Abstract
Background:
Patients in the ICU are thought to have abnormal circadian rhythms, but quantitative data are lacking.
Methods:
To investigate circadian rhythms in the ICU, we studied core body temperatures over a 48-h period in 21 patients (59 ± 11 years of age; eight men and 13 women).
Results:
The circadian phase position for 17 of the 21 patients fell outside the published range associated with morningness/eveningness, which determines the normative range for variability among healthy normal subjects. In 10 patients, the circadian phase position fell earlier than the normative range; in seven patients, the circadian phase position fell later than the normative range. The mean ± SD of circadian displacement in either direction (advance or delay) was 4.44 ± 3.54 h. There was no significant day-to-day variation of the 24-h temperature profile within each patient. Stepwise linear regression was performed to determine if age, sex, APACHE (Acute Physiology and Chronic Health Evaluation) III score, or day in the ICU could predict the patient-specific magnitude of circadian displacement. The APACHE III score was found to be significantly predictive of circadian displacement.
Conclusions:
The findings indicate that circadian rhythms are present but altered in patients in the ICU, with the degree of circadian abnormality correlating with severity of illness.
Almost all human physiologic processes exhibit circadian (ie, near-24-h) rhythms reflecting the synchronization of the body’s functions with each other and with the external environment. The suprachiasmatic nuclei in the hypothalamus contain the biologic clock that orchestrates circadian rhythms.1,2 In normal subjects, environmental time cues such as the light-dark cycle entrain (ie, synchronize) circadian rhythms, keeping them at a relatively constant 24-h period with a fixed temporal relationship to the environment. When left unsynchronized, the circadian pacemaker tends to drift, having an intrinsic period of about 24.2 h on average in humans.3
The circadian clock cannot be measured directly in humans, so surrogate markers have been used to measure its output. Under controlled circumstances, core body temperature (CBT), which is higher during the day than during the night, and plasma melatonin, which displays a pattern opposite to that of CBT, are considered reliable physiologic markers of circadian rhythmicity.4‐9 Plasma cortisol has also been used as a circadian indicator, but studies have cast doubt on the robustness of cortisol as a marker of circadian rhythmicity in humans.7,10
Limited data evaluating circadian rhythms in the ICU have suggested that these rhythms are considerably aberrant in patients.8,9,11‐18 Two retrospective studies11,12 evaluating circadian rhythms in patients in the ICU using CBT recordings showed an absence of circadian rhythmicity in 20% and 80% of patients in the ICU, respectively. The remaining patients showed large within-subject variability not normally observed in healthy individuals.12 A third study18 documented relatively stable circadian rhythms in CBT for comatose patients in the ICU, but did not report CBT rhythms for noncomatose patients in the ICU.
Such altered circadian rhythms may have important physiologic ramifications. For example, the efficacy, half-life, and toxicity of medications are influenced by circadian rhythms.19,20 Abnormal circadian rhythmicity is thought to be detrimental for recuperation from sepsis.8,21 Furthermore, sleep loss resulting from circadian rhythm disturbances may adversely affect respiratory muscle performance.22,23 As such, understanding circadian rhythms and their possible alterations in patients may be important for recovery in the ICU.24
The primary goals of the current study were to identify any abnormalities in the timing of the circadian rhythm as measured by CBT, while taking into account masking factors potentially affecting CBT, and to find demographic and/or clinical correlates of such abnormalities. We hypothesized that circadian rhythms in patients in the ICU would be significantly misaligned (temporally shifted) as compared with normal control subjects.
Materials and Methods
Subjects
CBT was measured in 28 noncomatose patients in the ICU at the University of Pennsylvania Medical Center (UPMC) in Philadelphia, Pennsylvania; in the ICU at Presbyterian Medical Center (PMC) in Philadelphia, Pennsylvania; and in the ICU at the University Medical Center Groningen (UMCG) in Groningen, The Netherlands. The facility at UPMC is a medical ICU with 12 acute-care beds and 12 intermediate-level-care (step-down) beds; the facility at PMC is a mixed medical and surgical ICU with 15 acute-care beds; and the facility at UMCG is a mixed medical and surgical ICU with 12 acute-care beds. To be enrolled in the study, patients had to be afebrile and not on any fever-reducing medications or sedatives. If mechanically ventilated, they also had to be on a stable ventilatory setting prior to enrollment. In these subjects, tube feeding was continuous during the day but there was no feeding during the night. This study was approved by the Institutional Review Board of the University of Pennsylvania under protocol number 280000, as well as by the Medical Ethical Committee of the UMCG under protocol number S-10-89G. Patients or their legally authorized representatives gave written consent prior to participation.
