Abstract
Background:
Interstitial lung disease (ILD) is a frequent complication of systemic sclerosis (SSc) and a major cause of SSc-related deaths. This study aimed to determine the influence of ILD on SSc in a population-based historical cohort study. The hypothesis was that patients with SSc who develop ILD have increased morbidity and mortality when compared with patients with SSc without ILD.
Methods:
Using the record linkage system of the Rochester Epidemiology Project in Olmsted County, Minnesota, this study identified the incidence of SSc between 1980 and 2010 and point prevalence on December 31, 2010 and determined the progression of organ involvement and its influence on outcome.
Results:
During the 30-year interval, we identified 64 incident cases of SSc: 57 women and seven men, median age 49.1 years (interquartile range [IQR], 39.8-67.6 years). There were 43 prevalent cases. ILD occurred in 19 cases, usually after the diagnosis of SSc (median, 2 years; IQR, 0-10 years), with only three cases occurring 6 to 24 months beforehand. Pulmonary arterial hypertension (PAH) was diagnosed in 14 cases, heart failure in 27 cases, and chronic kidney disease (CKD) in 21 cases. Seventeen patients died during the study period, with a median survival time after diagnosis of 22.9 years. ILD, PAH, and CKD were associated with an increased risk of death.
Conclusions:
The incidence of ILD associated with SSc was relatively low in this population-based cohort. ILD appeared to be a contributing factor to mortality. Other factors, including age, PAH, and CKD, were also associated with poor outcome.
Scleroderma is a heterogeneous group of rare conditions characterized by the presence of tightening, thickening, and nonpitting skin induration due to an excess of collagen fibers.1 Systemic sclerosis (SSc) is characterized by the association of sclerodermatous skin changes, Raynaud phenomenon, other vascular abnormalities, and lung and other organ involvment.2 SSc is a rare disease with an annual incidence of about 20 cases per million per year and a prevalence of about 240 cases per million in the United States.3 Incidence and prevalence vary among different populations studied.3,4 Prevalence is higher in women than in men and higher in blacks than in whites.5,6 Median survival was approximately 11 years in a Detroit Tri-County area survey and was shorter than expected when adjusted for age, sex, and race.6 Factors negatively affecting survival include male sex, older age at diagnosis, extent of skin involvement, and other organ involvement, especially the lung.6,7 Overall survival of SSc has improved over the past few decades.7,8 Renal disease accounts for some of the early mortality, but pulmonary disease has emerged as a major cause of death.8,9
Interstitial lung disease (ILD) refers to diffuse parenchymal infiltrative processes identified by chest imaging and/or restrictive physiology.10 The most common pulmonary manifestations of SSc are ILD and pulmonary arterial hypertension (PAH).11 The prevalence of SSc-associated ILD varies, depending on several factors: population studied, case definition, and sensitivity of methods of ascertainment.12 Although some degree of interstitial lung involvement is present in most cases in autopsy series,13 clinically relevant ILD is found in only approximately 40% of patients.14 Sequential measurements of pulmonary function in patients with SSc have shown remarkable variability in the progression of restrictive lung disease, ranging from an indolent course with stable pulmonary function to a rapidly progressive disease leading to respiratory failure and death.15 Of SSc-related deaths, 35% have been attributed to pulmonary fibrosis.9 A faster rate of decline in FVC % predicted has been associated with poor survival.16
Thus, although SSc is a rare disease, SSc-associated ILD is a major contributor to the morbidity and mortality associated with SSc. However, most studies of SSc-associated ILD come from registries and are subject to referral bias.17‐20 Little is known about the natural history of SSc-associated ILD in a population-based cohort. By identifying the incidence, point prevalence, and organ involvement, especially ILD, in SSc, the goal of this study was to determine the influence of ILD (and other organ involvement) on outcome of SSc. Our hypothesis was that patients with SSc who develop ILD have increased morbidity and mortality when compared with patients with SSc without ILD. The establishment of population-based epidemiologic evidence may provide a basis for the development of better strategies for the treatment of ILD to significantly lower the excess mortality occurring among individuals with SSc.
Materials and Methods
This was a historical, population-based, cohort study, designed using the resources of the Rochester Epidemiology Project (REP), a medical records linkage system in Olmsted County, Minnesota, that allows access to the complete medical records of the local population.21 This study was conducted in accordance with the amended Declaration of Helsinki and was approved by Mayo Clinic (10-008830) and Olmsted Medical Center (001-OMC-11) institutional review boards. Written informed consent was obtained from all patients.
