Abstract
Objectives
To measure the levels of serum CUB and zona pellucida-like domain-containing protein 1 (CUZD1) in patients with ovarian cancer (OvCa), benign gynecological conditions and healthy women and in a number of other cancer types (breast, colorectal, lung, prostate and testicular).
Design and methods
Serum CUZD1 levels were measured with a commercial enzyme-linked immunosorbent assay (ELISA). All specimens were analyzed in duplicate. Preliminary verification was performed in serum using 9 healthy women and 20 late stage (III–IV) OvCa patients. An independent cohort of serum samples was used to validate the verification results (18 late stage OvCa, 8 benign gynecological conditions and 8 healthy controls). The following specimens were used for the other cancer types of unknown stage – breast (n = 11), colorectal (n = 10), lung (n = 10), prostate (n = 15) and testicular (n = 10).
Results
Serum CUZD1 was significantly elevated in ovarian cancer patients (range 95–668μg/L) as compared to healthy controls (range 0.7 – 2.5μg/L). The independent cohort of OvCa samples confirmed the preliminary verification results. CUZD1 was also elevated in breast and lung cancer specimens and not in colorectal, prostate and testicular cancer specimens.
Conclusions
CUZD1 appears to be a highly promising novel serum biomarker for OvCa diagnosis. Its performance in the 2 independent cohorts examined, and in lung and breast cancer patients warrants further investigation.
Keywords: CUB and zona pellucida-like domain-containing protein 1, CUZD1, cancer biomarker, ovarian cancer, diagnostic, tumor marker
INTRODUCTION
Ovarian cancer (OvCa) occurs in 1 of 2,500 postmenopausal women in North America and is the most lethal gynecological malignancy. When OvCa is diagnosed at early stages, the 5-year survival rate is close to 90% [1], however, the vast majority of patients are identified when they have late stage disease where overall 5-year survival is 10–30% [2;3]. Unfortunately, other than definitive diagnosis by invasive surgery (laparoscopy or laparotomy), which carries its own morbidity and mortality, no diagnostic or screening test is presently suitable for the early diagnosis of ovarian carcinoma. Current screening modalities include pelvic examination, ultrasonography, and serum tumor markers [4]. Biannual pelvic examination, either during a routine physical examination or during evaluation of nonspecific abdominal/pelvic discomfort rarely detects early stage OvCa and therefore, has little impact on reducing cancer mortality. While it remains important in the assessment of women presenting with gynecological symptoms, it cannot be recommended as a first-line screening tool. Similarly, although transvaginal ultrasound is highly accurate in both the detection and the morphologic characterization of adnexal masses, the test’s ability to distinguish benign from malignant disease is more problematic [5].
The ability to detect human malignancy via a simple blood test has long been an objective in medical screening. The advantages of such an easy to use, relatively noninvasive and operator-independent test are self-evident. A variety of ovarian tumor markers have been studied. The most extensively investigated of these is carbohydrate antigen 125 (CA125), a large glycoprotein of unknown function and an FDA-approved serum marker for monitoring OvCa recurrence. Unfortunately, CA 125 lacks sufficient sensitivity as it is not elevated in 20–30% of patients with advanced disease [6] and serum levels are within the normal range in 50% of women with surgically documented stage I disease [7]. CA 125 also demonstrates poor specificity as it is elevated during menstruation or pregnancy and in other benign conditions such as endometriosis, peritonitis, or cirrhosis, particularly with ascites [8]. A major unmet need is the discovery of novel serological biomarkers to diagnose OvCa early so that they will have a strong impact on improving patient outcome through the administration of potentially curative surgery and/or treatment.
