Abstract
World Health Organization (WHO) group 2 pulmonary hypertension (PH) due to left-side heart disease (ie, heart failure or left-sided valvular heart disease) is the most common form of PH in western countries. Distinguishing patients with WHO group 2 PH, particularly the subset of patients with PH due to heart failure with preserved ejection fraction (HFpEF), from those with WHO group 1 pulmonary arterial hypertension (PAH) is challenging. Separating the two conditions is of vital importance because treatment strategies differ completely. Furthermore, therapies that are indicated for WHO group 1 PAH may be harmful in patients with WHO group 2 PH. We review the somewhat confusing PH nomenclature and the WHO classification system and rationale behind it. We then focus on left-side heart disorders that cause PH. An aging population and advances in the medical management of common cardiovascular disorders have caused the prevalence of heart failure to rise significantly, with more than one-half of patients having HFpEF. We review contemporary studies that focus on clinical and echocardiographic findings that help to distinguish HFpEF from PAH in the patient with PH. We discuss the typical, and sometimes atypical, hemodynamic profiles that characterize these two groups, review challenges in the interpretation of data obtained by right-sided heart catheterization, and highlight special maneuvers that may be required for accurate diagnosis. Finally, we review the largely disappointing studies on the use of PAH-specific therapies in patients with WHO group 2 PH, including the use of prostacyclins, endothelin receptor antagonists, and the more promising phosphodiesterase-5 inhibitors.
Pulmonary hypertension (PH) is more often due to left-side heart disease (LHD) than to pulmonary arterial hypertension (PAH).1,2 Yet, recognizing this subset of PH, especially in patients with preserved ejection fraction, is challenging. Failing to appreciate the left-side heart basis of PH may result in an unnecessary referral to a PH clinic, the administration of expensive treatments that may do more harm than good, and a delay in treatment of the true primary disease. We discuss how left-sided valvular heart disease and heart failure lead to PH and review their clinical significance. We then focus on the difficulties involved in distinguishing patients with World Health Organization (WHO) group 2 PH from those with WHO group 1 PAH and suggest a practical approach to this common problem. Finally, we briefly review prior and current studies examining the effect of PAH-specific therapies on clinical outcomes in patients with WHO group 2 PH.
What Is PH?
PH, defined by a mean pulmonary arterial pressure (PAP) ≥ 25 mm Hg,3,4 can be a consequence of an intrinsic disorder within the pulmonary arteries, resulting in elevated pulmonary vascular resistance (PVR); an increase in pulmonary venous pressure; an increase in pulmonary blood flow; or a combination of these elements.5 PH is a clinical and hemodynamic condition that most commonly is a consequence of another illness.
Clinical conditions associated with PH have been classified by the WHO into five major categories since the second World Symposium on PH convened in Evian, France, in 1998.6 The classification attempts to create categories of PH that have shared pathologic and clinical features as well as therapeutic approaches. The WHO last updated the classification following the 4th World Symposium held at Dana Point in 2008 (Table 1).7,8 The term “pulmonary hypertension” encompasses all five major categories in the WHO classification. PAH (WHO group 1) comprises idiopathic PH (previously primary PH), hereditary PH, and PH associated with a variety of clinical conditions, as detailed in Table 1. PH associated with LHD (ie, WHO group 2 PH), or postcapillary PH, refers to PH that results from elevated left-sided pressures. This category was first incorporated into the clinical classification in 1998 and was previously referred to as one of several causes of secondary PH. Other major causes of PH include lung diseases, hypoxia, or both (WHO group 3); chronic thromboembolic PH (WHO group 4); and a variety of hematologic, systemic, or metabolic disorders with unclear or multifactorial mechanisms (WHO group 5). Thorough and methodical evaluation is essential for patients with newly diagnosed PH because prognosis and treatment depend on the underlying cause.
Table 1.
—Updated World Health Organization Clinical Classification of PH (Dana Point, 2008)
| Group | Description |
| 1. PAH | Idiopathic (previously primary PH) |
| Heritable (mutations of BMPR2, ALK1, endoglin, or unknown) | |
| Drugs and toxins | |
| Associated with connective tissue disease, HIV, portal hypertension, congenital heart disease, schistosomiasis, chronic hemolytic anemia | |
| 1′ PVOD and/or PCH | |
| 2. PH owing to left heart disease | Systolic or diastolic dysfunctionValvular disease |
| 3. PH owing to lung disease | COPD, ILD, sleep-disordered breathing, high altitude |
| 4. CTEPH | |
| 5. PH with unclear, multifactorial mechanism | Hematologic, systemic, or metabolic disorders |
CTEPH = chronic thromboembolic pulmonary hypertension; ILD = interstitial lung disease; PAH = pulmonary arterial hypertension; PCH = pulmonary capillary hemangiomatosis; PH = pulmonary hypertension; PVOD = pulmonary venoocclusive disease. Adapted with permission from Simonneau et al.7
The hemodynamic definition of PH has two major components: mean PAP and the estimated or measured left-sided filling pressure of the heart as reflected by the left ventricular end-diastolic pressure (LVEDP), the left atrial pressure (LAP), or the pulmonary capillary wedge pressure (PCWP). The fourth World Symposium on Pulmonary Hypertension in 2008 recommended that a resting mean PAP ≥ 25 mm Hg, as measured by right-sided heart catheterization, should be considered elevated.3 This recommendation was based on an extensive review of the existing literature on healthy volunteers, which showed that normal resting mean PAP ranges from 8 to 20 mm Hg.3,4 The clinical significance of a mean PAP between 21 and 24 mm Hg is unclear. Exercise-induced PH, previously defined as mean PAP > 30 mm Hg during exercise, is a poorly understood entity. A review of right-sided heart catheterization studies found that PAP during exercise depends on exercise level and age and frequently exceeds 30 mm Hg, especially in elderly people.4 Because of a lack of standardized exercise protocols and uncertainty with regard to the definition of a normal pressure threshold, exercise-induced PH has been removed from the definition.
PH can be further categorized as either precapillary (PCWP or LVEDP ≤ 15 mm Hg) or postcapillary (PCWP or LVEDP > 15 mm Hg) in etiology.9 WHO group 1 PAH; group 3 PH due to lung disease, hypoxia, or both; group 4 chronic thromboembolic PH; and group 5 PH with an unclear or a multifactorial mechanism all result in precapillary PH and cannot be distinguished on the basis of hemodynamics alone. Early on, PH due to LHD may cause postcapillary PH with elevated left-sided filling pressure and a normal or mildly elevated PVR. However, as will be discussed in more detail later, a significant increase in PVR may subsequently develop in a subset of patients with this condition, which we will refer to as mixed PH.5,10 Distinguishing precapillary and postcapillary PH on the basis of left-sided filling pressures and PVR can be problematic, and additional maneuvers often are required during right-sided heart catheterization, as discussed later.
The survival of patients with PAH has improved considerably over the past 2 decades, which may reflect the effects of currently available PAH-specific therapies, a better understanding and closer monitoring of the disease, or differences in the patient population studied. Patients who initiate PAH-specific therapy within 6 months of diagnosis have been found to have 22% to 29% better 7-year survival than the National Institutes of Health registry cohort from the 1980s.11 However, PAH-specific medications are not indicated for all forms of PH, and identifying the specific cause of PH is necessary for directing therapy to the underlying cause of PH and for selecting appropriate candidates for PAH-specific therapy. Mislabeling a patient with PAH may result in ineffective and costly treatments that often have many side effects.
