Abstract
Whispering gallery mode resonators are small, radially symmetric dielectrics that trap light through continuous total internal reflection. The resonant condition at which light is efficiently confined within the structure is linked with refractive index, which has led to the development of sensitive label-free sensing schemes based on whispering gallery mode resonators. One resonator design uses inexpensive high index glass microspheres that offer intrinsically superior optical characteristics, but have proven difficult to multiplex and integrate with the fluidics for sample delivery and fluid exchange necessary for assay development. Recently, we introduced a fluorescence imaging approach that enables large scale multiplexing with microsphere resonators, thus removing one obstacle for assay development. Here we report an approach for microsphere immobilization that overcomes limitations arising from their integration with fluidic delivery. The approach is an adaptation of a calcium-assisted glass bonding method originally developed for microfluidic glass chip fabrication. Microspheres bonded to glass using this technique are shown to be stable with respect to fluid flow and show no detectable loss in optical performance. Measured Q-factors, for example, remain unchanged following sphere bonding to the substrate. The stability of the immobilized resonators is further demonstrated by transferring lipid films onto the immobilized spheres using the Langmuir-Blodgett technique. Bilayers of DOPC doped with GM1 were transferred onto immobilized resonators to detect the binding of cholera toxin to GM1. Binding curves generated from shifts in the whispering gallery mode resonance result in a measured Kd of 1.5 × 10−11 with a limit of detection of 3.3 pM. These results are discussed in terms of future assay development using microsphere resonators.
Introduction
Whispering gallery mode (WGM) resonators are small dielectric structures that confine light via continuous total internal reflection. Light evanescently coupled into WGM resonators can be efficiently trapped when the wavelength of light is an integer multiple of the distance circumnavigated around the resonator.1–4 Under these conditions, constructive interference leads to WGM resonances given by
| Eq. 1 |
where λr is the resonant wavelength, r is the radius of the resonator, neff is the effective refractive index, and m is an integer that indexes the mode number.2, 3
The small size and high quality factors (Q-factors) of WGM resonators have led to widespread studies in both fundamental and applied applications. These attributes seem especially well-suited for sensing applications where their small footprint and large Q-factor naturally lend themselves to the developments taking place in miniaturized detection platforms.1, 4, 5 Sensing applications with whispering gallery mode resonators take advantage of the link between effective refractive index and resonant wavelength as shown in Eq. 1.4–7 Binding of target analytes to recognition elements on the resonator surface can alter the effective refractive index, leading to a shift in the WGM resonant wavelength.7–9 This provides a sensitive, label-free approach for sensing which has been used to detect and quantify protein and nucleic acid biomarkers of disease.7–11
Whispering gallery mode biosensing platforms are predominantly built around either high index glass microsphere resonators or microfabricated planar ring resonators.12–15 The former uses commercially available or easily fabricated microspheres while the latter is produced on-chip using standard microfabrication techniques.12, 13 Microfabricated planar ring resonators have demonstrated multiplexing capabilities and are easily incorporated with the fluidics necessary for sample handling and delivery.16 Microsphere resonators, on the other hand, have historically suffered by comparison in these metrics but offer superior optical characteristics.17 Microspheres are formed from melts which yield exquisitely smooth surfaces, leading to Q-factors which can be orders of magnitude larger than other resonator designs.2, 14, 18 Large Q-factors translate into long effective path lengths which improves both the sensitivity and limits of detection, both of which are obviously desirable in sensing applications.4, 19 Moreover, microspheres are inexpensive, commercially available, and are offered in a range of sizes and materials.
For sensing, therefore, it is highly desirable to combine the multiplexing and fluidics capabilities of microring resonators with the optical properties of microsphere resonators. Recently, we reported a fluorescence imaging approach that enables the WGM resonances from each microsphere in a field of resonators to be simultaneously measured.7, 11 This enables large scale multiplexing which removes one of the barriers listed above when using microspheres in sensing applications.
In this approach, light is coupled into a large number of microspheres using the evanescent field created from total internal reflectance in a Dove prism. Each microsphere is labeled with the same fluorescent dye, which acts as marker signaling when a particular resonator comes into resonance. As the excitation wavelength from a tunable diode laser is scanned, a ring of enhanced fluorescence is observed around a microsphere when a WGM resonance is reached. This enables the WGM resonance from each sphere in a large field of view to be simultaneously measured using fluorescence imaging.7, 11
While this approach enables easily multiplexed WGM detection, developing sensing platforms remains problematic due to challenges associated with immobilizing microspheres. Typical biosensing assays require multiple fluid exchanges or mixing steps which can perturb spheres resting on a substrate through gravity alone. This creates problems since the resonant wavelength of a resonator is linked to the axis around which the WGM resonates.3, 5, 20 Any change in the WGM path around the sphere due to reorientations on the substrate, therefore, can shift the resonant wavelength and nullify an assay.
