis presented in Figure 4. We employed a simplified colorimetric zinc assay procedure. The results (Figure 4) show that the prostate cancer sample exhibited an extremely low zinc level, which was ~90–95% lower than the zinc levels of the BPH samples. Thus the relative zinc levels and the decrease in prostate cancer prostatic fluid parallels the citrate results in the same samples.
The potential utility of citrate and zinc analysis of prostatic fluid as specific and accurate biomarkers for prostate cancer
The fact that prostatic citrate and zinc levels are always markedly decreased in prostate malignancy does not, in itself, provide an unequivocal basis for the clinical application of prostatic fluid analysis for the screening of prostate cancer. There exists a number of attending issues that must be considered.
The consistency of the changes
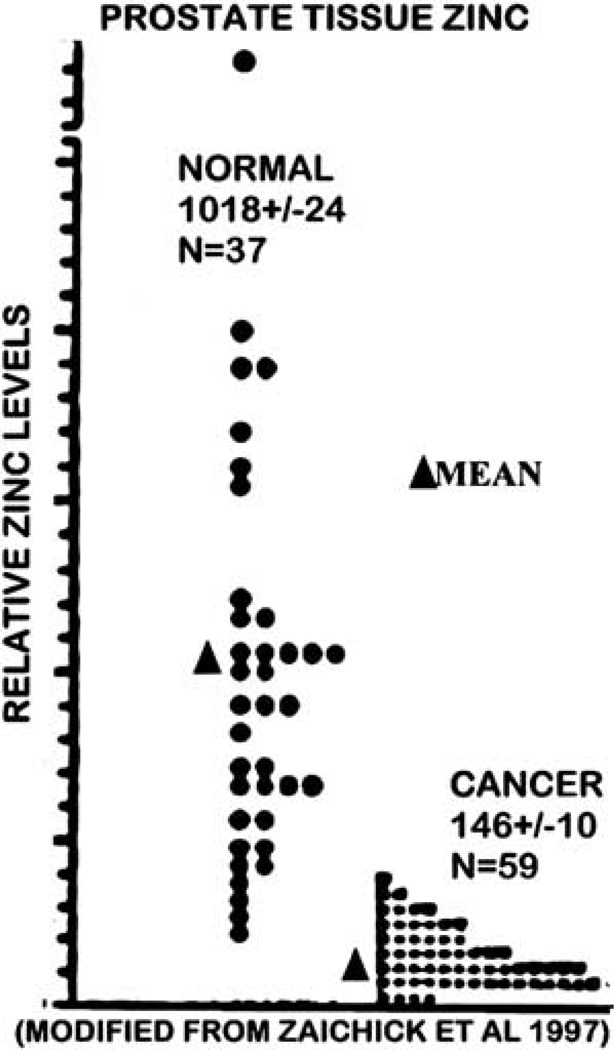
In the case of decreased zinc in prostate cancer, 17 reports since the original study of Mawson in 195215 have collectively shown a consistent 68% reduction (with a s.e. equal to only 3% of the mean) of zinc levels of cancerous prostate tissue compared to normal or BPH tissue.4 This consistent decrease occurs despite the fact that these are different studies, different subjects, different assay procedures, different stages of cancer and other variables. The same relationship exists when one analyzes the cellular zinc levels of malignant versus nonmalignant glands in situ. The consistency is further evident from another study of Zaichick et al. (Figure 6), which shows that cancer subjects always exhibit a decrease in zinc, and a ~90% decrease exists based on the mean values.
Figure 6.
Comparison of zinc levels in tissue samples of normal prostate versus prostate cancer.