PH Due to LHD
PH is increasingly recognized as a common and important complication of LHD, particularly in heart failure and valvular heart disease. Indeed, studies have consistently found that the presence of PH in patients with elevated left ventricular (LV) filling pressure predicts worse outcomes and higher mortality.12,13 Although the overall prevalence of PH due to LHD is unclear and varies according to definition and diagnostic methods, WHO group 2 PH is the most common cause of elevated PAP.1,2 Historically, mitral valve disease has probably been the best-described cause of PH.14‐16 In the current era, heart failure with preserved ejection fraction (HFpEF) is recognized as the predominant cause of elevated left-sided filling pressures resulting in PH.17 The estimated prevalence of HFpEF as measured by echocardiography in the general population is 11% to 27%.18,19 It is worth noting that echocardiographic findings of HFpEF often are subtle and can be missed.
Despite the high prevalence of WHO group 2 PH, the major focus of research on PH over the past decade has been on WHO group 1 PAH. Few investigators have focused on WHO group 2 PH; consequently, the pathophysiology of this condition remains poorly understood, and no specific therapy is available. Clinical and translational studies in this area are much needed and have the potential to positively affect large numbers of patients.
Pathophysiology of PH Due to LHD
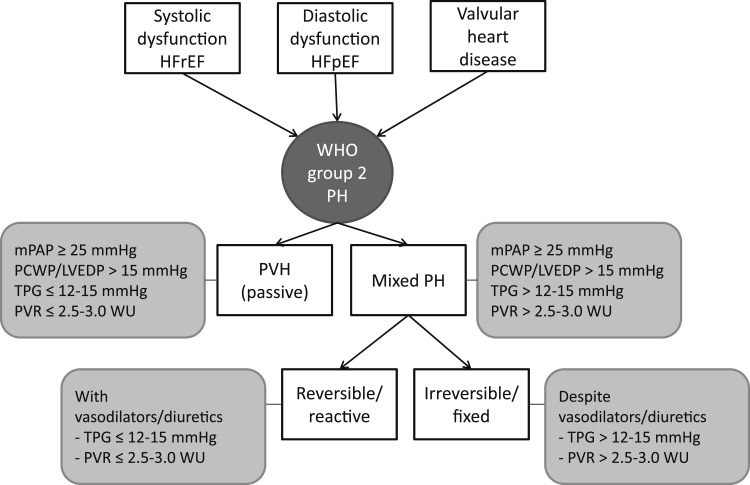
A spectrum of pathophysiologic changes, ranging from simple pulmonary venous congestion to significant structural and functional abnormalities of the pulmonary vasculature, occurs in WHO group 2 PH. In this review, we use the nomenclature proposed by the International Society for Heart and Lung Transplantation to define different pathophysiologic and hemodynamic forms of WHO group 2 PH (Fig 1).5 Early on, PH due to LHD occurs when left-sided ventricular or valvular disease produces an increase in LAP, which is transmitted passively into the pulmonary vascular tree. Hemodynamically, this results in an elevated PCWP and increased PAP, and in most cases, the transpulmonary gradient (TPG) is normal (< 12-15 mm Hg) and the PVR is not elevated (< 1.5 Wood units).4 A universal definition of a normal TPG has proven elusive, and, thus, a range is given.4,7 This situation is referred to herein as passive PH or pulmonary venous hypertension. However, many patients with LHD have long-standing and progressive elevation of left-sided heart pressures, resulting in a PAP that is higher than what would be expected from the elevated LAP alone.20‐22 This group of patients has an elevated TPG and PVR and is referred to as mixed, out of proportion, or disproportionate PH in the literature. In this review, we use the term mixed PH.5 Schwartzenberg et al23 and Guazzi and Borlaug24 recently showed that in more than one-half of patients with heart failure with reduced ejection fraction (HFrEF) and HFpEF the PVR is > 3 Wood units or TPG is > 15 mm Hg. In some cases, mixed PH is reversible (or reactive) with the administration of a systemic vasodilator or diuretic, implicating a pulmonary vasoconstrictive response to the elevation in left-sided pressures (see later in this article). In other cases, the PH is irreversible (or fixed), implicating vascular remodeling in the pathogenesis of this condition. It should be taken into consideration that patients with LHD and PH may have an additional precapillary component from another disorder, such as pulmonary embolism or untreated sleep-disordered breathing. As with other types of PH, a full diagnostic workup is essential.
Figure 1.
Etiologies and subcategories of WHO group 2 PH. HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; LVEDP = left ventricular end-diastolic pressure; mPAP = mean pulmonary arterial pressure; PCWP = pulmonary capillary wedge pressure; PH = pulmonary hypertension; PVH = pulmonary venous hypertension; PVR = pulmonary vascular resistance; TPG = transpulmonary gradient; WHO = World Health Organization; WU = Wood units.
Progress has been made in elucidating the pathophysiologic changes that occur in PH due to LHD, although they remain somewhat speculative. High resistance in the pulmonary arteries in patients with PH due to LHD is a result of long-standing elevation of left-sided pressures, dysregulation of vascular smooth muscle vessel tone (reversible), or pulmonary vascular remodeling (irreversible).5,21,25 Acute elevation of hydrostatic pressure in the pulmonary capillaries causes endothelial and alveolar cell membrane breaks (alveolar capillary stress failure).25,26 Chronic elevation of hydrostatic capillary pressures can result in remodeling with extracellular matrix thickening.27,28 Remodeling leads to a persistent reduction in alveolar-capillary membrane conductance and diffusing capacity of lung.29 Increases in pressure also result in remodeling, hypertrophy, and fibrous changes at the level of the pulmonary veins and arteries.20,30 Finally, patients with WHO group 2 PH have been shown to have endothelial dysfunction. Basal production of the pulmonary vascular vasodilator nitric oxide is relatively deficient,21 and the sensitivity of the pulmonary vasculature to other cyclic guanosine monophosphate-dependent vasodilators, such as brain natriuretic peptide, may be decreased.31 In addition, elevated levels of the pulmonary vasoconstrictor endothelin-1 have been demonstrated in patients with elevated left-sided heart pressures. Endothelin-1 causes proliferation and hypertrophy of vascular smooth muscle cells and, thus, likely contributes to the pulmonary vascular remodeling seen in patients with PH due to LHD.21,22 It should be noted that although endothelin-1 has been implicated in the pathogenesis of WHO group 2 PH, endothelin receptor antagonists have not been proven to be beneficial in clinical trials.32‐34 Lastly, various factors, like Platelet-derived growth factor, epidermal growth factor, and vascular endothelial growth factor have been implicated in PAH but not established in the pathogenesis of PH due to LHD.35
Persistent elevation of PAPs can lead to right ventricular failure from pressure overload. Initially, the right ventricle (RV) becomes hypertrophic in response to high PAPs and can generate much higher pressures than in the normal low afterload state. With time, RV hypertrophy may not be sufficient and the RV dilates, with a subsequent decrease in contractile function and symptoms of right-sided heart failure. The clinical manifestations of right-sided heart failure, including reduced LV filling from ventricular interdependence, hepatic and splanchnic congestion, impaired lung lymphatic drainage, and reduced renal sodium excretion, are themselves decompensatory and likely accelerate clinical deterioration.24
PH in Heart Failure
The prevalence of heart failure has been increasing as the population ages.36 It is currently estimated that 5.8 million people in the United States have either HFrEF or HFpEF.36 The exact proportion of patients with heart failure and PH varies depending on patient subsets, definitions of heart failure and PH, and the method used to estimate PAP. In a cohort of 379 patients with HFrEF, Ghio et al37 reported that 236 (62%) had a mean PAP > 20 mm Hg by right-sided heart catheterization. The prevalence of PH in HFpEF is in the range of 52% (defined as mean PAP > 25 mm Hg by right-sided heart catheterization)38 to 83% (defined as pulmonary artery systolic pressure > 35 mm Hg by echocardiographic estimates).39 Regardless of the type of heart failure, PH is an indicator of worse prognosis.37,40,41
The diagnosis of HFpEF is not always straightforward, and studies have found that it is indeed a major cause of unexplained dyspnea.42,43 As will be discussed later in this review, distinguishing PH due to HFpEF from PAH may be challenging because both groups of patients often have normal LVEF and no significant left-sided valvular disease on echocardiogram.44 Patients with HFpEF can have severe PH with elevated PVR, and this group poses the greatest diagnostic dilemma.45,46 The distinction between the two conditions is, however, critical because treatments that are indicated for PAH may be harmful in patients with PH related to HFpEF.
