Abstract
Cardiomyocyte-like cells have been reported in thoracic veins of rodents and other mammals, but their differentiation state and relationship to the muscle mass in the heart remain to be characterized. Here we investigated the distribution, ultrastructure, and the expression and developmental regulation of myofilament proteins in mouse and rat pulmonary and azygos venous cardiomyocytes. Tracing cardiomyocytes in transgenic mouse tissues with a lacZ reporter gene driven by cloned rat cardiac troponin T promoter demonstrated scattered distribution of cardiomyocytes discontinuous from the atrial sleeves. The longitudinal axis of venous cardiomyocytes is perpendicular to that of the vessel. These cells contain typical sarcomere structures and intercalated discs as shown in electron microscopic images and express cardiac isoforms of troponin T, troponin I and myosin. The expression of troponin I isoform genes and the alternative splicing of cardiac troponin T in thoracic venous cardiomyocytes are regulated during postnatal development in a precise synchrony with that in the heart. Nonetheless, the patterns of cardiac troponin T splicing in adult rat thoracic venous cardiomyocytes are slightly but clearly distinct from those in the atrial and ventricular muscles. The data indicate that mouse and rat thoracic venous cardiomyocytes residing in extra-cardiac tissue possess a physiologically differentiated state and an intrinsically preset developmental clock, which are apparently independent of the very different hemodynamic environments and functional features of the vessels and heart.
Keywords: cardiac muscle, myofilament protein, troponin isoform switch, development
INTRODUCTION
Cardiac and skeletal muscles are both striated muscles with many structural and functional similarities [1]. Cardiomyocytes in adult hearts of higher vertebrates are post-mitotic cells [2–5]. In contrast to skeletal muscle, cardiac muscle has little regenerative capacity due to a lack of local stem cells ([6] and references therein). Proper differentiation of cardiomyocytes requires a specific tissue environment, physiological activity, and mechanical load [7–10]. Understanding the requirements for cardiomyocyte differentiation is of major medical importance in exploring myocardial regeneration in the treatment of ischemic heart disease and other terminal heart failures due to losses of adult cardiomyocytes.
Twitch-type contractions of thoracic venous vessels have been observed in mammalian species for over two centuries, occurring independently of and asynchronously to the heart beats ([11] and references therein). More recent publications attributed these contractions to the presence of striated muscle in the vessel wall [12]. These muscles were shown to be morphologically similar to myocardium [13]. In large mammals including humans, the atrial myocardium extends into the vena cavae and pulmonary veins to form short “sleeves” [14]. Ectopic beats originating in the atrial sleeves are implicated as a possible source of atrial fibrillation [15–18].
Myocardium-like tissues were further found in distal regions of extra- and intrapulmonary veins of small rodents [19]. The physiological significance of cardiac muscle-like components in rodent pulmonary veins remains to be determined. A few hypotheses have been proposed, e.g., these muscle cells may function as a “throttle valve” for regulating pulmonary blood flow, and play a role in determining cardiac output [20]. Lineage tracing with in situ hybridization experiments illustrated that the pulmonary vein cardiomyocytes are not descendants of the atrial myocardium, but originated from pulmonary mesenchymal cells [21]. Despite the distinct developmental origins of atrial and pulmonary vein cardiomyocytes, they both exhibit non-pacemaker cell phenotypes [21].
Those previous studies did not characterize the detailed morphological, biochemical, and physiological properties of the thoracic venous cardiomyocytes. Here we examined tissue distribution, ultrastructural features, and expression and developmental regulation of myofilament protein isoforms of mouse thoracic venous cardiomyocytes. The spatial distribution of cardiomyocytes in thoracic veins was traced in transgenic mice expressing a lacZ transgene driven by a cloned cardiac troponin T (TnT) promoter. The venous cardiomyocytes are present in clusters discontinuous from the atrial muscle mass and contain cardiac-specific isoforms of myofilament proteins. The expression patterns of troponin I (TnI) and TnT isoforms in thoracic venous cardiomyocytes are synchronized with that in the heart during postnatal development. Nonetheless, differences in alternative splicing of cardiac TnT were found in the thoracic venous cardiomyocytes and that in atrial and ventricular muscles. The fully differentiated state and intrinsically preset developmental clock in the thoracic venous cardiomyocytes lay a foundation for further understanding their biological properties and potential function.
RESULTS
Scattered Distribution of cardiomyocytes in Mouse thoracic veins
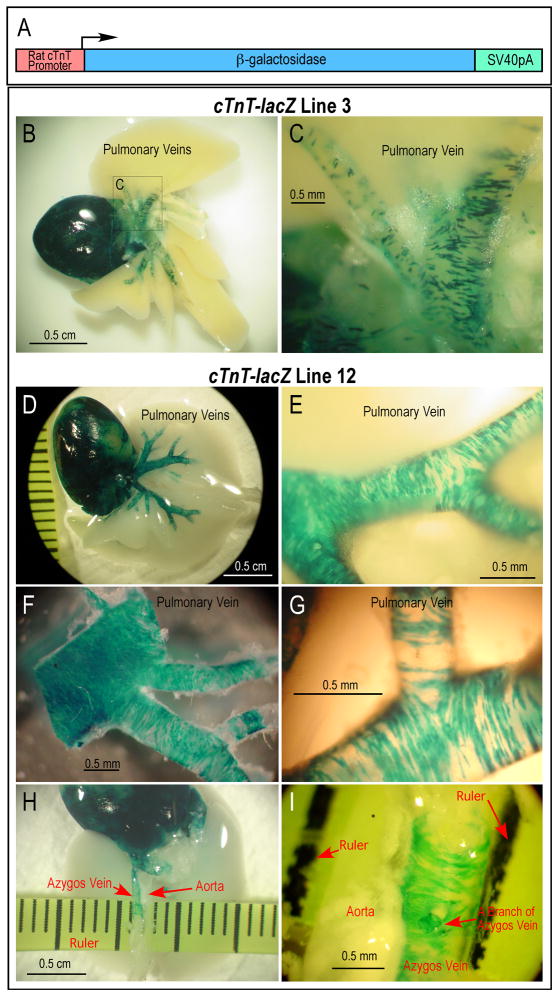
A cTnT-lacZ transgene construct encoding β-galactosidase under the control of a cloned rat cardiac TnT promoter (Fig. 1A) was described previously [22] and used to successfully regenerate four independent transgenic mouse lines. Using two of these mouse lines, we determined the venous distribution of cardiomyocytes by directly visualizing cardiomyocytes in thoracic tissues.
FIGURE 1. Abundant cardiomyocytes scattered in the wall of adult mouse pulmonary and azygos veins.
