Abstract
Recombinant proteins (RPs) are commonly expressed in bacteria followed by solubilization and chromatography. Purified RP yield can be diminished by losses at any step with very different changes in methods needed to try to improve yield. Time and labor can therefore be saved by first identifying the specific reason for low yield. The present study describes a new solid-state NMR approach to RP quantitation in whole cells or cell pellets without solubilization or purification. The approach is straightforward and inexpensive and only requires ~50 mL culture and a low-field spectrometer.
A common approach to produce recombinant protein (RP) begins with incorporation of recombinant DNA (rDNA) into bacteria followed by cell growth, expression and lysis, and finally chromatography to obtain pure RP. Assessment of RP quantity and purity after the expression, solubilization, and/or chromatography steps is typically done using SDS-PAGE that separates proteins by molecular weight (MW). For several different RPs in our laboratory, the RP gel band was not clearly observed after expression or solubilization and the final RP purified yield was unacceptably low, eg. 0.1 mg RP/L culture.1 One hypothesis to explain this result is poor RP expression followed by high-yield solubilization and chromatography. A second distinct hypothesis is high RP expression followed by poor solubilization and highyield chromatography. A third hypothesis is high RP expression and solubilization followed by chromatographic loss of RP. Distinguishing between these hypotheses is important because: (1) the corrective changes to the experimental protocol to improve RP yield are very different for each hypothesis; and (2) implementing these changes is often time- and labor-intensive. For example, low protein expression might be improved by codon changes in the rDNA or by varying induction time whereas poor solubilization might be improved by comprehensive screening of lysis buffers which differ in additives such as denaturants and detergents.
The present study focuses on distinguishing between the first low expression and the second poor solubilization hypotheses. The third chromatographic loss hypothesis is typically straightforwardly tested by comparing the relative RP gel band intensities of washes vs elutions from the chromatographic column. RP expression is typically examined by first boiling an aliquot of cells in buffer containing SDS buffer with subsequent SDS-PAGE of solubilized protein. The RP quantity is estimated by comparison of the intensity of the RP band to the intensities of bands of native bacterial proteins. There are a few reports of more accurate quantitation. 2 This approach relies on a RP MW which is fortuitously different from the MWs of any of the abundant bacterial proteins. Alternatively, the quantity of the solubilized RP could be much higher than the quantities of any of these native proteins, ie. high RP expression and solubility.
An assumption of the approach is that most of the RP is solubilized by boiling. However, the largest RP fraction in cells is typically solid inclusion body (IB) aggregates that can be difficult to solublilize. It is therefore important to develop alternative approaches for RP quantitation in either whole cells or cell extracts enriched in IB solids. One potential method is IR spectroscopy of IBs and is based on the hypothesis of an increased fraction of β sheet for the RP in IBs relative to the native structure, perhaps because of partial amyloid structure in the IB.3 However, the fractional increase in β sheet structure is likely highly variable among RPs in IBs with at least one RP in IBs showing retention of a large fraction of native helical structure.4
The present study describes an alternate solid-state NMR (SSNMR) approach to quantify RP in whole bacterial cells and cell extracts enriched in IBs. The approach does not depend on the structure(s) of the RPs in IBs. We note that there have been earlier applications of SSNMR to whole bacterial cells and cell extracts with a typical goal of elucidation of details of atomicresolution structure.5–11 The new method has been tested with five different RPs whose amino acid sequences are given in the SI. The generality of the approach is supported by use of different plasmid and Escherichia coli (E. coli) strain types.
One RP is Human proinsulin (HPI) which is the precursor to the hormone insulin.12 Folded HPI is a monomer with an α helical core.13 Three RPs (Hairpin, Fgp41, and Fgp41+) are different ectodomain segments of the HIV gp41 protein.1,14,15 Gp41 is an integral HIV membrane protein and the N-terminal ~175 residues of gp41 are the ectodomain that lies outside the virus. The ectodomain is subdivided into the ~20 N-terminal fusion peptide (FP) residues that bind to membranes and the larger C-terminal region that folds as a helical hairpin with a 180° turn.16 There is further assembly of three hairpins to form a molecular trimer with sixhelix-bundle (SHB) structure that is hyperthermostable. Hairpin, Fgp41, and Fgp41+ likely all form SHB structure with sequence differences among constructs as well as lack of FP and most of the loop in Hairpin. The fifth RP (FHA2) is the full ectodomain of the HA2 subunit of the hemagglutinin protein of the influenza virus.17–19 HA2 has similar topology and membrane fusion function as gp41 but there is little sequence homology between HA2 and gp41.20 Membrane-associated FHA2 has folded SHB structure. Previous efforts to solubilize each of these RPs from bacteria were consistent with a large fraction of RP in IBs.