All adult patients, including women and racial/ethnic minorities, with newly diagnosed SSc between January 1, 1980, and December 31, 2010, were included. REP computer tools were used to search for potential SSc cases. Patients with at least one of the following key words were identified: circumscribed scleroderma; diffuse disease of connective tissue; morphea, not otherwise specified; other hypertrophic and atrophic conditions of the skin; sclerodactylia; scleroderma, localized; scleroderma, lung; scleroderma, not otherwise specified; sclerosis, systemic, progressive; syndrome, CREST (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly and telangiectasia); and SSc. Patients who did not consent were excluded. All patient medical records were then reviewed longitudinally, beginning at patient age 18 years (or date of migration to Olmsted County, Minnesota, for those who became residents after age 18) and continuing until death, until relocation out of Olmsted County or loss to follow-up, or until December 31, 2010.
A rheumatologist (T. G. O.) confirmed the diagnosis of SSc based on both the criteria established by the American College of Rheumatology (ACR) from 19802 (“narrow criteria”) and the subsequent criteria by LeRoy et al22 that distinguishes diffuse and limited cutaneous SSc and includes SSc sine scleroderma (“broad criteria”).23 Two pulmonologists (P. R. B., J. H. R.) confirmed the diagnosis of ILD, which was defined by the presence of diffuse parenchymal opacities on chest imaging (high-resolution CT scan or chest radiograph) not attributable to cardiac disease, infection, exposures, or other identifiable causes.10 The index date for the diagnosis of SSc was the date of diagnosis of SSc found in the medical records. Residency status of Olmsted County was cross-checked at the time of diagnosis between January 1, 1980, and December 31, 2010 (incident case); prevalent cases were counted as the number of cases on December 31, 2010, who were residents on that date, independently as to whether they were given a diagnosis of SSc earlier as a resident of Olmsted County.
Outcome variables included deaths, hospital admissions, immunosuppressive therapy, other medications, supplemental oxygen, and lung transplant. Covariates included age, sex, ILD, PAH, congestive heart failure (CHF), and chronic kidney disease (CKD). ILD was defined by expert consensus as stated previously. PAH was defined by mean pulmonary artery pressure > 25 mm Hg at rest by right-sided cardiac catheterization or estimated right ventricular systolic pressure > 50 mm Hg by echocardiogram (in the absence of right-sided cardiac catheterization). CHF was defined as either systolic and/or diastolic heart failure. CKD was defined as an estimated glomerular filtration rate < 60 mL/min/1.73 m2 using the Chronic Kidney Disease Epidemiology Collaboration equation and/or evidence of kidney damage for at least 3 months.24,25
All variables were collected by chart review. A manual of definitions was established. The date of an event was defined as the first time mentioned in the medical record (if present) or the last time not observed (if absent). Information not available was coded as missing. To ensure accuracy and reliability, data were reviewed by two independent extractors (P. R. B., D. N. S.) after specific training to access the REP database. A separate pulmonologist (J. H. R.), rheumatologist (T. G. O.), and radiologist (D. L. L.) reviewed each case independently. In the case of a disagreement, a consensus was reached. Study data were collected and managed using REDCap (Research Electronic Data Capture)26 tools hosted at Mayo Clinic.25
Demographics were expressed as median with interquartile range (IQR) for quantitative data and as number with percentage for qualitative data. When calculating incidence (from January 1, 1980, to December 31, 2010) and prevalence (on December 31, 2010), the denominators were obtained using age- and sex-specific person-years for the population of Olmsted County residents estimated from decennial census data with interpolation between census years. Incidence rates were directly age and sex adjusted to the population structure of white people in the United States in 2000.27 Ninety-five percent CIs were calculated assuming a Poisson distribution.28 The Fisher exact test was used to compare small samples among cases with and without ILD. Kaplan-Meier29 and Cox30 proportional-hazards regression models were used to analyze survival in SSc. For the Cox proportional-hazards models, binary time-dependent covariates were used to assess whether the development of ILD, PAH, CHF, or CKD was associated with worse survival. Analyses were performed using SAS software (version 9; SAS Institute Inc) (A. C. H., D. R. S.).