PH and Restrictive Cardiomyopathy
Restrictive cardiomyopathies (as from amyloidosis, sarcoidosis, or prior radiation therapy) should always be considered in the differential diagnosis of patients presenting with elevated left-sided pressures and normal LV systolic function. Although certain echocardiographic findings, including Doppler tissue velocities, may suggest the diagnosis47,48; further testing, including invasive hemodynamics, endomyocardial biopsy, and additional imaging, may be necessary to establish the diagnosis and to differentiate this condition from constrictive pericarditis. Restrictive cardiomyopathy is frequently difficult to treat and may result in severe PH,49 although this phenomenon has not been well studied. Further evaluation and management of these patients is beyond the scope of this review.
PH in Valvular Heart Disease
Aortic Valve:
PH is present in 28% to 56% of patients with severe aortic stenosis, depending on patient selection criteria and definition of PH used.50 The prognosis of patients with severe aortic stenosis and PH is dismal.13,51 Surgical aortic valve replacement is the recommended treatment of patients with severe aortic stenosis in the appropriate clinical setting.52 Perioperative complications associated with aortic valve replacement are greater when PH is present preoperatively.53 Valve replacement is an effective treatment of the PH associated with this condition, however, and a significant decrease in PAP can be seen immediately after surgery. Some patients will have persistent PH, and these patients have decreased long-term survival.53 Transcatheter aortic valve replacement is an emerging therapeutic option, particularly in patients with severe aortic stenosis and PH who are at high risk for surgical valve replacement.54
PH can also develop in patients with aortic regurgitation. Surgical repair of aortic regurgitation is recommended when symptoms develop or when LV dilation occurs. There does not appear to be an increased risk of mortality or operative complications in patients with aortic regurgitation with severe PH compared with those with mild or no PH, and in most cases, the PAP normalizes with aortic valve replacement.55 Again, there is no current literature to support the use of PAH-specific therapies in patients with aortic regurgitation.
Mitral Valve:
PH commonly develops in patients with mitral valve disease because of chronically elevated LAP due to either an increased pressure gradient across the stenotic mitral valve or a regurgitant systolic jet. It has been known for > 40 years that severe PH can develop in patients with mitral valve disease (systolic PAP > 100 mm Hg) with very high PVR (> 6 Wood units).15,56 This results from a combination of backward transmission of elevated LAP and pulmonary arteriolar vasoconstriction and remodeling.16,57 PVR is reduced dramatically after correction of valvular lesions,15,56,58 and the PVR can continue to fall for months after surgery.59 The American College of Cardiology/American Heart Association guidelines recommend transcutaneous or surgical intervention in patients with mitral stenosis and PH (pulmonary arterial systolic pressure > 50 mm Hg).60 The reported operative mortality in patients with PH undergoing mitral valve replacement is highly variable and ranges from 6% to 31%.58 PAH-specific therapy can cause clinical deterioration and pulmonary edema in patients with elevated left-sided heart pressure. This could hypothetically occur as a consequence of either increased right ventricular output and LV filling from pulmonary vasodilation (decreased right ventricular afterload) or pulmonary venodilation with a consequent increase in capillary pressure that is partly related to an increased V wave. There are, case reports that have described the use of PAH-specific therapies in patients with PH follow surgery. One report was of a patient with persistent PH following mitral valve repair who experienced improved symptoms and hemodynamics with epoprostenol therapy.61 The use of sildenafil in a patient with persistent PH following mitral valve replacement has also been reported, and the therapy had a favorable outcome.62
Distinguishing PH Due to HFpEF From PAH
PAH can be easily differentiated from PH due to HFrEF or valvular heart disease on the basis of clinical features and echocardiogram. However, it can be difficult to differentiate PAH from PH due to HFpEF because LV systolic function is preserved in both and because both may have abnormal diastolic parameters.63 Distinguishing PH due to HFpEF from PAH is vital because the management is dramatically different for the two conditions. PAH-specific therapies may worsen heart failure symptoms and increase hospitalizations when used in patients with PH due to LHD.32,64 On the other hand, misclassifying and not identifying a patient with PAH in a timely manner will delay treatment that can significantly improve symptoms, exercise tolerance, and probably survival. HFpEF is being increasingly recognized as a major cause of PH, and the diagnostic challenges and implications of separating this entity from PAH have been acknowledged but not extensively studied. Several epidemiologic studies have started to look at clinical features, echocardiographic findings, and hemodynamic differences that may reliably distinguish the two conditions.
Clinical Features
Symptoms of PH are nonspecific but include dyspnea, fatigue, dizziness, and chest pain. Risk factors that have been associated with PH due to HFpEF differ from the conditions that are typically associated with PAH and are shown in Table 2.38,44,45,65 Additionally, orthopnea and paroxysmal nocturnal dyspnea generally are not features of PAH and suggest a primary left-sided heart etiology.
Table 2.
—Clinical Features and Risk Factors Distinguishing PAH From PH Due to HFpEF
| PAH6 | PH Due to HFpEF |
| Family history | Older age38,65 |
| Use of anorexigens, amphetamines | Systemic hypertension45,65 |
| Connective tissue disease; systemic sclerosis, SLE, MCTD | Diabetes45,65 |
| HIV | Coronary artery disease45,65 |
| Liver disease, portal hypertension | Atrial fibrillation38 |
| Congenital heart disease | Obesity38,45,65 |
| Schistosomiasis | Hyperlipidemia45 |
| Orthopnea, PND | |
| Exaggerated increase in BP with exercise66 | |
| ECG: right axis, RAE, RVH67,68 | ECG: left axis, LAE, LVH69 |
LAE = left atrial enlargement; LVH = left ventricular hypertrophy; MCTD = mixed connective tissue disease; PND = paroxysmal nocturnal dyspnea; RAE = right atrial enlargement; RVH = right ventricular hypertrophy; SLE = systemic lupus erythematosus.
Simple clinical tests may also be helpful in distinguishing the two etiologies of PH. ECG in advanced PAH typically reveals a right axis deviation, right atrial enlargement, and right ventricular hypertrophy,67,68 whereas in patients with HFpEF, the typical findings include evidence of left atrial enlargement and LV hypertrophy.69 The chest radiograph in both scenarios may show enlarged pulmonary arteries70; however, pulmonary vascular congestion and pleural effusions generally are not seen in patients with PAH but are found frequently in patients with LHD.71 Finally, most patients will have a CT scan of the chest at one point or another during the evaluation. Interstitial septal thickening and ground glass changes consistent with chronic pulmonary edema are more consistent with pulmonary vascular hypertension than with PAH. Again, pleural effusions are indicative of a left-sided process.
