Abstract
Background:
There is significant heterogeneity within the primary graft dysfunction (PGD) syndrome. We aimed to identify distinct grade 3 PGD phenotypes based on severity of lung dysfunction and patterns of resolution.
Methods:
Subjects from the Lung Transplant Outcomes Group (LTOG) cohort study with grade 3 PGD within 72 h after transplantation were included. Latent class analysis (LCA) was used to statistically identify classes based on changes in PGD International Society for Heart & Lung Transplantation grade over time. Construct validity of the classes was assessed by testing for divergence of recipient, donor, and operative characteristics between classes. Predictive validity was assessed using time to death.
Results:
Of 1,255 subjects, 361 had grade 3 PGD within the first 72 h after transplantation. LCA identified three distinct phenotypes: (1) severe persistent dysfunction (class 1), (2) complete resolution of dysfunction within 72 h (class 2), and (3) attenuation, without complete resolution within 72 h (class 3). Increased use of cardiopulmonary bypass, greater RBC transfusion, and higher mean pulmonary artery pressure were associated with persistent PGD (class 1). Subjects in class 1 also had the greatest risk of death (hazard ratio, 2.39; 95% CI, 1.57-3.63; P < .001).
Conclusions:
There are distinct phenotypes of resolution of dysfunction within the severe PGD syndrome. Subjects with early resolution may represent a different mechanism of lung pathology, such as resolving pulmonary edema, whereas those with persistent PGD may represent a more severe phenotype. Future studies aimed at PGD mechanism or treatment may focus on phenotypes based on resolution of graft dysfunction.
Primary graft dysfunction (PGD) is a form of acute lung injury occurring within 72 h of lung transplantation.1 PGD is defined according to clinical parameters, including radiographic and oxygenation criteria. PGD is graded from 0 to 3, with 3 being the most severe form.2 The incidence of PGD ranges from 10% to 30%, and it is the leading cause of early mortality in lung transplantation. Therefore, improved understanding of the fundamental mechanisms of PGD could have a major impact on lung transplant outcomes.3
A limitation of the PGD clinical definition is the heterogeneity of severity and duration inherent in the syndromic criteria. PGD presentation may vary considerably, ranging from transient infiltrates and mild hypoxia, to persistent severe lung injury requiring extracorporeal support.4 In fact, since the publication of the initial International Society for Heart & Lung Transplantation consensus definition in 2005, authors have used varying PGD definitions, including worst grade of PGD within the 72 h after transplantation5,6 or grade 2 or 3 PGD occurring at 48 or 72 h after transplantation.7 Refined phenotyping within the PGD syndrome definition may facilitate risk stratification of recipients after transplantation, research on therapeutic interventions to reduce PGD, and study of the mechanism of development of PGD.
In this study, we aimed to identify distinct phenotypes within the PGD syndrome definition based on the timing of onset and resolution of PGD using latent class analysis (LCA), a statistical method used to identify classes within a heterogeneous syndrome, based on observed measurements varying over time.8 To determine the construct validity of these classes, we evaluated differences in frequencies of known risk factors of PGD and risk of death among the classes.
Materials and Methods
Study Population
Subjects were enrolled as part of the ongoing, multicenter, prospective Lung Transplant Outcomes Group cohort study.9‐15 Subjects were enrolled from 10 US transplant centers from March 2002 through December 2010. Patients undergoing lung or combined heart-lung transplantation at a participating center were eligible for inclusion in the Lung Transplant Outcomes Group study if their age at the time of transplantation was between 18 and 80 years and they provided informed consent. For the current study, we included all subjects with at least one episode of grade 3 PGD within 72 h of transplantation, based on our prior study demonstrating greatest mortality and lung injury biomarker differences with grade 3 PGD occurring at any time point.5 Standardized case-report forms were used at all centers to collect recipient, donor, and operative clinical parameters. Additional donor and mortality information was verified using data from the United Network for Organ Sharing. Lung allocation scores were obtained from United Network for Organ Sharing. Mortality data are incomplete because one center did not have institutional review board approval for collection of death data. The institutional review boards at each of the participating centers approved this study (e-Appendixes 1 (601.9KB, pdf) and 2 (601.9KB, pdf) ).
