In textbooks of physiology and in medical school classrooms around the world, hypertension is described as a disease characterized by vascular dysfunction, renal impairment, and altered sympathetic outflow. T lymphocytes are never mentioned, and the notion that hypertension may be an autoimmune disease is not even entertained. Yet, increasing evidence, dating back as early as the 1960s, supports a role of inflammation and immunity in the genesis of hypertension. Cells of both the innate and adaptive immune systems infiltrate the vasculature and kidney in various animal models of hypertension, and efforts to suppress inflammation reduce end-organ damage and in some instances lower blood pressure. Mice and rats lacking T cells are partially protected against both angiotensin II and salt-sensitive hypertension and adoptive transfer of T cells restores blood pressure in these animals. It is currently thought that cytokines released by inflammatory cells, including interleukin 17 (IL17), tumor necrosis factor alpha (TNFα, and IL6 lead to vascular dysfunction and remodeling and promote renal sodium retention, leading to blood pressure elevation1, 2.
Unfortunately, despite this growing body of evidence, almost all studies to date have been performed in experimental animals, and it is unclear if adaptive immunity plays any role in human hypertension. While there is considerable evidence in humans that inflammation is important in specialized cases such as pulmonary hypertension3 or pre-eclampsia (hypertension of pregnancy)4, data that the immune system contributes to the pathophysiology of essential hypertension in non-pregnant adults is scarce. In 2005, Bautista et al.5 found a positive correlation between blood pressure and serum levels of TNFα and IL6 in 196 relatively healthy subjects. IL6 functions both upstream and downstream of another highly pro-inflammatory cytokine, IL17, which is secreted from specialized subsets of T cells. We found that serum IL17 levels were positively correlated with blood pressure in 112 diabetic patients6. In a small study of 8 patients with psoriasis or rheumatoid arthritis and grade 1 essential hypertension, treatment with the immunosuppressant drug, mycophenolate mofetil, reduced blood pressure and urinary excretion of TNFα7.
In this issue of Hypertension, Park et al8 provide interesting new data that suggest that T cells might indeed contribute to human hypertension. The authors used flow cytometry to demonstrate that hypertensive patients have an increased fraction of circulating CD8+ cytotoxic T cells bearing surface markers of immunosenescence. This phenotype is characterized by the loss of CD28 and the acquisition of CD57. These individuals also have increases in circulating levels of the CXCR3 chemokines, monokine induced by gamma interferon (MIG), interferon gamma-induced protein 10 (IP-10), and interferon-inducible T cell alpha chemoattractant (I-TAC). The authors showed that the circulating fraction of CD8+ T cells producing perforin, granzyme B, interferon gamma (IFNγ), or TNFα was increased in hypertensive patients compared to controls. Of note, there was no difference in IL17 producing CD8+ T cells between the two groups. Immunostaining of kidney sections from hypertensive patients with nephrosclerosis revealed an increase in both CD4+ and CD8+ subsets along with an increase in I-TAC in the proximal and distal tubules compared to normotensive control subjects.
The increase in CD28−/CD8+ T cells is particularly interesting as loss of the CD28 receptor is one of the most prominent changes associated with aging in humans9. Gain of the CD57 receptor is thought to occur in the later stages of CD28− T cell differentiation and may most accurately predict replicative senescence of CD8+ T cells10. CD28 is an important co-stimulatory receptor that binds to CD80 or CD86 on antigen presenting cells such as activated dendritic cells. This co-stimulatory signal is essential for activation of naïve T cells, clonal expansion, and differentiation into effector T cells11. At birth, virtually all human T cells express CD28. However, by age 80, about 10-15% of peripheral CD4+ T cells and 50-60% of CD8+ T cells lack CD28 expression. This loss of CD28 expression and gain of CD57 expression has been attributed to repeated antigenic stimulation. When T cells encounter an appropriate activation signal, they are induced to proliferate and differentiate into effector or memory T cells. With each repetitive stimulation/proliferation round, CD28 expression is progressively and eventually irreversibly downregulated, leading to the accumulation of CD28− cells with shortened telomeres, indicative of their reduced replicative lifespan. Of note, this phenomenon occurs in humans and non-human primates but not in mice, underscoring the importance of human studies in this area. CD28− T cells express high levels of adhesion molecules (like LFA-1) and cytolytic molecules (such as perforin and granzyme B) leading to enhanced cytotoxicity9.