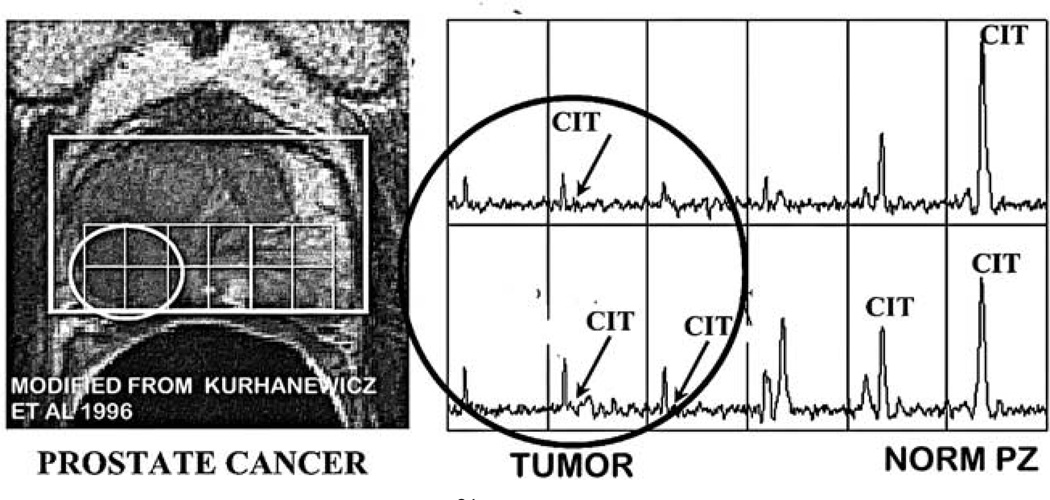
The same consistency exists regarding the decrease in citrate levels in malignancy.3–5 The most compelling evidence is obtained by the in situ imaging of the prostate gland by magnetic resonance spectroscopy imaging (MRSI) for the detection of citrate.16–18 As seen in Figure 7, a peripheral zone tumor is identified by the absence of detectable citrate compared to the opposite normal peripheral zone lobe that contains high citrate levels. There are now over forty MRS reports that consistently show the decreased citrate associated with malignancy; and the virtual absence of identification of malignancy with high citrate levels.
Figure 7.
In situ MRSI image (modified from Kurhanewicz et al24) of a subject with prostate cancer showing the marked reduction of citrate in the peripheral zone malignant locus as contrasted with the high citrate content of the normal peripheral zone glandular tissue.
Will these consistent tissue changes be reflected as consistent changes in prostatic fluid?
It must be recognized that the measurements of zinc and citrate in prostate tissue include the composite of the levels in the glandular epithelium and in the glandular luminal content, which is prostatic fluid. The normal peripheral zone tissue concentration of citrate, which is about 12mM, includes the luminal prostatic fluid that has a concentration of about 100mM. For zinc, the tissue concentration is about 3mM, and the prostatic fluid concentration is about 9mM. Consequently, the large decrease in the tissue levels of citrate and zinc must be due to a major decrease in prostatic fluid levels along with glandular epithelial cell levels. In fact this is evident from the parallelisms of the prostatic fluid changes in citrate and zinc that correlates with the tissue levels. For example, note the identical profiles of tissue and prostatic fluid zinc changes in Figures 5 and 6. This also demonstrates that the collection of expressed prostatic fluid represents the conditions that exist in the peripheral zone; that is, the peripheral zone secretion is the major contributor to prostatic fluid. This is why the decrease in citrate and in zinc is evident even in the presence of BPH; that is, the central zone glands and hyperplastic glands do not significantly contribute secretory products or volume to prostatic fluid production.
Will prostatic fluid identify early stage prostate cancer and at-risk individuals as well as advanced prostate cancer?
A potential advantage to the prostatic fluid biomarkers is the possible identification of individuals with early stage cancer or at-risk for prostate cancer. The basis for this expectation is that the genetic/metabolic transformation (Figure 2) occurs early in the development of malignancy; that is, exists in a premalignant stage. For example Cooper and Farid regarding citrate changes19 and Habib et al. regarding zinc changes20 reported that these changes were early events in prostate cancer development. This is further verified by the in situ MRS studies, which consistently reveal that the volume of prostate tissue that exhibits the decrease in citrate exceeds the volume of the histopathological identified malignant locus. This is due to the presence of a large population of cells that have been metabolically transformed as ‘premalignant cells’ that are not histologically distinguished from normal cells. This is consistent with the now-recognized ‘field effect’ in cancer biology, which demonstrates that genetic/epigenetic alternations occur in an area that is considerably larger than the histological identifiable area of cancer. It is important to recognize that histolopathological identification does not represent earliest or initial events in the development of malignancy. One must not confuse histopathological identification and characterizing of cancer progression from early stage cancer to advanced stage cancer with identification of initial/early events of malignancy that exist prior to histopathological changes.