Our laboratory has recently published a study where we mined publicly-available bioinformatic databases, in order to identify genes that were either uniquely, or highly expressed in one or two tissues (as opposed to genes that are ubiquitously expressed in many tissues) [9]. CUZD1 (CUB and zona pellucida-like domain-containing protein 1) was identified in this study as being pancreas-specific. Our laboratory has documented that CUZD1 is a novel pancreatic cancer serum biomarker (Chrystoja et al, manuscript in preparation). To date, there are only a handful of studies published in the literature related to CUZD1. Also called Transmembrane protein UO-44, the gene is located on chromosome 10q26.13 and encodes a 607 amino acid trans-membrane protein. In 2001, Huynh et al isolated a uterine and ovarian specific, tamoxifen and estrogen-induced rat UO-44 cDNA through differential display and cDNA library screening [10]. The authors found that the UO-44 gene was observed only in the uterus and ovary of rats. In 2004, the same group reported the cloning and characterization of four novel splice variants of the human ortholog of UO-44 (HuUO-44) [11]. While the authors found in 2001 that the Rat UO-44 was highly expressed in the ovaries and uterus, the human ortholog HuOU-44 was found highly expressed in the pancreas. The addition of HuUO-44 antisera markedly inhibited the NIH-OVCAR3 ovarian cancer cell attachment and proliferation. Hence, the authors postulated that HuUO-44 may be involved in ovarian cancer cell attachment and proliferation. In 2007, Leong et al observed that the overexpression of Hu-UO-44 in ovarian cancer cells significantly conferred resistance to cisplatin [12].
Taken together, all studies related to CUZD1/UO-44 suggest that it is a transmembrane-associated protein, expressed in the uterus, ovaries and pancreas. Primary translation products of UO-44 gene encode a polypeptide of 607 amino acids, which contains a secretory signal sequence, two CUB domains, a zona pellucida domain and a transmembrane region [13]. To date, no data exists examining the levels of CUZD1/UO-44 in the blood of ovarian cancer patients (or other cancer types) to determine if it has diagnostic potential. In this study, we investigated the serum levels of CUZD1 in healthy controls and ovarian cancer patients, as well as in other cancer types including breast, colorectal, lung, prostate and testicular. Herein, we report significantly elevated levels of CUZD1 in the serum of ovarian cancer patients, supporting CUZD1 as a novel serum biomarker for ovarian cancer. The protein is also elevated in lung and breast cancer patients.
MATERIALS AND METHODS
Patients and specimens
Preliminary verification study was performed using sera from 20 confirmed late stage (III–IV) epithelial ovarian cancer cases, 11 breast, 10 colorectal, 10 lung, 15 prostate and 10 testicular cancer cases (of unknown stage) from Toronto, Canada. The control cohort consisted of 9 men and 9 women with no previous history of malignancy. For the OvCa validation study, an independent cohort of samples was used (n = 18 late stage OvCa patients, 8 benign gynecological conditions and 8 healthy women) which were analyzed on a different day. All samples were collected and stored in an identical fashion (−80°C) until analysis. Samples were collected with informed consent and Institutional Review Board approval.
Measurement of CUZD1 and CA125 in serum
Using a commercially available sandwich ELISA assay from USCN LifeSciences, Missouri City, USA for CUZD1, the levels of this protein in serum were measured in duplicate for all specimens, as per the manufacturer’s recommendations. Briefly, pre-coated polystyrene strips were loaded with 100 μL of serum or standards and incubated for 2 hours at 37°C with gentle shaking. After washing the strips, 100 μL of ‘detection reagent A’ was added and the strips were incubated for an additional hour at 37°C with gentle shaking. After washing, 100 μL of ‘detection reagent B’ was added and the strips were incubated for 30 minutes at 37°C with gentle shaking. After a final washing, 100 μL of 3,3,5,5′-Tetramethylbenzidine substrate solution was added and the strips were incubated for 15 minutes at at 37°C with gentle shaking. The chromogenic reaction was stopped with the addition of 50 μL of hydrochloric acid solution. Absorbance was measured with the Wallac EnVision 2103 Multilabel Reader (Perkin Elmer) at 450 nm standardized to background absorbance at 620 nm. CA 125 levels were measured using the Roche Elecsys automated ELISA platform. All serum samples for CA125 and CUZD1 analysis were diluted 5-fold (or more) with 60g/L of bovine serum albumin before analysis. Final concentrations were calculated by multiplying with the dilution factor.
