Abstract
Background
Extracellular superoxide dismutase (Ec-SOD) may protect the heart against myocardial infarction (MI) because of its extended half-life and capacity to bind heparan sulfate proteoglycans on cellular surfaces. Accordingly, we used direct gene transfer to increase systemic levels of Ec-SOD and determined whether this gene therapy could protect against MI.
Methods and Results
The cDNA for human Ec-SOD was incorporated into a replication-deficient adenovirus (Ad5/CMV/Ec-SOD). Injection of this virus produced a high level of Ec-SOD in the liver, which was redistributed to the heart and other organs by injection of heparin. Untreated rabbits (group I) underwent a 30-minute coronary occlusion and 3 days of reperfusion. For comparison, preconditioned rabbits (group II) underwent a sequence of six 4-minute-occlusion/4-minute-reperfusion cycles 24 hours before the 30-minute occlusion. Control-treated rabbits (group III) were injected intravenously with Ad5/CMV/nls-LacZ, and gene-therapy rabbits (group IV) were injected with Ad5/CMV/Ec-SOD 3 days before the 30-minute occlusion. Both groups treated with Ad5 received intravenous heparin 2 hours before the 30-minute occlusion. Infarct size (percent risk area) was similar in groups I (57±6%) and III (58±5%). Ec-SOD gene therapy markedly reduced infarct size to 25±4% (P<0.01, group IV versus group III), a protection comparable to that of the late phase of ischemic preconditioning (29±3%, P<0.01 group II versus group I).
Conclusions
Direct gene transfer of the cDNA encoding membrane-bound Ec-SOD affords powerful cardioprotection, providing proof of principle for the effectiveness of antioxidant gene therapy against MI.
Keywords: myocardial infarction, ischemia, genes, antioxidants, viruses
Considerable evidence indicates that reactive oxygen species (ROS), such as superoxide anion (·O2–) and hydrogen peroxide (H2O2), contribute importantly to myocardial ischemia/reperfusion injury.1 When one examines the role of ROS, it is important to distinguish 2 forms of myocardial ischemia/reperfusion injury: reversible postischemic dysfunction (myocardial stunning) and cell death (myocardial infarction [MI]). Because of the fundamental differences between these 2 processes, pathogenetic and pathophysiological information pertaining to one cannot be extrapolated to the other. Indeed, although the role of ROS in myocardial stunning is widely accepted,2 intense controversy persists regarding whether ROS also participate in lethal ischemia/reperfusion injury (MI).1 A number of studies have shown that intravenous administration of antioxidant enzymes (eg, superoxide dismutase [SOD] and catalase) can reduce infarct size; however, other studies have failed to demonstrate a protective effect.1 The reasons underlying this discrepancy are unknown. We hypothesized that the inability of antioxidant enzymes to access the intracellular space after intravenous administration may have contributed to these variable results, and we postulated that the extracellular isoform of SOD (Ec-SOD), which binds to heparan sulfate proteoglycans on cellular surfaces, might provide for more consistent cardioprotection than freely soluble isoforms of SOD.
Another problem with using antioxidant enzymes to protect against MI is that they need to be given parenterally and have short plasma half-lives. These limitations can potentially be overcome by using gene therapy to create an endogenous source of antioxidant protection. Although numerous studies of ischemia/reperfusion injury have used antioxidant enzymes prepared by recombinant techniques,2–7 none has used gene therapy to protect intact animals against MI.
The goal of the present study was to compare the protection against MI afforded by a recombinant adenovirus (Ad5) that overexpresses Ec-SOD with that afforded by the late phase of ischemic preconditioning (PC). Although the underlying mechanisms responsible for cardioprotection may be different, the late PC group was included as an internal positive control against which to compare the efficacy of antioxidant gene therapy. We previously reported that Ad5-mediated gene transfer of Ec-SOD protects against myocardial stunning in conscious rabbits.8 In both the previous and current studies, the liver was targeted for gene transfer to exploit the efficiency with which Ad5 transfects hepatocytes after intravenous injection and to preclude the possibility of an inflammatory response against Ad5 in the heart. A conscious rabbit model of MI9,10 was used to overcome limitations inherent in open-chest preparations that could interfere with the study of ischemia/reperfusion injury.11
Methods
Surgical Preparation
The rabbit model of MI has been described previously.9,10 Briefly, pentobarbital-anesthetized New Zealand White male rabbits (weight 2.1±0.2 kg each) were instrumented under sterile conditions with a balloon occluder around a major branch of the left coronary artery, a 10-MHz pulsed Doppler ultrasonic crystal in the center of the region to be rendered ischemic, and bipolar ECG leads on the chest wall. Rabbits were allowed to recover for at least 14 days after surgery. All rabbits were purchased from Myrtles Rabbitry (Thompson Station, Tenn), and the study was performed under protocols approved by the Animal Care and Use Committee at the University of Louisville in accordance with the Guide for the Care and Use of Laboratory Animals (Department of Health and Human Services, publication No. [NIH] 86-23).