In tissues from transgenic mouse lines bearing a β-galactosidase reporter gene driven by a cloned cardiac TnT promoter, lacZ activity revealed by X-gal staining of whole mount thoracic tissue blocks demonstrates cardiomyocytes in thoracic veins. A. Schematic illustration of the cardiac TnT promoter-driven lacZ transgene. B, C. Distribution of lacZ-expressing cells in heart and pulmonary veins from the medium-level expression transgenic mouse line 3. D, E, F, G. Distribution of lacZ-expressing cells in pulmonary vein and branches in the high-level expression mouse line 12. F and G are high magnification micrographs of pulmonary vein branches after removing surrounding lung tissue to show the scattered cluster distribution of cardiomyocytes. H and I show the similar distribution of lacZ-expressing cells in azygos vein. The images demonstrate the discontinuity of thoracic venous cardiomyocytes from the atrial myocardium and their alignment perpendicular to the vessel axis.
The medium-level expression transgenic mouse line 3 showed robust and highly specific X-gal staining in the heart, and multiple clusters of X-gal-stained cardiomyocytes in pulmonary veins and their branches (Fig. 1B and C). The high expression transgenic mouse line 12 showed a wider coverage of X-gal staining in the pulmonary veins and branches (Fig. 1D – G). X-gal-stained cardiomyocytes were also clearly seen in the azygos vein (a vein in the systemic circulation, which runs up the right side of the thoracic vertebral column and transporting blood into the superior vena cava), in contrast to the lack of staining in the adjacent descending aorta (Fig. 1H and I). The different levels of lacZ activity in the two mouse lines may be attributable to transgene copy number or chromosomal insertion sites. Nevertheless, both lines showed specific expression in the thoracic venous cardiomyocytes, clearly distinct from the surrounding tissues.
The X-gal-stained whole-mount heart and lung tissue expressing β-galactosidase under the control of cardiac TnT promoter allowed detection of cardiomyocytes in thoracic veins. Similar to previous observations in rats [19], cardiomyocytes distribute deep into the mouse pulmonary vein branches in the lung (Fig. 1). The X-gal-stained cells are distributed in clusters, which was clearly shown even in the high expression mouse line 12. The spatial distribution data from both mouse lines further indicated that the venous cardiomyocytes are largely oriented perpendicularly to the axis of the vessels (Fig. 1C, E, G and I).
In both of the transgenic mouse lines, discontinuity of venous cardiomyocytes from the atrial myocardium, and from each other in distal portions of the main pulmonary veins and in their branches, is readily apparent (Fig. 1). The discontinuation between venous cardiomyocytes and heart muscle mass is in agreement with their divergent developmental origins [21]. This observation is of particular significance by suggesting biological, and potentially functional, differences between these cells and the muscle mass of the heart.
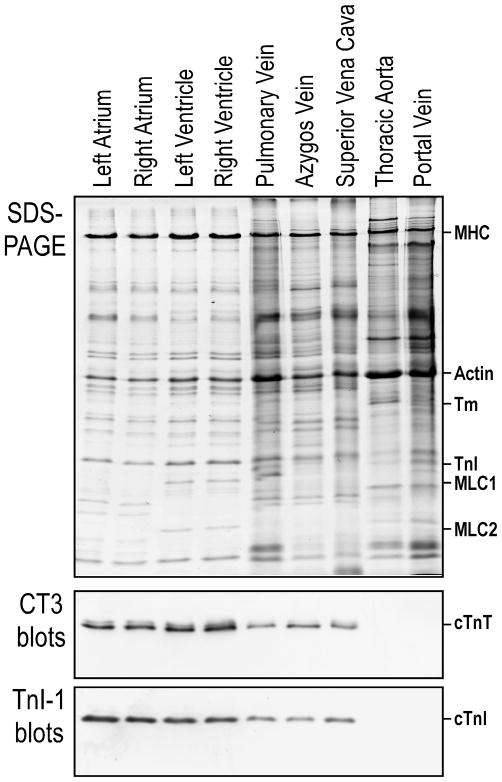
The SDS-PAGE densitometry and Western blot using anti-cardiac TnT mAb CT3 (Fig. 2) detected no significant difference between the transgenic and wild type adult mouse ventricles, atria, pulmonary veins and azygos veins. Therefore, the expression of lacZ in transgenic mice driven by cloned cardiac TnT gene promoter did not alter the expression of endogenous cardiac TnT and the overall protein profiles in the cardiomyocyte-containing tissues.
FIGURE 2. Cardiac TnT gene promoter-directed expression of lacZ in transgenic mice did not affect the expression of endogenous cardiac TnT or the overall protein profile in cardiomyocyte-containing tissues.
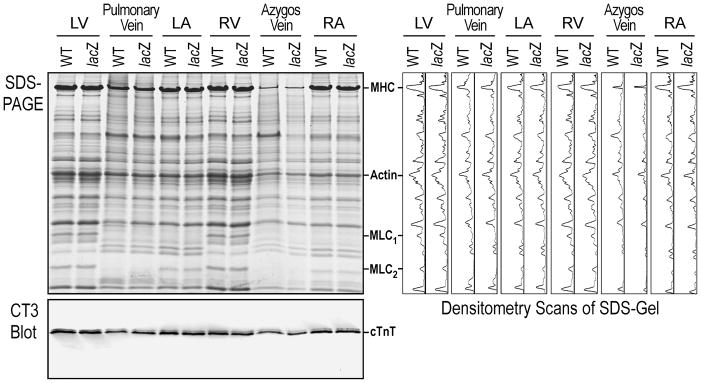
The SDS-PAGE densitometry and Western blot using mAb CT3 detected no significant difference between the transgenic and wild type (WT) adult mouse ventricles, atria, pulmonary vein and azygos vein. LV, left ventricle; RV, right ventricle; LA, left atrium; RA, right atrium; MHC, myosin heavy chain; MLC, myosin light chain; cTnT, cardiac TnT.
The comparisons further showed that the overall protein profiles, such as myosin light chain isoforms, in pulmonary and azygos veins are different from that of either ventricular or atrial tissue (Fig 2), suggesting that the venous cardiomyocytes are of unique differentiation states.
Thoracic venous cardiomyocytes contain typical sarcomeres and are interconnected via intercalated discs
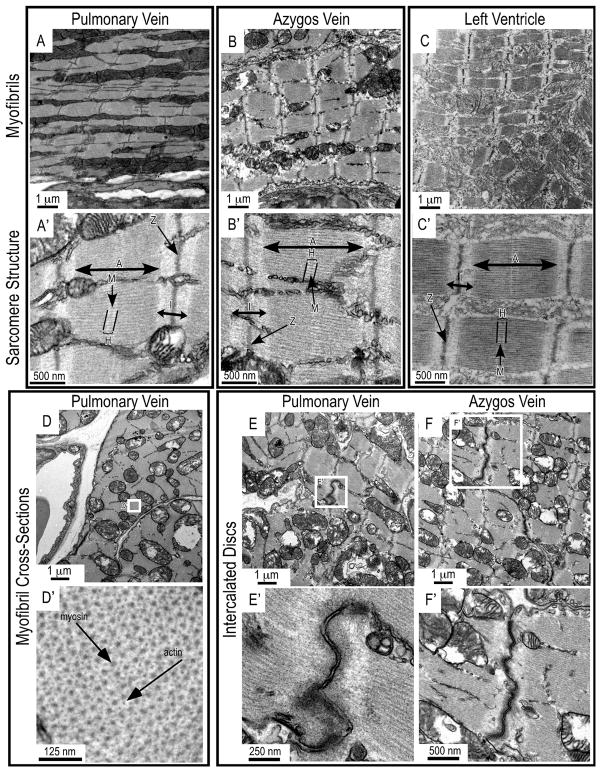
Transmission electron microscopic images revealed the ultrastructure of the striated muscle cells in mouse pulmonary and azygos veins. In the main branches of pulmonary vein and the upper portion of azygos vein of adult mice, myofibrils in these cells resemble those in the ventricular myocytes, and the sarcomere structures in the three tissues are strikingly similar (Fig. 3A–C). These data are consistent with the previously observed composition of cardiomyocyte-like cells in rat pulmonary veins [13, 23, 24].