Data analysis and statistics
Statistical analysis was performed using SPSS software, version 15.0 (SPSS Inc., Chicago, IL). Normal distribution was evaluated using Shapiro-Wilk test and by inspection of the Q-Q plot. For descriptive purposes, the results were expressed as median and interquartile range (25th and 75th percentiles) since the variable was not normally distributed. Kruskal-Wallis test was performed for comparison between more than two groups and Mann-Whitney U test with Bonferroni’s correction was performed for comparison between two groups. A p-value less than 0.05 was considered to be statistically significant. Spearman’s rank correlation coefficient was used to assess the correlations among CUZD1 and CA125 (GraphPad Software Inc., La Jolla, CA).
RESULTS
Increased Serum CUZD1 and CA125 in Ovarian Cancer Patients
In the initial evaluation, CUZD1 displayed significant discriminating power (p < 0.00001) between 9 healthy women and 20 late stage OvCa patients. The distribution of CUZD1, as measured by ELISA, in cases and controls are shown in Figure 1A. Serum CUZD1 was significantly elevated in ovarian cancer (mean ± standard deviation) compared to healthy controls (334 ± 183μg/L vs. 1.6 ± 0.7μg/L), as was serum CA125 (1565 ± 1388U/mL vs. 12 ± 5.2U/mL; p<0.001). The specific serum biomarker levels for these samples are highlighted in Table 1.
Figure 1. CUZD1 is a novel serological biomarker for OvCa.
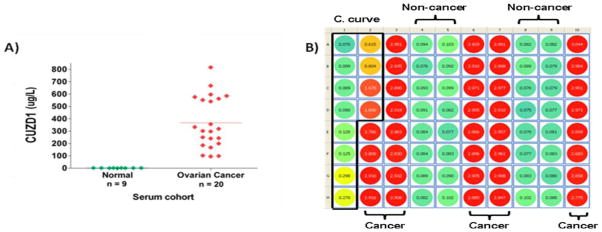
(A) A scatter plot of results for CUZD1 protein levels in 9 serum samples from healthy women and 20 late stage OvCa patients. Horizontal lines represent mean values. (B) Raw absorbance reading at 450nm (corrected at 620nm) for serum CUZD1 levels in an independent cohort (18 late stage OvCa, 8 benign gynaecological conditions and 8 healthy controls) using the ELISA kit. All standards and samples were analyzed in duplicate, loaded vertically per column in ascending order. Wells A1-D2 contain the calibration curve (C. curve), wells E2-H3, A6-H7 and A10-H10 contain the 18 OvCa serum samples, diluted 5-fold. Wells A4-H5 and A8-H9 contain benign and healthy serum samples. It is evident that OvCa specimens are well above the highest standard of 20μg/L (wells C2 and D2) (meaning CUZD1 > 100ug/L in all cancer samples, given the 5-fold dilution, while the non-cancer samples had CIZD1 levels < 1ug/L).
Table 1.
ELISA serum results for CA 125 and CUZD1 in 9 healthy control female serum samples and 20 late stage ovarian cancer samples.