How molecules like perforin and granzyme B contribute to hypertension is not clear. Perforin creates pores in plasma membranes resulting in membrane permeabilization/destabilization. Granzymes can cleave both extracellular and intracellular proteins. Extracellular effects include matrix remodeling, detachment of cells, and induction of cell death by lack of extracellular contact. Entrance of granzyme B into cells is facilitated by perforin. Once intracellular, granzyme B causes rapid caspase-dependent apoptosis12. Perhaps by inducing matrix remodeling and cell death, these molecules contribute to the vascular remodeling and microvascular rarefaction seen in hypertension.
A prior study from our group showed that the CD28:CD80/86 costimulation axis is critical in the development and maintenance of hypertension. We found that treatment with abatacept, a fusion protein that inhibits this axis, prevented angiotensin II and DOCA-salt induced hypertension and reversed already established hypertension. This effect was mimicked by genetic deletion of CD80/8613. At first glance, it seems paradoxical that we observed a critical role for CD28 signaling in hypertension while the current study by Park et al. found an increase in T cells lacking CD28 in hypertensive patients. One possible explanation for this apparent paradox is that the accumulation of CD28− T cells in humans might occur because of repeated antigenic stimulation with the yet to be defined “neoantigen(s)” of hypertension and that the presence of these CD28− cells is a consequence rather than a cause of hypertension. Another possibility is that while these cells are not involved in the initial break of tolerance, they may represent an important amplification mechanism leading to more severe, sustained disease. In keeping with this notion, patients with autoimmune diseases such as rheumatoid arthritis, Graves’ disease, or multiple sclerosis have elevated numbers of CD28− T cells. There is also precedence for CD28− CD4+ T cells in atherosclerotic lesions resulting in plaque instability, acute coronary syndromes, and stroke9.
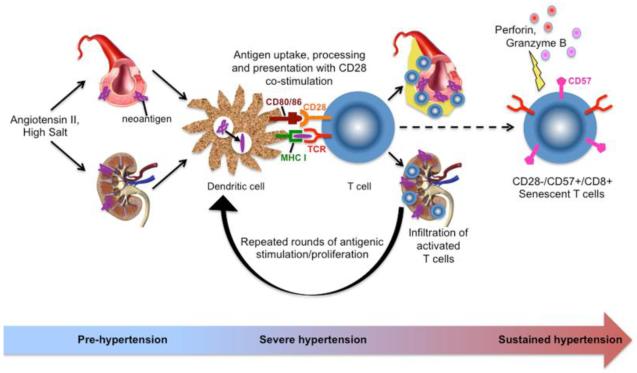
Putting this together, a working hypothesis can be formulated as shown in the Figure. Hypertensive stimuli such as angiotensin II or high salt causes mild hypertension, resulting in vascular damage, mild oxidative stress, and the formation of neoantigen(s). These neoantigens are processed and presented by innate immune cells and, through T cell costimulation via CD28, lead to T activation and their infiltration into target organs such as the vasculature and kidneys. This leads to further vascular damage, endothelial dysfunction, salt and water retention, and more severe hypertension. This, in turn, results in the formation of additional neoantigens and repeated antigenic stimulation of T cells, eventually leading to loss of CD28, gain of CD57, and formation of cytotoxic, pro-inflammatory senescent CD8+ T cells. These cells then, through production of perforin, granzyme B, and cytokines/chemokines, amplify and sustain the hypertensive response and the end organ damage associated with hypertension (Figure). An obvious implication of such a scenario is that accumulation of these cells might explain the increasing prevalence of hypertension with aging.
Figure.