Recordings
CBT recordings were made for 48 h at a rate of one sample every 5 min. CBT was measured with a temperature-sensing Foley urinary catheter (C.R. Bard, Inc) in 11 patients and with a Mallinckrodt 12F temperature-sensing rectal probe (Mallinckrodt Inc) in 10 patients. Urinary bladder temperature monitoring is a well-investigated and validated method to determine CBT, with a low likelihood of being accidentally extruded and a high degree of accuracy.4,25,26 Rectal temperature probes have also been successfully used and validated in numerous investigations of CBT.26,27
During the 48-h study period, patients were monitored for hypothermia or fever (exclusion criteria), and they did not receive any antibiotics, nonsteroidal antiinflammatory drugs, aspirin, corticosteroids or any other medications that would have suppressed a potential fever. For all patients in the ICU, APACHE (Acute Physiology and Chronic Health Evaluation) III scores were assessed on the first day of CBT measurement.
Analyses
First, hourly averages of CBT were computed for each subject. The first 24 h were labeled “day 1” and the second 24 h were labeled “day 2.” Subsequently, the data were analyzed with repeated-measures analysis of variance (ANOVA) in a time (24 levels) by day (2 levels) design, to evaluate the day-to-day variation of the 24-h CBT profile.
Contingent upon the absence of significant day-to-day variation, a harmonic regression model of two sinusoids, with a fundamental period of 24 h and a harmonic period of 12 h, respectively, was fitted to the individual patient’s original 48-h time series (ie, the 5-min samples, not the hourly averages).27,28 As a marker of the circadian phase position of the rhythm in CBT, the clock time of the CBT minimum in the regression model was assessed. In addition, the CBT range was determined as the difference between the minimal and the maximal temperatures in the regression model.
For each patient, circadian phase position values were compared with a database of healthy, extreme morning- and evening-type individuals studied under constant-routine conditions.27‐30 This database had been established previously to expose the boundaries of interindividual variability in circadian phase among healthy individuals, and may, therefore, serve as a reference for evaluating circadian abnormalities. For patients in the current study who had a circadian phase position of the CBT minimum that was outside the reference interval, which ranged from 04:38 to 06:45, the absolute time difference between the observed circadian phase position and the nearest boundary of the reference interval was computed as a measure of the magnitude of circadian displacement. This circadian displacement was a primary outcome measure of the study.
To assess associations of demographic and medical variables with the degree of circadian displacement, correlation analysis (Pearson’s r) and stepwise linear regression were performed. Finally, one-way ANOVA was employed to detect differences in circadian displacement between mechanically ventilated and nonventilated patients, and among disease categories: renal insufficiency, myasthenia gravis, COPD exacerbation, and ARDS.
Results
Seven of the 28 patients were excluded from the analyses because a fever (CBT > 38°C) developed in the 5 days before or during the study period, leaving a group of 21 patients in the ICU. Of these 21 patients, nine were hospitalized in the ICU at the UPMC in Philadelphia, Pennsylvania; six were hospitalized in the ICU at PMC in Philadelphia, Pennsylvania; and six were hospitalized in the ICU at the UMCG in Groningen, The Netherlands. Seventeen of the 21 patients were on mechanical ventilation; all were tube fed. Table 1 summarizes the demographic and clinical information of the study population.
Table 1.
—Subject Demographics and Clinical Information
| Demographics and Clinical Information | Data |
| No. subjects | 21 |
| Male (female) | 8 (13) |
| Age, mean ± SD, min-max, y | 59 ± 11, 33-75 |
| APACHE III score, mean ± SD, min-max | 49 ± 22, 29-95 |
| Mechanically ventilated | 17 |
| Renal insufficiency | 10 |
| Myasthenia gravis | 3 |
| COPD exacerbation | 6 |
| ARDS | 2 |
| First day of CBT recording,a mean ± SD, min-max | 19.9 ± 18.9, 2-45 |
Data are presented as No. subjects unless indicated otherwise. APACHE = Acute Physiology and Chronic Health Evaluation; CBT = core body temperature; max = maximum; min = minimum.
Relative to day of ICU admission.
Repeated-measures ANOVA of the hourly averages across the 48 h of CBT recording in all 21 patients showed no significant effects for day (F[1,20] = 0.045, P = .84) and for time by day interaction (F[23,460] = 0.809, P = .54). Thus, there was little day-to-day variation of the 24-h CBT profile within each patient. Analyses were, therefore, continued by fitting a harmonic regression model to each patient’s CBT time series. The 24-h rhythm component of CBT was statistically significant31 in each subject (P < .001). The range between the minimum and maximum of CBT was found to be 0.82 ± 0.60°C. The normative range for body temperature in healthy subjects under constant routine conditions (experimental conditions even more controlled than those in the ICU) is 0.5°C, and in ambulatory healthy subjects it goes up to about 1°C.32,33 The mean ± SD for the circadian phase position, as estimated by the timing of the CBT minimum in the regression model, was 10:07 (HH:MM) ± 417 min.