Echocardiography
Echocardiographic findings can be used to help separate PAH from PH due to HFpEF but often are subtle (Table 3). By definition, both groups of patients have elevated PAP, normal LV function, and no significant valvular heart disease. Patients with PH due to HFpEF more often have left atrial enlargement and less often have right atrial enlargement compared with patients with PAH.65 LV hypertrophy is more suggestive of PH due to HFpEF,75 whereas right ventricular hypertrophy favors PAH.65 Early diastolic mitral annular tissue Doppler velocity (E′) has been shown to correlate with invasive measures of LV relaxation independent of preload.78 E′ tends to be depressed because of intrinsic left ventricular disease in patients with HFpEF, indicating impaired relaxation.76 The ratio of early mitral flow velocity (E) to E′ is widely used as a noninvasive measure of LVEDP and LAP.76,78 Patients with impaired relaxation and elevated LAP have an elevated E but reduced E′, and an E/E′ ratio of > 15 is associated with a mean LAP > 15 mm Hg.78 It should be noted that patients with PAH can have impaired LV filling due to left ventricular interaction mediated by the interventricular septum.63 However, an E/E′ ratio of < 8 has been shown to differentiate patients with advanced idiopathic PAH from PH due to heart disease.76,77 Opotowsky et al74 recently derived a simple echocardiographic prediction rule to differentiate patients with high PVR as the primary cause of PH from patients with pulmonary venous hypertension. The prediction rule ranged from −2 to +2, with a higher score suggesting PH due to pulmonary vascular disease. According to the rule, an E/E′ ratio of > 10 and large left atrium each give −1 point, whereas a small left atrial size and right ventricular outflow tract acceleration time < 80 milliseconds give +1 point. The prediction rule had an area under the curve of 0.921 for PH with high PVR.
Table 3.
—Typical Echocardiographic Findings in PAH and PH Due to HFpEF
Exercise
Exertional dyspnea and reduced exercise capacity commonly occur in patients with PAH and those with PH due to HFpEF. In both groups, exercise capacity may be limited by an inability to recruit additional pulmonary vasculature or because of failure of pulmonary vascular dilation during exercise, thereby placing an additional load on the RV and preventing the cardiac output from increasing appropriately. Such patients also frequently exhibit a variety of gas exchange abnormalities during cardiopulmonary exercise testing, including an impaired ventilatory efficiency (eg, expired volume per unit time (e)/oxygen consumption (o2) ratio of > 34) during exercise.79,80 Of note, elevated PCWP and PAPs during exercise may develop in some patients with HFpEF who do not have PH at rest.43 Indeed, in patients with HFpEF, diastolic LV dysfunction with increased end-diastolic stiffness, a steep diastolic pressure-volume relation, and high PCWP at a low workload likely plays a key role in exercise limitation.81‐83 This theory is supported by the finding that diastolic dysfunction is strongly and inversely associated with exercise tolerance.84,85
Investigators have identified certain differences in exercise physiology between patients with PH due to HFpEF and patients with PAH. Importantly, exercise capacity that is more impaired than would be expected from the degree of PH alone is in favor of HFpEF as the main underlying cause,65 as is an exaggerated hypertensive response to exercise.66 Patients with PAH have an increase in dead space ventilation because arteriolar obstruction results in decreased perfusion to well-ventilated areas. This is manifested by decreased end-tidal CO2 at rest and during exercise.86 End-tidal CO2 has been found to be significantly lower in patients with PAH than in patients with PH due to HFpEF and can be used to help differentiate between the two conditions.87
Cardiac Catheterization
Right-sided heart catheterization is critical to distinguish between PAH and PH due to HFpEF. Unlike patients with PAH, patients with PH due to HFpEF generally have elevated left-sided filling pressure (PCWP, LVEDP, or both). There are, however, many potential pitfalls to keep in mind when using hemodynamics to distinguish between PAH and PH due to HFpEF (Table 4). Routine hemodynamic assessment is not always adequate, and additional procedures often are needed, particularly when there is a discrepancy between clinical risk factors and hemodynamics and when LV filling pressure is borderline elevated.
Table 4.
—Typical Hemodynamics and Pitfalls in Distinguishing PAH From PH Due to HFpEF
| PAH (mPAP > 25 mm Hg) | PH Due to HFpEF (mPAP > 25 mm Hg) | Pitfalls and Additional RHC Maneuvers |
| Normal PCWP/LVEDP | Elevated PCWP/LVEDP | PAH may have mild elevation in left-sided filling pressures.63 |
| HFpEF may have normal left-sided filling pressures after aggressive diuresis. Consider fluid or exercise challenge.88 | ||
| PCWP may not be an accurate estimate of LAP or LVEDP. Consider direct measurement of LVEDP.89 | ||
| PVR > 2.5-3 WU | PVR ≤ 2.5-3 WU (passive) | It is a misconception that patients with PH due to LHD will not have an elevated PVR. |
| PVR > 2.5-3 WU (mixed) |
LAP = left atrial pressure; LVEDP = left ventricular end-diastolic pressure; mPAP = mean pulmonary arterial pressure; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; RHC = right-sided heart catheterization; WU = Wood units. See Table 1 and 3 legends for expansion of other abbreviations.
Left-sided heart pressures are the most important and most challenging variables to obtain and interpret when distinguishing PAH from PH due to HFpEF. First, PCWP does not always accurately estimate LAP, and a more direct measurement with LVEDP may be needed.89 Second, critical errors can be made in waveform interpretation. Certain conditions make waveform interpretation more challenging, as when patients exhibit large swings in intrathoracic pressure due to advanced lung disease or obesity or when large V waves are present. A recent study showed that using the digital PCWP read instead of the end-expiration PCWP (when the influence of intrathoracic pressure on intracardiac pressure measurement is least) results in a significant underestimation of LVEDP and, thus, misclassification of patients as having PAH rather than PH due to HFpEF.90 Large V waves, which are commonly associated with significant mitral regurgitation but may also be seen in patients with high LVEDP,91 will also drive up the mean PCWP. There is no consensus about how best to calculate the mean PCWP in the setting of large V waves. Third, patients with PH due to HFpEF may have a normal resting PCWP and LVEDP after aggressive diuresis. On the other hand, patients with PAH may have slightly elevated PCWP because of the enlarged RV that impinges on the left ventricle and causes increased LV filling pressures (ventricular interdependence).63 Finally, it is worth emphasizing that long-standing elevation of LV filling pressures can result in arterial remodeling and a significant increase in PVR, as discussed previously.
Additional maneuvers can be used to unmask impaired relaxation of the left ventricle. Provocative maneuvers, including fluid challenge or exercise, can be done during the right-sided heart catheterization when the PCWP, LVEDP, or both are normal or mildly elevated (due to pharmacologic unloading, recent diuresis, or both), but there is a high clinical suspicion for pulmonary venous hypertension. There are no standardized protocols for either of those procedures. A typical amount of fluid administered is 500 to 1,000 mL.88 A significant increase in LV filling pressure (LVEDP or PCWP) during exercise or fluid challenge increases the likelihood of PH due to LHD, although no definition exists on how much increase is pathologic. Finally, a systemic vasodilator challenge with nitroprusside can be helpful in patients being evaluated for PH due to LHD. Normalization or near normalization of PAPs and PCWP supports the diagnosis of reversible WHO group 2 PH. The effects of reversibility with nitroprusside on response to medical therapy have not been studied in WHO group 2 PH. However, reversibility may be predictive of better outcome after heart transplantation.92,93 It should be noted that some patients with LHD have an irreversible component to their PH, and the PAP in such patients will not normalize acutely. While taking all of the previous discussion into consideration, we recommend that right-sided heart catheterizations for evaluation of PH be performed in expert centers.
Distinguishing PH due to HFpEF from PAH relies on a thorough clinical assessment and the accurate interpretation of complex echocardiographic and hemodynamic data. Thenappan et al65 examined clinical risk factors, echocardiographic findings, and hemodynamics that might distinguish between the two groups. They found that a model incorporating older age, the presence of systemic hypertension and coronary artery disease, the absence of right atrial enlargement, higher aortic systolic pressure, higher mean right atrial pressure, and higher cardiac output best differentiated PH due to HFpEF from PAH, with an impressive area under the receiver operating characteristic curve of 0.97. In this study, the authors used a hemodynamic definition of PH due to HFpEF that consisted of the presence of signs and symptoms of heart failure, an LVEF ≥ 50%, a PCWP or LVEDP > 15 mm Hg, and a PVR > 2.5 Woods units and/or TPG > 12 mm Hg. It should be noted that definitions of PH in HFpEF vary and that no “gold standard” exists. Accordingly, different hemodynamic definitions may yield different results.