| Sample ID | Cohort | Notes/Comments | CA125 (U/mL) | CUZD1 (ug/L) |
|---|---|---|---|---|
| N1 | Control | Healthy | 13 | 0.7 |
| N3 | Control | Healthy | 12 | 2.3 |
| N4 | Control | Healthy | 6 | 0.9 |
| N5 | Control | Healthy | 14 | 0.8 |
| N6 | Control | Healthy | 7 | 1.7 |
| N7 | Control | Healthy | 5 | 2.1 |
| N9 | Control | Healthy | 20 | 2.5 |
| N10 | Control | Healthy | 16 | 1.7 |
| N11 | Control | Healthy | 17 | 1.8 |
| 5 | Case | Confirmed ovarian cancer | 1639 | 585 |
| 26 | Case | Confirmed ovarian cancer | 788 | 242 |
| 11 | Case | Confirmed ovarian cancer | 1623 | 551 |
| 24 | Case | Confirmed ovarian cancer | 795 | 318 |
| 10 | Case | Confirmed ovarian cancer | 591 | 167 |
| 9 | Case | Confirmed ovarian cancer | 1894 | 356 |
| 2 | Case | Confirmed ovarian cancer | 1029 | 240 |
| 6 | Case | Confirmed ovarian cancer | 3310 | 540 |
| 14 | Case | Confirmed ovarian cancer | 3320 | 576 |
| 20 | Case | Confirmed ovarian cancer | 550 | 102 |
| 19 | Case | Confirmed ovarian cancer | 576 | 95 |
| 18 | Case | Confirmed ovarian cancer | 4282 | 668 |
| 21 | Case | Confirmed ovarian cancer | 2407 | 297 |
| 3 | Case | Confirmed ovarian cancer | 760 | 185 |
| 13 | Case | Confirmed ovarian cancer | 550 | 98 |
| 25 | Case | Confirmed ovarian cancer | 2400 | 562 |
| 15 | Case | Confirmed ovarian cancer | 1210 | 250 |
| 23 | Case | Confirmed ovarian cancer | 1637 | 333 |
| 16 | Case | Confirmed ovarian cancer | 760 | 199 |
| 1 | Case | Confirmed ovarian cancer | 1183 | 301 |
For CUZD1, a median of 1.7μg/L and 300μg/L was observed for healthy controls and ovarian cancer cases, respectively, with a median control:case ratio of 174. As well, a mean of 1.6μg/L and 334μg/L was observed for healthy controls and ovarian cancer cases, respectively, with a mean control:case ratio of 207. For CA125, a median of 13U/mL and 1388U/mL was observed for healthy controls and ovarian cancer cases respectively, with a median control:case ratio of 107. As well, a mean of 12U/mL and 1565U/mL was observed for healthy controls and ovarian cancer cases respectively, with a mean control:case ratio of 130. The differences in medians between OvCa cases and controls for both CUZD1 and CA125 were highly significant by the Mann-Whitney U test (p<0.001).
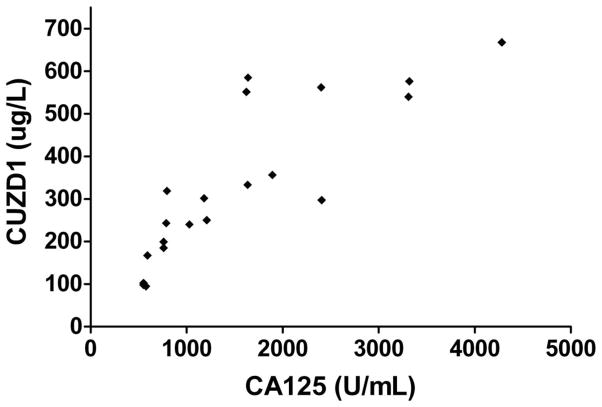
Serum CUZD1 was found to be significantly correlated with serum CA125 in the 20 ovarian cancer patients (Figure 2). The Spearman correlation coefficient between CUZD1 and CA125 was 0.90 (95% confidence interval of 0.75–0.96; p<0.0001).
Figure 2. CUZD1 and CA125 correlation.
Correlation between CUZD1 and CA125 in 20 sera from late stage OvCa patients. Spearman correlation was 0.90 with 95% confidence intervals of 0.75–0.96 (p < 0.001).