Infarction Protocol
Throughout the protocol, left ventricular (LV) systolic wall thickening and ECG were monitored continuously on a chart recorder. Diazepam (4 mg/kg IP) was administered 20 minutes before the onset of ischemia to prevent stress. The infarction protocol consisted of a 30-minute coronary artery occlusion followed by 3 days of reperfusion (Figure 1). Successful occlusions were verified by observation of ST-segment elevations and changes in the QRS complex on the ECGs and by paradoxical systolic wall thinning on the ultrasonic crystal recordings.
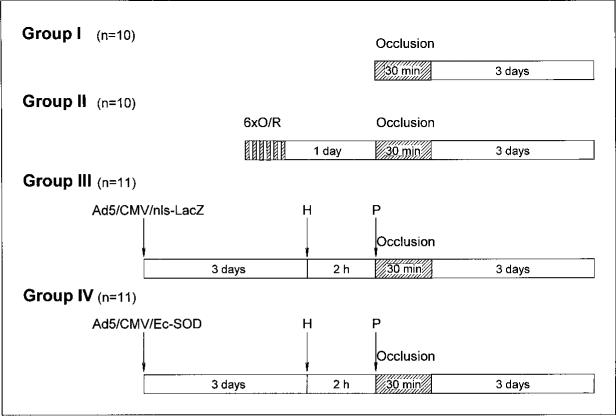
Figure 1.
Experimental protocol. Four groups of rabbits were subjected to 30-minute coronary occlusion followed by 3 days of reperfusion. Three days earlier, rabbits in groups III and IV had been injected with recombinant Ad5. Group III received irrelevant reporter virus (Ad5/CMV/ nls-LacZ), whereas group IV received virus carrying cDNA encoding human Ec-SOD (Ad5/CMV/ Ec-SOD). Groups III and IV received intravenous heparin (H) 2 hours before and protamine (P) immediately before 30-minute coronary occlusion. Twenty-four hours before 30-minute coronary occlusion, rabbits in group II were preconditioned with 6 cycles of 4-minute coronary occlusion/4-minute reperfusion (6xO/R). All rabbits were euthanatized 3 days after coronary occlusion for infarct-size determination.
Ischemic PC
Ischemic PC was induced in group II with a sequence of six 4-minute coronary occlusions interspersed with 4 minutes of reperfusion performed 24 hours before the 30-minute occlusion (Figure 1). The proper execution of the occlusion/reperfusion protocol was evidenced by a 4- to 5-hour period of myocardial stunning in each animal.11
Adenoviral Vectors
The constructions of the nuclear-localized LacZ reporter virus (Ad5/CMV/nls-LacZ) and the recombinant adenovirus that expresses the human cDNA encoding Ec-SOD12 were reported previously.8 Each viral isolate was plaque purified, verified by restriction analysis, and evaluated for its potential to overexpress enzymatic activity. Purified viral clones were propagated in 293 cells, isolated, concentrated, and titered by plaque assay according to Graham and Prevec.13
Expression of Ec-SOD
Ad5-mediated expression of Ec-SOD at the mRNA level was confirmed by infection of COS cells at an MOI of 10 and harvesting of the cells 2 days later for Northern analysis. Five rabbits were used to assess Ec-SOD expression in vivo. One was an untreated rabbit that exemplified group I, whereas the remaining 4 rabbits were treated with 2×108 pfu/kg of Ad5/CMV/Ec-SOD 3 days before euthanasia to simulate the gene-therapy protocol (group IV). Total RNA was extracted from samples by standard procedures (RNAeasy, Qiagen), separated by electrophoresis, and blotted onto a nylon membrane. A 32P-labeled riboprobe specific for human Ec-SOD (nucleotides [nt] 1020 through 138912) was prepared with commercial reagents (Maxiscript, Ambion). Northern blots were imaged with a Storm 840 phosphorimager (Molecular Dynamics) and quantified with integrated software.