For these reasons the prostatic fluid changes along with the prostate tissue changes in citrate and zinc will be evident in early and advanced stage cancer and with small volume malignant loci as well as with large tumors. It is further evident from the consistent major zinc decrease of ~70% that persistently exists in the 17 reports described above. The large population of subjects represented in that collection of studies must have included the myriad of developed cancer representing different stages, different Gleason grades and different tumor sizes. Yet, all the clinical evidence demonstrates that by the time that tumors are identifiable, a large and significant population of cells will exist as transformed premalignant cells in which the citrate and zinc changes already exist.
What is the expected reliability/accuracy of the prostatic fluid biomarker for prostate cancer?
To address this issue, we will consider those conditions that might introduce false-positive and false-negative results.
Positive diagnosis for prostate cancer—low citrate or low zinc
Negative diagnosis for prostate cancer—high citrate or high zinc
Potential causes of false-positive results: low citrate/zinc, absence of prostate cancer
Prostatitis
There is evidence that prostatic fluid from subjects with prostatitis will exhibit a decrease in citrate concentration. Chen et al.21 reported that prostatitis prostatic fluid samples exhibited citrate levels in the range of 51–67mM compared to normal sample values of ~131mM. However these values still fall into the expected ranges of the noncancer group. Similar results were reported by Kavanagh et al.22 It is also relevant that Zaichick et al.14 did not observe any significant decrease in the zinc level of prostatitis samples (Figure 5); whereas Kavanagh et al.22 reports a decrease in zinc. Consequently, prostatitis might present a potential issue that needs to be considered and addressed in future studies.
Premalignant stage (‘at-risk’ subjects)
As we have described, the neoplastic malignant cell exhibits the metabolic transformation as an early event that precedes histopathological evidence of malignancy; that is, the premalignant stage. Also, histological examination of biopsy cores often does not include detectable malignant loci, which leads to a negative result. However the volume of tissue that exhibits the decrease in citrate or zinc will exceed the volume of the identifiable malignant locus. Under these conditions, the citrate or zinc decrease will be interpreted as a false-positive result. However, the result would be more correctly interpreted as a possible at-risk individual.
Potential causes of false-negative results: high citrate/zinc, presence of prostate cancer
BPH
One might suggest that the elevated central zone levels of citrate and zinc due to BPH might increase the prostatic fluid levels of citrate and zinc. This could mask the decrease levels derived from the peripheral zone. However, the current evidence indicates that even in the presence of BPH, the prostatic fluid will represent the conditions of the peripheral zone as we have discussed.
Transition zone cancer
Transition zone malignancy, like peripheral zone malignancy, exhibits a decrease in citrate23 and possibly in zinc. However, it is likely that this would not be manifested in a corresponding decrease in the prostatic fluid. Therefore, a normal prostatic fluid composition with high citrate and zinc might not detect transition zone cancer. It is estimated that peripheral zone cancer represents about 80% and transition zone cancer represents about 15% of all prostate cancer cases.
Other potential prostatic fluid biomarkers
In addition to zinc and citrate, there exist other electrolytes that might also be suitable biomarkers. Presently, the changes in calcium, magnesium and potassium levels as represented in Figure 3 are largely conceptual. If the predicted changes do occur, these electrolytes should be investigated as potential biomarkers. For example, the anticipated change in potassium concentration from ~60mM in normal prostatic fluid to ~5mM in prostate cancer prostatic fluid could be an ideal biomarker. Further studies of prostatic fluid composition and changes in prostate cancer and other conditions are needed.