CUZD1 levels were further measured in an independent cohort of 18 late stage OvCa patients (mean ± standard deviation of CA125 = 1329 ± 380U/mL), 8 benign conditions (CA125 < 35U/mL) and 8 healthy women (CA125 < 35U/mL). Figure 1B shows the raw absorbance readings at 450nm, corrected for background at 620nm from an ELISA plate for these samples. It is evident that the wells containing the 18 OvCa samples have values well above the highest standard in the calibration curve (>20μg/L). Therefore, the respective CUZD1 concentrations in these OvCa specimens are much higher than 100ug/L (specimens were diluted 1:5 prior to loading on the wells; similar data to the preliminary verification study). The specimens corresponding to the 8 benign conditions and 8 normal controls had mean CUZD1 levels of < 1ug/L. The results shown in Figure 1, with a combined group of 38 late stage ovarian cancer patients and 25 non-cancer patients, confirm that CUZD1 has a very powerful discriminatory power between normal/benign and late stage OvCa patients.
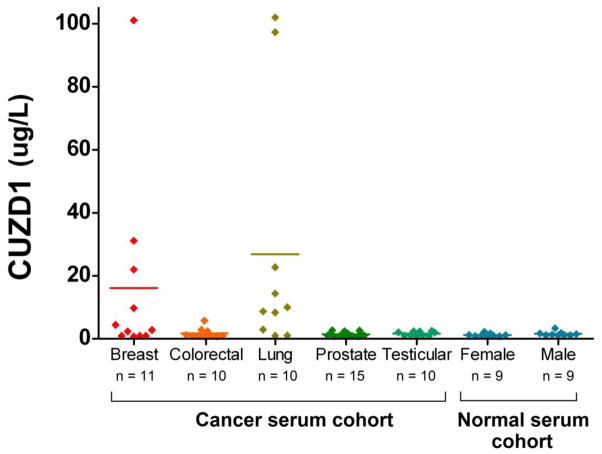
Serum Levels of CUZD1 in Other Cancer Types
CUZD1 levels were examined in the serum of 5 other cancer types (breast, colorectal, lung, prostate and testicular) along with normal male (n = 9) and female (n = 9) sera. Figure 3 displays a scatter plot of the values obtained for CUZD1. Sera from both breast cancer and lung cancer displayed higher levels of CUZD1 than the other 3 cancer types. The differences between lung cancer and male and female controls were significant by the Kruskal-Wallis test (p=0.0025) but the differences between breast cancer patients and female controls were not significant (p > .05).
Figure 3. CUZD1 expression in other cancer types.
A scatter plot of the results for CUZD1 protein levels in serum of various types of malignancies and healthy normal controls. Serum CUZD1 is elevated in several of breast and lung cancer patients while it is low in colorectal, prostate, and testicular cancer. For statistical comparison between groups, please see the ‘Results” section. Horizontal lines represent mean values..
DISCUSSION
The precise function and biological relevance of CUZD1 to ovarian cancer remain largely unknown. The limitations of CA125 as a diagnostic marker are well described and the need to identify a novel biomarker is well appreciated in the clinical community. In this study, we report for the first time elevated CUZD1 levels in the serum of ovarian cancer patients, as well as in breast and lung cancer patients. Our data provides strong evidence that CUZD1 represents a novel serological marker for ovarian cancer as this marker performed equally as well as CA125 in two independent cohorts of samples consisting of healthy controls and ovarian cancer cases. More studies on this highly promising biomarker are warranted. Whether CUZD1 has the ability to act as a diagnostic, prognostic, predictive, or therapeutic tool remains to be seen as validation in larger cohorts are needed to confirm the clinical utility of this marker. It will be particularly interesting to evaluate CUZD1 levels in early stage and prediagnostic serum samples from ovarian cancer patients.
Highlights.
Ovarian cancer is the most lethal gynecological malignacy.
No adequate screening or diagnostic methods exist for early stage ovarian cancer diagnosis.