Definition of PGD
PGD grade was determined according to International Society for Heart & Lung Transplantation criteria, with grade 3 defined by a Pao2/Fio2 ratio < 200 and the presence of diffuse parenchymal infiltrates in the allograft(s) on chest radiographs.2 A PGD grade was assigned for postoperative day 0 (6 h after transplantation), day 1 (24 h), day 2 (48 h), and day 3 (72 h after transplantation). All chest radiographs were interpreted independently by two trained physicians blinded to the clinical variables, with adjudication of disagreements. The classification κ for agreement on grade 3 PGD was 0.96.
Statistical Analysis
LCA is a statistical method used to define groups of subjects (latent classes) based on observed measures that are similar for subjects within classes.8 LCA produces predicted probabilities of each class for each subject based on timing of onset and resolution of PGD. Final class membership is determined by assigning subjects to the class with the highest predicted probability. Therefore, each subject is assigned to a single class; classes are discrete and mutually exclusive. PGD grades (0-3) at 6, 24, 48, and 72 h after transplantation were used to generate latent classes. Resolution of PGD was considered a change from grade 3 to 0, and attenuation was considered a change from grade 3 to grade 2 or 1. Clinical characteristics were not used in the model. We generated three classes in our primary analysis; however, we performed a sensitivity analysis with four classes to ensure the accuracy of our model. Multiple imputation was used to account for missing data within the clinical risk factors,16 except lung allocation score, which was complete for those transplanted after May 2005. There were no missing PGD grades in the dataset. A base case analysis was done using nonimputed data to evaluate the effect of imputation on our analysis. χ2 testing and one-way analysis of variance were used to analyze differences in recipient, donor, and operative characteristics among the classes. Cox proportional hazards models and Kaplan-Meier analysis models were used to evaluate the association between each class and time to death, using all-cause mortality.
Results
Of 1,255 subjects enrolled, 361 subjects (29%; 95% CI, 26%-31%) developed grade 3 PGD within 72 h after transplantation. More than one-half of the subjects with grade 3 PGD received cardiopulmonary bypass (CPB), 43% had idiopathic pulmonary fibrosis as the pretransplant diagnosis, and 67% had bilateral transplant procedures (Table 1). Subjects from 10 centers were included in the study; the distribution among centers is listed in Table 1.
Table 1.
—Demographic Characteristics of the Study Populationa
| Characteristics | Total Cohort (N = 361) |
| Grade 3 PGD within 72 h | 361 (100) |
| Grade 3 PGD at 72 h | 119 (33) |
| CPB | 194 (53) |
| Diagnosis | |
| COPD | 93 (26) |
| CF | 38 (11) |
| IPF | 154 (43) |
| PPH | 20 (6) |
| Sarcoid | 24 (7) |
| Other | 31 (9) |
| Bilateral transplant | 241 (67) |
| Center | |
| University of Pennsylvania | 91 (25) |
| University of Chicago | 9 (2) |
| Columbia University | 73 (20) |
| University of Alabama | 28 (8) |
| Vanderbilt University | 27 (7) |
| Stanford University | 34 (9) |
| Johns Hopkins University | 7 (2) |
| University of Michigan | 50 (14) |
| University of Pittsburgh | 35 (10) |
| Duke University | 7 (2) |
| Female recipient | 163 (45) |
| Female donor | 161 (45) |
| Recipient race | |
| White | 280 (78) |
| Black | 56 (16) |
| Hispanic | 14 (4) |
| Asian | 7(2) |
| Other | 4 (1) |
| Donor race | |
| White | 241 (67) |
| Black | 69 (19) |
| Hispanic | 37 (10) |
| Other | 14 (4) |
| Recipient age, mean ± SD, y | 52.9 ± 12.3 |
| Donor age, mean ± SD, y | 35 ± 13.9 |
| BMI of recipient, mean ± SD, kg/m2 | 26.4 ± 5.2 |
| Crystalloids, mean ± SD, mL | 1,279 ± 1,845 |
| PRBC, mean ± SD, mL | 1,023.4 ± 1,411 |
Data given as No. (%) unless otherwise indicated. CF = cystic fibrosis; CPB = cardiopulmonary bypass; IPF = idiopathic pulmonary fibrosis; PGD = primary graft dysfunction; PPH = primary pulmonary hypertension; PRBC = packed RBC.