Results
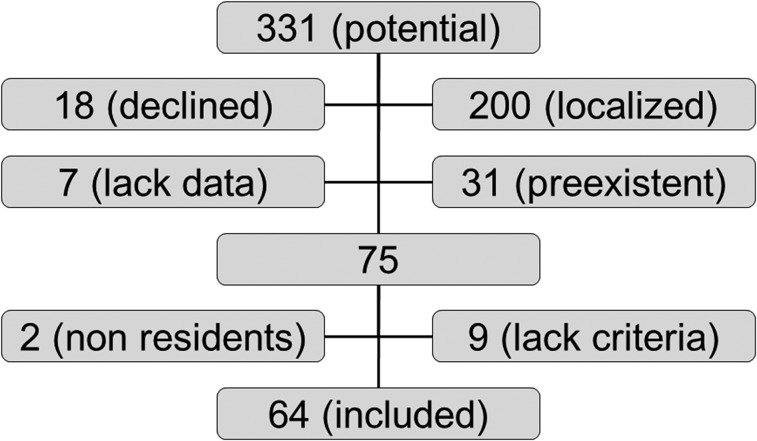
Of 331 potential SSc cases identified, 18 patients (5.4%) declined to have their chart reviewed for research. Out of the 313 remaining cases, 200 were localized forms of scleroderma and 31 were prevalent cases; data were lacking in seven cases (Fig 1). Of the 75 remaining cases, two patients were nonresidents for census purpose (members of the clergy), and nine did not meet SSc criteria after independent review by a rheumatologist (T. G. O.). Median follow-up time was 8.1 years (IQR, 3.2-13.4 years).
Figure 1.
Flow diagram showing the number of individuals at each stage of study. Localized = localized form of scleroderma; preexistent = systemic sclerosis diagnosed before the study period.
During the 30-year interval, we identified 64 new cases of SSc that met the predefined criteria. There were significantly more women than men with SSc (female to male ratio = 57:7). Other characteristics of the patients are provided (Tables 1, 2). No asbestos exposures, drug toxicities, or environmental factors were noted. The overall incidence rate was 2.43 cases per 100,000 (95% CI, 1.83-3.02) using broad criteria and 1.37 per 100,000 (95% CI, 0.92-1.81) using narrow criteria (Table 3). On December 31, 2010, there were 43 prevalent cases of SSc, which corresponds to a point prevalence of 39.92 per 100,000 (95% CI, 27.87-51.96) (Table 3).
Table 1.
—Demographics of Patients With Scleroderma
| Demographic | Variable |
| Cases, No. | 64 |
| Age at diagnosis, median (IQR), y | 49.1 (39.8-67.6) |
| Sex | |
| Female | 57 (89) |
| Male | 7 (11) |
| Ethnicity | |
| Not Hispanic | 45 (70) |
| Unknown | 19 (30) |
| Race | |
| White | 55 (86) |
| Asian | 6 (9) |
| Black | 1 (2) |
| Unknown | 2 (3) |
| Smoking status | |
| Never | 36 (56) |
| Previous | 20 (31) |
| Current | 8 (13) |
| Diagnosis criteria | |
| Not ACR | 28 (44) |
| With ACR | 36 (56) |
| Type of connective tissue disease | |
| Undifferentiated | 5 (8) |
| Scleroderma | 47 (73) |
| Mixed | 12 (19) |
| Subset of systemic sclerosis | |
| Limited cutaneous | 47 (73) |
| Diffuse cutaneous | 9 (14) |
| Sine scleroderma | 8 (13) |
Data are presented as No. (%) unless indicated otherwise. ACR = American College of Rheumatology2; IQR = interquartile range.
Table 2.