We present “HC” and “HCN” SSNMR approaches to RP quantitation which respectively require double-resonance 1H/13C and triple-resonance 1H/13C/15N SSNMR spectrometers and probes. For the HC approach, two samples are prepared that are denoted “RP+lab” and “RP−lab” and the bacteria respectively have a plasmid with or with the RP rDNA insert. The preparation of either sample includes addition of a 13CO-labeled amino acid to the expression medium. For the present study, this is 13CO-Leu. The SSNMR (RP+lab–RP−lab) 13CO difference intensity should therefore be the signal of the labeled (lab) 13CO nuclei of the RP. Comparison with a standard curve of 13CO intensity vs mole 13CO allows for conversion to mass RP/L bacterial culture which is a common metric of RP expression. Variation in cell mass between the RP+lab and RP−lab samples is accounted for by matching the intensities of the two samples in the 0–90 ppm aliphatic region. This aliphatic 13C signal serves as an internal standard because it is due to natural abundance (na) nuclei whose numbers should be comparable in a RP+ and a RP− cell.
The second HCN approach applies rotational-echo double-resonance (REDOR) SSNMR to one RP+lab sample labeled with either a 13CO,15N-amino acid or a 13CO-amino acid and a 15N-amino acid.21 Separate S0 and S1 REDOR data are acquired with 1 ms dephasing. All 13C signals are detected in S0 whereas there is specific attenuation in S1 of signals of directly-bonded 13CO-15N spin pairs because of the ~1 kHz dipolar coupling. The ΔS=S0–S1 13CO spectrum is therefore dominated by these pairs.22 The ΔS 13CO signal intensity is converted to RP mass/L culture using a method analogous to that of the HC approach. Relative to HC, the HCN RP quantitation has the advantage of one rather than two samples. The HC variant has the advantage of requiring a double-rather than triple-resonance SSNMR spectrometer and probe. Triple-resonance SSNMR instruments are less common and can have lower 13C sensitivity.
The SI provides detailed protocols for sample preparation and SSNMR. The ~2-day experiment is mostly unattended. The approach is inexpensive with only ~50 ml culture volume and ~10 mg labeled amino acid. There is also a protocol to suppress scrambling of the 13CO and/or 15N labels to other amino acid types.23 A “whole cell” (WC) sample is the centrifugation pellet of the bacterial culture. Cell lysis is done prior to centrifugation for the “insoluble cell pellet” (ICP) sample. The RP+lab ICP is therefore enriched in IB RP. The 9.4 T magnetic field, 8 kHz MAS frequency, and ~50 kHz rf fields are moderate and accessible for many NMR facilities including those with a SSNMR probe (~$100,000 cost) on an otherwise liquid-state NMR instrument.
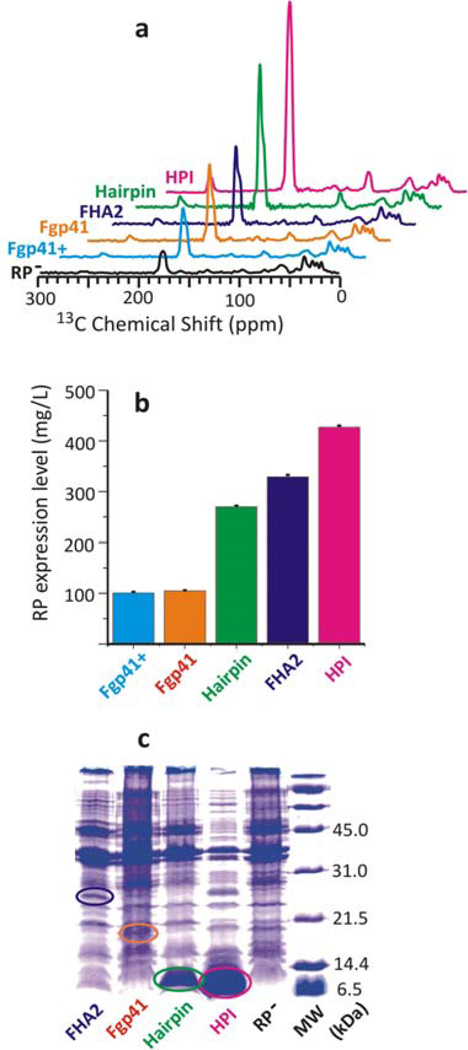
Fig. 1 displays results from HC RP quantitation. Panel a displays 13C spectra of the 13CO-Leu RP−lab and RP+lab samples. Although there are differences in plasmid and E. coli strain types among the samples, the corresponding spectra have similar aliphatic 13C signal intensities in the 0–90 ppm region. The isotropic 13CO signals of the spectra are near 175 ppm with much weaker spinning sideband 13CO signals near 95 and 255 ppm. Relative to RP−lab, there are much larger 13CO signals in the RP+lab spectra which supports significant expression of all the RPs. There