All subjects had grade 3 PGD within 72 h.
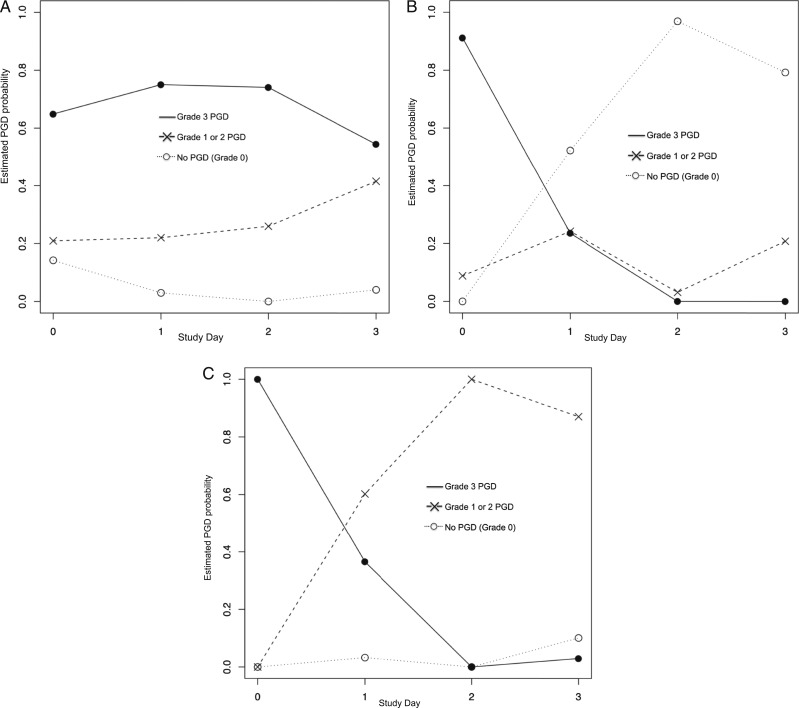
The results of our primary three-class model are presented in Figure 1. Class 1 (n = 197) (Fig 1A) represents a phenotype of persistent, severe dysfunction, in which the majority of subjects have grade 3 PGD at time zero and continue to have grade 3 PGD at 72 h. Class 2 (n = 25) (Fig 1B) demonstrates a phenotype of complete resolution of dysfunction by day 2. In class 3 (n = 139) (Fig 1C), subjects start with grade 3 PGD at time zero, but the majority move to grade 1 or 2 by day 2, indicating an improvement in severity of lung dysfunction but not a complete resolution.
Figure 1.
Three-class model generated from latent class analysis. The y axis indicates the probability of International Society for Heart & Lung Transplantation PGD grade on a given day. The x axis represents the postoperative day. A given probability of PGD grade for each day is represented by symbols connected by corresponding lines (● = grade 3, X = grade 1 or 2, ○ = grade 0). The line with the highest probability generated from the model indicates that members of that class are most likely to have a particular grade on a particular day. A, Latent class 1 (55% of grade 3 PGD population): Grade 3 PGD present on day 0 and persisted to day 3. Grade 3 had the highest probability on all study days in this class. B, Latent class 2 (7% of grade 3 PGD population): Grade 3 PGD present on day 0 that resolved completely to grade 0 by day 3. On day 0, grade 3 had the highest probability, but the probability of grade 3 decreased by day 1, and on days 2 and 3, grade 0 had the highest probability. C, Latent class 3 (39% of grade 3 population): Grade 3 PGD present on day 0 that attenuated to lower grades 1 and 2, but did not completely resolve, by day 3. On day 0, grade 3 had the highest probability, but decreased by day 1, and on days 2 and 3, intermediate grades had the highest probability. PGD = primary graft dysfunction.