FIGURE 3. Myofibrils, sarcomeres and intercalated discs in mouse thoracic venous cardiomyocytes.
Transmission electron microscopic images of cardiomyocytes in the main branches of pulmonary vein and the upper portion of azygos vein of adult mice are shown with left ventricular muscle control. Myofibril and sarcomere structures are seen in transverse sections of pulmonary vein (A, A′) and azygos vein (B, B′) with similar, but not identical, appearance when compared with that in ventricular muscle (C, C′). Cross sections showed the normal arrangements of sarcomeric myosin thick and actin thin filaments (D, D′). Intercalated discs are found at the end-to-end junctions of cardiomyocytes in pulmonary vein (E, E′) and azygos vein (F, F′). In the sarcomere structure: A, A-band; I, I-band; H, H-zone; M, M-line.
Cross sections of mouse pulmonary vein myofibrils further showed the typical integrative pattern of myosin thick and actin thin filaments (Fig. 3D). Similar to the myocardium, intercalated discs are seen to interconnect cardiomyocytes in mouse pulmonary and azygos veins (Fig. 3E and F).
High level of cardiac myofilament proteins in adult mouse thoracic veins
Using monoclonal antibodies (mAbs) recognizing cardiac TnT (CT3 [25]) and cardiac TnI (TnI-1 [26]), two myofilament proteins highly specific to adult cardiac muscle [27], the Western blots in Fig. 4 detected high levels of cardiac TnT and cardiac TnI in pulmonary vein, azygos vein, and superior vena cava of adult mouse. In contrast, these cardiomyocyte-specific molecular markers were not detectable in the total protein extracts from thoracic aorta and portal vein (Fig. 4). The high levels cardiac TnT and cardiac TnI in thoracic veins indicated the presence of highly differentiated cardiomyocytes in these non-cardiac tissues.
FIGURE 4. High levels of cardiac TnT and cardiac TnI in mouse thoracic venous tissues.
SDS-PAGE and Western blots using mAbs CT3 and TnI-1 detected cardiac myofilament-specific troponin subunits in mouse thoracic venous vessels, but not thoracic aorta and portal vein. Atrial and ventricular muscles were included as controls. MHC, myosin heavy chain; Tm, tropomyosin; cTnT, cardiac TnT; cTnI, cardiac TnI; MLC, myosin light chain.
Cardiac α-myosin heavy chain (MHC) promoter-directed transgenes are highly expressed in thoracic veins
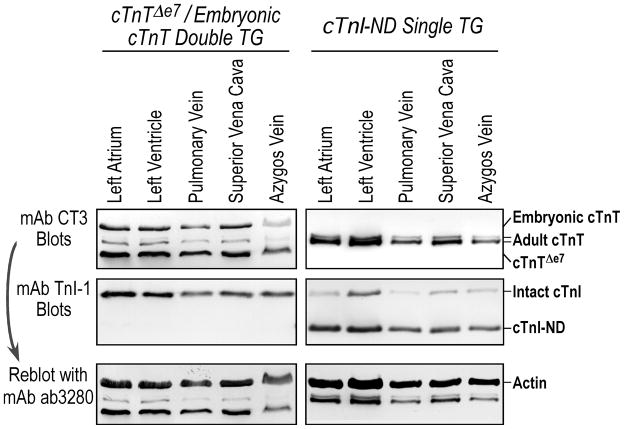
In addition to the data that endogenous cardiac muscle specific genes, i.e., cardiac TnT and cardiac TnI, are expressed in thoracic venous tissues (Fig. 4), we further demonstrated in two previously characterized transgenic mouse lines [28, 29] that three independent cardiac α-MHC promoter-driven transgene alleles are expressed at high levels in thoracic veins. The mAb CT3 immunoblots in Fig. 5 showed that, together with the endogenous cardiac TnT isoforms, the transgene-encoded TnT variants were readily detected in heart as well as in thoracic vein tissue extracts from the transgenic mice. Matching levels of expression were observed in the heart and thoracic veins for each of the two transgene alleles. Similarly, transgene-encoded N-terminally truncated TnI (TnI-ND) was detected in heart and thoracic vein tissue extracts of the transgenic mice as shown in the mAb TnI-1 blot (Fig. 5).
FIGURE 5. Active expression of transgenes driven by the adult cardiac muscle-specific α-MHC promoter in mouse thoracic veins.
The mAb CT3 and mAb TnI-1 Western blots of cardiac muscles and thoracic veins from a single transgenic mouse line bearing an α-MHC promoter-driven transgene encoding N-terminally truncated cardiac TnI (cTnI-ND) and a double transgenic mouse line bearing two α-MHC promoter-driven transgenes encoding splicing variants of cardiac TnT (cTnTΔe7: exon 7-deleted cardiac TnT, eTnT: embryonic cardiac TnT) showed high levels of transgene expression in thoracic veins, similar to that in the heart. The bottom panels are the mAb CT3 blots reprobed with anti-actin mAb ab3280 to verify the amounts of sample loading.
Developmentally regulated expression of troponin and isoforms in thoracic venous cardiomyocytes is in synchrony with that in the heart
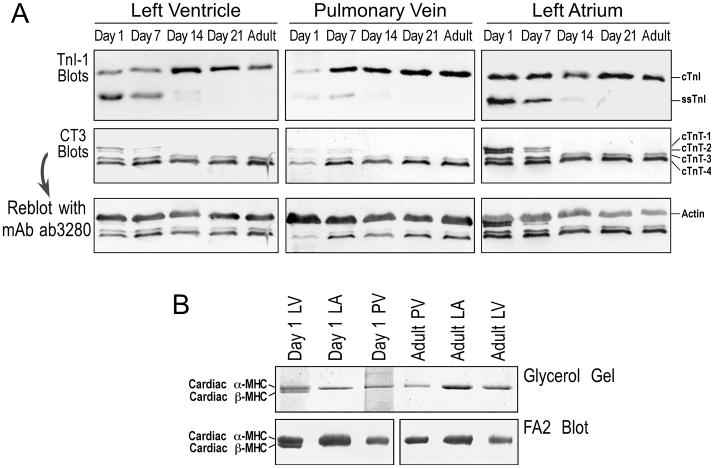
Previous studies have documented that TnI and TnT both undergo isoform switching during postnatal development [30–33]. Our developmental studies found the same slow to cardiac TnI switch during postnatal development in mouse pulmonary vein, occurring in precise synchrony with the switching in the ventricle and atrium (Fig. 6A).