Treatment
Currently, the mainstay of treatment of PH due to LHD is to optimize the management of underlying heart failure, valvular disease, or both. For patients with heart failure and clinical evidence of volume overload, this includes consuming a low-sodium diet and using diuretics. Patients with HFrEF should be treated with angiotensin-converting enzyme inhibitors and β-blockers because both have been shown to reduce mortality. Aldosterone antagonists have demonstrated efficacy in HFrEF as well, particularly in patients with severe heart failure and after a myocardial infarction.94 In patients with HFpEF, management of all contributing conditions, including systemic hypertension, diabetes, obesity, and sleep apnea, is essential. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers should be considered in patients with HFpEF, especially in those with symptomatic atherosclerosis or diabetes. β-Blockers and calcium channel blockers should be especially considered in patients with hypertension or atrial fibrillation.94 Detailed discussion of these approaches is beyond the scope of this article. Areas of ongoing investigation include the role of pulmonary vasodilators and mechanical support in the treatment of WHO group 2 PH, and these topics are discussed next.
Pulmonary Vasodilator Therapies
Trials of PAH-specific therapies in heart failure have been largely disappointing (Table 5). Prostacyclins are not recommended for patients with HFrEF because of a clinical trial that was terminated early because of a strong trend toward decreased survival in patients treated with epoprostenol.64 In addition, epoprostenol therapy was not associated an increase in distance walked or quality of life. Another trial investigated the effects of endothelin receptor antagonists in HFrEF.32 Patients with EF < 35% were randomized to receive bosentan or placebo for 26 weeks. Safety concerns, particularly a high incidence of elevated liver function tests, led to the early termination of this trial, and bosentan exhibited no apparent benefit.
Table 5.
—Randomized Placebo Controlled Trials on the Efficacy of Pulmonary Vasodilators in HF
| Medication | Disease | No. Patients | Primary End Point | Outcome |
| Epoprostenol64 | HFrEF | 471 | Survival | Strong trend toward decreased survival |
| Bosentan33 | HFrEF | 1,600 | Survival, HF hospitalizations | No improved survival |
| Bosentan34 | HFrEF | 94 | Systolic PAP | No improved hemodynamics |
| Bosentan32 | HFrEF | 370 | Change in clinical status | Terminated because of safety concerns |
| Sildenafil95 | HFrEF | 34 | Peak o2 | Increased peak o2 |
| Reduced PVR | ||||
| Increased CO | ||||
| Reduced hospitalizations | ||||
| Improved 6MWD | ||||
| Sildenafil96 | HFrEF | 46 | Exercise capacity | Reduced systolic PAP |
| Improved exercise ventilation | ||||
| Sildenafil97 | HFpEF | 44 | mPAP | Decreased mPAP |
In contrast, two trials suggested a role for phosphodiesterase-5 inhibitors in WHO group 2 PH. One study of patients with HFrEF and PH showed that inhibition of phosphodiesterase-5 with sildenafil improved exercise capacity and quality of life.95 The first clinical trial showing a benefit of treatment of PH in patients with HFpEF compared 1 year of sildenafil therapy with placebo and assessed for hemodynamic improvement in 44 patients with heart failure, diastolic dysfunction, EF > 50%, and pulmonary artery systolic pressure > 40 mm Hg.97 Treatment with sildenafil led to an improvement in pulmonary hemodynamics and right ventricular performance as well as to LV relaxation.
Ongoing Clinical Trials
Two trials investigating drugs targeting the nitric oxide and cyclic guanine monophosphate pathway in the HFpEF population are currently under way. The RELAX (Evaluating the Effectiveness of Sildenafil at Improving Health Outcomes and Exercise Ability in People with Diastolic Heart Failure) trial is evaluating the effect of 6 months of sildenafil vs placebo on exercise capacity, functional status, and ventricular function in patients with diastolic heart failure (EF > 50%). The DILATE (A Study to Test the Effects of Riociguat in Patients with Pulmonary Hypertension Associated With Left Ventricular Diastolic Dysfunction) trial is investigating the efficacy, safety, and pharmacokinetic profile of the novel agent riociguat, a stimulator of soluble guanylate cyclase, on mean PAP in patients with symptomatic PH due to LV diastolic dysfunction.
There are two other ongoing trials examining the utility of endothelin receptor antagonists in HFpEF-associated PH. The BADDHY (Safety and Efficacy of Bosentan in Patients with Diastolic Heart Failure and Secondary Pulmonary Hypertension) trial is investigating the effect of 12 weeks of bosentan on 6-min walk distance, hemodynamics, and quality of life in patients with PH and a PCWP > 15 mm Hg. The Safety and Efficacy Trial to Treat Diastolic Heart Failure Using Ambrisentan will investigate the safety of ambrisentan given for 16 weeks, with secondary outcomes being 6-min walk distance, WHO functional class, and 36-Item Short Form Health Survey scores.
Mechanical Support
Mechanical support in PH associated with HFrEF has been another area of study. Consistently, studies have shown that left ventricular assist device (LVAD) support reverses fixed or medically unresponsive PH and allows patients with HFrEF and PH to be eligible for orthotopic heart transplantation.98‐101 Posttransplant survival for patients with HFrEF and PH treated with LVAD does not differ from those patients without PH who receive LVAD.102,103 Thus, in addition to other medical therapy, patients with HFrEF may benefit from further reduction of pulmonary pressures with mechanical support.