CUZD1 appears to be a highly promising novel serological biomarker for ovarian cancer.
CUZD1 may be a biomarker for breast and lung cancer.
Acknowledgments
This work is supported by Proteomic Methods Inc., Toronto, Canada. E.P. Diamandis is funded by the Early Detection Research Network of NIH (EDRN; Grant #5U01CA152755).
Abbreviations
- CA 125
carbohydrate antigen 125
- CUZD1
CUB and zona pellucida-like domain-containing protein 1
- ELISA
enzyme-linked immunosorbent assay
- HuUO-44
human ortholog of UO-44
- OvCa
ovarian cancer
- SN
sensitivity
- SP
specificity
- UO-44
uterine-ovarian-specific gene 44
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Konstantinopoulos PA, Spentzos D, Cannistra SA. Gene-expression profiling in epithelial ovarian cancer. Nat Clin Pract Oncol. 2008;5:577–87. doi: 10.1038/ncponc1178. [DOI] [PubMed] [Google Scholar]
- 3.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–29. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 4.Bast RC, Jr, Urban N, Shridhar V, et al. Early detection of ovarian cancer: promise and reality. Cancer Treat Res. 2002;107:61–97. doi: 10.1007/978-1-4757-3587-1_3. [DOI] [PubMed] [Google Scholar]
- 5.Higgins RV, van NJ, Jr, Woods CH, Thompson EA, Kryscio RJ. Interobserver variation in ovarian measurements using transvaginal sonography. Gynecol Oncol. 1990;39:69–71. doi: 10.1016/0090-8258(90)90401-6. [DOI] [PubMed] [Google Scholar]
- 6.Xu Y, Shen Z, Wiper DW, et al. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA. 1998;280:719–23. doi: 10.1001/jama.280.8.719. [DOI] [PubMed] [Google Scholar]
- 7.van Nagell JRJ, Pavlik EJ. Ovarian cancer screening. Clin Obstet Gynecol. 2012;55:43–51. doi: 10.1097/GRF.0b013e3182460c0d. [DOI] [PubMed] [Google Scholar]
- 8.Morgan RJ, Jr, Copeland L, Gershenson D, et al. NCCN Ovarian Cancer Practice Guidelines. The National Comprehensive Cancer Network Oncology (Williston Park) 1996;10:293–310. [PubMed] [Google Scholar]
- 9.Prassas I, Chrystoja CC, Makawita S, Diamandis EP. Bioinformatic identification of proteins with tissue-specific expression for biomarker discovery. BMC Med. 2012;10:39. doi: 10.1186/1741-7015-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huynh H, Ng CY, Lim KB, et al. Induction of UO-44 gene expression by tamoxifen in the rat uterus and ovary. Endocrinology. 2001;142:2985–95. doi: 10.1210/endo.142.7.8247. [DOI] [PubMed] [Google Scholar]
- 11.Leong CT, Ng CY, Ong CK, et al. Molecular cloning, characterization and isolation of novel spliced variants of the human ortholog of a rat estrogen-regulated membrane-associated protein, UO-44. Oncogene. 2004;23:5707–18. doi: 10.1038/sj.onc.1207754. [DOI] [PubMed] [Google Scholar]
- 12.Leong CT, Ong CK, Tay SK, Huynh H. Silencing expression of UO-44 (CUZD1) using small interfering RNA sensitizes human ovarian cancer cells to cisplatin in vitro. Oncogene. 2007;26:870–80. doi: 10.1038/sj.onc.1209836. [DOI] [PubMed] [Google Scholar]
- 13.Chen D, Xu X, Zhu LJ, et al. Cloning and uterus/oviduct-specific expression of a novel estrogen-regulated gene (ERG1) J Biol Chem. 1999;274:32215–24. doi: 10.1074/jbc.274.45.32215. [DOI] [PubMed] [Google Scholar]