Conclusion
We have presented the clinical and physiological basis and evidence in support of the plausibility that changes in prostatic fluid composition (particularly citrate and zinc) will provide biomarkers for the accurate screening/ detection of prostate cancer. This approach has the advantage of being a noninvasive procedure in which expressed prostatic fluid samples can be obtained by the DRE performed during the routine urology examination. Because of the extremely high concentrations of citrate, zinc, and other electrolytes, assays can be employed that will require as little as 1ul of expressed prostatic fluid or less. The opportunity exists for immediate and rapid assays that can be performed and completed in the urology suite while the patient is still being examined. This will provide the urologist with valuable information for initial assessment of the status of the patient.
This approach also has the advantage that the changes will be identified in early as well as advanced cancer, in subjects that are not positively diagnosed by the combination of PSA and biopsy, and in ‘at-risk’ individuals. Unlike PSA, BPH will not provide false-positive results. This has the potential to eliminate an estimated 500000 per year unnecessary biopsy procedures and to reduce the number of prostate cancer deaths that results when individuals with early stage malignancy are not identified by existing procedures.
We return to the critical issue and question posed by Stamey et al,2 ‘We urgently need a(…) marker that reflects prostate cancer in the current PSA range of 2 to 10 ng/ ml(…) In the meantime what are we to do in the face of such massive, unwarranted PSA screening?’ We respond that prostatic fluid markers can provide the solution to this problem. However, in order to go forward with the exploration and development of the potential utility and clinical application of prostatic fluid analysis for prostate cancer, an informed, open-minded medical community is required. In the absence of such an environment critically required funding to pursue developmental studies and clinical trials will not be available, and an important potential tool to combat prostate cancer will not see the light of day.
Acknowledgements
Data were obtained from studies supported by NIH grants CA79903, CA093443, CA71207.
References
- 1.Carter H, DeMarzo A, Lilja H. Detection, Diagnosis, and Prognosis of Prostate Cancer. Prostate Cancer Foundation Report to the Nation on Prostate Cancer. 2004:9–26. [Google Scholar]
- 2.Stamey TA, Caldwell M, McNeal JE, Nolley R, Hemenez M, Downs J. The prostate specific antigen era in the United States is over for prostate cancer: what happened in the last 20 years? J Urol. 2004;172:1297–1301. doi: 10.1097/01.ju.0000139993.51181.5d. [DOI] [PubMed] [Google Scholar]
- 3.Costello LC, Franklin RB. Citrate metabolism in prostate and other cancers. In: Singh K, Costello LC, editors. Mitochondria and Cancer. New York: Springer; (in press) [Google Scholar]
- 4.Costello LC, Franklin RB. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Mol Cancer. 2006;5:17. doi: 10.1186/1476-4598-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costello LC, Franklin RB. The metabolism of prostate malignancy: insights into the pathogenesis of prostate cancer and new approaches for its diagnosis and treatment. Oncol Spectr. 2001;2:452–157. [Google Scholar]
- 6.Franklin RF, Costello LC. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch Biochem Biophys. 2007;463:211–217. doi: 10.1016/j.abb.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franklin RB, Milon B, Feng P, Costello LC. Zinc and zinc transporter in normal prostate function and the pathogenesis of prostate cancer. Front Biosci. 2005;10:2230–2239. doi: 10.2741/1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schersten B. Uber das vorkommen der zitronensaure in geschlechtsdrusensekreton. Skand Arch Physiol. 1929;58:90. [Google Scholar]