—Clinical Characteristics of Patients With Scleroderma
| Characteristic | Absent, No. (%) | Present, No. (%) | Missing, No. (%) |
| Clinical data | |||
| Raynaud phenomenon | 0 (0) | 64 (100) | 0 (0) |
| Pitting nail | 9 (14) | 25 (39) | 30 (47) |
| Skin sclerosis | 33 (52) | 29 (45) | 2 (3) |
| Calcinosis | 50 (78) | 10 (16) | 4 (6) |
| Sclerodactyly | 10 (16) | 53 (83) | 1 (2) |
| Telangiectasia | 15 (23) | 49 (77) | 0 (0) |
| Digital ulcer | 31 (48) | 31 (48) | 2 (3) |
| Heartburn | 19 (30) | 45 (70) | 0 (0) |
| Dysphagia | 32 (50) | 31 (48) | 1 (2) |
| Dysmotility | 35 (55) | 20 (31) | 9 (14) |
| Diarrhea | 44 (69) | 16 (25) | 4 (6) |
| Myositis | 52 (81) | 8 (13) | 4 (6) |
| Hypertension | 29 (45) | 35 (55) | 0 (0) |
| Laboratory data | |||
| ANA | 2 (3) | 58 (91) | 4 (6) |
| Anti-Scl-70 | 34 (53) | 5 (8) | 25 (39) |
| ACA | 26 (41) | 21 (33) | 17 (26) |
| Organ Involvement | |||
| CKD | 37 (58) | 21 (33) | 6 (9) |
| CHF, systolic | 48 (75) | 5 (8) | 11 (17) |
| CHF, diastolic | 24 (38) | 22 (34) | 18 (28) |
| PAH | 34 (53) | 14 (22) | 16 (25) |
| ILD | 45 (70) | 19 (30) | 0 (0) |
ACA = anticentromere antibody; ANA = antinuclear antibody; anti-Scl-70 = antitopoisomerase I; CHF = congestive heart failure; CKD = chronic kidney disease; ILD = interstitial lung disease; PAH = pulmonary arterial hypertension.
Table 3.
—Incidence Using Broad and Narrow Criteria and Point Prevalence of Scleroderma per 100,000 Adjusted to the 2000 US White Population
| Female | Male | Overall | ||||
| Time Frame | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI |
| Incidence (broad criteria) | ||||||
| 1980-1989 | 3.27 | 1.42-5.13 | 0.00 | 0.00-0.00 | 1.69 | 0.73-2.65 |
| 1990-1999 | 4.89 | 2.80-6.98 | 0.26 | 0.00-0.76 | 2.65 | 1.54-3.76 |
| 2000-2010 | 4.21 | 2.52-5.89 | 1.12 | 0.22-2.01 | 2.71 | 1.74-3.68 |
| Overall | 4.17 | 3.09-5.25 | 0.56 | 0.15-0.98 | 2.43 | 1.83-3.02 |
| Incidence (narrow criteria) | ||||||
| 1980-1989 | 2.18 | 0.67-3.70 | 0.00 | 0.00-0.00 | 1.13 | 0.35-1.91 |
| 1990-1999 | 1.86 | 0.57-3.16 | 0.26 | 0.00-0.76 | 1.09 | 0.38-1.80 |
| 2000-2010 | 2.28 | 1.04-3.52 | 1.12 | 0.22-2.01 | 1.72 | 0.95-2.49 |
| Overall | 2.12 | 1.35-2.89 | 0.56 | 0.15-0.98 | 1.37 | 0.92-1.81 |
| Prevalence (broad criteria) | ||||||
| 12/31/2010 | 62.02 | 41.65-82.39 | 13.32 | 3.38-23.26 | 39.92 | 27.87-51.96 |
ILD occurred in 19 cases, usually after the diagnosis of SSc (median, 2 years; IQR, 0-10 years). Only three cases occurred 6 to 24 months before the diagnosis of SSc. PAH occurred in 14 cases. CKD occurred in 21 cases. Systolic CHF was present in five cases, and some degree of diastolic CHF was noted in 22 cases. Most patients had a chest radiograph (92%). About two-thirds had chest CT scan and pulmonary function testing (Table 4). Three patients had a bronchoscopy, and one patient underwent surgical lung biopsy showing diffuse alveolar damage. The presence of crackles, finding of fibrosis on chest radiographs, or typical findings by CT scan were seen more frequently in ILD. Other pulmonary symptoms (dyspnea, cough, clubbing) were not discriminative for ILD, nor was pulmonary function testing when available.
Table 4.