Experimental Design
Three days before MI, rabbits were randomized to 4 groups. Rabbits in the control-treated group (group III) and the gene-therapy group (group IV) were injected with 2×108 pfu/kg of recombinant adenovirus via ear vein (Figure 1). This amount was chosen because the same dose had previously protected conscious rabbits against myocardial stunning.8 In both previous and current studies, heparin was administered 2 hours before the first occlusion to release Ec-SOD from the liver. Protamine was injected before coronary occlusion to reverse the effects of heparin. Both groups III and IV were subjected to this same regimen (ie, Ad5 injection 3 days before coronary occlusion, heparin [2000 U/kg IV] 2 hours before coronary occlusion, and protamine [10 mg/kg IV] over the last 8 minutes before coronary occlusion).
Measurement of Region at Risk and Infarct Size
At the conclusion of the study, rabbits were treated with heparin (2000 U/kg IV), anesthetized with sodium pentobarbital (50 mg/kg IV), and euthanatized with a bolus of KCl. The heart was excised, mounted on a Langendorff apparatus, and washed extensively with heparinized saline (50 U/mL) to remove residual Ec-SOD. The repeated heparin treatments, in combination with the 3-day reperfusion period, helped to ensure accurate infarct size determinations by triphenyltetrazolium chloride (TTC) histochemistry.14 The size of the ischemic-reperfused region (region at risk) was determined by ligation of the coronary artery at the site of the previous occlusion and perfusion of the aortic root for 2 minutes with a 5% solution of phthalo blue dye at a pressure of 70 mm Hg.9,10 The heart was then cut into 6 to 7 transverse slices, which were incubated for 10 minutes at 37°C in a 1% solution of TTC in phosphate buffer (pH 7.4). All atrial and right ventricular tissues were removed, after which the slices were weighed, fixed in 10% neutral buffered formaldehyde, and photographed. The resulting 35-mm slides were projected at 103 magnification, and the borders of the infarcted, ischemic/ reperfused, and nonischemic regions were traced for digital planimetry with Adobe Photoshop software. Infarct size was calculated as a percentage of the region at risk.9,10
Statistical Analysis
Data are reported as mean±SEM. For intergroup comparisons, data were analyzed by either 1-way or 2-way repeated-measures ANOVA (time and group), as appropriate, followed by unpaired Student's t tests with the Bonferroni correction. The relationship between infarct size and risk region size was compared among groups with ANCOVA, with the size of the risk region as the covariate.10 The correlation between infarct size and risk-region size was assessed by linear regression with the least squares method. All statistical analyses were performed with the SAS software system.15
Results
Expression of Ec-SOD
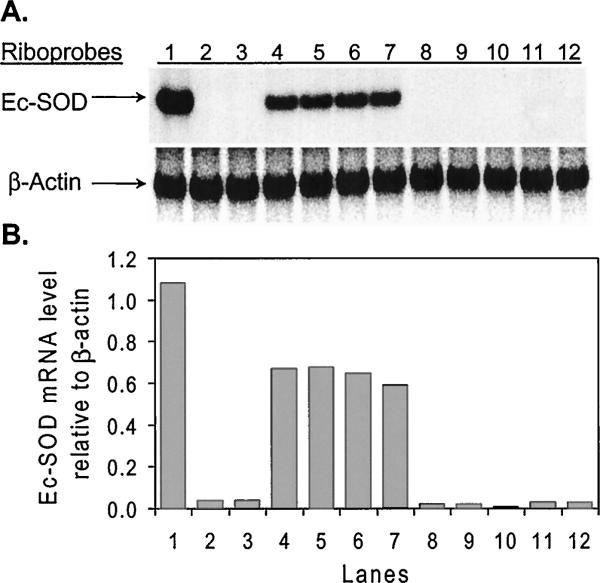
As shown in Figure 2A, mRNA from COS cells infected with Ad5/CMV/Ec-SOD yielded a discrete band when hybridized with a riboprobe specific for human Ec-SOD mRNA (lane 1). This positive control band corresponded to abundant message in livers from rabbits treated with Ad5/CMV/Ec-SOD (lanes 4 to 7). No signal was detected in the liver (lane 3) or the heart (lane 8) of a normal, untreated rabbit. As anticipated, no human Ec-SOD mRNA was detected in the hearts of rabbits treated with Ad5/CMV/Ec-SOD (lanes 9 to 12), even though abundant message was detected in livers from these same animals (lanes 4 to 7). As demonstrated previously,8 the recombinant Ec-SOD protein is expressed in the liver, secreted from hepatocytes, and then displaced by heparin for transport through the bloodstream to the heart and other organs. In Figure 2B, a quantitative analysis of the Northern blot demonstrates that intravenous injection of Ad5/CMV/Ec-SOD gave rise to reproducible levels of human Ec-SOD mRNA expression in the livers of experimental rabbits.
Figure 2.