FIGURE 6. Developmental regulation of troponin isoforms in mouse heart and thoracic venous cardiomyocytes.
A. Western blots using mAb TnI-I and mAb CT3 showed that the slow to cardiac TnI gene transition and the alternatively spliced cardiac TnT isoform switching occur in postnatal mouse pulmonary vein, atrium and ventricle in a synchronized manner. The third row panels are the mAb CT3 blots reprobed with anti-actin mAb ab3280 to verify the amounts of sample loading. B. Glycerol-SDS-PAGE and mAb FA2 Western blots detected only α-MHC in adult ventricle, atrium and pulmonary vein as well as neonatal atrium and pulmonary vein, whereas β-MHC was also found in day 1 postnatal ventricular muscle.
Similarly, a developmental high-to-low molecular weight isoform switch of cardiac TnT is found in mouse pulmonary vein, similar to that seen in the heart. Like the TnI isoform switch regulated at the level of gene transcription, the alternative RNA splicing-based regulation of cardiac TnT was also synchronized in the pulmonary vein and ventricular and atrial muscles (Fig. 6A).
Taken together, these data indicate that cardiomyocytes in mouse pulmonary vein possess cardiac-type gene regulation at the levels of both transcriptional control (e.g., the down-regulation of slow TnI gene and up-regulation of cardiac TnI gene) and alternative RNA splicing (e.g., the cardiac TnT variants).
Consistent with the cardiac α-MHC promoter-driven transgene expression in thoracic veins, the highly differentiated cardiac muscle phenotype of the thoracic venous cardiomyocytes was further demonstrated by the result that cardiac α-MHC was the predominant myosin heavy chain in adult mouse pulmonary and azygos veins as revealed by glycerol-SDS-PAGE and mAb FA2 Western blot (Fig. 6B). As in ventricle and atrium, only cardiac α-MHC was detected in the adult mouse pulmonary vein. While both α- and β-isoforms of MHC were found in neonatal ventricle, only α-MHC was detectable in neonatal atrium and pulmonary vein (Fig. 6B). This pattern of MHC isoform expression implies a closer developmental origin of pulmonary vein cardiomyocytes to atrial than to ventricular muscle.
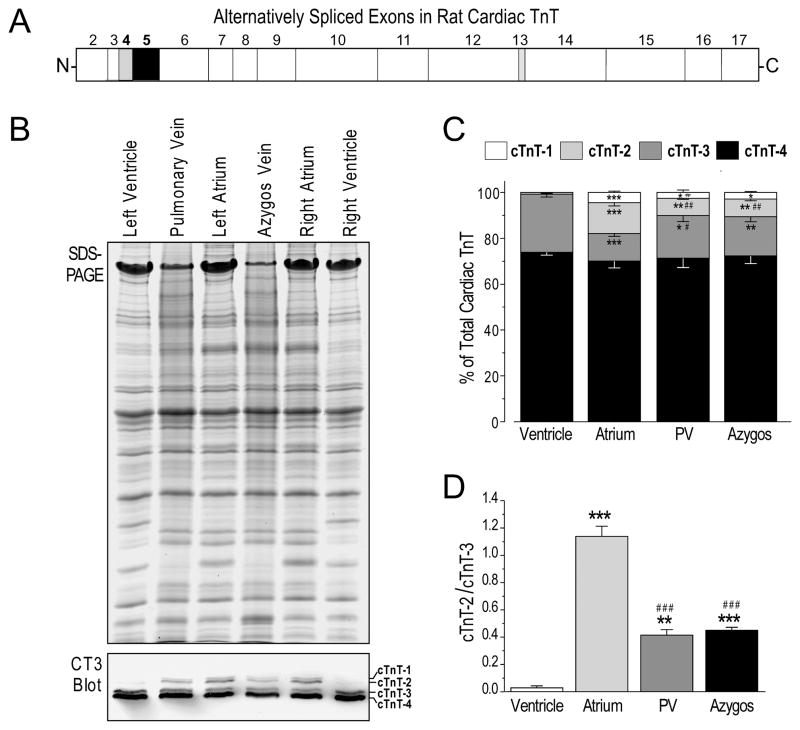
Distinct patterns of cardiac TnT alternative splicing in rat pulmonary vein, azygos vein, atria and ventricles
Taking advantage of the fact that alternative RNA splicing of cardiac TnT is differentially regulated in atria and ventricles of adult rat (Fig. 7), we further investigated similarities and differences between the differentiation states of thoracic venous cardiomyocytes and atrial and ventricular cardiac muscle.
FIGURE 7. The expression of alternatively spliced cardiac TnT isoforms in rat thoracic veins is distinct from that in atrial and ventricular muscles.
A. The regions of rat cardiac TnT encoded by different exons are outlined. Exons 4, 5 and 13 are alternatively spliced (30). While variations at the exon 13-encoded segment do not alter SDS-gel mobility, combinations of exon 4 and 5 splicing generate four protein variants detectable by Western blotting. B. SDS-PAGE gel and mAb CT3 immunoblot of protein extracts from adult (3–4 months old) rat ventricles, atria and thoracic veins detected four alternatively spliced cardiac TnT (cTnT) variants at visibly different ratios. C. Densitometry analysis of multiple copies of CT3 Western blots quantified the distinct ratios among the cTnT splicing variants in ventricular, atrial and thoracic venous tissues. D. The unique pattern of cTnT alternative splicing in thoracic veins is more clearly illustrated with the cTnT-2/cTnT-3 ratio. *, **, ***: P<0.05, P<0.01, and P<0.001, respectively, versus the ventricles, #, ##, ###: P<0.05, P<0.01, and P<0.001, respectively, versus atria, determined by Tukey test. The values are shown as mean ± SE. N = 5 rats for atria or ventricle samples, N = 3 rats for pulmonary vein or azygos vein.
mAb CT3 Western blots in Fig. 7B showed distinct patterns of cardiac TnT splicing in ventricular, atrial, pulmonary and azygos vein tissues of 3–4 months old rats. The two low molecular weight adult cardiac TnT splice forms (cTnT-3 and cTnT-4) are predominant in adult rat ventricles. In addition to cTnT-3 and cTnT-4, the two embryonic cardiac TnT splice forms (cTnT-1 and cTnT-2) [32] are also expressed at significant levels in adult rat atria and pulmonary and azygos veins. Close examination of the relative amounts of the alternative splice forms revealed statistically significant differences between atria and pulmonary and azygos veins. Pulmonary and azygos veins express more cTnT-3 and less cTnT-2 than that in the atria (Fig. 7C). Accordingly, the cTnT-2/cTnT-3 ratio in pulmonary and azygos veins was much lower than that in the atria (Fig. 7D).