—Pulmonary Features in Patients With Scleroderma
| No ILD (n = 45) | ILD (n = 19) | ||||||
| Pulmonary Feature | No | Yes | Missing | No | Yes | Missing | P Valuea |
| Symptoms | |||||||
| Dyspnea | 19 (42) | 22 (49) | 4 (9) | 6 (32) | 13 (68) | 0 (0) | .3997 |
| Cough | 17 (38) | 24 (53) | 4 (9) | 8 (42) | 10 (53) | 1 (5) | 1.0000 |
| Clubbing | 39 (87) | 2 (4) | 4 (9) | 15 (79) | 3 (16) | 1 (5) | .1604 |
| Crackles | 27 (60) | 14 (31) | 4 (9) | 2 (11) | 17 (89) | 0 (0) | 1.0000 |
| CXR | 3 (7) | 40 (89) | 2 (4) | 0 (0) | 19 (100) | 0 (0) | .5464 |
| Fibrosis | 25 (56) | 15 (33) | 5 (11) | 6 (32) | 13 (68) | 0 (0) | .0494b |
| CT scan | 18 (40) | 25 (56) | 2 (4) | 3 (16) | 15 (79) | 1 (5) | .0790 |
| Ground glass opacities | 21 (47) | 4 (9) | 20 (44) | 7 (37) | 8 (42) | 4 (21) | .0297b |
| Reticular opacities | 21 (47) | 4 (9) | 20 (44) | 3 (16) | 12 (63) | 4 (21) | .0001b |
| Traction bronchiectasis | 25 (56) | 0 (0) | 20 (44) | 8 (42) | 7 (37) | 4 (21) | .0003b |
| Honeycombing | 25 (56) | 0 (0) | 20 (44) | 12 (63) | 3 (16) | 4 (21) | .0461b |
| PFT | 15 (33) | 28 (62) | 2 (4) | 3 (16) | 15 (79) | 3 (5) | .2219 |
| TLC < 80% expected | 14 (31) | 2 (4) | 29 (65) | 5 (26) | 5 (26) | 9 (48) | .0687 |
| FVC < 80% expected | 17 (38) | 11 (24) | 17 (38) | 8 (42) | 7 (37) | 4 (21) | .7497 |
| Dlco < 60% expected | 20 (44) | 7 (16) | 18 (40) | 7 (37) | 7 (37) | 5 (26) | .1703 |
| Dlco adj < 60% expected | 6 (14) | 2 (4) | 37 (82) | 2 (11) | 5 (26) | 12 (63) | .1319 |
| Bronchoscopy | 40 (89) | 1 (2) | 4 (9) | 15 (78) | 2 (11) | 2 (11) | .2027 |
Data are presented as No. (%). CXR = chest radiography; Dlco = diffusing capacity of the lung for carbon monoxide; Dlco adj = diffusing capacity adjusted for hemoglobin (% expected); PFT = pulmonary function testing; TLC = total lung capacity (% expected). See Table 2 legend for expansion of other abbreviation.
Fisher exact test, missing data excluded.
Significant when P < .05.
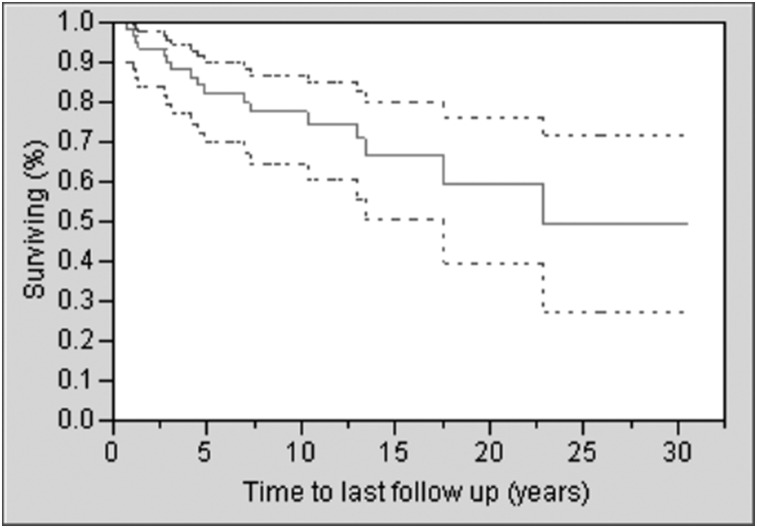
Seventeen patients died during the study period, with a median survival time after diagnosis of SSc of 22.9 years (Fig 2). SSc was the primary cause of death in nine cases, a contributing factor in four cases, and not related in four cases. PAH contributed to death in seven cases (six cases as primary cause), ILD in one case, and CKD in one case. There was no difference in immunosuppressive drugs, supplemental oxygen, antihypertensive medications, and other medications aimed at treating PAH or the need for hospitalization between those with and without ILD (data not shown). No patient underwent lung transplant. From analyses that adjusted for age, the presence of ILD, PAH, and CKD, but not CHF or smoking history, were found to be associated with an increased risk of death (Table 5).
Figure 2.
Survival plot of incident cases of scleroderma (with 95% CI).
Table 5.