Northern blot analysis. A, Total RNA hybridized with a riboprobe specific for human Ec-SOD mRNA. Lane 1: RNA from COS cells infected 2 days previously with Ad5/CMV/Ec-SOD. Lane 2: RNA from normal COS cells. Lane 3: RNA from liver of normal rabbit. Lanes 4 to 7: RNA isolated from livers of 4 rabbits infected 3 days earlier with Ad5/CMV/Ec-SOD. Lane 8: RNA from heart of normal rabbit. Lanes 9 to 12: RNA from hearts of 4 rabbits infected 3 days earlier with Ad5/CMV/Ec-SOD. B, Levels of human Ec-SOD mRNA relative to endogenous levels of b-actin mRNA. Each bar on graph corresponds to lane shown above in A.
Exclusions and Arrhythmias
Of the 51 rabbits instrumented, 13 were assigned to group I, 12 to group II, 15 to group III, and 11 to group IV. Four rabbits died of ventricular fibrillation during coronary occlusion (2 in group I and 2 in group II). Two rabbits in group III were excluded owing to technical problems with the postmortem analysis, and 3 rabbits (1 in group I and 2 in group III) were excluded because of failure of the balloon occluder. Thus, a total of 10 rabbits completed the experimental protocol in group I, 10 in group II, 11 in group III, and 11 in group IV. The incidence of ventricular fibrillation during the 30-minute occlusion and of ventricular tachycardia after reperfusion did not differ significantly between the control and treated groups.
Blood Pressure and Heart Rate
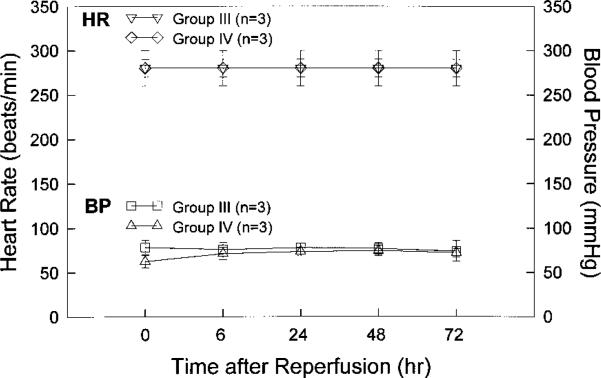
Blood pressure and heart rate were monitored in 3 rabbits from group III and 3 rabbits from group IV. Measurements were taken before coronary occlusion (time 0) and at 6, 24, 48, and 72 hours after reperfusion. As shown in Figure 3, heart rate was similar between the 2 groups at each time point. Blood pressure was also similar between groups at every time point after reperfusion, although it was slightly lower in group IV rabbits than in group III rabbits at the time of coronary occlusion. These results are consistent with previous studies indicating that Ec-SOD has no effect on blood pressure.4,6
Figure 3.
Heart rate and blood pressure in rabbits treated with recombinant Ad5. Heart rate (HR) and blood pressure (BP) were monitored via cannulation of major ear artery in 3 rabbits injected 3 days earlier with Ad5/CMV/nls-LacZ (group III) and in 3 rabbits similarly treated with Ad5/CMV/Ec-SOD (group IV). Heart rate and blood pressure were recorded before coronary occlusion (time 0) and at 6, 24, 48, and 72 hours after reperfusion.
Region at Risk and Infarct Size
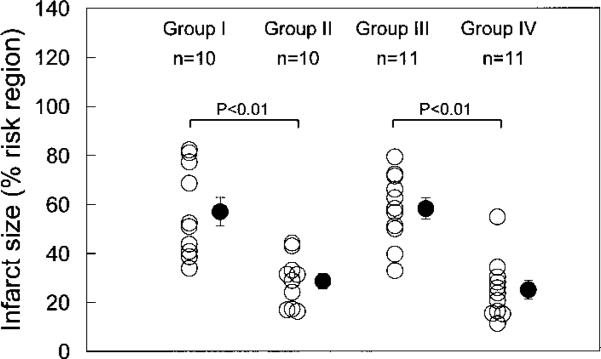
On the day of the 30-minute occlusion, there were no significant differences in baseline systolic thickening fraction among the 4 groups (39.7±2.5%, 35.7±5.1%, 35.4±2.5%, and 35.7±2.6% in groups I, II, III, and IV, respectively). Similarly, there were no appreciable differences among groups with respect to LV weight, region at risk, or region at risk as a percentage of LV weight (Table). However, mean infarct size was 50% smaller in the ischemic PC group (group II) than in the untreated group (group I) (28.6±3.2% versus 56.9±5.9% of the region at risk, respectively; P<,0.01; Figure 4), indicating a late PC effect against MI. In the gene-therapy group (group IV), the average infarct size was 57% smaller than in the control-treated group (group III) (25.1±4.3% versus 58.3±5.0%, respectively; P<,0.01; Figure 4), indicating that the expression of Ec-SOD (as opposed to nls-LacZ) was responsible for the marked cardioprotective effect. The similarity in infarct size between the ischemic PC group (28.6±3.2%) and the gene-therapy group (25.1±4.3%) indicates that the protective effect of gene therapy was comparable to that induced by the late phase of ischemic PC. The similarity in infarct size between the untreated group (56.9±5.9%) and the control-treated group (58.3±5.0%) indicates that neither the administration of an irrelevant adenoviral vector nor the injections of heparin and/or protamine had significant effects on infarct size.