While N-terminal variations of TnT are known to have functional significances [34], the relatively small difference in the ratios of alternatively spliced cardiac TnT isoforms in rat ventricular, atrial and thoracic venous cardiomyocytes may not have a large functional impact. However, the clearly detectable differences do indicate differences in differentiation states.
DISCUSSION
The presence of striated muscle cells in thoracic veins has been an intriguing finding from the beginning. Despite many revisits, the developmental origin of these cells, their differentiation state and physiological function are still not yet understood. While most of earlier publications only examined pulmonary vein myocardium, our study also includes cardiomyocytes in superior vena cava and azygos vein. The present study contributes several interesting observations.
Abundance of cardiomyocytes in mouse and rat pulmonary and azygos veins
While α-MHC-lacZ transgenic mice were used previously to show the presence of cardiomyocytes in the pulmonary vein [24], the density and distribution of these cells in the vessel wall were not characterized. Using the lacZ reporter gene driven by cloned cardiac TnT promoter as a visible marker for cardiomyocytes [22], we traced the distribution of cardiomyocytes in pulmonary veins and other thoracic vessels. The results in Fig. 1 demonstrate large numbers of cardiomyocytes that form clusters in the wall of mouse pulmonary and azygos veins. The electron microscopic images also indicated high densities of cardiomyocytes in mouse pulmonary and azygos veins, with interconnections through intercalated discs (Fig. 3). Consistent with the presence of cardiomyocyte-like cells in venules of the rat lung [19], our data detected a significant presence of cells with strong expression of cardiac TnT promoter-directed lacZ expression the branches of mouse pulmonary vein (Fig. 1).
The abundance of cardiomyocytes in the thoracic veins indicates a unique tissue environment that sustains the fully differentiated state of adult cardiomyocytes. The physical proximity of these cells and vascular smooth muscle cells suggests smooth muscle and cardiomyocyte precursors develop adjacent to each other while maintaining their differentiated phenotypes. A previous report demonstrated that exogenously introduced cardiomyocytes could take up residence in a venous smooth muscle environment and retain their contractility [35], thus cues that promote differentiation of the venous cardiomyocytes and the maintenance of their differentiated state may be autonomous in these cells.
Thoracic venous cardiomyocytes are discontinuous from atrial myocardium
The presence of atrial sleeves in the region of thoracic veins adjacent to the atria is well documented [14]. In the two cTnT-lacZ transgenic mouse lines, we traced the distribution of cardiomyocytes in the wall of pulmonary and azygos veins. Mouse line 3 with high cardiac muscle specificity in lacZ expression clearly showed the scattered distribution of cardiomyocytes in the branches of pulmonary vein (Fig. 1C). Whereas mouse line 12 represented a more sensitive detection of cardiomyocytes in the thoracic vessels, the distribution of X-gal-stained cells in the branches of pulmonary vein and azygos vein also showed discontinuation from the atrial muscle mass (Fig. 1E, G and I). Together, the spatial distribution of lacZ-positive cardiomyocytes in the vessel walls convincingly demonstrated the presence of cardiomyocytes in the form of clusters of various sizes discontinuous from the atrial myocardium, most apparently in the distal portion of pulmonary veins and their branches. The discontinuous spatial discontinuity is consistent with the previous notion that myocardial and venous cardiomyocytes have distinct developmental origins [21].
Earlier studies in dog and rabbit indicated an electrical continuum between the left atrium, the proximal pulmonary vein and, possibly, distal regions of pulmonary veins, but their arrhythmogenic contribution was contradictory [36]. Our results demonstrated an histological discontinuity between the cardiomyocytes in the branches of mouse pulmonary veins and the atrial muscle mass, which is also clearly seen in mouse azygos vein (Fig. 1). Therefore, at least in rodents, fully differentiated cardiomyocytes can exist in the wall of thoracic veins separately from cardiomyocytes in the heart. This histological discontinuity would suggest no coordination between heart and thoracic vein electrical activity and contraction, which would, therefore, exclude the hypothesis that the venous cardiomyocytes function in providing a venous anti-backflow valve.
Thoracic venous cardiomyocytes are highly differentiated cardiac muscle cells
Electron microscopic images showed the ultrastructure of mouse thoracic venous cardiomyocytes. The normal appearance of myofibrils, sarcomere structure and myofilament arrangement are similar to that in cardiac muscle (Fig. 3). The presence of intercalated discs between thoracic venous cardiomyocytes further demonstrates the similarities between thoracic venous cardiomyocytes and myocardial myocytes.
The expression of myofilament proteins TnT and TnI are restricted to differentiated striated muscle cells and cardiac TnT and cardiac TnI are established molecular markers for mature cardiac muscle [27]. Western blots using specific mAbs revealed high levels of cardiac isoforms of TnT and TnI in mouse and rat thoracic venous vessels, including pulmonary veins, superior vena cava, and azygos vein (Fig. 4). The Western blots using mAb TnI-1 recognizing all TnI isoforms [26] did not detect fast or slow skeletal muscle TnI in the venous tissue samples (Figs. 4 and 5). Non-thoracic veins, such as the portal vein, and arteries including thoracic aorta have neither TnT nor TnI expression (Fig. 1). These results are consistent with previous histological and ultrastructural studies that indicate the presence of cardiomyocyte-like cells in the pulmonary and azygos veins [24, 37].
The expression of cardiac TnT promoter-driven transgene encoding β-galactosidase in transgenic mice confirms the expression of cardiac muscle-specific genes in thoracic veins (Fig. 1). To further demonstrate the cardiac muscle-specific cellular environment of mouse thoracic venous cardiomyocytes, transgenes constructed using the extensively characterized cardiac α-MHC promoter highly specific to adult cardiac muscle [38] also expressed at high levels in the pulmonary and azygos veins of transgenic mice (Fig. 5).
While previous morphological and ultrastructural data suggest that the striated muscle cells found in thoracic veins are cardiomyocyte-like cells, our data are the first to reveal their cardiomyocyte identity at the level of myofilament proteins. Altogether, the data from gene regulation, cellular environment, and ultrastructure clearly indicate that pulmonary and azygos vein cardiomyocytes have fully differentiated cardiac muscle phenotypes.
Synchronized developmental changes in mouse thoracic venous cardiomyocytes and heart muscle
The results that thoracic venous cardiomyocytes and myocardium show synchronized switching of cardiac TnT and TnI isoforms during postnatal development (Fig. 6) indicate similar postnatal maturation cues. It was previously known that during mouse embryonic development, the cardiomyocytes in pulmonary vein did not arise from migrations of cardiac cells from the developing atrium. Instead, atrial and pulmonary vein cardiomyocytes arise independently of each other before becoming physically adjacent [21]. The similar developmental regulation of cardiac myofilament protein gene expression in the thoracic venous and myocardial cardiomyocytes (Fig 6), however, suggests their common origin from ancestor cells that have committed to the cardiomyocyte lineage. These observed similarities highlight a homologous relationship between myocardial and thoracic venous cardiomyocytes.