The individual subjects’ circadian phase positions are shown in Figure 1. Substantial interindividual variability in the timing of the CBT rhythm was observed: Circadian phase position values spanned almost the entire 24 h of the day. A Kolmogorov-Smirnov test confirmed that the distribution of circadian phase positions of the CBT rhythm was not significantly different from a uniform distribution across the 24 h of the day (Z = 1.12, P = .16). This indicates that the minimum of CBT was not consistently anchored in the early-morning hours, as is typical for healthy normal individuals, but could be positioned at any hour of the day. This finding demonstrates that the timing of circadian rhythms was abnormal in the patients in the ICU.
Figure 1.
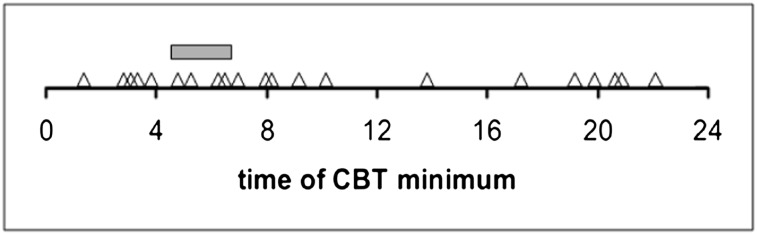
Subjects’ circadian phase positions. Each triangle indicates an individual patient’s clock time of the estimated CBT minimum, plotted against clock time (in hours). Data from all 21 patients in the ICU included in the study are shown. The gray bar indicates the reference interval for healthy normal subjects (04:38-06:45), taken from a database of healthy extreme morning- and evening-type individuals studied under constant-routine conditions.27 The vast majority of healthy normal subjects would be expected to have circadian phase positions inside this relatively narrow reference interval. In contrast, the circadian phase positions of patients in the ICU were distributed over the entire 24 h of the day. CBT = core body temperature.
For 17 of the 21 patients, the circadian phase position fell outside the reference interval for healthy normal subjects of between 04:38 and 06:45 (gray bar in Fig 1). For these 17 patients, the mean ± SD of circadian displacement, defined as the absolute deviation from the nearest boundary of the reference interval, was 4.44 ± 3.54 h. For 10 of them, the circadian phase position was closest to the early boundary of the reference interval (ie, 04:38), which suggests that the circadian rhythm in these patients was relatively advanced. For the remaining seven patients, the circadian phase position was closest to the late boundary of the reference interval (ie, 06:45), which suggested that the circadian rhythm in these patients was relatively delayed.
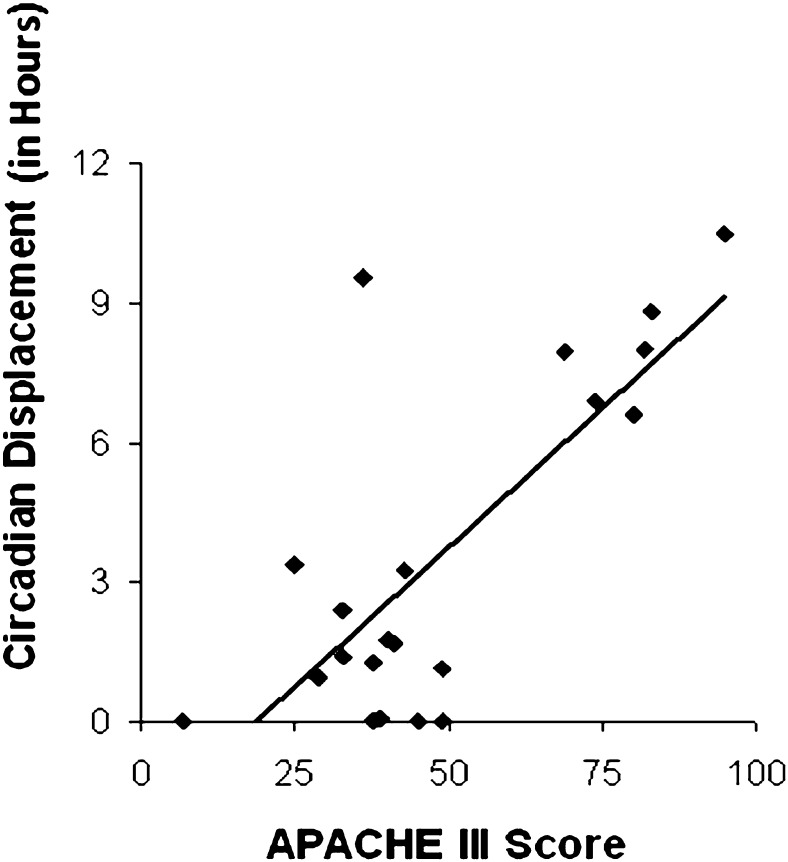
Stepwise linear regression yielded the APACHE III score as the only variable to be significantly predictive of circadian displacement (r = 0.750; F[1,19] = 24.4; P < .001). Higher APACHE III scores were associated with larger circadian displacements, as shown in Figure 2. One-way ANOVA revealed a trend for an effect of mechanical ventilation (F[1,19] = 3.81; P = .066): the mean ± SE of circadian displacement was 0.61 ± 0.35 h for the four nonventilated subjects, and 4.30 ± 0.90 h for the 17 mechanically ventilated subjects. No statistically significant differences in circadian displacement were found among the different disease categories (F[3,17] = 0.33; P = .81).