—Risk of Death Among Patients With ILD, PAH, CHF, or CKD, or Who Smoked
| Covariate | HR | 95% CI | P Value |
| ILD | 2.90 | 1.16-7.26 | .023 |
| PAHa | 4.78 | 1.83-12.49 | .002 |
| CHF | 1.19 | 0.44-3.27 | .733 |
| CKD | 2.78 | 1.21-6.40 | .016 |
| Smokingb | 1.69 | 0.64-4.50 | .291 |
Proportional hazards analysis was used modeling ILD, PAH, CHF, CKD, and smoking as binary, time-dependent covariates. Each model was adjusted for age. HR = hazard ratio. See Table 2 legend for expansion of other abbreviations.
Not secondary to ILD.
Smoking is represented as ever vs never smoked.
Survival did not differ between the broad and narrow criteria defining SSc, including undifferentiated and mixed connective tissue disease. However, a trend toward a reduced median survival time, from 22.9 to 17.6 years (P = .057 by Wilcoxon test), was observed between the 17 cases of undifferentiated and mixed connective tissue disease and the 47 pure cases of SSc, defined either by the broad or the narrow criteria. Among those 47 cases of SSc were 16 cases of ILD.
Discussion
In this population-based study, the overall incidence of SSc was 2.43 per 100,000 (95% CI, 1.83-3.02) and was stable over time. We confirmed the hypothesis that patients with SSc who develop ILD have increased morbidity and mortality when compared with patients with SSc without ILD. The overall incidence of SSc was higher using the broad criteria (LeRoy et al22) than the narrow criteria (ACR),2 which may have underestimated the actual incidence of SSc. When compared with the literature, incidence and prevalence were similar to the findings of most recent studies in the United States4,6 but were higher than that reported previously in the same population from Minnesota.31,32 The apparent increase in incidence is likely due to a better definition of the disease with the establishment of ACR criteria in 1980. As shown in Table 3, the incidence remained stable over time after these criteria were established. The point prevalence on December 31, 2010, was 39.92 per 100,000 (95% CI, 27.87-51.96), which is similar to studies in the United States using the same ACR criteria.4,6 Although the prevalence of SSc was considered to be higher in the United States than in Japan and Europe,4 the results of this study are close to those of a study in a district of northern Italy,33 challenging the concept of a west-east gradient. The probability of survival was higher than reported previously,6‐9 but similar to a Canadian study in which the overall 5-year and 10-year survival rates were 90% and 82%, respectively,34 emphasizing a trend toward an apparent better outcome of SSc, possibly explained in part by the use of broader criteria. Among the visceral complications, ILD, PAH, CHF, and CKD occurred most frequently. When adjusted for age, the risk of death was not only influenced by the presence of PAH or CKD, but also by ILD itself. Although ILD is now considered a major cause of death in SSc,8,9 the review of the causes of death in the current study suggests that ILD is more a contributing factor than an immediate cause of death in these patients. In this study, we found that PAH was the principal cause of poor outcome among those with SSc. The lengthy time span of our study may be one reason why PAH was a predominant cause of death. Of the SSc-related fatalities from the large EUSTAR database, 35% were attributed to ILD, 26% to PAH, and 26% to cardiac causes.9 Proteinuria, PAH by echocardiography, and reduced FVC were among the independent risk factors for mortality.9 The difference in our study can be explained by a different design (population- vs referral center-based), and a different time frame (the EUSTAR database was initiated in 2004). Nevertheless, both studies emphasize the importance of ILD, PAH, and CKD. Cardiac causes certainly play a role as well, but could not be further established in our study because of the limited number of patients.
This population-based study included only new cases of SSc diagnosed by a rheumatologist while the patient was a resident in Olmsted County. It may have not included some cases that already existed but were not yet diagnosed. Seven patients lacking sufficient information were excluded. The study was limited to cases evaluated by a rheumatologist and/or a pulmonologist at the time when the diagnosis was considered. Formes fruste or fulminant cases of the disease may have been missed. The diagnosis of ILD was designated by consensus of two of the authors based on their review of medical records and imaging studies, with the exclusion of those patients whose lung infiltrates were attributable to other causes. The diagnosis of PAH in some patients was based on estimated right ventricular systolic pressure obtained by echocardiography. The relatively low number of patients with SSc (n = 64) and even lower number of patients with concomitant ILD (n = 19) limited the analyses and justify caution in interpreting the results. Olmsted County, with a population of 144,248 in 2010, lies 90 miles southeast of Minneapolis/Saint Paul. In 2000, the population was 90% white and largely middle class. Therefore, the results may not be generalizable to locations with higher minority populations and may be similar only to other white populations in the United States.