Adult thoracic venous cardiomyocytes are distinct from atrial or ventricular cardiomyocytes
Despite the high degree of similarity between the thoracic venous cardiomyocytes and myocardial myocytes, we also found evidence for differences in their structure and differentiation states. For example, the electron microscopic structure data showed that the venous cardiomyocytes have partially parallel fusion of myofibrils (Fig. 3A and B). In comparison to that of ventricular myocytes, there was no significant difference in sarcomere length or A-band and I-band widths (Fig. 3). The expression of alternatively spliced cardiac TnT isoforms in adult rat pulmonary and azygos veins is different from that in the ventricles and atria as shown by their relative levels (Fig. 7).
An earlier study reported at the mRNA level that the pulmonary venous cardiomyocytes had an atrial pattern of cardiac-specific gene expression and suggested that cardiomyocytes migrated into the pulmonary vein from the left atrium [39]. However, more recent studies demonstrated independent developmental origins of atrial and venous cardiomyocytes [21]. Consistently, our present study showed that while several cardiac muscle-specific transgenes examined were all active in thoracic venous cardiomyocytes, the ratios of α-MHC promoter-directed transgene products and the proteins encoded by endogenous cardiac TnT or cardiac TnI genes differ reproducibly across the heart and vessel tissues tested (data not shown). Together with the ultrastructural and alternative splicing differences, the results suggest a distinct differentiation state of the venous cardiomyocytes compared to that of myocardial myocytes.
Similar phenotypes of cardiomyocytes in venous vessels of systemic and pulmonary circulations
A previous study reported that the cardiomyocytes in systemic (superior vena cava and azygos vein) and pulmonary veins are developed from different progenitors (Nkx2.5-negative and Nkx2.5-positive, respectively) [21]. However, our results suggest that their cardiomyocyte contents are identical in development and the differentiated phenotypes as shown by the switching of TnI isoforms (Fig. 6A), cardiac TnT alternative splicing (Fig. 7), and the myosin isoform content (Fig. 6B). The previous observation for the different origins of systemic and pulmonary venous cardiomyocytes was only based on the presence or absence of Nkx2.5. We have reported that deletion of Nkx2.5 binding site from a cardiac TnT promoter did not affect cardiac specific expression [40–42]. Considering the well-accepted combinatory mechanism for transcription factors to control cardiac gene expression, it is not surprising to see the identical phenotype of contractile protein expression in the cardiomyocytes residing in the systemic and pulmonary venous vessels. Nonetheless, our finding that the cardiomyocytes in systemic and pulmonary venous vessels are both distinct from atrial or ventricular myocytes in gene expression phenotypes (Fig. 7) indicated a role of tissue environment in the differentiation of cardiomyocytes.
In conclusion, our study demonstrated that abundant mouse and rat thoracic venous cardiomyocytes residing in an extra-cardiac tissue possess a physiologically differentiated state and an intrinsically preset developmental clock, which are apparently independent of the very different hemodynamic functions of the venous vessels and heart. Besides studies on the role of pulmonary vein and superior vena cava cardiomyocytes in originating atrial fibrillation [15–18, 43], further investigations on cardiomyocytes in thoracic veins will help understand gene regulation, differentiation, and function of cardiomyocytes in health and diseases.
MATERIALS AND METHODS
All experimental protocols using animals were approved by the Institutional Animal Care and Use Committees.
Transgenic mouse lines
For tracing differentiated cardiomyocytes in vivo, four independent transgenic lines carrying a β-galactosidase reporter gene driven by a cloned rat cardiac TnT promoter (cTnT-lacZ) were generated. The transgene was constructed as described previously [22]. Injection of zygotes and embryo implantation into pseudopregnant mothers were done at the Transgenic Animal Facility of the Carver College of Medicine, University of Iowa. Transgenic founders and their progeny were genotyped by PCR using template DNA extracted from tissue biopsies. Two lines (cTnT-lacZ-3 and cTnT-lacZ-12) were used in the present study.
A transgenic mouse line over-expressing an N-terminally truncated cardiac TnI [28] and a double transgenic mouse line over-expressing two cardiac TnT splicing variants [29] were described previously. The transgenes are driven by a cloned α-myosin heavy chain (α-MHC) promoter [38].
X-gal staining of transgenic mouse tissues
Two- to three-month-old cTnT-lacZ transgenic mice were heparinized and euthanized 30 minutes later by intraperitoneal injection of pentobarbital (100 mg/kg). The mouse was placed on a heating pad to maintain body temperature at 37°C. After opening the thorax cavity, the left and right ventricles were cannulated to perfuse systemic and pulmonary circulations simultaneously with filtered Krebs-Henseleit solution (118 mM NaCl, 25 mM NaHCO3, 4.7 mM KCl, 1.2 mM KH2PO4, 2.25 mM MgSO4, 2.25 mM CaCl2, and 11 mM D-glucose, oxygenated with 5% CO2 and 95% O2 at 37°C and pH 7.4) using a peristaltic pump (BioRad, Hercules, CA) at a flow rate of 6.4 mL/min. Blood was flushed out from incisions made in the left and right atria. Perfusion fixation was then carried out with filtered 3.7% formaldehyde in phosphate-buffered saline (PBS) at 37°C and a flow rate of 6.4 mL/min until the visceral tissues hardened. Thereafter, Krebs-Henseleit solution was briefly perfused in to remove excess fixative, before perfusion with X-gal reaction solution (0.1% 5-bromo-4-chloro-indolyl-galactopyranoside (X-gal)/5 mM potassium ferrocyanide/5 mM potassium ferricyanide/0.002% IGEPAL/0.01% sodium deoxycholate in Krebs-Henseleit solution) at 37°C. Color development was monitored and, when necessary, permitted to progress further by dissecting out the heart-lung block and gently agitating it in X-gal reaction solution at 37°C. After three 5-minute rinses in PBS at room temperature, the tissue block was post-fixed in 3.7% formaldehyde in PBS at room temperature for one hour, or at 4°C overnight.
Whole-mount tissues were imaged with a ProgRes C3 camera using ProgRes Mac Capture Pro software (Jenoptik, Jena, Germany), or with a Sony CyberShot 3.3 megapixel camera mounted on a Leica StereoZoom 6 microscope (Wetzlar, Germany).
Transmission electron microscopy
Main branches of pulmonary vein and upper portion of azygos vein were dissected from adult mice. The tissues were fixed in 3% paraformaldehyde/0.2% glutaraldehyde in PBS. Subsequent embedding, sectioning and observation were performed in the Central Microscopy Research Facility at the University of Iowa for transmission electron microscopy analysis using a JEOL JEM-1230 transmission electron microscope.