are also differences among the 13CO intensities of the different RP+lab samples which support RP-dependent variation in expression. Panel b displays the expression levels in mg RP/L culture as determined from: (1) measurements of IAl− and ICO –, respectively the integrated aliphatic and isotropic 13CO intensities of the RP− lab spectrum; (2) measurement of the corresponding IAl+ and ICO+ of the RP+lab spectrum; and (3) calculation of the 13CO intensity from 13CO-Leus in the RP using IAl0×[(ICO+/IAl+)–(ICO−/IAl−)] where the IAl0 is the value for a typical sample and IAl0×(ICO+/IAl+) and IAl0×(ICO−/IAl−) are 13CO intensities normalized to NMR sample mass. The expression level is calculated using (3) and (i) an experimentally-determined µmole 13CO/ICO conversion factor; (ii) MWRP/NLeu where NLeu is the number of Leus in the RP sequence; and (iii) NMR sample is from cells in ~25 mL culture volume.
Figure 1.
HC variant of RP quantitation. Panel a displays 13C SSNMR spectra of 13CO-Leu ICP samples. Panel b displays the RP expression levels calculated from the (RP+lab–RP−lab) difference intensities of the 13CO (~175 ppm) region. The differences between the ordering of spectral intensities in panel a and the ordering of expression levels in panel b are largely due to different ratios of NLeu/N tot in the RP sequences. Besides the displayed uncertainties based on spectral noise, there is ~10% sample-to-sample variation in RP expression, cf. SI. Panel c displays SDS-PAGE of most of the samples and the marked bands may correspond to the RPs.
The SSNMR-determined expression levels (panel b) are 100–450 mg RP/L culture. These levels are very high relative to the reported ~5 mg/L purified yields for Fgp41, FHA2, and HPI.1,12,18 The most common current approach to assess RP expression is SDS-PAGE. Panel c displays SDS-PAGE of boiled ICPs. Relative to the back ground, there are clear bands for HPI and Hairpin and much fainter and more ambiguous bands for FHA2 and Fgp41. The variation of the RP band intensities in the SDS-PAGE is more reflective of differences in RP IB solubilization than differences in expression levels. FHA2 and Fgp41 are membrane proteins while HPI and Hairpin are not, so the membrane RP IBs appear to be less well-solubilized. The SSNMR approach has the important advantage of being independent of IB solubilization.
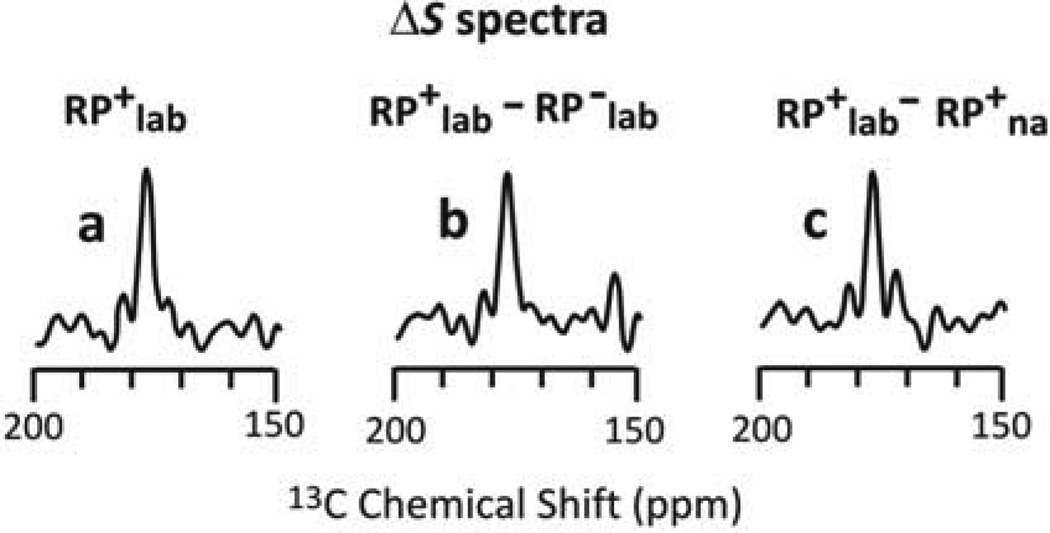
The HCN approach is based on the ΔS=S0–S1 13CO REDOR difference spectrum of the RP+lab ICP sample. This spectrum is dominated by directly-bonded lab 13CO-15N spin pairs in the IB RP. For Fig. 2a, RP≡Fgp41, lab≡13CO,15N-Leu, and the ΔS spectrum is mostly due to the N-terminal Ls of the six LL dipeptides in the Fgp41 sequence. One control is the RP−lab ΔS spectrum which is dominated by LL dipeptides of proteins other than Fgp41 produced during expression. However, there is no ΔS(RP−lab) signal, cf. SI, or equivalently, Fig. 2b shows a ΔS(RP+lab)–ΔS(RP−lab) spectrum very similar to the ΔS(RP+lab) spectrum that must therefore be dominated by the IB Fpg41 signals. Another control is the ΔS(RP+na) spectrum of a sample prepared with unlabeled Leu and reflecting signals of na 13CO-15N spin pairs. However, there is little ΔS(RP+na) signal as reflected in Fig. 2c ΔS(RP+lab)– ΔS(RP+ na) spectrum that is similar to the ΔS(RP+lab) spectrum.