Immobilizing microsphere resonators on a substrate, however, presents challenges for WGM sensing since the circumference of the sphere supporting the resonance must remain pristine. The fluorescence imaging scheme enabling large scale multiplexing mentioned above requires that light be launched into spheres from a common substrate. This precludes embedding the spheres in adhesives or other films that would disrupt the coupling of light around the sphere. One can envision loading spheres into microfabricated arrays of wells to hold them in place, but this turns out to be problematic in practice. First, the poor monodispersity of spheres complicates matching well size with sphere dimensions for stable immobilization. The larger issue, however, arises from complications associated with efficiently coupling light into the immobilized spheres. It is very difficult to load arrays with spheres such that they are both tightly held and in good contact with the substrate, which is necessary for efficient coupling of light into the resonator through evanescent field excitation.
Here we report a method for immobilizing microsphere resonators on glass substrates adapted from a calcium-assisted glass-to-glass bonding method developed for microfluidic glass chip fabrication.21 The method creates a stable contact between the high index glass microsphere resonators and a glass substrate, enabling efficient coupling of light into the immobilized microspheres. Measurement of resonator Q-factors confirms that the immobilization method does not degrade the WGM resonance or greatly perturb the interface between the sphere and the substrate. To demonstrate the robustness of the immobilized microresonators, lipid bilayers were transferred onto the substrate bound microspheres using a combination of the Langmuir-Blodgett (LB) and Langmuir Schaeffer (LS) techniques. Bilayers of the lipid DOPC doped with 5 mol% ganglioside GM1 were formed using this sequential transfer method where each leaflet was transferred at 25 mN/m. GM1 binds cholera toxin, the oligomeric protein secreted by Vibrio cholera which causes the dehabilitating diarrhea associated with cholera.22 Here we demonstrate the sensing capabilities of the bilayer draped over immobilized microspheres by tracking changes in their WGM resonant wavelength upon the addition of cholera toxin. Analysis of the resulting binding curves yields a measured Kd of 1.5 × 10−11, consistent with literature values, and a detection limit of 3.3 pM.23, 24 The reported bonding scheme, therefore, is shown not to perturb the optical properties of the resonators while immobilizing them sufficiently onto the substrate for assay development and implementation.
Results and Discussion
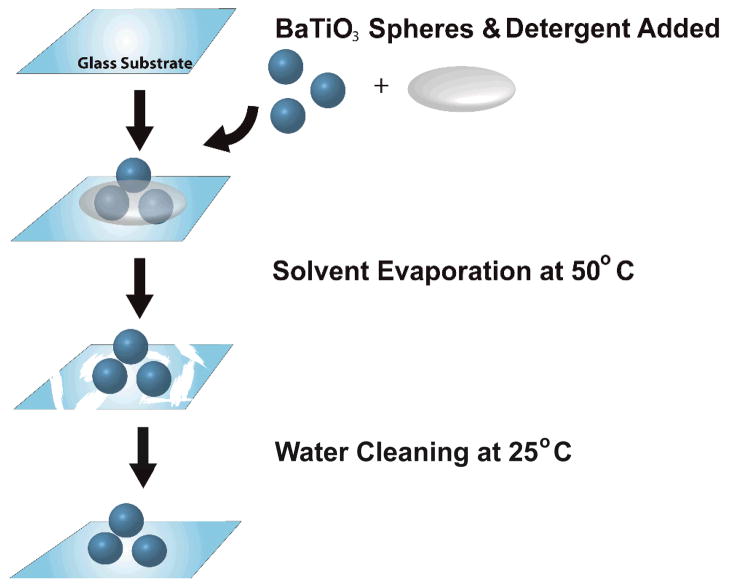
High index barium titanate (BaTiO3) microspheres were bonded to glass substrates using the steps outlined schematically in Fig. 1. A glass substrate was first rubbed with a slurry of basic detergent (Alconox) containing 8.0 mM Ca2+. The spheres were then deposited on the glass substrate and incubated at 50°C for approximately 20 minutes until dry. The spheres, now bonded to the substrate, were incubated in 18 MΩ water overnight to remove the residual salts and unbound spheres from the substrate surface.
Figure 1.
Schematic of the calcium-assisted procedure for immobilizing WGM microsphere resonators to a glass substrate. A drop containing 0.125% (v/v) Alconox and 0.125% (v/v) calcium acetate solution is placed along with a cluster of microspheres onto a clean glass substrate. The sample is then placed in a 50°C oven for 20 minutes. Evaporation of the solvent leaves a salt residue which is removed by bathing in deionized water overnight at room temperature, leaving a clean glass substrate with microspheres bound to the surface.
Conceptually, one can envision the divalent calcium ion as acting as a bridge between the negatively charged surface groups on the microspheres and substrate, thus leading to stable bond formation.21 However, as the original report showed, a more complicated mechanism is almost certainly needed since other divalent cations do not lead to bond formation. Regardless of the mechanism, this approach does successfully immobilize BaTiO3 spheres on the glass substrate. The bonding scheme outlined in Fig. 1 uses a lower temperature, which was found in the original study to lead to reversible bond formation. However, as will be shown, this gentler bonding method is more than sufficient to immobilize spheres for the assay employed in this report. Additionally, this immobilization method can be used to adhere dye functionalized spheres without loss of dye function. This is advantageous for the WGM imaging approach which uses fluorescence to detect sphere resonances.