LV Weight, Risk Region, and Infarct Size
| Group | LV Wt, g | Infarct Wt, g | Infarct, % of LV | Risk Region Wt, g | Risk Region, % of LV | Infarct, % of Risk Region |
|---|---|---|---|---|---|---|
| I | 4.73±0.36 | 0.49±0.09 | 9.8±1.4 | 0.84±0.13 | 17.0±1.7 | 56.9±5.9 |
| II | 4.30±0.19 | 0.20±0.03* | 4.7±0.7* | 0.68±0.07 | 15.8±1.2 | 28.6±3.2* |
| III | 3.68±0.27 | 0.36±0.06 | 10.0±1.8 | 0.61±0.08 | 16.1±2.2 | 58.3±5.0 |
| IV | 3.97±0.31 | 0.19±0.05t | 4.8±1.2† | 0.72±0.11 | 17.7±2.2 | 25.1±4.3† |
Data are mean±SEM. Wt indicates weight.
P<0.05 vs group I
P<0.05 vs group III.
Figure 4.
Myocardial infarct size in 4 groups. Infarct size is expressed as percentage of region at risk of infarction. Group I: normal rabbits subjected to 30 minutes’ coronary occlusion. Group II: rabbits protected by late ischemic PC induced 24 hours before coronary occlusion. Group III: control rabbits injected with irrelevant Ad5 vector 3 days before coronary occlusion. Group IV: rabbits protected by antioxidant gene therapy administered 3 days before coronary occlusion. Open circles represent individual rabbits, and solid circles represent mean±SEM for each group.
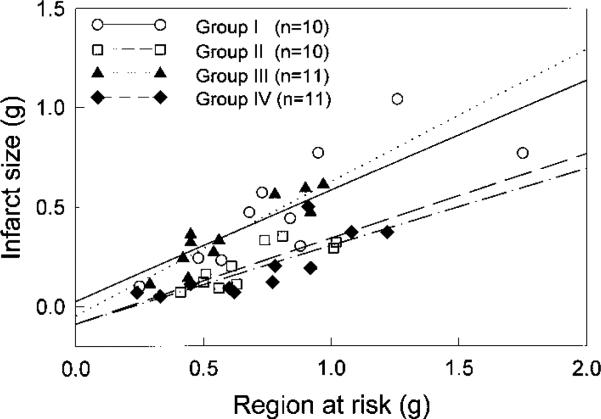
In all groups, the size of the infarction was positively and linearly related to the size of the region at risk (Figure 5). As expected,10 the regression line was shifted to the right in the ischemic PC group compared with the control groups (groups I and III) (P50.05 by ANCOVA; Figure 5). In the group pretreated with antioxidant gene therapy (group IV), the regression line was again significantly shifted to the right compared with the control groups (P<,0.05 by ANCOVA; Figure 5) and was virtually indistinguishable from that of the ischemic PC group, indicating both that for any given size of the region at risk, the infarct size was reduced by antioxidant gene therapy and that the magnitude of this effect was similar to that induced by ischemic PC.
Figure 5.
Relationship between size of region at risk and size of MI. Graph shows individual values and linear regression lines for 4 groups. In all groups, infarct size was positively and linearly related to risk region size. Linear regression equations and R values were as follows: group I, y=0.026+0.56x, R=0.80; group II, y=–0.087+0.43x, R=0.83; group III, y=–0.047+0.67x, R=0.92; group IV, y=–0.087+0.39x, R=0.79. ANCOVA demonstrated that regression line for group II was significantly different from that of groups I and III and that regression line for group IV was significantly different from that of groups I and III, respectively (P<,0.05 for each comparison).