Figure 2.
13CO ΔS spectra based on the S0 and S1 spectra of three different ICP samples. The RP+lab and RP−lab plasmids respectively had and lacked the Fgp41 insert. The lab and na expression media respectively contained 13CO, 15N-Leu and unlabeled Leu. Panel a ΔS (RP+lab=S0–S1 signal represents directly-bonded 13CO-15N spin pairs of the RP+lab sample. Panel b ΔS=ΔS(RP+lab)–ΔS(RP−lab) is from spin pairs of IB Fgp41. Panel c ΔS=ΔS(RP+lab)–ΔS(RP+na) is from lab spin pairs of the RP+lab sample. The similar peak intensities, shifts, and lineshapes of the three spectra support a dominant contribution to the ΔS(RP+lab) spectrum of 13CO signals of labeled Ls of the LL dipeptides of IB Fgp41.
The HCN approach to quantitation of RP expression is detailed in the SI. For a particular RP+lab sample, the HC and HCN expression levels typically agree to within a factor of 2. Quantitative labeling of the RP is assumed for both approaches so the levels are likely lower limits of expression but probably within a factor of ~2. Incomplete labeling will have a larger effect on HCN quantitation because the ΔS signal is only observed from dipeptides with both residues labeled.
Most of folded Fgp41 is a thermostable six-helix bundle which includes the six LL dipeptides.16 The ΔS spectrum was previously obtained for 13CO,15N-Leu Fgp41 that had been purified, refolded, and reconstituted in membranes.1 There was a single peak with 178 ppm shift and 3 ppm width which is consistent with folded helical structure. The Fig. 2 ΔS(RP+lab) spectrum of Fgp41 in IBs is very similar and supports formation of folded Fgp41 structure in the IBs. For other RPs in IBs, the ΔS spectral widths are sometimes much broader, eg. ~7 ppm for HPI, cf. SI. This breadth is consistent with unfolded RP structure in the IBs. SSNMR quantitation of RP expression by either the HC or HCN approaches is independent of the degree of RP folding in the IBs.
For all the RPs of the present study, the SSNMR spectra demonstrated high expression, ie. ≥100 mg IB RP/L culture, so the main obstacle to purified RP is solubilization of the IBs. For other RPs that are produced at much lower levels, SSNMR could also be applied to optimize RP production. 13CO-RP+lab samples would be prepared with different growth and/or expression parameters and expression levels determined from the 13CO intensities. In summary, this paper describes general, inexpensive, rapid, and straightforward SSNMR approaches to RP quantitation in whole cells and cell extracts without purification.
Supplementary Material
ACKNOWLEDGMENT
Drs. R. Mackin , Y-K Shin, and K. Sackett provided plasmids.
Funding Sources
National Institutes of Health Grant A147153
ABBREVIATIONS
- Fgp41
Fgp41+, and Hairpin, HIV gp41 ectodomains
- FHA2
Influenza hemagglutinin ectodomain
- HPI
human proinsulin
- IB
inclusion body
- ICP
insoluble cell pellet
- REDOR
rotational-echo double-resonance
- RP
recombinant protein
- SHB
six-helix bundle
- SSNMR
solid-state NMR
- WC
Whole cell
Footnotes
ASSOCIATED CONTENT
Supporting Information
Additional NMR spectra, cell growth and labeling, sample preparation, plasmids and protein sequences, NMR methods and analysis. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interests.