SDS-polyacrylamide gel electrophoresis (PAGE) and Western blotting
Adult and postnatal developing mice were euthanized and tissue samples were rapidly isolated from left and right atria, left and right ventricles, aortic arch, superior vena cava, pulmonary vein, azygos vein and portal vein.
Adult male Sprague-Dawley rats were euthanized and the heart and thoracic veins were isolated for studies of troponin isoform expression.
The tissues were rinsed in PBS, blotted to remove excess liquid, and snap-frozen on dry ice. Total protein was extracted by homogenization in SDS-PAGE sample buffer containing 2% SDS and 1% β-mercaptoethanol using a high-speed mechanical homogenizer or a high power sonicator. The tissue lysates were stored at −80°C in aliquots. As described previously [44], the SDS-gel sample loading was normalized to the band intensity of either MHC or actin. Duplicate gels were transferred to nitrocellulose membranes for Western analysis using mAb TnI-1 against a conserved C-terminal epitope on troponin I (TnI) [26], mAb CT3 recognizing cardiac TnT and slow skeletal muscle TnT that are distinct in their molecular weights [25], or mAb FA2 against cardiac MHC [45].
After image scanning to document the results, some nitrocellulose membranes probed with mAb CT3 were re-probed using anti-actin antibody to confirm the amount of sample loading. The dried membranes were rehydrated in a blocking buffer containing TBS and 1% BSA at room temperature for 30 min and then incubated with mouse anti-actin mAb ab3280 (Abcam) diluted in TBS containing 0.1% BSA at 4°C overnight. The washing, secondary antibody incubation and substrate reaction were carried out same as described above.
Data analysis
Densitometry analysis of SDS-gel and Western blot was performed on images scanned at 600 dpi. Statistical analysis was performed for all quantitative data to establish significance of differences (P<0.05). Densitometry scans of SDS-PAGE gels using ImageJ software were also compared for the overall protein profiles of mouse atria, ventricles, pulmonary vein and azygos vein extracts.
Acknowledgments
We thank Hui Wang and Geoff Cady for technical assistance. This work was supported by NIH grants AR048816, HL086720, and HL098945 to J-PJ.
References
- 1.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 2.Chien KR, Olson EN. Converging pathways and principles in heart development and disease: CV@CSH. Cell. 2002;110:153–162. doi: 10.1016/s0092-8674(02)00834-6. [DOI] [PubMed] [Google Scholar]
- 3.Claycomb WC. Control of cardiac muscle cell division. Trends Cardiovasc Med. 1992;2:231–236. doi: 10.1016/1050-1738(92)90030-V. [DOI] [PubMed] [Google Scholar]
- 4.MacLellan WR, Schneider MD. Genetic dissection of cardiac growth control pathways. Annu Rev Physiol. 2000;62:289–319. doi: 10.1146/annurev.physiol.62.1.289. [DOI] [PubMed] [Google Scholar]
- 5.Soonpaa MH, Field LJ. Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circ Res. 1998;83:15–26. doi: 10.1161/01.res.83.1.15. [DOI] [PubMed] [Google Scholar]
- 6.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadoshima J, Izumo S. Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine/paracrine mechanism. EMBO J. 1993;12:1681–1692. doi: 10.1002/j.1460-2075.1993.tb05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sedmera D, Pexieder T, Rychterova V, Hu N, Clark EB. Remodeling of chick embryonic ventricular myoarchitecture under experimentally changed loading conditions. Anat Rec. 1999;254:238–252. doi: 10.1002/(SICI)1097-0185(19990201)254:2<238::AID-AR10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 9.Sedmera D, Thompson RP, Kolar F. Effect of increased pressure loading on heart growth in neonatal rats. J Mol Cell Cardiol. 2003;35:301–309. doi: 10.1016/s0022-2828(03)00011-7. [DOI] [PubMed] [Google Scholar]
- 10.Tobita K, Garrison JB, Liu LJ, Tinney JP, Keller BB. Three-dimensional myofiber architecture of the embryonic left ventricle during normal development and altered mechanical loads. Anat Rec A Discov Mol Cell Evol Biol. 2005;283:193–201. doi: 10.1002/ar.a.20133. [DOI] [PubMed] [Google Scholar]
- 11.Brunton TLaFJ. Note on independent pulsation of pulmonary veins and vena cava. Proc Royal Soc London. 1876;25:174–176. [Google Scholar]
- 12.Burch GE, Romney RB. Functional anatomy and throttle valve action on the pulmonary veins. Am Heart J. 1954;47:58–66. doi: 10.1016/0002-8703(54)90211-2. [DOI] [PubMed] [Google Scholar]
- 13.Klavins JV. Demonstration of striated muscle in the pulmonary veins of the rat. J Anat. 1963;97:239–241. [PMC free article] [PubMed] [Google Scholar]
- 14.Nathan H, Gloobe H. Myocardial atrio-venous junctions and extensions (sleeves) over the pulmonary and caval veins. Anatomical observations in various mammals. Thorax. 1970;25:317–324. doi: 10.1136/thx.25.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chard M, Tabrizchi R. The role of pulmonary veins in atrial fibrillation: a complex yet simple story. Pharmacol Ther. 2009;124:207–218. doi: 10.1016/j.pharmthera.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Chen SA, Hsieh MH, Tai CT, Tsai CF, Prakash VS, Yu WC, Hsu TL, Ding YA, Chang MS. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation. 1999;100:1879–1886. doi: 10.1161/01.cir.100.18.1879. [DOI] [PubMed] [Google Scholar]
- 17.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 18.Jais P, Haissaguerre M, Shah DC, Chouairi S, Gencel L, Hocini M, Clementy J. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation. 1997;95:572–576. doi: 10.1161/01.cir.95.3.572. [DOI] [PubMed] [Google Scholar]
- 19.Kramer AW, Jr, Marks LS. The occurrence of cardiac muscle in the pulmonary veins of Rodenita. J Morphol. 1965;117:135–149. doi: 10.1002/jmor.1051170202. [DOI] [PubMed] [Google Scholar]
- 20.Nathan H, Eliakim M. The junction between the left atrium and the pulmonary veins. An anatomic study of human hearts. Circulation. 1966;34:412–422. doi: 10.1161/01.cir.34.3.412. [DOI] [PubMed] [Google Scholar]