Summary
PH due to LHD is the most common type of PH encountered in western countries. The severity ranges from mild to severe disease in which the PVR is commonly significantly elevated as a result of remodeling of the pulmonary vasculature. Distinguishing WHO group 1 PAH from WHO group 2 PH may be challenging and should integrate clinical, echocardiographic, and hemodynamic information, ideally in centers with expertise. There is no consensus on what level, if any, of PAP or PVR should be considered disproportionate or out of proportion with the underlying cardiac conditions. At this time, the fundamentals of therapy for WHO group 2 PH are to optimize treatment of underlying conditions. Clinical studies on PAH-specific therapies have been disappointing, although small studies suggest that phosphodiesterase-5 inhibitors may be beneficial. More studies are required and some are currently under way to explore whether a subset of patients, particularly patients with higher pressure and PVR suggestive of pulmonary vascular remodeling, may benefit from therapies that are currently used for WHO group 1 PAH. A better understanding of the different phenotypes of PH due to LHD and their respective pathophysiologies is required so that much-needed new therapeutic approaches can be developed.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Drs Hansdottir and Groskreutz are the principal investigator and subinvestigator, respectively, at the University of Iowa for multicenter studies on pulmonary arterial hypertension sponsored by Actelion Pharmaceuticals US, Inc; Bayer AG; Gilead; INO Therapeutics LLC; and United Therapeutics Corporation. Dr Gehlbach has reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Abbreviations
- E
early mitral flow velocity
- E′
mitral annular tissue Doppler velocity
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- LAP
left atrial pressure
- LHD
left-side heart disease
- LV
left ventricular
- LVAD
left ventricular assist device
- LVEDP
left ventricular end-diastolic pressure
- PAH
pulmonary arterial hypertension
- PAP
pulmonary arterial pressure
- PCWP
pulmonary capillary wedge pressure
- PH
pulmonary hypertension
- PVR
pulmonary vascular resistance
- RV
right ventricle
- TPG
transpulmonary gradient
- WHO
World Health Organization
Footnotes
Funding/Support: Dr Groskreutz received support from the National Heart, Lung, and Blood Institute [K08HL089392]. Dr Gehlbach receives support from the National Heart, Lung, and Blood Institute [K23HL088020].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Oudiz RJ. Pulmonary hypertension associated with left-sided heart disease. Clin Chest Med. 2007;28(1):233-241 [DOI] [PubMed] [Google Scholar]
- 2.Strange G, Playford D, Stewart S, et al. Pulmonary hypertension: prevalence and mortality in the Armadale echocardiography cohort. Heart. 2012;98(24):1805-1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badesch DB, Champion HC, Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;541):S55-S66 [DOI] [PubMed] [Google Scholar]
- 4.Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J. 2009;34(4):888-894 [DOI] [PubMed] [Google Scholar]
- 5.Fang JC, DeMarco T, Givertz MM, et al. World Health Organization pulmonary hypertension group 2: pulmonary hypertension due to left heart disease in the adult—a summary statement from the Pulmonary Hypertension Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2012;31(9):913-933 [DOI] [PubMed] [Google Scholar]
- 6.Fishman AP. Clinical classification of pulmonary hypertension. Clin Chest Med. 2001;22(3):385-391 [DOI] [PubMed] [Google Scholar]
- 7.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;541):S43-S54 [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin VV, Archer SL, Badesch DB, et al. ; American College of Cardiology Foundation Task Force on Expert Consensus Documents; American Heart Association; American College of Chest Physicians; American Thoracic Society, Inc; Pulmonary Hypertension Association ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53(17):1573-1619 [DOI] [PubMed] [Google Scholar]
- 9.Galiè N, Hoeper MM, Humbert M, et al. ; ESC Committee for Practice Guidelines (CPG) Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30(20):2493-2537 [DOI] [PubMed] [Google Scholar]
- 10.Galiè N, Hoeper MM, Humbert M, et al. ; Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT) Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34(6):1219-1263 [DOI] [PubMed] [Google Scholar]
- 11.Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142(2):448-456 [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee NA, Lewis GD. What is the prognostic significance of pulmonary hypertension in heart failure? Circ Heart Fail. 2011;4(5):541-545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben-Dor I, Goldstein SA, Pichard AD, et al. Clinical profile, prognostic implication, and response to treatment of pulmonary hypertension in patients with severe aortic stenosis. Am J Cardiol. 2011;107(7):1046-1051 [DOI] [PubMed] [Google Scholar]
- 14.Alexopoulos D, Lazzam C, Borrico S, Fiedler L, Ambrose JA. Isolated chronic mitral regurgitation with preserved systolic left ventricular function and severe pulmonary hypertension. J Am Coll Cardiol. 1989;14(2):319-322 [DOI] [PubMed] [Google Scholar]
- 15.Braunwald E, Braunwald NS, Ross J, Jr, Morrow AG. Effects of mitral-valve replacement on the pulmonary vascular dynamics of patients with pulmonary hypertension. N Engl J Med. 1965;273:509-514 [DOI] [PubMed] [Google Scholar]
- 16.Semler HJ, Shepherd JT, Wood EH. The role of vessel tone in maintaining pulmonary vascular resistance in patients with mitral stenosis. Circulation. 1959;19(3):386-394 [DOI] [PubMed] [Google Scholar]
- 17.Hoeper MM, Barberà JA, Channick RN, et al. Diagnosis, assessment, and treatment of non-pulmonary arterial hypertension pulmonary hypertension. J Am Coll Cardiol. 2009;541):S85-S96 [DOI] [PubMed] [Google Scholar]
- 18.Kuznetsova T, Herbots L, López B, et al. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2(2):105-112 [DOI] [PubMed] [Google Scholar]
- 19.Fischer M, Baessler A, Hense HW, et al. Prevalence of left ventricular diastolic dysfunction in the community. Results from a Doppler echocardiographic-based survey of a population sample. Eur Heart J. 2003;24(4):320-328 [DOI] [PubMed] [Google Scholar]
- 20.Delgado JF, Conde E, Sánchez V, et al. Pulmonary vascular remodeling in pulmonary hypertension due to chronic heart failure. Eur J Heart Fail. 2005;7(6):1011-1016 [DOI] [PubMed] [Google Scholar]
- 21.Moraes DL, Colucci WS, Givertz MM. Secondary pulmonary hypertension in chronic heart failure: the role of the endothelium in pathophysiology and management. Circulation. 2000;102(14):1718-1723 [DOI] [PubMed] [Google Scholar]
- 22.Snopek G, Pogorzelska H, Rywik TM, Browarek A, Janas J, Korewicki J. Usefulness of endothelin-1 concentration in capillary blood in patients with mitral stenosis as a predictor of regression of pulmonary hypertension after mitral valve replacement or valvuloplasty. Am J Cardiol. 2002;90(2):188-189 [DOI] [PubMed] [Google Scholar]
- 23.Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol. 2012;59(5):442-451 [DOI] [PubMed] [Google Scholar]
- 24.Guazzi M, Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation. 2012;126(8):975-990 [DOI] [PubMed] [Google Scholar]
- 25.West JB, Mathieu-Costello O. Vulnerability of pulmonary capillaries in heart disease. Circulation. 1995;92(3):622-631 [DOI] [PubMed] [Google Scholar]
- 26.Tsukimoto K, Mathieu-Costello O, Prediletto R, Elliott AR, West JB. Ultrastructural appearances of pulmonary capillaries at high transmural pressures. J Appl Physiol. 1991;71(2):573-582 [DOI] [PubMed] [Google Scholar]
- 27.Kay JM, Edwards FR. Ultrastructure of the alveolar-capillary wall in mitral stenosis. J Pathol. 1973;111(4):239-245 [DOI] [PubMed] [Google Scholar]