Figure 2.
Scatter plot of APACHE III score vs circadian displacement (in hours) in all 21 subjects, with trend line. Higher APACHE III scores were associated with greater circadian displacement (r = 0.750, P < .001). APACHE = Acute Physiology And Chronic Health Evaluation.
Discussion
The main finding of this study was that, although circadian rhythms were detectable in the CBT recordings of all 21 patients in the ICU investigated, they showed abnormal circadian phase positions. To minimize masking effects on CBT measurements, we required the patients to be afebrile, off any fever-reducing medications, and on a stable ventilatory setting (if mechanically ventilated) prior to enrollment in the investigation. As such, most patients were studied several weeks into their hospital stay (20 days on average), and were potentially in the recovery period of their illness. This limited the overall severity of illness as measured by APACHE III scores. Even so, we observed substantial circadian displacement in the patients. Moreover, circadian displacement was greatest in patients with the highest APACHE III scores. Of the 21 patients, only four had a circadian phase position of the CBT minimum that fell inside the reference interval for healthy normal subjects as bracketed by extreme morning- and evening-type individuals.27
Our finding of altered circadian phase positions in ICU patients is consistent with the results of Gehlbach et al,9 who documented abnormally timed circadian rhythm in the melatonin profiles of 11 of 15 patients who were mechanically ventilated and IV-sedated and admitted to the ICU 1 to 3 days earlier. Although the melatonin profiles were derived from urinary sampling of a melatonin metabolite, which limits the temporal resolution of the data, the circadian rhythm was found to be stable across 2 measurement days. Similarly, Tweedie and colleagues11 found that the timing of circadian rhythm in CBT varied substantially among 15 patients who spent at least 8 days in the ICU. In this study, the peak of the circadian rhythm also varied by several hours from day to day within subjects. Our data differed from those of Tweedie et al11 in that we observed only small day-to-day variation in the timing of the CBT rhythm within patients. This may be a consequence of the various steps we took (eg, exclusion of febrile patients and those with fever-reducing medications) to limit masking effects on CBT recordings.
Other ICU studies have documented circadian disruptions of a different nature. Dauch and Bauer12 recorded the CBT profiles of 31 patients suffering from severe cerebral damage and showed only 20% to have a sinusoidal shape in their CBT profile. However, this result was not supported by proper statistical analysis, because the CBT measurements were transformed into rank numbers before analysis (the time of day with the lowest temperature was ranked as 1, the time of day with the second lowest temperature was ranked as 2, and so forth). No consideration was given to the absolute temperatures, or to the range of oscillation as actually observed. Paul and Lemmer34 recorded the tympanic temperature and sampled plasma melatonin in 24 analgo-sedated patients. They did not detect significant circadian rhythms in body temperature in any of their patients, but tympanic temperature is less accurate in capturing circadian rhythmicity than is CBT.35 For plasma melatonin, they reported that compared with those of healthy control subjects, the 24-h profiles were greatly disturbed, with elevated daytime levels and reduced nighttime levels.34 This would appear to indicate that circadian rhythmicity in the analgo-sedated patients was phase shifted and/or dampened, but the authors suggested that their results could have been confounded by medications used such as benzodiazepines. Mundigler et al8 examined melatonin profiles in critically ill septic and nonseptic patients and observed significant disruptions in the circadian rhythm of the nonseptic patients. In this study, as in the study of Gehlbach et al,9 the melatonin profiles were derived from a urinary metabolite. The observations of Mundigler et al8 may have been systematically biased by having patients wear eye masks to block the melatonin-suppressing effect of light exposure during the hours of 10:00 pm to 6:00 am, but not at other times of the day.8 Regardless of the methodologic limitations of these previous studies, they all point to circadian abnormalities in patients in the ICU.
Our finding that the circadian phase position of the CBT rhythm was more aberrant with greater APACHE III scores suggests that the severity of illness may directly or indirectly contribute to changes in the circadian rhythms in patients in the ICU. Altered circadian phase positions in patients in the ICU may also result from abnormal temporal cues (zeitgebers) in the ICU environment, which can cause desynchronization of the circadian pacemaker. Indeed, studies have suggested that zeitgebers are abnormal in the ICU.24,36 Light patterns, in particular, appear to be different for patients in the ICU compared with normal control subjects.35,36 Ambient light is a relatively potent zeitgeber in human beings, but if patients in the ICU receive insufficient and/or improperly timed light, changes in the circadian rhythmicity, such as those observed in the current study, may result.