- 21.Mommersteeg MT, Brown NA, Prall OW, de Gier-de Vries C, Harvey RP, Moorman AF, Christoffels VM. Pitx2c and Nkx2.5 are required for the formation and identity of the pulmonary myocardium. Circ Res. 2007;101:902–909. doi: 10.1161/CIRCRESAHA.107.161182. [DOI] [PubMed] [Google Scholar]
- 22.Wang Q, Reiter RS, Huang QQ, Jin JP, Lin JJ. Comparative studies on the expression patterns of three troponin T genes during mouse development. Anat Rec. 2001;263:72–84. doi: 10.1002/ar.1078. [DOI] [PubMed] [Google Scholar]
- 23.Ludatscher RM. Fine structure of the muscular wall of rat pulmonary veins. J Anat. 1968;103:345–357. [PMC free article] [PubMed] [Google Scholar]
- 24.Mueller-Hoecker J, Beitinger F, Fernandez B, Bahlmann O, Assmann G, Troidl C, Dimomeletis I, Kaab S, Deindl E. Of rodents and humans: a light microscopic and ultrastructural study on cardiomyocytes in pulmonary veins. Int J Med Sci. 2008;5:152–158. doi: 10.7150/ijms.5.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z, Biesiadecki BJ, Jin JP. Selective deletion of the NH2-terminal variable region of cardiac troponin T in ischemia reperfusion by myofibril-associated mu-calpain cleavage. Biochemistry. 2006;45:11681–11694. doi: 10.1021/bi060273s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin JP, Yang FW, Yu ZB, Ruse CI, Bond M, Chen A. The highly conserved COOH terminus of troponin I forms a Ca2+-modulated allosteric domain in the troponin complex. Biochemistry. 2001;40:2623–2631. doi: 10.1021/bi002423j. [DOI] [PubMed] [Google Scholar]
- 27.Jin JP, Zhang Z, Bautista JA. Isoform diversity, regulation, and functional adaptation of troponin and calponin. Crit Rev Eukaryot Gene Expr. 2008;18:93–124. doi: 10.1615/critreveukargeneexpr.v18.i2.10. [DOI] [PubMed] [Google Scholar]
- 28.Barbato JC, Huang QQ, Hossain MM, Bond M, Jin JP. Proteolytic N-terminal truncation of cardiac troponin I enhances ventricular diastolic function. J Biol Chem. 2005;280:6602–6609. doi: 10.1074/jbc.M408525200. [DOI] [PubMed] [Google Scholar]
- 29.Biesiadecki BJ, Elder BD, Yu ZB, Jin JP. Cardiac troponin T variants produced by aberrant splicing of multiple exons in animals with high instances of dilated cardiomyopathy. J Biol Chem. 2002;277:50275–50285. doi: 10.1074/jbc.M206369200. [DOI] [PubMed] [Google Scholar]
- 30.Jin JP. Alternative RNA splicing-generated cardiac troponin T isoform switching: a non-heart-restricted genetic programming synchronized in developing cardiac and skeletal muscles. Biochem Biophys Res Commun. 1996;225:883–889. doi: 10.1006/bbrc.1996.1267. [DOI] [PubMed] [Google Scholar]
- 31.Jin JP, Lin JJ. Rapid purification of mammalian cardiac troponin T and its isoform switching in rat hearts during development. J Biol Chem. 1988;263:7309–7315. [PubMed] [Google Scholar]
- 32.Jin JP, Lin JJ. Isolation and characterization of cDNA clones encoding embryonic and adult isoforms of rat cardiac troponin T. J Biol Chem. 1989;264:14471–14477. [PubMed] [Google Scholar]
- 33.Saggin L, Gorza L, Ausoni S, Schiaffino S. Troponin I switching in the developing heart. J Biol Chem. 1989;264:16299–16302. [PubMed] [Google Scholar]
- 34.Wei B, Jin JP. Troponin T isoforms and posttranscriptional modifications: Evolution, regulation and function. Arch Biochem Biophy. 2011;505:144–154. doi: 10.1016/j.abb.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai W, Hale SL, Kloner RA. Development of a spontaneously beating vein by cardiomyocyte transplantation in the wall of the inferior vena cava in a rat: a pilot study. J Vasc Surg. 2007;45:817–820. doi: 10.1016/j.jvs.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang TM, Chiang CE, Sheu JR, Tsou CH, Chang HM, Luk HN. Homogenous distribution of fast response action potentials in canine pulmonary vein sleeves: a contradictory report. Int J Cardiol. 2003;89:187–195. doi: 10.1016/s0167-5273(02)00474-6. [DOI] [PubMed] [Google Scholar]
- 37.Cullinan V, Campbell JH, Mosse PR, Campbell GR. The morphology and cell culture of the striated musculature of the rat azygos vein. Cell Tissue Res. 1986;243:185–191. doi: 10.1007/BF00221867. [DOI] [PubMed] [Google Scholar]
- 38.Subramaniam A, Jones WK, Gulick J, Wert S, Neumann J, Robbins J. Tissue-specific regulation of the alpha-myosin heavy chain gene promoter in transgenic mice. J Biol Chem. 1991;266:24613–24620. [PubMed] [Google Scholar]
- 39.Jones WK, Sánchez A, Robbins J. Murine pulmonary myocardium: developmental analysis of cardiac gene expression. Dev Dyn. 1994;200:117–128. doi: 10.1002/aja.1002000204. [DOI] [PubMed] [Google Scholar]
- 40.Wang Q, Sigmund CD, Lin JJ. Identification of cis elements in the cardiac troponin T gene conferring specific expression in cardiac muscle of transgenic mice. Circ Res. 2000;86:478–484. doi: 10.1161/01.res.86.4.478. [DOI] [PubMed] [Google Scholar]
- 41.Wang Q, Li-Chun Lin J, Jung-Ching Lin J. A novel TCTG(G/C) direct repeat and an A/T-rich HMG2-binding site control the expression of the rat cardiac troponin T gene. J Mol Cell Cardiol. 2002;34:1667–79. doi: 10.1006/jmcc.2002.2116. [DOI] [PubMed] [Google Scholar]
- 42.Harlan SM, Reiter RS, Sigmund CD, Lin JL, Lin JJ. Requirement of TCTG(G/C) Direct Repeats and Overlapping GATA Site for Maintaining the Cardiac-Specific Expression of Cardiac troponin T in Developing and Adult Mice. Anat Rec (Hoboken) 2008;291:1574–1586. doi: 10.1002/ar.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin WS, Prakash VS, Tai CT, Hsieh MH, Tsai CF, Yu WC, Lin YK, Ding YA, Chang MS, Chen SA. Pulmonary vein morphology in patients with paroxysmal atrial fibrillation initiated by ectopic beats originating from the pulmonary veins: implications for catheter ablation. Circulation. 2000;101:1274–81. doi: 10.1161/01.cir.101.11.1274. [DOI] [PubMed] [Google Scholar]
- 44.Feng HZ, Wei B, Jin JP. Deletion of a genomic segment containing the cardiac troponin I gene knocks down expression of the slow troponin T gene and impairs fatigue tolerance of diaphragm muscle. J Biol Chem. 2009;284:31798–31806. doi: 10.1074/jbc.M109.020826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin JP, Malik ML, Lin JJ. Monoclonal antibodies against cardiac myosin heavy chain. Hybridoma. 1990;9:597–608. doi: 10.1089/hyb.1990.9.597. [DOI] [PubMed] [Google Scholar]