- 28.Lee YS. Electron microscopic studies on the alveolar-capillary barrier in the patients of chronic pulmonary edema. Jpn Circ J. 1979;43(10):945-954 [DOI] [PubMed] [Google Scholar]
- 29.Guazzi M. Alveolar gas diffusion abnormalities in heart failure. J Card Fail. 2008;14(8):695-702 [DOI] [PubMed] [Google Scholar]
- 30.Parker F, Weiss S. The nature and significance of the structural changes in the lungs in mitral stenosis. Am J Pathol. 1936;12(5):573-598 [PMC free article] [PubMed] [Google Scholar]
- 31.Melenovsky V, Al-Hiti H, Kazdova L, et al. Transpulmonary B-type natriuretic peptide uptake and cyclic guanosine monophosphate release in heart failure and pulmonary hypertension: the effects of sildenafil. J Am Coll Cardiol. 2009;54(7):595-600 [DOI] [PubMed] [Google Scholar]
- 32.Packer M, McMurray J, Massie BM, et al. Clinical effects of endothelin receptor antagonism with bosentan in patients with severe chronic heart failure: results of a pilot study. J Card Fail. 2005;11(1):12-20 [DOI] [PubMed] [Google Scholar]
- 33.Ferguson JJ. Meeting highlights: highlights of the 51st annual scientific sessions of the American College of Cardiology. Atlanta, Georgia, USA. March 17-20, 2002. Circulation. 2002;106(7):E24-E30 [DOI] [PubMed] [Google Scholar]
- 34.Kaluski E, Cotter G, Leitman M, et al. Clinical and hemodynamic effects of bosentan dose optimization in symptomatic heart failure patients with severe systolic dysfunction, associated with secondary pulmonary hypertension—a multi-center randomized study. Cardiology. 2008;109(4):273-280 [DOI] [PubMed] [Google Scholar]
- 35.Hassoun PM, Mouthon L, Barberà JA, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;541):S10-S19 [DOI] [PubMed] [Google Scholar]
- 36.Lloyd-Jones D, Adams R, Carnethon M, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):e21-e181 [DOI] [PubMed] [Google Scholar]
- 37.Ghio S, Gavazzi A, Campana C, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37(1):183-188 [DOI] [PubMed] [Google Scholar]
- 38.Leung CC, Moondra V, Catherwood E, Andrus BW. Prevalence and risk factors of pulmonary hypertension in patients with elevated pulmonary venous pressure and preserved ejection fraction. Am J Cardiol. 2010;106(2):284-286 [DOI] [PubMed] [Google Scholar]
- 39.Lam CSP, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53(13):1119-1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kjaergaard J, Akkan D, Iversen KK, et al. Prognostic importance of pulmonary hypertension in patients with heart failure. Am J Cardiol. 2007;99(8):1146-1150 [DOI] [PubMed] [Google Scholar]
- 41.Aronson D, Eitan A, Dragu R, Burger AJ. Relationship between reactive pulmonary hypertension and mortality in patients with acute decompensated heart failure. Circ Heart Fail. 2011;4(5):644-650 [DOI] [PubMed] [Google Scholar]
- 42.Penicka M, Bartunek J, Trakalova H, et al. Heart failure with preserved ejection fraction in outpatients with unexplained dyspnea: a pressure-volume loop analysis. J Am Coll Cardiol. 2010;55(16):1701-1710 [DOI] [PubMed] [Google Scholar]
- 43.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3(5):588-595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shapiro BP, McGoon MD, Redfield MM. Unexplained pulmonary hypertension in elderly patients. Chest. 2007;131(1):94-100 [DOI] [PubMed] [Google Scholar]
- 45.Robbins IM, Newman JH, Johnson RF, et al. Association of the metabolic syndrome with pulmonary venous hypertension. Chest. 2009;136(1):31-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willens HJ, Kessler KM. Severe pulmonary hypertension associated with diastolic left ventricular dysfunction. Chest. 1993;103(6):1877-1883 [DOI] [PubMed] [Google Scholar]
- 47.Rajagopalan N, Garcia MJ, Rodriguez L, et al. Comparison of new Doppler echocardiographic methods to differentiate constrictive pericardial heart disease and restrictive cardiomyopathy. Am J Cardiol. 2001;87(1):86-94 [DOI] [PubMed] [Google Scholar]
- 48.Choi EY, Ha JW, Kim JM, et al. Incremental value of combining systolic mitral annular velocity and time difference between mitral inflow and diastolic mitral annular velocity to early diastolic annular velocity for differentiating constrictive pericarditis from restrictive cardiomyopathy. J Am Soc Echocardiogr. 2007;20(6):738-743 [DOI] [PubMed] [Google Scholar]
- 49.Fenton MJ, Chubb H, McMahon AM, Rees P, Elliott MJ, Burch M. Heart and heart-lung transplantation for idiopathic restrictive cardiomyopathy in children. Heart. 2006;92(1):85-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cam A, Goel SS, Agarwal S, et al. Prognostic implications of pulmonary hypertension in patients with severe aortic stenosis. J Thorac Cardiovasc Surg. 2011;142(4):800-808 [DOI] [PubMed] [Google Scholar]
- 51.Malouf JF, Enriquez-Sarano M, Pellikka PA, et al. Severe pulmonary hypertension in patients with severe aortic valve stenosis: clinical profile and prognostic implications. J Am Coll Cardiol. 2002;40(4):789-795 [DOI] [PubMed] [Google Scholar]
- 52.Bonow RO, Carabello BA, Chatterjee K, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of patients With Valvular Heart Disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52(13):e1-e142 [DOI] [PubMed] [Google Scholar]
- 53.Melby SJ, Moon MR, Lindman BR, Bailey MS, Hill LL, Damiano RJ., Jr Impact of pulmonary hypertension on outcomes after aortic valve replacement for aortic valve stenosis. J Thorac Cardiovasc Surg. 2011;141(6):1424-1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodés-Cabau J, Webb JG, Cheung A, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55(11):1080-1090 [DOI] [PubMed] [Google Scholar]
- 55.Naidoo DP, Mitha AS, Vythilingum S, Chetty S. Pulmonary hypertension in aortic regurgitation: early surgical outcome. Q J Med. 1991;80(291):589-595 [PubMed] [Google Scholar]
- 56.Zener JC, Hancock EW, Shumway NE, Harrison DC. Regression of extreme pulmonary hypertension after mitral valve surgery. Am J Cardiol. 1972;30(8):820-826 [DOI] [PubMed] [Google Scholar]
- 57.Goodale F, Jr, Sanchez G, Friedlich AL, Scannell JG, Myers GS. Correlation of pulmonary arteriolar resistance with pulmonary vascular changes in patients with mitral stenosis before and after valvulotomy. N Engl J Med. 1955;252(23):979-983 [DOI] [PubMed] [Google Scholar]
- 58.Vincens JJ, Temizer D, Post JR, Edmunds LH, Jr, Herrmann HC. Long-term outcome of cardiac surgery in patients with mitral stenosis and severe pulmonary hypertension. Circulation. 1995;929):II137-II142 [DOI] [PubMed] [Google Scholar]
- 59.Fawzy ME, Hassan W, Stefadouros M, Moursi M, El Shaer F, Chaudhary MA. Prevalence and fate of severe pulmonary hypertension in 559 consecutive patients with severe rheumatic mitral stenosis undergoing mitral balloon valvotomy. J Heart Valve Dis. 2004;13(6):942-947, discussion 947-948. [PubMed] [Google Scholar]
- 60.Bonow RO, Carabello BA, Chatterjee K, et al. ; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118(15):e523-e661 [DOI] [PubMed] [Google Scholar]
- 61.Elliott CG, Palevsky HI. Treatment with epoprostenol of pulmonary arterial hypertension following mitral valve replacement for mitral stenosis. Thorax. 2004;59(6):536-537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bomma C, Ventura HO, Daniel G, Patel H. Adjunctive sildenafil for the treatment of pulmonary hypertension after mitral valve replacement. Congest Heart Fail. 2006;12(6):347-348 [DOI] [PubMed] [Google Scholar]
- 63.Gan CT, Lankhaar JW, Marcus JT, et al. Impaired left ventricular filling due to right-to-left ventricular interaction in patients with pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. 2006;290(4):H1528-H1533 [DOI] [PubMed] [Google Scholar]
- 64.Califf RM, Adams KF, McKenna WJ, et al. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: The Flolan International Randomized Survival Trial (FIRST). Am Heart J. 1997;134(1):44-54 [DOI] [PubMed] [Google Scholar]