In the context of circadian abnormalities, it is noteworthy that patients in the ICU have abnormal sleep-wake patterns.13,37‐42 Patients in the ICU tend to sleep in short bouts approximately evenly dispersed over day and night. Whether the sleep-wake pattern influences circadian rhythmicity directly is under debate,43 but sleep is also associated with shielding from light exposure due to eyelid closure. Thus, even if light levels in the ICU are not disruptive to circadian rhythms per se, diurnal sleep bouts may still result in abnormal light exposure and consequently disrupt circadian rhythmicity.
Moreover, altered circadian rhythmicity may play a role in the pathogenesis of abnormal sleep-wake patterns. Environmental noise has been shown to be disruptive to sleep in the ICU,13,44,45 but it is not the only sleep-disturbing factor.13 In view of the normal regulatory relationship between circadian rhythmicity and sleep propensity,1,46,47 our finding of significantly altered CBT rhythms in patients in the ICU could help further explain why sleep is abnormal in the ICU. If sleep is beneficial for clinical recovery, as is widely believed, then it may be worthwhile to investigate means to normalize circadian rhythms in the ICU, because this may lead to reduced sleep disruption.
Knowledge of the circadian phase position in critically ill patients may also have direct physiologic and therapeutic implications. For instance, pulmonary and peripheral muscle strength vary across the circadian cycle.48,49 Patients with COPD show circadian fluctuations in pulmonary function, with circadian differences between peak and trough values of FEV1 and peak expiratory flow rates of 25% to 50%.19,48 It may, thus, be useful to wean patients who are mechanically ventilated, especially those with COPD, when respiratory muscle strength is at its circadian peak. This time may be predictable based on the temporal relationship between the pulmonary function rhythm and the CBT rhythm. Patients at risk of respiratory failure with little physiologic reserve may benefit particularly from circadian rhythm-tailored weaning strategies. Healing may be impacted by circadian rhythms,50 and alignment of central and peripheral oscillators by zeitgebers, such as feeding regimes, may benefit patients.51,52
Drug efficacy and half-life depend on circadian timing. Therefore, chronotherapy (the administration of drugs at specific circadian times) may benefit patients in the ICU by potentially enhancing drug efficacy and/or decreasing toxicity.19,20 Because CBT rhythms appeared to be relatively stable across days in our 48-h study period, and are comparatively easy and inexpensive to measure, CBT recordings may be useful as a circadian marker in future research to evaluate the efficacy of circadian-based drug delivery strategies.
We used only one circadian phase marker in our study. We selected CBT rather than plasma melatonin as a marker because melatonin secretion is suppressed by light exposure, and light patterns in the ICU are abnormal and elevated at night.53 Moreover, sleep (and, therefore, eye closure) in the ICU is fragmented and distributed across the entire 24-h day.13 As a consequence, there is considerable potential for masking (confounding) effects on plasma melatonin profiles because of light exposure in the ICU. Furthermore, the effects on melatonin levels of medications typically used to treat patients in the ICU have not been studied comprehensively. Our successful use of CBT profiles to mark the circadian phase represents an important methodologic advance, given the need for investigators to identify biologically valid and reproducible measurements pertaining to sleep and circadian rhythmicity. Nonetheless, CBT cannot be examined as a circadian phase marker in patients with fever or hypothermia. For this reason, CBT and melatonin-based measurements will likely be complementary in examining circadian rhythms. It should also be noted that we used two different techniques to measure CBT, which may have increased the variability in the results.
Although several factors cause masking effects on CBT profiles, the most important are typically absent from the ICU environment. Ambient temperature in the ICU varies little because of active climate control. Physical activity and postural changes influence CBT,54 but patients in the ICU are generally not physically active and typically undergo postural changes by standardized protocols (and the patients were not upright). The onset of sleep is associated with a gradual drop in CBT,55 and the occurrence of wakefulness gradually reverses this effect; however, the sleep-related effect can only develop fully if sleep is consolidated. In patients in the ICU, sleep and wakefulness are very fragmented and almost evenly distributed over the 24 h of the day13; therefore, masking from sleep is unlikely to occur systematically. Food intake has also been found to affect CBT,56 but when patients in the ICU are tube fed, this effect should be minimal. All the patients in this study were tube fed according to the same algorithms (during the day, not at night). Nonetheless, both feeding51,52,57 and eye closure58‐60 can affect and entrain circadian rhythms. Finally, hypothermia and fever (or fever-reducing medications) may mask circadian rhythmicity measured by CBT, but these effects were avoided because we selected patients who were neither hypothermic nor febrile. The patients did not develop fever during the 48 h they were studied.