REFERENCES
- 1.Vogel EP, Curtis-Fisk J, Young KM, Weliky DP. Biochemistry. 2011;50:10013–10026. doi: 10.1021/bi201292e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miles AP, Saul A. Protein Protocols Handbook. 3rd ed. Totowa: Humana; 2009. pp. 487–496. [Google Scholar]
- 3.Gross-Selbeck S, Margreiter G, Obinger C, Bayer K. Biotech. Prog. 2007;23:762–766. doi: 10.1021/bp070022q. [DOI] [PubMed] [Google Scholar]
- 4.Curtis-Fisk J, Spencer RM, Weliky DP. J. Am. Chem. Soc. 2008;130:12568–12569. doi: 10.1021/ja8039426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, Balazs YS, Thompson LK. Biochemistry. 1997;36:1699–1703. doi: 10.1021/bi962578k. [DOI] [PubMed] [Google Scholar]
- 6.Kim SJ, Cegelski L, Stueber D, Singh M, Dietrich E, Tanaka KSE, Parr TR, Far AR, Schaefer J. J. Mol. Biol. 2008;377:281–293. doi: 10.1016/j.jmb.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ieronimo M, Afonin S, Koch K, Berditsch M, Wadhwani P, Ulrich AS. J. Am. Chem. Soc. 2010;132:8822–8823. doi: 10.1021/ja101608z. [DOI] [PubMed] [Google Scholar]
- 8.Fu RQ, Wang XS, Li CG, Santiago-Miranda AN, Pielak GJ, Tian F. J. Am. Chem. Soc. 2011;133:12370–12373. doi: 10.1021/ja204062v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reckel S, Lopez JJ, Lohr F, Glaubitz C, Dotsch V. Chembiochem. 2012;13:534–537. doi: 10.1002/cbic.201100721. [DOI] [PubMed] [Google Scholar]
- 10.Zhou XX, Cegelski L. Biochemistry. 2012;51:8143–8153. doi: 10.1021/bi3012115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang SL, Ishii Y. J. Am. Chem. Soc. 2012;134:2848–2851. doi: 10.1021/ja212190z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackin RB, Choquette MH. Prot. Expr. Purif. 2003;27:210–219. doi: 10.1016/s1046-5928(02)00643-5. [DOI] [PubMed] [Google Scholar]
- 13.Yang YW, Hua QX, Liu J, Shimizu EH, Choquette MH, Mackin RB, Weiss MA. J. Biol. Chem. 2010;285:7847–7851. doi: 10.1074/jbc.C109.084921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sackett K, Nethercott MJ, Shai Y, Weliky DP. Biochemistry. 2009;48:2714–2722. doi: 10.1021/bi8019492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sackett K, Nethercott MJ, Epand RF, Epand RM, Kindra DR, Shai Y, Weliky DP. J. Mol. Biol. 2010;397:301–315. doi: 10.1016/j.jmb.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang ZN, Mueser TC, Kaufman J, Stahl SJ, Wingfield PT, Hyde CC. J. Struct. Biol. 1999;126:131–144. doi: 10.1006/jsbi.1999.4116. [DOI] [PubMed] [Google Scholar]
- 17.Curtis-Fisk J, Preston C, Zheng ZX, Worden RM, Weliky DP. J. Am. Chem. Soc. 2007;129:11320–11321. doi: 10.1021/ja073644g. [DOI] [PubMed] [Google Scholar]
- 18.Curtis-Fisk J, Spencer RM, Weliky DP. Prot. Expr. Purif. 2008;61:212–219. doi: 10.1016/j.pep.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim CS, Epand RF, Leikina E, Epand RM, Chernomordik LV. J. Biol. Chem. 2011;286:13226–13234. doi: 10.1074/jbc.M110.181297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Skehel JJ, Wiley DC. Proc. Natl. Acad. Sci. U.S.A. 1999;96:8967–8972. doi: 10.1073/pnas.96.16.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gullion T, Schaefer J. J. Magn. Reson. 1989;81:196–200. [Google Scholar]
- 22.Yang J, Parkanzky PD, Bodner ML, Duskin CG, Weliky DP. J. Magn. Reson. 2002;159:101–110. doi: 10.1016/s1090-7807(02)00033-2. [DOI] [PubMed] [Google Scholar]
- 23.Tong KI, Yamamoto M, Tanaka T. J. Biomol. NMR. 2008;42:59–67. doi: 10.1007/s10858-008-9264-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.