Another advantage of the microsphere bonding scheme results from the flexibility inherent in this approach when designing assays. Immobilizing microsphere resonators onto a substrate opens new opportunities for functionalizing the resonators using techniques that are not compatible for use with free spheres. For example, Langmuir-Blodgett (LB) and related techniques offer extraordinary capabilities for creating highly ordered films on substrate surfaces. These techniques provide exquisite layer-by-layer control over film properties such as composition, packing, and constituent orientation which has generated interest in using this control to tailor assay properties.25, 26 As illustrated in Fig. 2, the LB method involves the transfer of films from an air-water interface onto a substrate surface using a dipping method. As suggested in Fig. 2, here we show that the bonded spheres are sufficiently immobilized to withstand LB film transfer, thus creating new opportunities for assay development using microsphere resonators.
Figure 2.
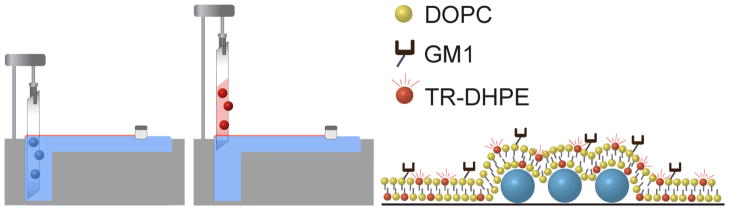
Schematic of the Langmuir-Blodgett trough used to transfer lipid films onto a substrate. A 50 uL aliquot of the lipid solution is dispersed on the water subphase and a moving barrier compresses the film to the desired surface pressure. A glass substrate with bonded microspheres is slowly pulled through the air-water interface, transferring a lipid monolayer onto the substrate surface. For these experiments, a second monolayer is transferred onto the first using the Langmuir-Schaffer technique, creating a bilayer as shown schematically in the right panel.
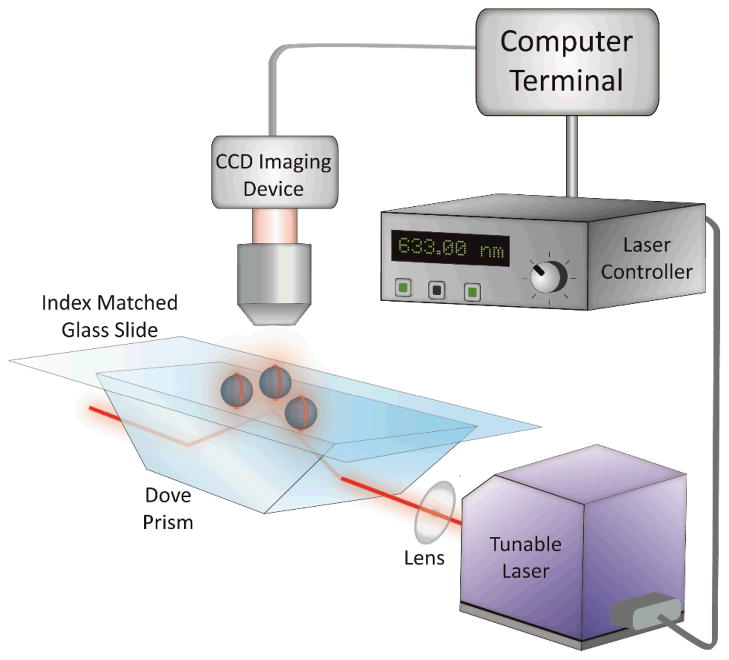
To confirm that the immobilization procedure does not interfere with or degrade WGM resonances of the microspheres, fluorescence imaging experiments were first performed using LB films doped with a fluorescent marker. For these experiments, immobilized microspheres were coated with a DOPC bilayer containing 0.25 mol% TR-DHPE, transferred using the LB/LS method. The fluorescent lipid analog, TR-DHPE, was added to act as a fluorescent reporter of the WGM resonances. The immobilized spheres were mounted on a Dove prism, as shown in Fig. 3, where light from a tunable diode laser experiences total internal reflection at the sample interface. As the wavelength of the diode laser was scanned, the associated evanescent field launches light into the immobilized spheres. WGM resonances are detected as an enhanced ring of fluorescence around the particular microsphere resonator, which is detected from above using fluorescence imaging. The fluorescence was collected and imaged onto a CCD camera as shown in Fig. 3.
Figure 3.
Schematic of the instrumentation used for the fluorescence imaging of WGM resonances. Light from a tunable diode laser is directed through a Dove prism, which creates an evanescent field at the substrate interface. The evanescent field couples light into the immobilized microspheres and WGM resonances are detected through fluorescence imaging of a dye marker located on their surface. As the excitation wavelength is tuned, an enhanced ring of fluorescence is observed around the spheres as a WGM resonance is reached. The fluorescence is collected and imaged onto a CCD camera.