Discussion
The use of in vivo gene transfer to elevate systemic levels of therapeutic proteins has considerable potential, particularly if therapeutic levels can be achieved without adverse side effects. The present study demonstrates that gene therapy with Ec-SOD protects conscious rabbits against MI, indicating that this approach can be effectively used to enhance endogenous antioxidant defenses and limit lethal myocellular injury during ischemia/reperfusion in vivo. Although many studies have found that antioxidant enzymes can protect the heart against ischemia/reperfusion injury (reviewed in Bolli1), to the best of our knowledge, this is the first demonstration that the heart can be protected against MI with the use of antioxidant gene therapy, which can potentially provide a sustained supply of antioxidant protein(s). The present study complements earlier work from our laboratory demonstrating that Ec-SOD gene therapy attenuates myocardial stunning in conscious rabbits.8 Together, these studies suggest that gene therapy with Ec-SOD offers considerable potential for protecting the heart against both reversible (myocardial stunning) and irreversible (MI) ischemia/reperfusion injury. Because Ec-SOD is released into the systemic circulation and because this release can be precisely manipulated with heparin and protamine, these studies also suggest a general methodological approach for controlling gene therapy at the posttranslational level and for simultaneously protecting multiple tissues from ROS-mediated injury.
Methodological Considerations
The conscious rabbit model used in the present study is well characterized with respect to the infarct-sparing effects of ischemic PC9,10 and avoids a number of factors that could interfere with the assessment of postischemic myocardial dysfunction (eg, anesthesia, trauma, temperature fluctuations, abnormal hemodynamics, elevated catecholamines, and cytokine release11). Most importantly, the use of a conscious model avoids the exaggerated oxyradical formation observed in open-chest animals,11 which could possibly overwhelm the antioxidant therapy under investigation.
The choice of antioxidant enzyme was also a critical factor in the present study. Many previous animal studies examining antioxidant enzymes used continuous intravenous infusion of recombinant Cu/Zn-SOD or Mn-SOD protein and thus examined the function of intracellular enzymes in the extracellular space. However, careful examination of the distribution kinetics of Cu/Zn-SOD indicates that the interstitial levels of this enzyme (rather than the plasma levels) are primarily responsible for protection against myocardial ischemia/reper-fusion injury.7 This being the case, it was reasonable to consider an isoform of SOD that has natural affinity for the interstitial space. The selection of Ec-SOD for these studies was also influenced by the fact that it is the only isoform of SOD that is secreted from cells and is therefore uniquely suited for hepatic production and systemic distribution.
The possibility of an inflammatory response against the first-generation Ad5 vector prompted us to target gene transfer to an organ other than the heart. Unlike the heart, the liver has a profound regenerative capacity, and remarkably high frequencies of Ad5-mediated transfection (>90%) can be obtained by intravenous injection without compromising hepatic function.16 Not only is the liver an opportune target for Ad5, but this strategy served to alleviate concerns regarding the possibility of inflammation in the heart and its potential impact on ischemic PC and MI.
Microsphere measurements of collateral flow were not undertaken in the present study. However, pilot experiments in our laboratory have demonstrated that rabbits have negligible capability to recruit collateral flow to ischemic regions of myocardium. Minimal collateral flow in ischemic rabbit myocardium has also been reported by others,17 and thus it is unlikely that differences in collateral flow could account for the cardioprotective effects of either late PC or antioxidant gene therapy.
Previous Studies of the Cardioprotective Effects of Ec-SOD
Numerous studies have examined whether Cu/Zn-SOD and Mn-SOD can limit tissue damage after coronary occlusion.1 However, comparatively few investigations have examined the effect of Ec-SOD on myocardial ischemia/reperfusion injury, and fewer still have been conducted in intact animals. Wahlund et al3 reported that Ec-SOD reduced creatine kinase release in rats subjected to 10 minutes of coronary occlusion and 24 hours of reperfusion. Hatori et al4 found that retroin-fusion of purified, recombinant Ec-SOD protein into the great cardiac vein decreased the size of MIs in open-chest pigs subjected to coronary occlusion. Chen et al6 found that hearts isolated from transgenic mice overexpressing human Ec-SOD exhibited enhanced postischemic myocontractile function. This cardioprotective effect is consistent with our present results; furthermore, the 5-fold elevation in Ec-SOD levels obtained by transgenesis in the study by Chen et al6 is comparable to that obtained in our rabbit model of gene therapy.8 The present study differs from previous investigations3,4,6 in that we examined Ec-SOD produced as a result of gene therapy, and we used a conscious animal model of MI. Thus, the present study may bear some clinical relevance, particularly because gene therapy has the potential to provide a sustained source of antioxidant enzyme over a period of months or even years.