Table 2 demonstrates the distribution of subject characteristics among the three classes. In class 1, where the subjects have severe, persistent dysfunction, there was more frequent use of CPB (61% vs 40% in class 2 and 46% in class 3; P = .01); increased volume of intraoperative, packed RBC transfusion (1,142.3 ± 1,547 mL compared with 694 ± 983.1 mL in class 2 and 914.1 ± 1,255.5 mL in class 3; P = .003); and increased mean pulmonary artery pressure (PAP) prior to transplantation (34.5 mm Hg vs 29.7 mm Hg in class 2 and 29 mm Hg in class 3; P = .001). There was also an increased frequency of donors with a smoking history (defined as ≥ 20 pack-years) in this class (51% vs 44% in class 3 and 24% in class 2; P = .03). There was no difference in pretransplant diagnosis or lung allocation score among the classes.
Table 2.
—Frequencies of Demographic Characteristics Among Classes in the Three-Class Model
| Characteristics | Class 1 (n = 197) | Class 2 (n = 25) | Class 3 (n = 139) | P Valuea |
| Recipient | ||||
| Recipient age, y | 52.9 ± 12.3 | 51.5 ± 14.6 | 53 ± 12.0 | .41 |
| Native disease | .45 | |||
| COPD | 50 (26) | 3 (12) | 40 (29) | |
| CF | 17 (9) | 5 (20) | 16 (12) | |
| IPF | 81 (41) | 13 (52) | 60 (43) | |
| PPH | 11 (6) | 1 (4) | 8 (6) | |
| Sarcoid | 17 (9) | 1 (4) | 6 (4) | |
| Other | 20 (10) | 2 (8) | 9 (6) | |
| Female recipient | 92(47) | 10 (40) | 61 (44) | .76 |
| Prior pregnancy | 73 (79) | 6 (60) | 41 (67) | .15 |
| Recipient race | .29 | |||
| White | 152 (77) | 18 (72) | 110 (79) | |
| Black | 32(16) | 6 (24) | 18 (13) | |
| Hispanic | 4 (2) | 1 (4) | 9 (6) | |
| Asian | 6 (3) | 0 (0) | 1 (< 1) | |
| Other | 3 (2) | 0 (0) | 1 (< 1) | |
| BMI recipient | 26.8 ± 5.5 | 24.6 ± 5.0 | 26.1 ± 4.8 | .25 |
| LAS (n = 312) | 46.9 ± 15.9 | 47.6 ± 14.2 | 45.7 ± 15 | .65 |
| Donor | ||||
| Female donor | 91(46) | 14 (56) | 56 (41) | .28 |
| Donor age, y | 35.6 ± 14.6 | 27.8 ± 10.4 | 35.7 ± 13.2 | .08 |
| Donor race | .45 | |||
| White | 128 (65) | 17 (68) | 96 (69) | |
| Black | 43 (22) | 6 (24) | 20 (15) | |
| Hispanic | 17 (9) | 2 (8) | 18 (13) | |
| Other | 9 (5) | 0 (0) | 5 (4) | |
| Donor ventilation days | .73 | |||
| One | 58 (30) | 6 (24) | 31 (22) | |
| Two | 54 (27) | 6 (24) | 43 (31) | |
| Three | 36 (19) | 7 (28) | 31 (22) | |
| Four | 49 (25) | 6 (24) | 34 (24) | |
| Donor smoking | 100 (51) | 6 (24) | 61(44) | .03 |
| Donor mode of death | .02 | |||
| Trauma | 79 (40) | 10 (40) | 61(44) | |
| Stroke | 83 (42) | 9 (36) | 60 (43) | |
| Anoxia | 10 (5) | 3 (12) | 15 (11) | |