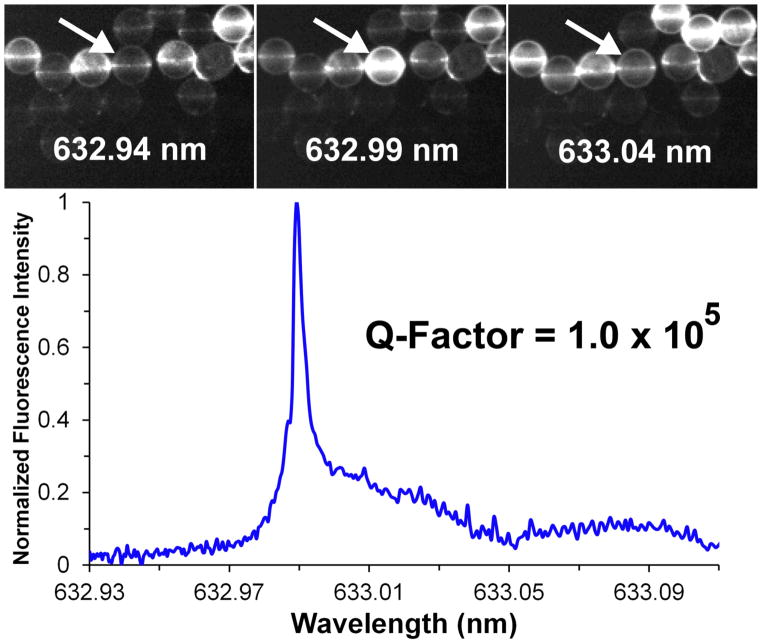
Figure 4 shows a typical series of fluorescence images taken on the same field of microspheres as the excitation wavelength from the tunable diode laser is scanned. These spheres have been immobilized onto the substrate surface using the scheme in Fig. 1 and exhibit bright fluorescence rings indicative of WGM resonances. The excitation spectrum for each resonator can be extracted from a series of fluorescence images collected as a function of excitation wavelength. A typical excitation spectrum is shown in Fig. 4. The Q-factor calculated from this spectrum is 1.0 × 105 which is comparable to measured Q-factors for spheres resting on substrates through gravity alone. This demonstrates that the immobilization process has little effect on the quality of the WGM resonance.
Figure 4.
(top) Representative fluorescence images of 45 μm spheres extracted from a series of images taken as the wavelength of the diode laser is scanned between 632.93 nm and 633.10 nm. The arrow denotes a sphere that undergoes a large change in fluorescence, indicating a WGM resonance near 632.99 nm. (bottom) Excitation spectrum of the WGM resonance for the indicated sphere, extracted from the series of fluorescence images by integrating the emission from the sphere in each image.
Having shown that the immobilization procedure does not degrade resonator optical properties, the utility of membrane coated microspheres for bioassay development was explored. Bioassays require rapid fluid exchanges and the immobilized resonators need to maintain both close contact with the substrate surface and not reorient during these processes. To test the stability of the immobilized microspheres during rapid fluid exchanges, the flow cell in Fig. 5 was fabricated.
Figure 5.
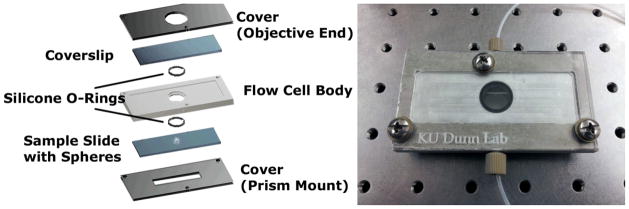
(left) Schematic of the flow cell used for fluidic exchange and WGM imaging. The bottom cover accepts the Dove prism used to launch light into the small resonators which are imaged from above through the transparent window. An image of the flow cell is shown on the right.
The glass substrate supporting the immobilized microspheres rests on a rigid bottom plate that is notched to accept the Dove prism. An aluminum flow cell body that contains the solution inlet and outlet ports is sandwiched between the sample substrate and top coverslip using two silicone o-rings to form a water tight seal. The thickness of the flow cell body dictates the dead volume of the cell which is 500 uL. Screws connect a rigid top cover with the bottom PMMA plate and compress the o-rings. An image of the assembled flow cell is shown in Fig. 5.
Studies using the flow cell shown in Fig. 5 indicate that the immobilized spheres remain stable at all flow rates studied (up to a maximum flow of 3 mL/min). This demonstrates that the bonding method described above results in resonators compatible with assay fluidics. As shown above, the resonator immobilization scheme does not degrade resonator optical properties while rigidly holding the spheres on the substrate surface under high flow rates. This approach, therefore, removes a significant barrier encountered when integrating microsphere resonators with the fluidics necessary for assay development using the WGM imaging method.