Conclusions
In conclusion, the incidence of ILD associated with SSc was relatively low in this population-based cohort. Not only ILD, but PAH and CKD, were all contributors to poor outcome. Whether the presence of ILD justifies a more aggressive management of SSc remains open to debate.
Acknowledgments
Author contributions: Dr Bauer is guarantor of the manuscript and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Bauer: contributed to the concept and design of the study; collection, analysis, and interpretation of data; and review and final approval of the manuscript.
Dr Schiavo: contributed to the collection, analysis, and interpretation of data and drafting, review, and final approval of the manuscript.
Dr Osborn: contributed to the analysis of each case from a rheumatology standpoint and review and final approval of the manuscript.
Dr Levin: contributed to the analysis of each case from a radiology standpoint and review and final approval of the manuscript.
Dr St. Sauver: contributed to concept and design of the study, analysis and interpretation of data, and review and final approval of the manuscript.
Mr Hanson: contributed to the statistical analysis and interpretation of data and review and final approval of the manuscript.
Mr Schroeder: contributed to statistical analysis and interpretation of data and review and final approval of the manuscript.
Dr Ryu: contributed to design of the study, analysis and interpretation of data, and review and final approval of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Bauer was the recipient of the 2011 Walter and Leonor Annenberg Career Development Award in Pulmonary Medicine, Mayo Foundation. Drs Schiavo, Osborn, Levin, St. Sauver, and Ryu and Messrs Hanson and Schroeder have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: Funding covered the cost for protected time for data retrieval, interpretation of data and drafting of the manuscript, and statistical analyses. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Other contributions: This work was performed at Mayo Clinic, Rochester, Minnesota.
Abbreviations
- ACR
American College of Rheumatology
- CHF
congestive heart failure
- CKD
chronic kidney disease
- ILD
interstitial lung disease
- IQR
interquartile range
- PAH
pulmonary arterial hypertension
- REP
Rochester Epidemiology Project
- SSc
systemic sclerosis
Footnotes
Funding/Support: This publication was made possible by Center for Translational Science Activities (CTSA) [Grant UL1 TR000135] from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH) and by Rochester Epidemiology Project [Grant R01 AG034676] from the National Institute on Aging. This work was also funded by the 2011 Walter and Leonor Annenberg Career Development Award in Pulmonary Medicine.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009;360(19):1989-2003 [DOI] [PubMed] [Google Scholar]
- 2.Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum. 1980;23(5):581-590 [DOI] [PubMed] [Google Scholar]
- 3.Mayes MD. Scleroderma epidemiology. Rheum Dis Clin North Am. 2003;29(2):239-254 [DOI] [PubMed] [Google Scholar]
- 4.Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum. 2008;37(4):223-235 [DOI] [PubMed] [Google Scholar]
- 5.Steen VD, Oddis CV, Conte CG, Janoski J, Casterline GZ, Medsger TA., Jr Incidence of systemic sclerosis in Allegheny County, Pennsylvania. A twenty-year study of hospital-diagnosed cases, 1963-1982. Arthritis Rheum. 1997;40(3):441-445 [DOI] [PubMed] [Google Scholar]
- 6.Mayes MD, Lacey JV, Jr, Beebe-Dimmer J, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003;48(8):2246-2255 [DOI] [PubMed] [Google Scholar]
- 7.Ferri C, Valentini G, Cozzi F, et al. ; Systemic Sclerosis Study Group of the Italian Society of Rheumatology (SIR-GSSSc) Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore). 2002;81(2):139-153 [DOI] [PubMed] [Google Scholar]
- 8.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis. 2007;66(7):940-944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69(10):1809-1815 [DOI] [PubMed] [Google Scholar]
- 10.Ryu JH, Daniels CE, Hartman TE, Yi ES. Diagnosis of interstitial lung diseases. Mayo Clin Proc. 2007;82(8):976-986 [DOI] [PubMed] [Google Scholar]