| Other | 25 (13) | 3 (12) | 3 (2) | |
| Operative | ||||
| Bilateral transplant | 135 (69) | 15 (60) | 91 (66) | .64 |
| CPB | 120 (61) | 10 (40) | 64 (46) | .01 |
| Intraoperative nitric oxide | 122 (62) | 16 (64) | 88 (63) | .95 |
| Intraoperative crystalloids, mean ± SD, mL | 1,056.3 ± 1,593 | 1,550 ± 2,114 | 1,547.8 ± 2,083.2 | .002 |
| Reperfusion Fio2, mean ± SD | 67.1 ± 28.7 | 71.8 ± 26.4 | 66.7 ± 29.8 | .73 |
| Tidal volume, mean ± SD, mL/kg | 7.0 ± 3.2 | 8.0 ± 2.6 | 7.0 ± 2.6 | .05 |
| PRBC, mean ± SD, mL | 1,142.3 ± 1,547 | 694 ± 983.1 | 914.1 ± 1,255.5 | .003 |
| PAP, mean ± SD, mm Hg | 34.5 ± 16.4 | 29.7 ± 12.9 | 29 ± 12 | .001 |
Data given as No. (%) unless otherwise indicated. LAS = lung allocation score; PAP = pulmonary artery pressure at the time of transplantation. See Table 1 legend for expansion of other abbreviations.
P values were calculated using χ2 and t tests.
Class 2, in which dysfunction resolves by day 2, was the smallest class, with only 7% of subjects. Subjects in class 2 received a significantly higher volume of intraoperative crystalloids (1,550 ± 2,114 mL) compared with class 1 (1,056.3 ± 1,593 mL; P = .002). Additionally, class 2 had the lowest volume of blood transfused and the lowest frequency of smoking donors.
Class 3 (39% of PGD) represents a phenotype of severe dysfunction, with subsequent attenuation, but not complete resolution, by 48 h. Subjects in class 3 had a higher volume of intraoperative blood transfusion than class 2, but a lower volume than class 1. Additionally, class 3 had a higher frequency of donor smoking than class 2 (44% vs 24%), although lower than class 1 (44% vs 51%). Again, class 3 had a higher incidence of CPB than class 2 (46% vs 40%) and lower than class 1 (46% vs 61%). Subjects in class 3 have an intermediate severity phenotype.
Association With Mortality
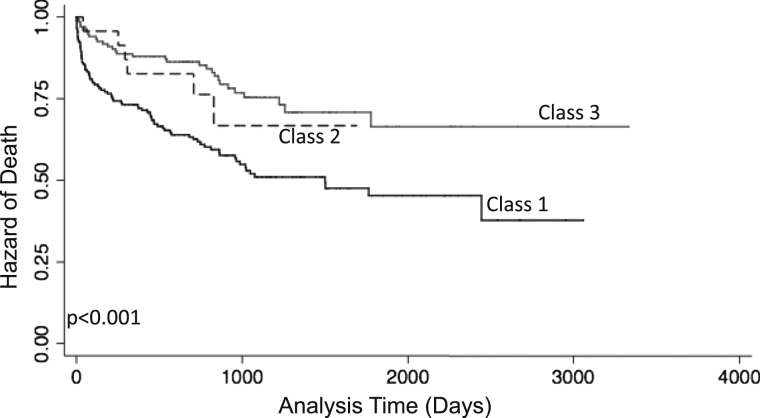
To determine construct validity of our latent classes in mortality prediction, we evaluated the relationship between each class and time to death (Fig 2). Of the 336 subjects with mortality information, 118 (35%) died. Class 1 had the greatest risk of death (hazard ratio [HR], 2.39; 95% CI, 1.57-3.63; P < .001) when compared with class 3. Class 2 had a no difference in risk for death (HR, 1.36; 95% CI, 0.57-3.28; P = .69); however, this comparison was limited by small numbers in class 2. Subjects in class 1 also had a higher risk of 90-day mortality (OR, 3.69; 95% CI, 1.65-8.27; P = .001) when compared with class 3.