To illustrate this new capability, lipid films containing the ganglioside GM1 were transferred onto immobilized microsphere resonators to detect the presence of cholera toxin (CTx) using WGM imaging. CTx is secreted by Vibrio cholera and leads to the debilitating diarrhea associated with cholera infection. CTx is a 85 kDa protein containing one active alpha subunit and five binding beta subunits which bind GM1, an acidic glycosphingolipid found ubiquitously in the outer leaflet of cellular membranes.22 The high affinity of CTx beta subunit (CTxB), a 11.4 kDa protein, for GM1 is considered a model for protein-sugar interactions with measured Kd values ranging from nanomolar to picomolar depending on the particular system studied.24 In this approach a 57 kDa non-toxic pentameric CTxB fluorescently labeled with Alexa 555 (CTxB-A555) was detected.
A monolayer of DOPC containing 5 mol% GM1 was transferred onto a microsphere/substrate platform at a surface pressure of 25 mN/m using the LB method. A second monolayer at the same surface pressure and composition was deposited on the first using the Langmuir Schaeffer (LS) method, creating a bilayer covering the entire surface area of the substrate. At this surface pressure, both GM1 and the dye marker distribute homogeneously in the DOPC lipid film matrix. To confirm that the membranes incorporating GM1 were capable of CTxB binding, bulk fluorescence imaging of the bilayers were measured following the addition of fluorescently labeled CTxB-A555.
Figure 6 presents fluorescence images of a DOPC/GM1 bilayer following increasing additions of CTxB-A555. The images are taken in the same region of the bilayer, which was allowed to react with the indicated dose of CTxB-A555 for 5 minutes, flushed with PBS buffer, and imaged. As seen in Fig. 6, exposure of the bilayer to increasing aliquots of CTxB-A555 leads to increased fluorescence intensity, as CTxB-A555 binds to the GM1. Control studies using substrates coated with pure DOPC bilayers, lacking GM1, exhibited constant fluorescence signals over the same CTxB-A555 dosing levels. This suggests that the increase in fluorescence observed arises from specific interactions between GM1 and the CTxB-A555.
Figure 6.
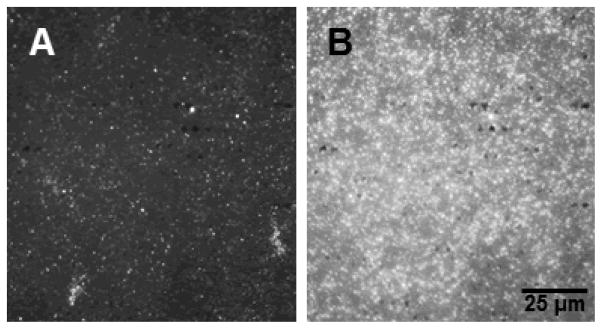
Bulk fluorescence assay of the binding of fluorescently labeled CTxB (CTxB-A555) to DOPC/GM1 bilayers transferred onto a glass substrate at 25 mN/m. Fluorescence images of the same region of the bilayer are shown following incubation with (A) 27.6 pM CTxB-A555 and (B) 110 pM CTxB-A555. The increase in fluorescence indicates specific binding of CTxB-A555 at GM1 sites in the bilayer. Control studies of bilayers composed of DOPC but lacking GM1 (not shown) do not show any significant fluorescence after incubation with CTxB-A555.
Having confirmed that CTxB-A555 specifically binds to the GM1 in the transferred membranes, studies were carried out to explore the WGM response of the membrane coated microspheres. For this study, TR-DHPE was incorporated into the lipid bilayer at 0.25 mol% to provide a fluorescence marker for the WGM imaging. To ensure consistency with the bulk fluorescence studies, the fluorescently labeled CTxB-A555 was also used in these binding studies. However, long pass filters were inserted to remove any residual fluorescence from the A555 marker. Using the WGM fluorescence imaging approach outlined in Fig. 3, WGM excitation spectra were collected as a function of CTxB-A555 dose. Figure 7 shows a typical series of WGM excitation spectra collected as a function of CTxB-A555 concentration. Prior to the addition of CTxB-A555, this particular microsphere resonator had a WGM resonance centered at 632.98 nm with a Q-factor of 1.0 × 105. With the addition of 2.8 pM CTxB-A555, the peak red-shifts 6.33 pm as CTxB-A555 binds to the GM1/DOPC bilayer on the sphere surface which changes the effective refractive index around the resonator (Eq. 1). The peak continues to red shift as additional CTxB-A555 is added as shown in Fig. 7.
Figure 7.