Conclusions
In conclusion, we demonstrated that the circadian rhythm of CBT in critically ill patients in the ICU to be considerably shifted relative to normal control subjects. Patients with higher APACHE III scores showed greater circadian phase displacement. However, circadian rhythmicity was relatively stable in patients over 48 h of recording. Increased knowledge and consideration of patients’ circadian rhythmicity may have a positive impact on therapeutic interventions (eg, drug administration, weaning) and the quality of sleep in the ICU. Although the causes of circadian abnormalities were not elucidated by the current study, and further research is necessary, the finding of abnormal circadian rhythms in the ICU strongly suggests that appropriately timed patient care and treatment strategies aimed at realigning circadian rhythms may be beneficial for clinical recovery in critically ill patients.
Acknowledgments
Author contributions: Dr Schwab is the guarantor for the manuscript.
Dr. Gazendam: contributed to the studies in The Netherlands, analysis of the data, and writing of much of the manuscript.
Dr. Van Dongen: contributed to the statistical analyses and the writing of the manuscript.
Ms. Grant: contributed to the domain knowledge, review of the literature, and editing of the manuscript.
Dr. Freedman: contributed to the performance of the studies and review of the data at the University of Pennsylvania.
Dr. Zwaveling: contributed to the review of the data and editing of the manuscript.
Dr. Schwab: contributed to the design of the study, review of the data, and writing and editing of a significant portion of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: This work was performed at UPMC, PMC, and UMCG.
Abbreviations
- ANOVA
analysis of variance
- APACHE
Acute Physiology and Chronic Health Evaluation
- CBT
core body temperature
- PMC
Presbyterian Medical Center
- UMCG
University Medical Center Groningen
- UPMC
University of Pennsylvania Medical Center
Footnotes
Funding/Support: This work and Dr Schwab were supported by National Institutes of Health [Award K24 HL67948]. Dr Van Dongen and Ms Grant were supported by Congressionally Directed Medical Research Program [Award W81XWH-05-1-0099].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Czeisler CA, Weitzman Ed, Moore-Ede MC, Zimmerman JC, Knauer RS. Human sleep: its duration and organization depend on its circadian phase. Science. 1980;210(4475):1264-1267 [DOI] [PubMed] [Google Scholar]
- 2.Chan MC, Spieth PM, Quinn K, Parotto M, Zhang H, Slutsky AS. Circadian rhythms: from basic mechanisms to the intensive care unit. Crit Care Med. 2012;40(1):246-253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284(5423):2177-2181 [DOI] [PubMed] [Google Scholar]
- 4.Nierman DM. Core temperature measurement in the intensive care unit. Crit Care Med. 1991;19(6):818-823 [DOI] [PubMed] [Google Scholar]
- 5.Shilo L, Dagan Y, Smorjik Y, et al. Patients in the intensive care unit suffer from severe lack of sleep associated with loss of normal melatonin secretion pattern. Am J Med Sci. 1999;317(5):278-281 [DOI] [PubMed] [Google Scholar]
- 6.Duffy JF, Dijk DJ. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms. 2002;17(1):4-13 [DOI] [PubMed] [Google Scholar]
- 7.Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17(2):181-193 [DOI] [PubMed] [Google Scholar]
- 8.Mundigler G, Delle-Karth G, Koreny M, et al. Impaired circadian rhythm of melatonin secretion in sedated critically ill patients with severe sepsis. Crit Care Med. 2002;30(3):536-540 [DOI] [PubMed] [Google Scholar]
- 9.Gehlbach BK, Chapotot F, Leproult R, et al. Temporal disorganization of circadian rhythmicity and sleep-wake regulation in mechanically ventilated patients receiving continuous intravenous sedation. Sleep. 2012;35(8):1105-1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheer FA, Van Paassen B, Van Montfrans GA, et al. Human basal cortisol levels are increased in hospital compared to home setting. Neurosci Lett. 2002;333(2):79-82 [DOI] [PubMed] [Google Scholar]
- 11.Tweedie IE, Bell CF, Clegg A, Campbell IT, Minors DS, Waterhouse JM. Retrospective study of temperature rhythms of intensive care patients. Crit Care Med. 1989;17(11):1159-1165 [DOI] [PubMed] [Google Scholar]
- 12.Dauch WA, Bauer S. Circadian rhythms in the body temperatures of intensive care patients with brain lesions. J Neurol Neurosurg Psychiatry. 1990;53(4):345-347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001;163(2):451-457 [DOI] [PubMed] [Google Scholar]