Finally, previous work from our laboratory described an Ec-SOD gene-therapy regimen capable of reducing myocardial stunning by ≈50%.8 That same study determined that Ad5/CMV/Ec-SOD (2×108 pfu/kg IV) increased total liver SOD activity by 4.4-fold and total myocardial SOD activity by 2-fold over normal. Subsequent heparin administration led to a 2-fold increase in total plasma SOD activity and increased total SOD activity in the heart by a factor of 5.4-fold over normal. The present investigation does not formally exclude the possibility that this dose of Ad5 vector might have been cardioprotective by itself without heparin injection. However, our previous pilot studies8 suggested that only an intermediate level of cardioprotection would be obtained at this low dose of virus without the manipulations involving heparin and protamine.
Present Study
The present report builds on previous work8 by applying the same Ec-SOD gene-therapy regimen in a conscious animal model of MI. As shown in Figure 2, Northern analysis of rabbits treated 3 days previously with Ad5/CMV/Ec-SOD revealed consistent levels of human Ec-SOD mRNA in liver tissue. As summarized in Figure 4, the same strategy that protected against myocardial stunning in our previous study8 also protected the heart against irreversible ischemia/reperfusion injury, reducing the size of MI by >50%. This level of cardioprotection was comparable to that found in rabbits protected by the late phase of ischemic PC. Interestingly, the cardioprotective mechanism underlying the late phase of ischemic PC appears to depend on enhanced production of nitric oxide.10 Cardioprotection by Ec-SOD may also involve nitric oxide, because elevations in this enzyme will decrease ambient levels of ·O2–, thus protecting endogenous nitric oxide from the diffusion-limited reaction with ·O2– to form peroxynitrite.
Taken together with our previous study,8 the finding that Ec-SOD gene therapy affords robust protection against MI implicates ROS as important mediators not only of reversible contractile dysfunction (myocardial stunning) but also of irreversible ischemia/reperfusion injury (MI). Thus, the present observations significantly broaden the potential clinical usefulness of Ec-SOD gene therapy and also have important pathophysiological implications regarding the role of ROS in lethal ischemia/reperfusion injury.
The effectiveness of SOD in limiting infarct size has been questioned repeatedly.1 Indeed, although our results are consistent with previous investigations of Ec-SOD,3,4,6 they are seemingly in contrast with several previous studies of Cu/Zn-SOD and Mn-SOD that failed to detect significant cardioprotection against infarction (reviewed in Bolli1). Numerous methodological differences in the experimental design of these studies make it impossible to identify the precise reason(s) for the discrepancy. One plausible explanation involves the properties of the antioxidant enzymes examined. Whereas Cu/Zn-SOD and Mn-SOD are freely diffusible in the plasma compartment and interstitial space within the myocardium, Ec-SOD binds to heparan sulfate proteoglycans18 present on the endothelial glycocalyx, in the extracellular matrix, and on the sarcolemma of cardiomyocytes, thereby providing effective protection against ·O2– in the interstitium and on vulnerable cellular surfaces. These strategic locations of the enzyme may enable it to inactivate ·O2– before it can cause extensive tissue damage. The failure of previous studies to detect a protective action of Cu/Zn-SOD and Mn-SOD against MI may have been caused, at least in part, by the relative inability of these proteins to gain access to injurious ·O2–.
Conclusions
The present study demonstrates that gene therapy can be used to protect conscious rabbits from MI with the use of a single antioxidant enzyme (Ec-SOD). The efficacy of Ec-SOD in protecting the myocardium against ischemia/reperfusion injury contrasts with the lack of consistent protection observed with Cu/Zn-SOD or Mn-SOD and might be attributed to the extended half-life and/or the extracellular-binding properties of this unique antioxidant enzyme.18 The present results not only implicate ROS in the genesis of lethal ischemia/reperfusion injury in the conscious animal but also have significant clinical implications for the development of novel cardioprotective strategies.
Acknowledgments
This study was supported in part by a medical research grant (96-413S) from the Jewish Hospital Foundation, Louisville, Ky (Dr French), and by NIH grants R01HL-58582 (Dr French), R01HL-43151 (Dr Bolli), and R01HL-55757 (Dr Bolli).