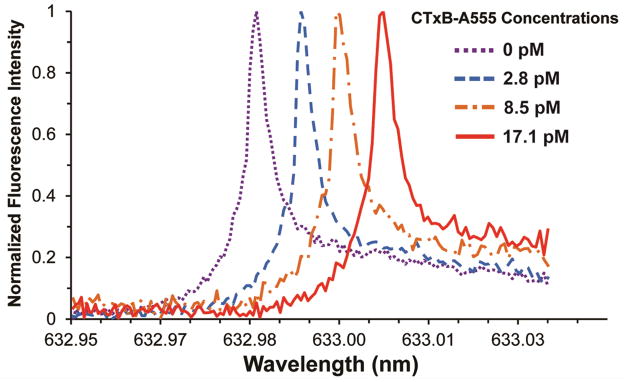
Representative WGM excitation spectra extracted from the same microsphere resonator at four different CTxB-A555 concentrations. Specific binding of CTxB-A555 to the GM1 containing bilayer coated around the microsphere, changes the effective refractive index and shifts the WGM resonance.
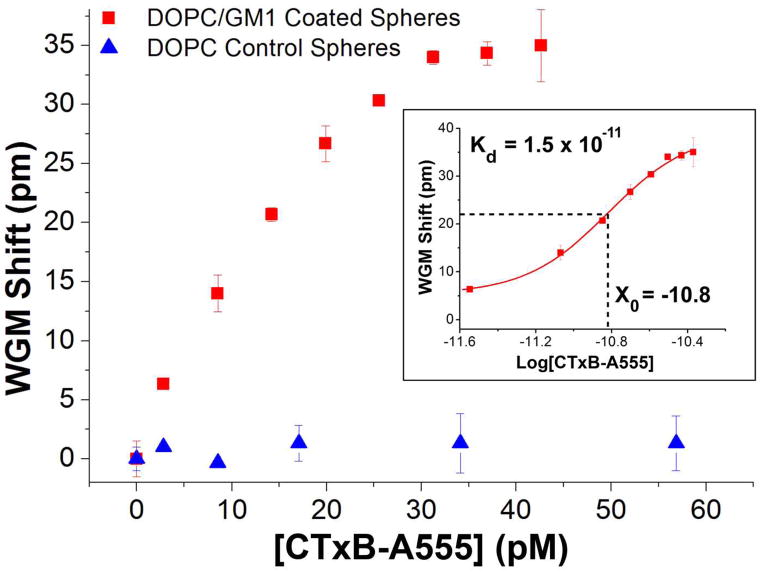
The results in Fig. 7 are summarized by the binding curve shown in Fig. 8. Figure 8 plots the WGM resonance shifts as a function of CTxB-A555 concentration, exhibiting the classic binding curve shape expected. The binding curve saturates at approximately 4.0 × 10−11 M CTxB-A555 and fitting the curve yields a measured Kd value of 1.5 × 10−11. This value is in the range of the reported values (7.3 × 10−10 to 4.6 × 10−12) measured using surface plasmon resonance.23, 24
Figure 8.
CTxB-A555 binding curves measured by tracking shifts in the WGM resonance with CTxB-A555 concentration. For resonators coated with DOPC bilayers incorporating GM1 (red squares) the binding curve shows a saturation response with a detection limit of 3.3 pM. This is compared with control studies using spheres coated with pure DOPC bilayers (blue triangles), which show negligible shifts with CTxB-A555 addition. The inset shows a log plot of the data from which a Kd of 1.5 × 10−11 is calculated, which is consistent with literature values.
These measurements show that microspheres can be efficiently immobilized on glass substrates using the calcium bonding method with no loss in optical performance. The spheres remain stable under fluid exchange and the bond is sufficiently strong to enable lipid film transfer onto their surface using the LB/LS technique. Using bilayers incorporating GM1 transferred onto the spheres, we show that WGM fluorescence imaging measurements can quantify CTxB-A555 binding with picomolar detection limits. These studies, therefore, illustrate the utility of the calcium bonding approach for integrating high-Q microsphere resonators with the fluidics necessary for assay development. The simple immobilization procedure does not require expensive equipment or elaborate fabrication processes, thus making the approach generally applicable. This, therefore, remove a significant barrier for the development of sensitive, multiplexed biosensors using the superior optical properties of high-Q microsphere resonators.
Experimental
Microsphere Immobilization
High refractive index (n = 1.9), barium titanate (BaTiO3) glass microspheres (45 μm ± 7% diameter, Mo-Sci, Rolla, MO) were cleaned in a 5% Contrad solution. The spheres were rinsed in an ethanol/water (30/70 v/v) solution and stored in absolute ethanol. Spheres were exchanged into an aqueous PBS solution prior to use. A glass bonding solution was prepared with 0.125% w/v calcium acetate (Fisher Scientific, Hampton, NH) and 0.125% w/v powdered detergent (Alconox Inc., White Plains, NY) in nanopure H2O. As shown in Fig. 1, approximately 100 uL of the bonding solution was placed on a clean glass cover slip (Fisher Scientific, Pittsburg, PA) and allowed to deprotonate the glass surface for ~5 minutes. Approximately 5 μL of the clean microspheres in PBS solution were transferred to the sample slide and allowed to dry for 20 minutes at 50°C. The sample slide was washed thoroughly with nanopure H2O to remove any unbound spheres and excess salts from the surface prior to monolayer transfer.