- 11.Guillevin L, Mouthon L. Pulmonary involvement in systemic scleroderma [in French]. Bull Acad Natl Med. 2011;195(1):79-90 [PubMed] [Google Scholar]
- 12.Renzoni EA. Interstitial lung disease in systemic sclerosis. Monaldi Arch Chest Dis. 2007;67(4):217-228 [DOI] [PubMed] [Google Scholar]
- 13.D’Angelo WA, Fries JF, Masi AT, Shulman LE. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med. 1969;46(3):428-440 [DOI] [PubMed] [Google Scholar]
- 14.Highland KB, Silver RM. New developments in scleroderma interstitial lung disease. Curr Opin Rheumatol. 2005;17(6):737-745 [DOI] [PubMed] [Google Scholar]
- 15.McNearney TA, Reveille JD, Fischbach M, et al. Pulmonary involvement in systemic sclerosis: associations with genetic, serologic, sociodemographic, and behavioral factors. Arthritis Rheum. 2007;57(2):318-326 [DOI] [PubMed] [Google Scholar]
- 16.Assassi S, Sharif R, Lasky RE, et al. ; GENISOS Study Group Predictors of interstitial lung disease in early systemic sclerosis: a prospective longitudinal study of the GENISOS cohort. Arthritis Res Ther. 2010;12(5):R166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kane GC, Varga J, Conant EF, Spirn PW, Jimenez S, Fish JE. Lung involvement in systemic sclerosis (scleroderma): relation to classification based on extent of skin involvement or autoantibody status. Respir Med. 1996;90(4):223-230 [DOI] [PubMed] [Google Scholar]
- 18.Chang B, Wigley FM, White B, Wise RA. Scleroderma patients with combined pulmonary hypertension and interstitial lung disease. J Rheumatol. 2003;30(11):2398-2405 [PubMed] [Google Scholar]
- 19.Benan M, Hande I, Gul O. The natural course of progressive systemic sclerosis patients with interstitial lung involvement. Clin Rheumatol. 2007;26(3):349-354 [DOI] [PubMed] [Google Scholar]
- 20.Fischer A, Swigris JJ, Groshong SD, et al. Clinically significant interstitial lung disease in limited scleroderma: histopathology, clinical features, and survival. Chest. 2008;134(3):601-605 [DOI] [PubMed] [Google Scholar]
- 21.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266-274 [DOI] [PubMed] [Google Scholar]
- 22.LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15(2):202-205 [PubMed] [Google Scholar]
- 23.Hachulla E, Launay D. Diagnosis and classification of systemic sclerosis. Clin Rev Allergy Immunol. 2011;40(2):78-83 [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fadem SZ, Rosenthal B. CKD-EPI & MDRD study equation calculator - (with SI units). Nephron Information Center website. http://nephron.org/MDRD_GFR.cgi Accessed July 12, 2012
- 26.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergstralh EJ, Offord KP, Chu CP, Beard CM, O’Fallon WM. Calculating incidence, prevalence and mortality rates for Olmsted County, Minnesota residents: An update. Technical Report Series No. 49. Rochester, MN: Section of Biostatistics, Mayo Clinic; January 1992. [Google Scholar]
- 28.Good IJ. Some statistical applications of Poisson’s work. Stat Sci. 1986;1(2):157-170 [Google Scholar]
- 29.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Am Sta Assoc. 1958;53(282):457-481 [Google Scholar]
- 30.Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health. 1999;20:145-157 [DOI] [PubMed] [Google Scholar]
- 31.Kurland LT, Hauser WA, Ferguson RH, Holley KE. Epidemiologic features of diffuse connective tissue disorders in Rochester, Minn., 1951 through 1967, with special reference to systemic lupus erythematosus. Mayo Clin Proc. 1969;44(9):649-663 [PubMed] [Google Scholar]
- 32.Michet CJ, Jr, McKenna CH, Elveback LR, Kaslow RA, Kurland LT. Epidemiology of systemic lupus erythematosus and other connective tissue diseases in Rochester, Minnesota, 1950 through 1979. Mayo Clin Proc. 1985;60(2):105-113 [DOI] [PubMed] [Google Scholar]
- 33.Lo Monaco A, Bruschi M, La Corte R, Volpinari S, Trotta F. Epidemiology of systemic sclerosis in a district of northern Italy. Clin Exp Rheumatol. 2011;29(2 suppl 65):S10-S14 [PubMed] [Google Scholar]
- 34.Al-Dhaher FF, Pope JE, Ouimet JM. Determinants of morbidity and mortality of systemic sclerosis in Canada. Semin Arthritis Rheum. 2010;39(4):269-277 [DOI] [PubMed] [Google Scholar]