- 14.Lebelle PDJ, Prevot E. The temperature rhythms delay of intensive care patients after surgery. Sleep. 2001;24:A200-A201 [Google Scholar]
- 15.Gazendam JAC. VDH, Freedman NS. The circadian rhythm of core body temperature in the intensive care unit. J Intensive Care Med. 2002;28:S153 [Google Scholar]
- 16.Olofsson K, Alling C, Lundberg D, Malmros C. Abolished circadian rhythm of melatonin secretion in sedated and artificially ventilated intensive care patients. Acta Anaesthesiol Scand. 2004;48(6):679-684 [DOI] [PubMed] [Google Scholar]
- 17.Drouot X, Cabello B, d’Ortho MP, Brochard L. Sleep in the intensive care unit. Sleep Med Rev. 2008;12(5):391-403 [DOI] [PubMed] [Google Scholar]
- 18.Lazreg TBNW, Skhouri H, Saiid R, Dogui M. Altered circadian rhythms in rectal temperature and circulating blood cells in intensive care unit patients. Biol Rhythm Res. 2001;42(4):337-347 [Google Scholar]
- 19.D’Alonzo GE, Smolensky MH, Feldman S, et al. Twenty-four hour lung function in adult patients with asthma. Chronoptimized theophylline therapy once-daily dosing in the evening versus conventional twice-daily dosing. Am Rev Respir Dis. 1990;142(1):84-90 [DOI] [PubMed] [Google Scholar]
- 20.Focan C. Circadian rhythms and cancer chemotherapy. Pharmacol Ther. 1995;67(1):1-52 [DOI] [PubMed] [Google Scholar]
- 21.Carlson DE, Chiu WC. The absence of circadian cues during recovery from sepsis modifies pituitary-adrenocortical function and impairs survival. Shock. 2008;29(1):127-132 [DOI] [PubMed] [Google Scholar]
- 22.Cooper KR, Phillips BA. Effect of short-term sleep loss on breathing. J Appl Physiol. 1982;53(4):855-858 [DOI] [PubMed] [Google Scholar]
- 23.Chen HI, Tang YR. Sleep loss impairs inspiratory muscle endurance. Am Rev Respir Dis. 1989;140(4):907-909 [DOI] [PubMed] [Google Scholar]
- 24.Freedman NS, Kotzer N, Schwab RJ. Patient perception of sleep quality and etiology of sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 1999;159(4 pt 1):1155-1162 [DOI] [PubMed] [Google Scholar]
- 25.Lilly JK, Boland JP, Zekan S. Urinary bladder temperature monitoring: a new index of body core temperature. Crit Care Med. 1980;8(12):742-744 [DOI] [PubMed] [Google Scholar]
- 26.Lefrant JY, Muller L, de La Coussaye JE, et al. Temperature measurement in intensive care patients: comparison of urinary bladder, oesophageal, rectal, axillary, and inguinal methods versus pulmonary artery core method. Intensive Care Med. 2003;29(3):414-418 [DOI] [PubMed] [Google Scholar]
- 27.Kerkhof GA, Van Dongen HP. Morning-type and evening-type individuals differ in the phase position of their endogenous circadian oscillator. Neurosci Lett. 1996;218(3):153-156 [DOI] [PubMed] [Google Scholar]
- 28.Dawson D, Lushington K, Lack L, Campbell S, Matthews C. The variability in circadian phase and amplitude estimates derived from sequential constant routines. Chronobiol Int. 1992;9(5):362-370 [DOI] [PubMed] [Google Scholar]
- 29.Mongrain V, Carrier J, Dumont M. Circadian and homeostatic sleep regulation in morningness-eveningness. J Sleep Res. 2006;15(2):162-166 [DOI] [PubMed] [Google Scholar]
- 30.Duffy JF, Dijk DJ, Hall EF, Czeisler CA. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J Investig Med. 1999;47(3):141-150 [PMC free article] [PubMed] [Google Scholar]
- 31.Van Dongen HP, Olofsen E, VanHartevelt JH, Kruyt EW. A procedure of multiple period searching in unequally spaced time-series with the Lomb-Scargle method. Biol Rhythm Res. 1999;30(2):149-177 [DOI] [PubMed] [Google Scholar]
- 32.Minors DS, Waterhouse JM. The use of constant routines in unmasking the endogenous component of human circadian rhythms. Chronobiol Int. 1984;1(3):205-216 [DOI] [PubMed] [Google Scholar]
- 33.Rietveld WJ, Minors DS, Waterhouse JM. Circadian rhythms and masking: an overview. Chronobiol Int. 1993;10(4):306-312 [DOI] [PubMed] [Google Scholar]
- 34.Paul T, Lemmer B. Disturbance of circadian rhythms in analgosedated intensive care unit patients with and without craniocerebral injury. Chronobiol Int. 2007;24(1):45-61 [DOI] [PubMed] [Google Scholar]
- 35.Meyer TJ, Eveloff SE, Bauer MS, Schwartz WA, Hill NS, Millman RP. Adverse environmental conditions in the respiratory and medical ICU settings. Chest. 1994;105(4):1211-1216 [DOI] [PubMed] [Google Scholar]