Monolayer Transfer
Dioleoylphosphatidylcholine (DOPC) and ganglioside GM1 (GM1) were obtained at >99% purity (Avanti Polar Lipids, Alabaster, AL) and used without further purification. The fluorescent lipid probe, Texas-Red dihexadecanoyl-sn-glycero-3-phosphoethanolamine (TR-DHPE) (Life Technologies, Carlsbad, CA), was diluted in methanol to obtain appropriate working concentrations. DOPC/GM1 (95:5 mol%) solutions were prepared at 1 mg/mL concentrations in a 65:35 volume mixture of chloroform and methanol. Lipid mixtures prepared for WGM assays were further doped with 0.25 mol% TR-DHPE to enable WGM fluorescence imaging. Approximately 50 μL of the appropriate lipid solution was dispersed on a 18MΩ water subphase in a Langmuir-Blodgett trough (Type 611, Nima Technology, Coventry, England). The solvent was allowed to evaporate for at least 15 min prior to initiating compression/expansion cycles to anneal the film. Each monolayer was subjected to two compression/expansion cycles between surface pressures of 10 mN/m and 40 mN/m with the barrier rate held constant at 100 cm2/min. Following the last expansion, the monolayers were compressed to 25 mN/m and held for ~10 min prior to transfer onto the substrate. Bilayers were transferred on to the immobilized glass microspheres at dipping velocity of 1 mm/min by the Langmuir-Blodgett/Langmuir-Schaeffer method resulting Y-type bilayers. All bilayers were transferred and imaged at 22°C.
Assay Preparation
Prepared glass cover slips were fit into the custom flow cell. Refractive index matching fluid (n=1.514 immersion oil, Olympus, Center Valley, PA) was used between the prism surface and sample slide. A syringe pump (Harvard apparatus, Holliston, MA) was used to fill the flow cell chamber with a PBS solution (MP Biomedicals, Solon, OH). A second syringe pump and fluidic controller (Warner Instrument Corp., Hamden, CT) was used to inject aliquots of purified recombinant cholera toxin beta labeled with A555 (CTxB-A555) (Molecular Probes, Eugene, OR) into the flow chamber. Each injection was allowed in incubate in the flow chamber for 5 minutes before being flushed with PBS and imaged.
TIR Fluorescence imaging
Fluorescence imaging assays of cholera toxin binding to supported bilayers of DOPC/GM1 utilized CTxB-A555 (Molecular Probes). Binding of the CTxB-A555 to the lipid bilayer was imaged with an inverted microscope (Olympus IX71) equipped with a 60x PlanAPO objective (1.45 NA, Olympus). The 514 nm line from an argon ion laser (Innova 90, Coherent Inc., Santa Clara, CA) was coupled into the microscope through the objective using a total internal reflection illumination configuration. Emission from the bound CTxB-A555 was collected with the same objective, filtered to remove the excitation light (Chroma), and imaged onto a cooled CCD (Coolsnap K4). Image collection was controlled with Slidebook software (Intelligent Imaging Innovations).
WGM Fluorescence Imaging
The tunable output from a Vortex II TLB-6900 external cavity diode laser (New Focus, Santa Clara, CA) was directed into a N-BK7 Dove prism (n = 1.52, Edmund Optics, Barrington, NJ), on which the sample was mounted. Total internal reflection at the substrate interface creates an evanescent field, which was used to launch light into the immobilized microspheres. At a WGM resonance, an enhanced ring of fluorescence from TR-DHPE was observed from the microspheres, which were imaged from above. The fluorescence was collected through a 10X UMPlanFL (0.3 NA) objective (Olympus, Center Valley, PA), filtered to remove the residual excitation (Chroma, Bellows Falls, VT), and imaged onto a cooled CCD camera (Coolsnap K4, Roper Scientific, Tuscon, AZ). A LabView program controlled scanning of the laser system, which was synchronized with Slidebook image collection software (Intelligent Imaging Innovations, Denver, CO).
Conclusion
High index glass microspheres were immobilized onto glass substrates using a calcium-assisted bonding method. The bonding method was shown not to degrade the optical properties of the immobilized resonators which was confirmed through characterization of resonator Q-factors. The immobilized resonators were stable over the flow rates necessary for assay development and amenable to lipid film transfer using the Langmuir-Blodgett and Langmuir-Schaeffer methods. Bilayers transferred on to immobilized spheres using the sequential transfer of monolayers of DOPC doped with 5 mol% GM1 were fabricated to detect CTxB-A555 which binds to GM1. CTxB-A555 binding to GM1 was characterized by measuring shifts in the WGM resonance. Analysis of the resulting binding curves yields a measured Kd of 1.5 × 10−11 which is consistent with previous SPR measurements. The measured detection limit of 3.3 pM is competitive with other approaches and the small size of the microspheres reduces the detection footprint and amount of material needed. The calcium bonding method, therefore, is shown to lead to stably immobilized resonators that are compatible with the fluidics necessary for assay development. This removes a significant barrier for integrating microsphere resonators with assay fluidics for WGM detection. The superior optical properties of spheres coupled with their availability in a wide range of sizes and materials opens new opportunities for cost effective assay development and deployment.