Hypothetical scheme for activation and differentiation of CD8+ T cells in hypertension. Hypertensive stimuli such as angiotensin II or high salt causes mild hypertension, resulting in vascular damage, mild oxidative stress, and the formation of neoantigen(s). These promote T cell activation and infiltration of target organs such as the vasculature and kidneys, leading to further vascular damage, endothelial dysfunction, salt and water retention, and more severe hypertension. Repeated antigenic stimulation of T cells eventually leads to loss of CD28, gain of CD57, and formation of cytotoxic, pro-inflammatory senescent CD8+ T cells. These cells then, through production of perforin, granzyme B, and cytokines/chemokines, amplify and sustain the hypertensive response and the end organ damage associated with hypertension.
Some limitations of the current study by Park et al. are that although the hypertensive and control groups were age-matched, the hypertensive patients had higher body mass indices, fasting glucose, creatinine, and cholesterol/triglyceride levels that may have confounded the results. Adiposity, in particular, is associated with a systemic inflammatory state, which can, in part, be responsible for the higher level of senescent T cells seen in the hypertensive group. Also, even though the blood is the easiest place to look, particularly in humans, it is likely not the best place to identify which subsets of T cells are important in the pathophysiology of hypertension. The authors did do a limited analysis of kidney sections but a thorough characterization of T cell subsets in various target organs (such as the vessel wall, perivascular fat, and kidney) and secondary lymphoid organs may be more revealing in terms of identifying the key players responsible for hypertension. Nevertheless, the finding of senescent CD28 null cells in hypertensive patients is intriguing and warrants further investigation. It is unclear what therapeutic approaches might be used to correct any pathology that they cause, but immunomodulatory therapy directed toward the inflammatory cytokines that they produce might be considered.
Acknowledgments
Sources of Funding: Supported by NIH R01HL039006, P01HL058000, P01HL095070, P01GM015431 and R01HL105294.
Footnotes
Disclosures: None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Brands MW, Banes-Berceli AK, Inscho EW, Al-Azawi H, Allen AJ, Labazi H. Interleukin 6 knockout prevents angiotensin II hypertension: role of renal vasoconstriction and janus kinase 2/signal transducer and activator of transcription 3 activation. Hypertension. 2010;56:879–884. doi: 10.1161/HYPERTENSIONAHA.110.158071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El Chami H, Hassoun PM. Immune and inflammatory mechanisms in pulmonary arterial hypertension. Progress in cardiovascular diseases. 2012;55:218–228. doi: 10.1016/j.pcad.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn H, Park J, Gilman-Sachs A, Kwak-Kim J. Immunologic characteristics of preeclampsia, a comprehensive review. Am J Reprod Immunol. 2011;65:377–394. doi: 10.1111/j.1600-0897.2010.00913.x. [DOI] [PubMed] [Google Scholar]
- 5.Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens. 2005;19:149–154. doi: 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- 6.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol. 2006;17:S218–225. doi: 10.1681/ASN.2006080918. [DOI] [PubMed] [Google Scholar]
- 8.Park S, Youn J-C, Yu HT, Lim BJ, Koh MJ, Lee J, Chang D-Y, Choi Y-S, Lee S-H, Kang S-M, Jang Y, Yoo O-J, Shin E-C. Immunosensescent CD8+ T cells and CXCR3 Chemokines Are Increased in Human Hypertension. Hypertension. 2013 doi: 10.1161/HYPERTENSIONAHA.113.00689. [DOI] [PubMed] [Google Scholar]
- 9.Weng NP, Akbar AN, Goronzy J. CD28(−) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28− and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134:17–32. doi: 10.1111/j.1365-2567.2011.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bu DX, Lichtman AH. T cells and blood vessels: costimulation turns up the pressure. Circulation. 2010;122:2495–2498. doi: 10.1161/CIRCULATIONAHA.110.991059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bots M, Medema JP. Granzymes at a glance. J Cell Sci. 2006;119:5011–5014. doi: 10.1242/jcs.03239. [DOI] [PubMed] [Google Scholar]
- 13.Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, Weyand CM, Harrison DG, Guzik TJ. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation. 2010;122:2529–2537. doi: 10.1161/CIRCULATIONAHA.109.930446. [DOI] [PMC free article] [PubMed] [Google Scholar]