- 9.Huggins C. The prostatic secretions. Harvey Lect. 1946;42:148–193. [Google Scholar]
- 10.Kavanagh JP. Sodium, potassium, calcium, magnesium, zinc, citrate and chloride content of human prostatic and seminal fluid. J Re-prod Fertil. 1985;75:35–41. doi: 10.1530/jrf.0.0750035. [DOI] [PubMed] [Google Scholar]
- 11.Averna TA, Kline EE, Smith AY, Sillerud LO. A decrease in 1H nuclear magnetic resonance spectroscopically determined citrate in human seminal fluid accompanies the development of prostate adenocarcinoma. J Urol. 2005;173:433–138. doi: 10.1097/01.ju.0000148949.72314.d7. [DOI] [PubMed] [Google Scholar]
- 12.Kline EE, Treat EG, Averna TA, Davis MS, Smith AY, Sillerud LO. Citrate concentrations in human seminal fluid and expressed prostatic fluid determined via 1H nuclear magnetic resonance spectroscopy outperform prostate specific antigen in prostate cancer detection. J Urol. 2006;176:2274–2279. doi: 10.1016/j.juro.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 13.Serkova NJ, Gamito EJ, Jones RH, O’Donnell C, Brown JL, Green S, et al. The metabolites citrate, myo-inositol, and spermine are potential age-independent markers of prostate cancer in human expressed prostatic secretions. Prostate. 2008;68:620–628. doi: 10.1002/pros.20727. [DOI] [PubMed] [Google Scholar]
- 14.Zaichick VY, Sviridova TV, Zaichick SV. Zinc concentration in human prostatic fluid: normal, chronic prostatitis, adenoma and cancer. Int Urol Nephrol. 1996;28:687–694. doi: 10.1007/BF02552165. [DOI] [PubMed] [Google Scholar]
- 15.Mawson CA, Fischer MI. The occurrence of zinc in the human prostate gland. Can J Med Sci. 1952;30:336–339. doi: 10.1139/cjms52-043. [DOI] [PubMed] [Google Scholar]
- 16.Costello LC, Franklin RB, Narayan P. Citrate in the diagnosis of prostate cancer. Prostate. 1999;38:237–245. doi: 10.1002/(sici)1097-0045(19990215)38:3<237::aid-pros8>3.0.co;2-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costello L, Franklin RB, Kurhanewicz J. The metabolic characterization of prostate malignancy by magnetic resonance spectroscopy. In: Pertino J, editor. Encyclopedia of Cancer. 2nd edn. vol 3. USA: Elsevier Science; 2002. pp. 167–177. [Google Scholar]
- 18.Kurhanewicz J, Swanson MG, Nelson SJ, Vigneron DB. Combined magnetic resonance imaging and spectroscopic imaging approach to molecular imaging of prostate cancer. J Magn Reson Imaging. 2002;16:451–463. doi: 10.1002/jmri.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper JE, Farid I. The role of citric acid in the physiology of the prostate: lactic/citrate ratios in benign and malignant prostatic homogenates as an index of prostatic malignancy. J Urol. 1964;92:533–536. doi: 10.1016/S0022-5347(17)64003-5. [DOI] [PubMed] [Google Scholar]
- 20.Habib FK, Mason MK, Smith PH, Stitch SR. Cancer of the prostate: early diagnosis by zinc and hormone analysis. Br J Cancer. 1979;39:700–704. doi: 10.1038/bjc.1979.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Xu Z, Zhao H, Jiang X. Citrate in expressed prostatic secretions has the feasibility to be used as a useful indicator for the diagnosis of category IIIB prostatitis. Urol Int. 2007;78:230–234. doi: 10.1159/000099343. [DOI] [PubMed] [Google Scholar]
- 22.Kavanagh JP, Darby C, Costello CB. The response of seven prostatic fluid components to prostatic disease. Int J Androl. 1982;5:487–496. doi: 10.1111/j.1365-2605.1982.tb00280.x. [DOI] [PubMed] [Google Scholar]
- 23.Zakian KL, Eberhardt S, Hricak H, Shukla-Dave A, Kleinman S, Muruganandham M, et al. Transition zone prostate cancer: metabolic characteristics at 1H MR spectroscopic imaging-initial results. Radiology. 2003;229:241–247. doi: 10.1148/radiol.2291021383. [DOI] [PubMed] [Google Scholar]
- 24.Kurhanewicz J, Vigneron DB, Hricak H, Narayan P, Carroll P, Nelson SJ. Three dimensional hydrogen-1 MR spectroscopic imaging of the in situ human prostate with (0.24-0.7-cm3) high spatial resolution. Radiology. 1996;198:795–805. doi: 10.1148/radiology.198.3.8628874. [DOI] [PubMed] [Google Scholar]