Acknowledgments
K.P.A. gratefully acknowledges support from the NIH Dynamic Aspects of Chemical Biology Training Grant (T32 GM08545). We gratefully acknowledge support from NSF (CBET 1133814).
References
- 1.Vollmer F, Arnold S. Nature Methods. 2008;5:591–596. doi: 10.1038/nmeth.1221. [DOI] [PubMed] [Google Scholar]
- 2.Gorodetsky ML, Ilchenko VS. J Opt Soc Am B. 1999;16:147–154. [Google Scholar]
- 3.Knight JC, Cheung G, Jacques F, Birks TA. Opt Lett. 1997;22:1129–1131. doi: 10.1364/ol.22.001129. [DOI] [PubMed] [Google Scholar]
- 4.Vollmer F, Yang L. Nanophotonics. 2012;1:267. doi: 10.1515/nanoph-2012-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soria S, Berneschi S, Brenci M, Cosi F, Nunzi Conti G, Pelli S, Righini GC. Sensors. 2011;11:785–805. doi: 10.3390/s110100785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan XD, White IM, Shopova SI, Zhu HY, Suter JD, Sun YZ. Anal Chim Acta. 2008;620:8–26. doi: 10.1016/j.aca.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huckabay HA, Dunn RC. Sens Actuators, B. 2011;160:1262–1267. [Google Scholar]
- 8.Arnold S, Khoshsima M, Teraoka I, Holler S, Vollmer F. Opt Lett. 2003;28:272–274. doi: 10.1364/ol.28.000272. [DOI] [PubMed] [Google Scholar]
- 9.Vollmer F, Braun D, Libchaber A, Khoshsima M, Teraoka I, Arnold S. Appl Phys Lett. 2002;80:4057–4059. [Google Scholar]
- 10.Vollmer F, Arnold S, Braun D, Teraoka I, Libchaber A. Biophys J. 2003;85:1974–1979. doi: 10.1016/S0006-3495(03)74625-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huckabay HA, Wildgen SM, Dunn RC. Biosens Bioelectron. 2013 doi: 10.1016/j.bios.2013.01.072. In press. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Zhang Z, Qin S, Wang T, Liu F, Qiu M, Su Y. Appl Opt. 2009;48:F90–94. doi: 10.1364/AO.48.000F90. [DOI] [PubMed] [Google Scholar]
- 13.Qavi AJ, Bailey RC. Angew Chem, Int Ed. 2010;49:4608–4611. S4608/4601–S4608/4621. doi: 10.1002/anie.201001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiasera A, Dumeige Y, Feron P, Ferrari M, Jestin Y, Conti GN, Pelli S, Soria S, Righini GC. Laser Photonics Rev. 2010;4:457–482. [Google Scholar]
- 15.Vollmer F, Arnold S, Keng D. Proc Natl Acad Sci U S A. 2008;105:20701–20704. doi: 10.1073/pnas.0808988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Washburn AL, Luchansky MS, Bowman AL, Bailey RC. Anal Chem. 2009;82:69–72. doi: 10.1021/ac902451b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan X, White IM, Zhu H, Suter JD, Oveys H. Laser Resonators and Beam Control IX. 2007:64520M–64520M. [Google Scholar]
- 18.Gorodetsky ML, Savchenkov AA, Ilchenko VS. Opt Lett. 1996;21:453–455. doi: 10.1364/ol.21.000453. [DOI] [PubMed] [Google Scholar]
- 19.Vahala KJ. Nature. 2003;424:839–846. doi: 10.1038/nature01939. [DOI] [PubMed] [Google Scholar]
- 20.Mazzei A, Götzinger S, Menezes LdS, Sandoghdar V, Benson O. Optics Communications. 2005;250:428–433. [Google Scholar]
- 21.Allen PB, Chiu DT. Anal Chem (Washington, DC, U S) 2008;80:7153–7157. doi: 10.1021/ac801059h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reed RA, Mattai J, Shipley GG. Biochemistry. 1987;26:824–832. doi: 10.1021/bi00377a025. [DOI] [PubMed] [Google Scholar]
- 23.MacKenzie CR, Hirama T, Lee KK, Altman E, Young NM. The Journal of biological chemistry. 1997;272:5533–5538. doi: 10.1074/jbc.272.9.5533. [DOI] [PubMed] [Google Scholar]
- 24.Kuziemko GM, Stroh M, Stevens RC. Biochemistry. 1996;35:6375–6384. doi: 10.1021/bi952314i. [DOI] [PubMed] [Google Scholar]
- 25.Davis F, Higson SPJ. Biosens Bioelectron. 2005;21:1–20. doi: 10.1016/j.bios.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Girard-Egrot A, Blum L. In: Nanobiotechnology of Biomimetic Membranes. Martin D, editor. Vol. 1. Springer US; 2007. pp. 23–74. [Google Scholar]