Figure 2.
Relationship of each class with time to death. P value generated from log-rank test.
Sensitivity Analysis
The chosen number of classes can affect latent-class modeling, so we also performed sensitivity analyses with a four-class model, which helped to further refine class 3 (e-Fig 1 (601.9KB, pdf) ). Class 3 (a pattern of partial resolution of dysfunction) was subdivided into two subclasses: one of early dysfunction that attenuated early (within 24 h) and early dysfunction that attenuated late (at 48 h) (e-Fig 1 (601.9KB, pdf) ). The pattern of early dysfunction that attenuated early (class 3a) resembled class 2, with a low frequency of CPB (39%) and a high volume of intraoperative, IV crystalloid infusion (1,680.5 ± 1,971 mL). The pattern of early dysfunction that attenuated late (class 3b) most closely resembled class 1, with a higher frequency of CPB (60%) and a higher mean PAP (31.0 ± 11.3 mm Hg). Therefore, a four-class model split the third class into a phenotype similar to class 1 or a phenotype similar to class 2 (e-Table 1 (601.9KB, pdf) ). The associations with mortality in the four-class model were similar to the three-class model, with class 1 having the highest risk of death (HR, 3.45; 95% CI, 1.90-6.25; P < .001) (e-Fig 2 (601.9KB, pdf) ).
Base Case Analysis
Multiple imputation was used to account for missing data in covariates. To evaluate the effect of imputation on our analysis, we repeated all analyses using complete data. Numbers of subjects with data for each variable are presented in e-Table 2 (601.9KB, pdf) . There were no significant differences between the two analyses as presented in e-Tables 2 (601.9KB, pdf) and 3 (601.9KB, pdf) .
Discussion
This study identified subphenotypes within a group of subjects with grade 3 PGD, a heterogeneous clinical syndrome. Overall, the two dominant phenotypes that emerged are those with severe, persistent lung dysfunction that either attenuates by day 2 or remains persistent at day 3, and those with transient dysfunction that either completely resolves or attenuates by day 1. The two main phenotypes identified are associated with different clinical variables, and have distinct risks of death, adding validity to our statistically derived phenotypes.
The definition of PGD was created to represent early allograft injury.1 Although construct validity of the syndrome definition has been demonstrated, there is controversy in the field over the appropriate grade and time point of PGD to use as an outcome. Other work has used severe, persistent lung dysfunction as the primary outcome.7 Our results provide further support for the use of subjects with severe, persistent lung dysfunction, who have dysfunction early and either attenuate later (by day 2) or do not resolve, as a useful phenotype for studies interested in the most severe PGD phenotype and the one most likely to reflect the pathophysiology of underlying diffuse alveolar damage. Subjects with this phenotype had the highest risk for death, and a greater frequency of characteristics thought to be associated with more severe dysfunction, including CPB, large volume of RBC transfusion, and higher mean PAP at the time of transplantation.14,17,18 These factors may be interrelated, and further work needs to be done to identify predictors associated with each class.
A small percentage of subjects who meet the criteria for grade 3 PGD within 72 h may have rapidly resolving graft dysfunction. Subjects with dysfunction that resolved or attenuated early received a greater volume of IV crystalloid infusion, which indicates that the pattern of early resolution of lung dysfunction may not represent classic alveolar damage mechanisms, and may be a result of volume overload. An alternative possibility is that subjects in class 2 are biologically more equipped to deal with early insults to the lung, and, therefore resolve rapidly. Further studies of biologic markers may help distinguish this phenotype from that of severe, persistent dysfunction. Overall, this phenotype occurred less frequently than the other phenotypes in our study; however, it is important to identify these subjects, as they have a lower risk for death, and in the future, may not require therapies targeting PGD.