- 65.Thenappan T, Shah SJ, Gomberg-Maitland M, et al. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction/clinical perspective. Circ Heart Fail. 2011;4(3):257-265 [DOI] [PubMed] [Google Scholar]
- 66.Fleg JL, O’Connor F, Gerstenblith G, et al. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J Appl Physiol. 1995;78(3):890-900 [DOI] [PubMed] [Google Scholar]
- 67.Rich S, Dantzker DR, Ayres SM, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987;107(2):216-223 [DOI] [PubMed] [Google Scholar]
- 68.Bossone E, Paciocco G, Iarussi D, et al. The prognostic role of the ECG in primary pulmonary hypertension. Chest. 2002;121(2):513-518 [DOI] [PubMed] [Google Scholar]
- 69.McMurray JJ, Carson PE, Komajda M, et al. Heart failure with preserved ejection fraction: clinical characteristics of 4133 patients enrolled in the I-PRESERVE trial. Eur J Heart Fail. 2008;10(2):149-156 [DOI] [PubMed] [Google Scholar]
- 70.Lupi E, Dumont C, Tejada VM, Horwitz S, Galland F. A radiologic index of pulmonary arterial hypertension. Chest. 1975;68(1):28-31 [DOI] [PubMed] [Google Scholar]
- 71.Milne EN. Forgotten gold in diagnosing pulmonary hypertension: the plain chest radiograph. Radiographics. 2012;32(4):1085-1087 [DOI] [PubMed] [Google Scholar]
- 72.Raymond RJ, Hinderliter AL, Willis PW, et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol. 2002;39(7):1214-1219 [DOI] [PubMed] [Google Scholar]
- 73.Bossone E, Duong-Wagner TH, Paciocco G, et al. Echocardiographic features of primary pulmonary hypertension. J Am Soc Echocardiogr. 1999;12(8):655-662 [DOI] [PubMed] [Google Scholar]
- 74.Opotowsky AR, Ojeda J, Rogers F, et al. A simple echocardiographic prediction rule for hemodynamics in pulmonary hypertension. Circ Cardiovasc Imaging. 2012;5(6):765-775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zile MR, Gottdiener JS, Hetzel SJ, et al. ; I-PRESERVE Investigators Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124(23):2491-2501 [DOI] [PubMed] [Google Scholar]
- 76.Willens HJ, Chirinos JA, Gomez-Marin O, et al. Noninvasive differentiation of pulmonary arterial and venous hypertension using conventional and Doppler tissue imaging echocardiography. J Am Soc Echocardiogr. 2008;21(6):715-719 [DOI] [PubMed] [Google Scholar]
- 77.Ruan Q, Nagueh SF. Clinical application of tissue Doppler imaging in patients with idiopathic pulmonary hypertension. Chest. 2007;131(2):395-401 [DOI] [PubMed] [Google Scholar]
- 78.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quiñones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30(6):1527-1533 [DOI] [PubMed] [Google Scholar]
- 79.Guazzi M, Myers J, Arena R. Cardiopulmonary exercise testing in the clinical and prognostic assessment of diastolic heart failure. J Am Coll Cardiol. 2005;46(10):1883-1890 [DOI] [PubMed] [Google Scholar]
- 80.Paolillo S, Farina S, Bussotti M, et al. Exercise testing in the clinical management of patients affected by pulmonary arterial hypertension. Eur J Prev Cardiol. 2012;19(5):960-971 [DOI] [PubMed] [Google Scholar]
- 81.Borlaug BA, Olson TP, Lam CS, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56(11):845-854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maeder MT, Thompson BR, Brunner-La Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56(11):855-863 [DOI] [PubMed] [Google Scholar]
- 83.Paulus WJ. Culprit mechanism(s) for exercise intolerance in heart failure with normal ejection fraction. J Am Coll Cardiol. 2010;56(11):864-866 [DOI] [PubMed] [Google Scholar]
- 84.Grewal J, McCully RB, Kane GC, Lam C, Pellikka PA. Left ventricular function and exercise capacity. JAMA. 2009;301(3):286-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17(5):1065-1072 [DOI] [PubMed] [Google Scholar]
- 86.Yasunobu Y, Oudiz RJ, Sun XG, Hansen JE, Wasserman K. End-tidal PCO2 abnormality and exercise limitation in patients with primary pulmonary hypertension. Chest. 2005;127(5):1637-1646 [DOI] [PubMed] [Google Scholar]
- 87.Hemnes AR, Pugh ME, Newman AL, et al. End tidal CO(2) tension: pulmonary arterial hypertension vs pulmonary venous hypertension and response to treatment. Chest. 2011;140(5):1267-1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fox BD, Shimony A, Langleben D, et al. High prevalence of occult left heart disease in scleroderma-pulmonary hypertension [published online ahead of print Dec 20, 2012]. Eur Respir J. doi:10.1183/09031936 [DOI] [PubMed] [Google Scholar]
- 89.Halpern SD, Taichman DB. Misclassification of pulmonary hypertension due to reliance on pulmonary capillary wedge pressure rather than left ventricular end-diastolic pressure. Chest. 2009;136(1):37-43 [DOI] [PubMed] [Google Scholar]
- 90.Ryan JJ, Rich JD, Thiruvoipati T, Swamy R, Kim GH, Rich S. Current practice for determining pulmonary capillary wedge pressure predisposes to serious errors in the classification of patients with pulmonary hypertension. Am Heart J. 2012;163(4):589-594 [DOI] [PubMed] [Google Scholar]
- 91.Pichard AD, Kay R, Smith H, Rentrop P, Holt J, Gorlin R. Large V waves in the pulmonary wedge pressure tracing in the absence of mitral regurgitation. Am J Cardiol. 1982;50(5):1044-1050 [DOI] [PubMed] [Google Scholar]
- 92.Costard-Jäckle A, Fowler MB. Influence of preoperative pulmonary artery pressure on mortality after heart transplantation: testing of potential reversibility of pulmonary hypertension with nitroprusside is useful in defining a high risk group. J Am Coll Cardiol. 1992;19(1):48-54 [DOI] [PubMed] [Google Scholar]
- 93.Chen JM, Levin HR, Michler RE, Prusmack CJ, Rose EA, Aaronson KD. Reevaluating the significance of pulmonary hypertension before cardiac transplantation: determination of optimal thresholds and quantification of the effect of reversibility on perioperative mortality. J Thorac Cardiovasc Surg. 1997;114(4):627-634 [DOI] [PubMed] [Google Scholar]
- 94.Lindenfeld J, Albert NM, Boehmer JP, et al. ; Heart Failure Society of America HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16(6):e1-e194 [DOI] [PubMed] [Google Scholar]
- 95.Lewis GD, Shah R, Shahzad K, et al. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116(14):1555-1562 [DOI] [PubMed] [Google Scholar]
- 96.Guazzi M, Samaja M, Arena R, Vicenzi M, Guazzi MD. Long-term use of sildenafil in the therapeutic management of heart failure. J Am Coll Cardiol. 2007;50(22):2136-2144 [DOI] [PubMed] [Google Scholar]
- 97.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124(2):164-174 [DOI] [PubMed] [Google Scholar]
- 98.Etz CD, Welp HA, Tjan TD, et al. Medically refractory pulmonary hypertension: treatment with nonpulsatile left ventricular assist devices. Ann Thorac Surg. 2007;83(5):1697-1705 [DOI] [PubMed] [Google Scholar]
- 99.Nair PK, Kormos RL, Teuteberg JJ, et al. Pulsatile left ventricular assist device support as a bridge to decision in patients with end-stage heart failure complicated by pulmonary hypertension. J Heart Lung Transplant. 2010;29(2):201-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pauwaa S, Bhat G, Tatooles AJ, et al. How effective are continuous flow left ventricular assist devices in lowering high pulmonary artery pressures in heart transplant candidates? Cardiol J. 2012;19(2):153-158 [DOI] [PubMed] [Google Scholar]
- 101.Zimpfer D, Zrunek P, Sandner S, et al. Post-transplant survival after lowering fixed pulmonary hypertension using left ventricular assist devices. Eur J Cardiothorac Surg. 2007;31(4):698-702 [DOI] [PubMed] [Google Scholar]
- 102.Torre-Amione G, Southard RE, Loebe MM, et al. Reversal of secondary pulmonary hypertension by axial and pulsatile mechanical circulatory support. J Heart Lung Transplant. 2010;29(2):195-200 [DOI] [PubMed] [Google Scholar]
- 103.Alba AC, Rao V, Ross HJ, et al. Impact of fixed pulmonary hypertension on post-heart transplant outcomes in bridge-to-transplant patients. J Heart Lung Transplant. 2010;29(11):1253-1258 [DOI] [PubMed] [Google Scholar]