- 36.Gazendam JACFN, Schwab RJ. Light/dark cycles are abnormal in the intensive care unit. Am J Respir Crit Care Med. 1999;159:A827 [Google Scholar]
- 37.Hilton BA. Quantity and quality of patients’ sleep and sleep-disturbing factors in a respiratory intensive care unit. J Adv Nurs. 1976;1(6):453-468 [DOI] [PubMed] [Google Scholar]
- 38.Broughton R, Baron R. Sleep patterns in the intensive care unit and on the ward after acute myocardial infarction. Electroencephalogr Clin Neurophysiol. 1978;45(3):348-360 [DOI] [PubMed] [Google Scholar]
- 39.Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in nine patients receiving postoperative care. Br Med J (Clin Res Ed). 1985;290(6474):1029-1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richards KC, Bairnsfather L. A description of night sleep patterns in the critical care unit. Heart Lung. 1988;17(1):35-42 [PubMed] [Google Scholar]
- 41.Cooper AB, Thornley KS, Young GB, Slutsky AS, Stewart TE, Hanly PJ. Sleep in critically ill patients requiring mechanical ventilation. Chest. 2000;117(3):809-818 [DOI] [PubMed] [Google Scholar]
- 42.Hardin KA. Sleep in the ICU: potential mechanisms and clinical implications. Chest. 2009;136(1):284-294 [DOI] [PubMed] [Google Scholar]
- 43.Danilenko KV, Cajochen C, Wirz-Justice A. Is sleep per se a zeitgeber in humans? J Biol Rhythms. 2003;18(2):170-178 [DOI] [PubMed] [Google Scholar]
- 44.Walder B, Francioli D, Meyer JJ, Lançon M, Romand JA. Effects of guidelines implementation in a surgical intensive care unit to control nighttime light and noise levels. Crit Care Med. 2000;28(7):2242-2247 [DOI] [PubMed] [Google Scholar]
- 45.Li SY, Wang TJ, Vivienne Wu SF, Liang SY, Tung HH. Efficacy of controlling night-time noise and activities to improve patients’ sleep quality in a surgical intensive care unit. J Clin Nurs. 2011;20(3-4):396-407 [DOI] [PubMed] [Google Scholar]
- 46.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1(3):195-204 [PubMed] [Google Scholar]
- 47.Lavie P, Zvuluni A. The 24-hour sleep propensity function: experimental bases for somnotypology. Psychophysiology. 1992;29(5):566-575 [DOI] [PubMed] [Google Scholar]
- 48.Hetzel MR, Clark TJ. Comparison of normal and asthmatic circadian rhythms in peak expiratory flow rate. Thorax. 1980;35(10):732-738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gauthier A, Davenne D, Gentil C, Van Hoecke J. Circadian rhythm in the torque developed by elbow flexors during isometric contraction. Effect of sampling schedules. Chronobiol Int. 1997;14(3):287-294 [DOI] [PubMed] [Google Scholar]
- 50.Idda ML, Kage E, Lopez-Olmeda JF, Mracek P, Foulkes NS, Vallone D. Circadian timing of injury-induced cell proliferation in zebrafish. PLoS ONE. 2012;7(3):e34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106(11):4453-4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schibler U, Ripperger J, Brown SA. Peripheral circadian oscillators in mammals: time and food. J Biol Rhythms. 2003;18(3):250-260 [DOI] [PubMed] [Google Scholar]
- 53.Fulbrook P. Core body temperature measurement: a comparison of axilla, tympanic membrane and pulmonary artery blood temperature. Intensive Crit Care Nurs. 1997;13(5):266-272 [DOI] [PubMed] [Google Scholar]
- 54.Minors DS, Waterhouse JM. Masking in humans: the problem and some attempts to solve it. Chronobiol Int. 1989;6(1):29-53 [DOI] [PubMed] [Google Scholar]
- 55.Kleitman NDA. The effect of the position of the body and of sleep on rectal temperature in man. Am J Physiol. 1933;104:340-343 [Google Scholar]
- 56.Minors DS, Rabbitt PM, Worthington H, Waterhouse JM. Variation in meals and sleep-activity patterns in aged subjects; its relevance to circadian rhythm studies. Chronobiol Int. 1989;6(2):139-146 [DOI] [PubMed] [Google Scholar]
- 57.Mendoza J. Circadian clocks: setting time by food. J Neuroendocrinol. 2007;19(2):127-137 [DOI] [PubMed] [Google Scholar]
- 58.Brainard GC, Sliney D, Hanifin JP, et al. Sensitivity of the human circadian system to short-wavelength (420-nm) light. J Biol Rhythms. 2008;23(5):379-386 [DOI] [PubMed] [Google Scholar]
- 59.McNeill DS, Altimus CM, Hattar S. Retina-clock relations dictate nocturnal to diurnal behaviors. Proc Natl Acad Sci U S A. 2008;105(35):12645-12646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Altimus CM, Güler AD, Villa KL, McNeill DS, Legates TA, Hattar S. Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc Natl Acad Sci U S A. 2008;105(50):19998-20003 [DOI] [PMC free article] [PubMed] [Google Scholar]