Our study has several limitations. We did not include clinical characteristics in the derivation of the latent classes; however, our approach favored an unbiased definition of phenotypes, and we have internally validated these phenotypes by demonstrating different frequencies of clinical characteristics among classes. Second, there were missing data in this study. However, we used multiple imputation to account for this, and point estimates in our nonimputed dataset were very similar to those in the imputed dataset. Additionally, like many key variables, PGD grades were complete in the dataset. Third, although we have published associations of biomarkers with PGD previously, there was insufficient biomarker information in this smaller study population of patients with grade 3 PGD to evaluate differences in markers of biologic associations between the classes. Fourth, we do not collect information on intensive care management of subjects with PGD, and, therefore, we are unable to account for differences in care among centers or differences in management over time, as subjects were enrolled over 8 years. However, lung-protective ventilation was initiated in 2000,19 before this study began, and there have been no major changes in therapy for PGD or acute lung injury since then. Fifth, we did not have access to information on bronchiolitis obliterans syndrome for this cohort. Finally, we did not have lung biopsies on any of the subjects in the cohort. Future work will focus on identifying pathologic differences among the classes and study of the relationship with long-term outcomes, including bronchiolitis obliterans syndrome.
In conclusion, LCA identified two main phenotypes of resolution in PGD. First, a pattern of severe, persistent dysfunction, which includes subjects with persistent dysfunction for 72 h and subjects with late attenuation of dysfunction; and second, a pattern of early resolution or attenuation of dysfunction within 24 h of transplantation. After validation of these phenotypes in another population, further work should address biologic associations and mechanisms within these phenotypes. Refinement of the syndrome definition is important to assist in research identifying biomarkers of lung dysfunction after transplantation, describing mechanisms of early allograft dysfunction, evaluating prognostic models of allograft dysfunction, and targeting subjects most likely to benefit from therapeutic interventions. The results of our study support the use of grade 3 PGD at 48 or 72 h as the primary outcome for studies with these aims, and may support the use of intermediate grades at 48 and 72 h to further identify subjects with lung injury.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Dr Shah had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Shah: contributed to statistical analyses and writing the manuscript, and served as principal author.
Dr Diamond: contributed to interpreting results, supervising patient enrollment, and editing the manuscript.
Dr Cantu: contributed to interpreting results, supervising patient enrollment, and editing the manuscript.
Dr Lee: contributed to interpretation of results, supervision of patient enrollment, and editing the manuscript.
Dr Lederer: contributed to interpretation of results, supervision of patient enrollment, and editing the manuscript.
Dr Lama: contributed to interpreting results, supervising patient enrollment, and editing the manuscript.
Dr Orens: contributed to interpreting results, supervising patient enrollment, and editing the manuscript.
Dr Weinacker: contributed to interpreting results, supervising patient enrollment, and editing the manuscript.
Dr Wilkes: contributed to interpreting results, supervising patient enrollment, and editing the manuscript.
Dr Bhorade: contributed to interpreting results, supervising patient enrollment, and editing the manuscript.
Dr Wille: contributed to interpreting results, supervising patient enrollment, and editing the manuscript.
Dr Ware: contributed to interpreting results, supervising patient enrollment, and editing the manuscript.
Dr Palmer: contributed to interpreting results, supervising patient enrollment, and editing the manuscript.
Dr Crespo: contributed to interpreting results, supervising patient enrollment, and editing the manuscript.
Dr Localio: contributed to interpreting results, supervising patient enrollment, and editing the manuscript.
Ms Demissie: contributed to interpreting results, supervising patient enrollment, and editing the manuscript.
Dr Kawut: contributed to interpreting results, supervising patient enrollment, and editing the manuscript.
Dr Bellamy: contributed to statistical analyses and editing the manuscript.