References
- 1.Bolli R. Oxygen-derived free radicals and myocardial reperfusion injury: an overview. Cardiovasc Drugs Ther. 1991;5(suppl 2):249–268. doi: 10.1007/BF00054747. [DOI] [PubMed] [Google Scholar]
- 2.Jeroudi MO, Triana JF, Patel BS, et al. Effect of superoxide dismutase and catalase, given separately, on myocardial “stunning.”. Am J Physiol. 1990;259:H889–H901. doi: 10.1152/ajpheart.1990.259.3.H889. [DOI] [PubMed] [Google Scholar]
- 3.Wahlund G, Marklund SL, Sjoquist PO. Extracellular-superoxide dismutase type C (EC-SOD C) reduces myocardial damage in rats subjected to coronary occlusion and 24 hours of reperfusion. Free Radic Res Commun. 1992;17:41–47. doi: 10.3109/10715769209061087. [DOI] [PubMed] [Google Scholar]
- 4.Hatori N, Sjoquist PO, Marklund SL, et al. Effects of recombinant human extracellular-superoxide dismutase type C on myocardial infarct size in pigs. Free Radic Biol Med. 1992;13:221–230. doi: 10.1016/0891-5849(92)90018-c. [DOI] [PubMed] [Google Scholar]
- 5.Omar BA, Gad NM, Jordan MC, et al. Cardioprotection by Cu, Zn-superoxide dismutase is lost at high doses in the reoxygenated heart. Free Radic Biol Med. 1990;9:465–471. doi: 10.1016/0891-5849(90)90123-z. [DOI] [PubMed] [Google Scholar]
- 6.Chen EP, Bittner HB, Davis RD, et al. Extracellular superoxide dismutase transgene overexpression preserves postischemic myocardial function in isolated murine hearts. Circulation. 1996;94(suppl II):II–412–II-417. [PubMed] [Google Scholar]
- 7.Omar BA, McCord JM. Interstitial equilibration of superoxide dismutase correlates with its protective effect in the isolated rabbit heart. J Mol Cell Cardiol. 1991;23:149–159. doi: 10.1016/0022-2828(91)90102-r. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Bolli R, Qiu Y, et al. Gene therapy with extracellular superoxide dismutase attenuates myocardial stunning in conscious rabbits. Circulation. 1998;98:1438–1448. doi: 10.1161/01.cir.98.14.1438. [DOI] [PubMed] [Google Scholar]
- 9.Qiu Y, Rizvi A, Tang X-L, et al. Nitric oxide triggers late preconditioning against MI in conscious rabbits. Am J Physiol. 1997;273:H2931–H2936. doi: 10.1152/ajpheart.1997.273.6.H2931. [DOI] [PubMed] [Google Scholar]
- 10.Takano H, Manchikalapudi S, Tang X-L, et al. Nitric oxide synthase is the mediator of late preconditioning against MI in conscious rabbits. Circulation. 1998;98:441–449. doi: 10.1161/01.cir.98.5.441. [DOI] [PubMed] [Google Scholar]
- 11.Li X-Y, McCay PB, Zughaib M, et al. Demonstration of free radical generation in the “stunned” myocardium in the conscious dog and identification of major differences between conscious and open-chest dogs. J Clin Invest. 1993;92:1025–1041. doi: 10.1172/JCI116608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hjalmarsson K, Marklund SL, Engstrom A, et al. Isolation and sequence of complementary DNA encoding human extracellular superoxide dismutase. Proc Natl Acad Sci U S A. 1987;84:6340–6344. doi: 10.1073/pnas.84.18.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham FL, Prevec L. Manipulation of adenovirus vectors. In: Murray EJ, editor. Methods in Molecular Biology: Vol. 7: Gene Transfer and Expression Protocols. Humana Press; Clifton, NJ: 1991. pp. 109–128. [DOI] [PubMed] [Google Scholar]
- 14.Shirato C, Miura T, Ooiwa H, et al. Tetrazolium artifactually indicates superoxide dismutase-induced salvage in reperfused rabbit heart. J Mol Cell Cardiol. 1989;21:1187–1193. doi: 10.1016/0022-2828(89)90695-0. [DOI] [PubMed] [Google Scholar]
- 15.SAS Institute . SAS/STAT User's Guide. Release 6.03. SAS Institute; Cary, NC: 1988. pp. 675–712. [Google Scholar]
- 16.Kashyap VS, Santamarina-Fojo S, Brown DR, et al. Apolipoprotein E deficiency in mice: gene replacement and prevention of atherosclerosis using adenovirus vectors. J Clin Invest. 1995;96:1612–1620. doi: 10.1172/JCI118200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen MV, Yang XM, Liu Y, et al. A new animal model of controlled coronary artery occlusion in conscious rabbits. Cardiovasc Res. 1994;28:61–65. doi: 10.1093/cvr/28.1.61. [DOI] [PubMed] [Google Scholar]
- 18.Karlsson K, Sandstrom J, Edlund A, et al. Pharmacokinetics of extracellular-superoxide dismutase in the vascular system. Free Radic Biol Med. 1993;14:185–190. doi: 10.1016/0891-5849(93)90009-j. [DOI] [PubMed] [Google Scholar]