Dr Christie: contributed to supervising data collection and the statistical analyses, interpreting the results, and editing the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Lederer consults for ImmuneWorks on lung transplantation and primary graft dysfunction. Dr Wilkes is the cofounder and Chief Scientific Officer of ImmuneWorks, and has received compensation for consulting on the company’s progress. The remaining authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendixes, e-Figures, and e-Tables can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- CPB
cardiopulmonary bypass
- HR
hazard ratio
- LCA
latent class analysis
- PGD
primary graft dysfunction
Footnotes
Funding/Support: This work was supported by the National Institutes of Health [Grants R01-HL087115 to J. D. C. and HL088263 to L. B. W.].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Christie JD, Kotloff RM, Pochettino A, et al. Clinical risk factors for primary graft failure following lung transplantation. Chest. 2003;124(4):1232-1241 [DOI] [PubMed] [Google Scholar]
- 2.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D; ISHLT Working Group on Primary Lung Graft Dysfunction Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24(10):1454-1459 [DOI] [PubMed] [Google Scholar]
- 3.Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Lung and Heart-Lung Transplant Report—2011. J Heart Lung Transplant. 2011;30(10):1104-1122 [DOI] [PubMed] [Google Scholar]
- 4.Dahlberg PS, Prekker ME, Herrington CS, Hertz MI, Park SJ. Medium-term results of extracorporeal membrane oxygenation for severe acute lung injury after lung transplantation. J Heart Lung Transplant. 2004;23(8):979-984 [DOI] [PubMed] [Google Scholar]
- 5.Christie JD, Bellamy S, Ware LB, et al. Construct validity of the definition of primary graft dysfunction after lung transplantation. J Heart Lung Transplant. 2010;29(11):1231-1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daud SA, Yusen RD, Meyers BF, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175(5):507-513 [DOI] [PubMed] [Google Scholar]
- 7.Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;364(15):1431-1440 [DOI] [PubMed] [Google Scholar]
- 8.Conaway M. Latent class analysis. : Armitage P, Colton T. Encyclopedia of Biostatistics. Hoboken, NJ: John Wiley & Sons Inc; 2005 [Google Scholar]
- 9.Diamond JM, Kawut SM, Lederer DJ, et al. ; Lung Transplant Outcomes Group Elevated plasma clara cell secretory protein concentration is associated with high-grade primary graft dysfunction. Am J Transplant. 2011;11(3):561-567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diamond JM, Lederer DJ, Kawut SM, et al. ; Lung Transplant Outcomes Group Elevated plasma long pentraxin-3 levels and primary graft dysfunction after lung transplantation for idiopathic pulmonary fibrosis. Am J Transplant. 2011;11(11):2517-2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang A, Studer S, Kawut SM, et al. ; Lung Transplant Outcomes Group Elevated pulmonary artery pressure is a risk factor for primary graft dysfunction following lung transplantation for idiopathic pulmonary fibrosis. Chest. 2011;139(4):782-787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman SA, Wang L, Shah CV, et al. ; Lung Transplant Outcomes Group Plasma cytokines and chemokines in primary graft dysfunction post-lung transplantation. Am J Transplant. 2009;9(2):389-396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiley JP. Advancing respiratory research. Chest. 2011;140(2):497-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christie JD, Shah CV, Kawut SM, et al. ; Lung Transplant Outcomes Group Plasma levels of receptor for advanced glycation end products, blood transfusion, and risk of primary graft dysfunction. Am J Respir Crit Care Med. 2009;180(10):1010-1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Covarrubias M, Ware LB, Kawut SM, et al. ; Lung Transplant Outcomes Group Plasma intercellular adhesion molecule-1 and von Willebrand factor in primary graft dysfunction after lung transplantation. Am J Transplant. 2007;7(11):2573-2578 [DOI] [PubMed] [Google Scholar]
- 16.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3-15 [DOI] [PubMed] [Google Scholar]
- 17.Kuntz CL, Hadjiliadis D, Ahya VN, et al. Risk factors for early primary graft dysfunction after lung transplantation: a registry study. Clin Transplant. 2009;23(6):819-830 [DOI] [PubMed] [Google Scholar]
- 18.Gammie JS, Cheul Lee J, Pham SM, et al. Cardiopulmonary bypass is associated with early allograft dysfunction but not death after double-lung transplantation. J Thorac Cardiovasc Surg. 1998;115(5):990-997 [DOI] [PubMed] [Google Scholar]
- 19.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301-1308 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement