Abstract
Choline is a crucial factor in the regulation of sperm membrane structure and fluidity, and this nutrient plays an important role in the maturation and fertilizing capacity of spermatozoa. Transcripts of phosphatidylethanolamine N-methyltransferase (PEMT) and choline dehydrogenase (CHDH), two basic enzymes of choline metabolism, have been observed in the human testis, demonstrating their gene expression in this tissue. In the present study, we explored the contribution of the PEMT and CHDH gene variants to sperm parameters. Two hundred oligospermic and 250 normozoospermic men were recruited. DNA was extracted from the spermatozoa, and the PEMT −774G>C and CHDH +432G>T polymorphisms were genotyped. The genotype distribution of the PEMT −774G>C polymorphism did not differ between oligospermic and normozoospermic men. In contrast, in the case of the CHDH +432G>T polymorphism, oligospermic men presented the CHDH 432G/G genotype more frequently than normozoospermic men (62% vs. 42%, P<0.001). The PEMT 774G/G genotype was associated with a higher sperm concentration compared to the PEMT 774G/C and 774C/C genotypes in oligospermic men (12.5±5.6×106 spermatozoa ml−1 vs. 8.3±5.2×106 spermatozoa ml−1, P<0.002) and normozoospermic men (81.5±55.6×106 vs. 68.1±44.5×106 spermatozoa ml−1, P<0.006). In addition, the CHDH 432G/G genotype was associated with higher sperm concentration compared to CHDH 432G/T and 432T/T genotypes in oligospermic (11.8±5.1×106 vs. 7.8±5.3×106 spermatozoa ml−1, P<0.003) and normozoospermic men (98.6±62.2×106 vs. 58.8±33.6×106 spermatozoa ml−1, P<0.001). In our series, the PEMT −774G>C and CHDH +432G>T polymorphisms were associated with sperm concentration. This finding suggests a possible influence of these genes on sperm quality.
Keywords: choline dehydrogenase, male infertility, phosphatidylethanolamine N-methyltransferase, phospholipids, sperm concentration
Introduction
Choline, phosphatidylcholine (PC), diacylcholine phosphoglycerides and betaine play a crucial role in lipid metabolism and transport, methylation processes and the biosynthesis of the neurotransmitter acetylcholine.1 PC constitutes the principal phospholipid of eukaryotic cellular membranes. A plasma membrane analysis of mammalian epididymal spermatozoa revealed an exclusively high concentration of the choline-plasmalogens in the PC fractions of phospholipids, which are localized mainly in the outer membrane lipid monolayer.2 This finding explains subsequent observations that alterations in PC concentration have been implicated in changes of sperm membrane fluidity.3
A main pathway for the synthesis of PC in hepatic tissue is the methylation of phosphatidylethanolamine with the catalytic action of phosphatidylethanolamine N-methyltransferase (PEMT).4 Two isoforms of PEMT have been identified: PEMT1, localized to the cytosolic surface of the endoplasmic reticulum, and PEMT2, localized to mitochondria-associated membranes (PEMT2).5, 6 Both isoforms are capable of catalysing all three methylation steps for the conversion of phosphatidylethanolamine to PC.7 PEMT1 generates the majority of PEMT activity in the liver,8 and PEMT2 is related to liver growth.9 The significance of the PEMT gene to choline metabolism has been confirmed by studies of PEMT knockout mice. These mice present with reduced plasma homocysteine10 and docosahexaenoic and arachidonic acid concentrations;11 membrane integrity loss;12 abnormal hepatic choline metabolite levels and severe hepatic steatosis;13 and noteworthy changes in the expression of genes regulating cell cycle, differentiation and neurogenesis.14 Apart from the liver, PEMT transcripts have been observed in the human testis, indicating the action of PC biosynthesis in this tissue.9 Indeed, epithelial cells of the epididymis synthesize choline, making it clear that this nutrient is a major biochemical marker of epididymal secretory function.15
Choline dehydrogenase (CHDH) also plays an important role in choline metabolism by catalysing the oxidation of choline to betaine in the inner mitochondrial membrane.16 Betaine acts as a methyl donor in the conversion of homocysteine to methionine.17 Testicular betaine concentrations have been found at 10 times that of concentrations in the liver, the organ thought to be the primary choline metabolism site. This finding suggests that betaine plays a critical role in testicular function.18 Furthermore, testis, liver and kidney tissues have the highest CHDH activity, and choline and betaine concentrations are the most altered by CHDH gene deletion at these sites.18 The significance of the CHDH gene in the male reproductive system has been confirmed by studies of CHDH knockout male mice. The impaired fertility of these mice is mainly due to diminished sperm motility, abnormal mitochondrial morphology, inner membrane polarisation and reduced ATP content.18
In humans, a great amount of variation in the PEMT and CHDH genes has been identified. Of the variants, PEMT rs12325817 (+744G→C) and CHDH rs12676 (−432G→T) have been found to affect PEMT and CHDH gene activity, respectively. For example, the PEMT −774G>C polymorphism is implicated in organ dysfunction in low choline diets,19 the CHDH +432G>T polymorphism is associated with an increased susceptibility to choline deficiency,19 and both of them are correlated with breast cancer risk.20 In the present study, considering the above findings and the presence of PEMT and CHDH transcripts in testis, we examined the potential association of the PEMT −774G>C and CHDH +432G>T polymorphisms with sperm parameters in normozoospermic and oligospermic men.
Materials and methods
Subjects
The study population consisted of 250 normozoospermic and 200 oligospermic Greek men who were referred to the In Vitro Fertilization Unit of the Department of Obstetrics and Gynecology of the Medical School of Ioannina in Greece for semen analysis. The stratification of men in the normozoospermic and oligospermic groups was based on sperm concentration.21 Specifically, men with a sperm concentration ≥20×106 spermatozoa ml−1 were characterized as normozoospermic.
A detailed medical history was obtained from all subjects. Information on reproductive history and general lifestyle was taken into account. Every participant completed a Block Food Frequency Questionnaire22 that assessed the intake of more than 100 food items in the year before the sampling. The dietary intakes of choline and betaine were estimated using a previously described protocol.23 Only men with choline and betaine intakes that qualified as normal according to the US Institute of Medicine standards24 participated in the current study. Men consuming choline-deficient and betaine-deficient diets were excluded from the study.
Men suffering from metabolic syndrome, hydrocele, hypogonadotropic hypogonadism, varicocele or obstructive syndromes of the seminal tract; men carrying microdeletions of the long arm of the Y-chromosome or karyotype abnormalities; and men taking spermatogenesis-impairing medication were excluded from the study.
Semen analysis was performed according to World Health Organization guidelines.21 The men were asked to abstain from sexual activity for 4 days prior to semen analysis. Two independent investigators performed a blind semen analysis. The average values of the two investigators were calculated. In the event of inconsistency (over 10% difference), a third assessment was done.
Blood samples were drawn from the participants for the measurement of serum follicle-stimulating hormone (FSH), luteinizing hormone (LH) and testosterone (T). Total T, serum FSH and LH concentrations were determined by chemiluminescent microparticle immunoassay. The coefficients of variation were 4% for total testosterone, 3.5% for LH and 4% for FSH. DNA was extracted from the sperm according to a previously described protocol.25
The Institutional Ethics Committee approved the study protocol in accordance with the Helsinki declaration. All participants gave informed consent.
Genotype analysis
Polymerase chain reaction was used to amplify the PEMT −774G>C and CHDH +432G>T polymorphisms. The primer pairs we used are listed as follows: PEMT 774F: 5′-ACT TCC TGG GTT GAA GCG ATT CTC-3′ PEMT 774R: 5′-TTT ATT CTC TGG CCG TGC CCA G-3′ CHDH 432F: 5′-AGT CAT CTC ATT CCC CTC CGT GGA TCA GA-3′ and CHDH 432R: 5′-TAG CAC CAG TTG TAC CTG TCG TCG CAC A-3′. The thermal cycling for the PEMT −774G>C polymorphism is described as follows: denaturation at 96 °C for 2 min, 30 cycles of 94 °C for 30 s, 60 °C for 1 min, 72 °C for 2 min and a final extension at 72 °C for 10 min. The thermal cycling for the CHDH +432G>T polymorphism is described as follows: denaturation at 94 °C for 1 min, 30 cycles at 94 °C for 30 s, 68 °C for 3 min and a final extension at 70 °C for 10 min.
The 224-base pair PEMT polymerase chain reaction products were digested with restriction enzyme endonuclease BsmBI, which cut the wild type G allele into two products of 132 and 92 base pairs. Likewise, the 370-base pair CHDH polymerase chain reaction products were digested with restriction enzyme endonuclease BssHII, which cut the wild type G allele into two products of 281 and 89 base pairs. The enzyme digestion products were separated by 3% agarose gel electrophoresis and visualized by exposure to ultraviolet light after ethidium bromide staining. All reactions were run in duplicates with negative and positive controls and blanks. The resulting genotypes for PEMT (G/C) 774 and CHDH (G/T) 432 and polymorphic sites were characterized as GG/GC/CC and GG/GT/TT, respectively.
Statistical analysis
Statistical analysis of the differences in allele and genotype frequencies was performed using the chi-square test. Statistical analysis was also performed for pooled samples (combination of heterozygotes and homozygotes). Normal distribution of continuous parameters was tested by the Kolmogorov–Smirnov test. Differences in continuous parameters were assessed by the t-test for independent variables, the non-parametric Mann–Whitney U test or the Kruskal–Wallis test as appropriate. Regression analysis was performed using choline and betaine intakes as independent variables to examine their effect on sperm concentration and motility, and the interaction effect between nutrient intake and the PEMT −774G>C and CHDH +432G>T genotypes. A P value of <0.05 was set as statistically significant. All results are reported as the mean±s.d. All analyses were carried out using the SPSS statistical package (version 14.0; SPSS Inc., Chicago, IL, USA).
Results
Clinical characteristics of the study population
The clinical characteristics of 250 normozoospermic men and 200 oligospermic patients are presented in Table 1.
Table 1. Characteristics of the study population.
| Normozoospermic men | Oligospermic patients | P value | |
|---|---|---|---|
| Number of patients | 250 | 200 | |
| Age of patients (year) | 36.9±7.3 | 36.1±6.6 | ns |
| Sperm concentration (×106 spermatozoa ml−1) | 76.1±53.3 | 9.9±6.1 | <0.001 |
| Sperm motility (%) | 54.2±17.1 | 37.9±16.9 | <0.001 |
| Sperm morphology (%) | 38.2±7.1 | 26.1±5.8 | <0.002 |
| Daily choline intake (mg) | 320.9±43.1 | 324.7±41.4 | ns |
| Daily betaine intake (mg) | 126.1±34 | 130.9±34.3 | ns |
| Body mass index (kg m−2) | 26.9±4.8 | 26.2±5.3 | ns |
| FSH (mIU ml−1) | 5.1±1.6 | 9.9±1.8 | <0.001 |
| LH (mIU ml−1) | 4.7±1.5 | 10.8±1.4 | <0.001 |
| T (ng dl−1) | 779±31 | 419±22 | <0.001 |
Abbreviations: FSH, follicle-stimulating hormone; LH, luteinizing hormone; ns, non-significant; T, testosterone.
Normozoospermic men: sperm concentration ≥20×106 spermatozoa ml−1; oligospermic men: sperm concentration <20×106 spermatozoa ml−1. Data are shown as mean±s.d.
Genotype analysis
The genotypic and allelic distribution of the PEMT −774G>C polymorphism did not differ between oligospermic and normozoospermic men (Table 2). The ratio between PEMT −774G/G to PEMT −774G/C and -774C/C carriers also did not differ between oligospermic and normozoospermic men (34%/66% vs. 40%/60%, P=0.191). However, the CHDH +432G>T genotype and allele frequencies were notably different between normozoospermic and oligospermic men (Table 2). Specifically, the frequency of both the CHDH 432T/T genotype and the CHDH +432T allele was increased in normozoospermic men compared to oligospermic men (P<0.001 and P<0.001, respectively). Additionally, the ratio between CHDH 432G/G to CHDH 432G/T and 432T/T carriers differed between oligospermic and normozoospermic men (62%/38% vs. 42%/58%, P<0.001).
Table 2. The genotypic and allelic distribution of the PEMT and CHDH polymorphisms in normozoospermic and oligospermic men.
| Polymorphism | Genotypes/alleles | Oligospermic men, n (%) | Normozoospermic men, n (%) | P value |
|---|---|---|---|---|
| PEMT −774G>C | GG | 68 (34.0) | 100 (40.0) | ns* |
| GC | 98 (49.0) | 115 (46.0) | ||
| CC | 34 (17.0) | 35 (14.0) | ||
| G | 234 (58.5) | 315 (63.0) | ns* | |
| C | 166 (41.5) | 185 (37.0) | ||
| CHDH +432G>T | GG | 124 (62.0) | 105 (42.0) | <0.001* |
| GT | 70 (35.0) | 103 (41.2) | ||
| TT | 6 (3.0) | 42 (16.8) | ||
| G | 318 (79.5) | 313 (62.6) | <0.001* | |
| T | 82 (20.5) | 187 (37.4) |
Abbreviations: CHDH, choline dehydrogenase; ns, non-significant; PEMT, phosphatidylethanolamine N-methyltransferase.
Normozoospermic men ≥20×106 spermatozoa ml−1; oligospermic men <20×106 spermatozoa ml−1. Data shown as number (n) and percentage (%). Both polymorphisms are in Hardy–Weinberg equilibrium.
Chi-square test analysis.
The gene frequencies for PEMT −774G>C and CHDH +432G>T polymorphisms were in Hardy–Weinberg equilibrium in oligospermic (χ2=0.02, P>0.05; χ2=1.09, P>0.05; respectively) and normozoospermic men (χ2=0.04, P>0.05; χ2=3.61, P>0.05, respectively).
Regression analysis was performed to investigate if the effect of PEMT −774G>C and CHDH +432G>T polymorphisms on sperm concentration and motility depended on choline and betaine intakes, respectively. We found that the effect of the PEMT −774G>C polymorphism on sperm concentration in oligospermic (β=0.043, P=0.463) and normozoospermic men (β=0.016, P=0.836) was independent of choline intake. Additionally, the effect of this polymorphism on sperm motility in oligospermic (β=0.022, P=0.703) and normozoospermic men (β=0.052, P=0.510) was independent of choline intake. Alternatively, the effect of the CHDH +432G>T polymorphism on sperm concentration in oligospermic (β=0.054, P=0.357) and normozoospermic men (β=−0.032, P=0.670) was independent of betaine intake, whereas the effect of this polymorphism on sperm motility in oligospermic (β=0.078, P=0.180) and normozoospermic men (β=0.127, P=0.091) was independent of choline intake. Additionally, the choline and betaine intakes did not show important differences between PEMT −774G>C and CHDH +432G>T genotypes, respectively (data not shown).
No differences in age were observed between normozoospermic men and oligospermic patients. The simultaneous analysis of the PEMT(G/C) 774 and CHDH(G/T) 432 genotypes/alleles, serum FSH, LH and T levels, and sperm morphology and body mass index revealed no correlations in normozoospermic nor oligospermic men. Additionally, no correlation was found between CHDH–PEMT genotypes and serum FSH, LH and T levels, indicating that altered CHDH–PEMT activity may not influence serum hormone levels.
Association of PEMT −774G>C polymorphism with sperm parameters
Our analysis of the PEMT −774G>C polymorphism revealed important associations between the gene variant and sperm concentration in normozoospermic men. Specifically, men with the 774G/G genotype had a sperm concentration of 81.5±55.6×106 spermatozoa ml−1, whereas those with 774G/C and 774C/C genotypes had a lower sperm concentration (68.1±44.5×106 spermatozoa ml−1, P<0.006) (Figure 1a). The analysis of each PEMT −774G>C genotype separately revealed noteworthy differences in the respective sperm concentrations (PEMT −774G/G: 81.5±55.6×106 spermatozoa ml−1, PEMT −774G/C: 70.2±46.6×106 spermatozoa ml−1, PEMT −774C/C: 67.0±44.2×106 spermatozoa ml−1, P<0.02).
Figure 1.
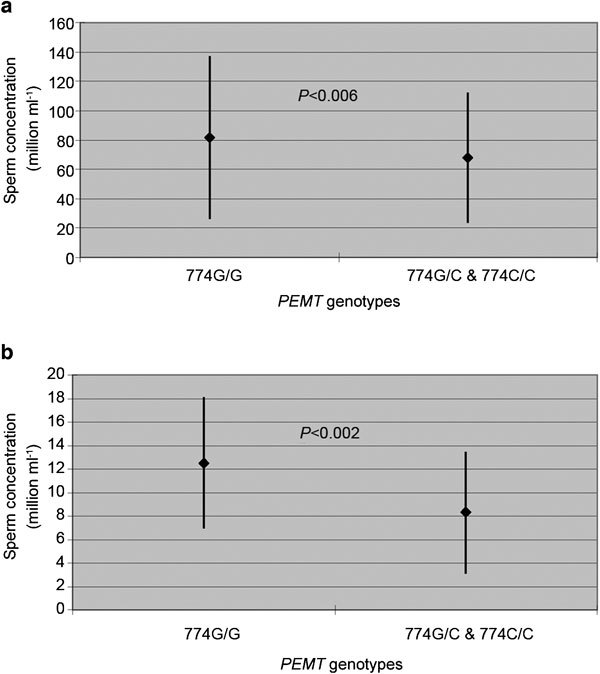
Association of the PEMT −774G>C genotypes with sperm concentration in (a) normozoospermic men (P<0.006) and (b) oligospermic men (P<0.002). Data are shown as mean±s.d. PEMT, phosphatidylethanolamine N-methyltransferase.
We observed a similar association in oligospermic men. Specifically, men with the 774G/G genotype had a sperm concentration of 12.5±5.6×106 spermatozoa ml−1, whereas those with the 774G/C and 774C/C genotypes had a lower sperm concentration (8.3±5.2×106 spermatozoa ml−1, P<0.002) (Figure 1b).
We found no associations between the PEMT −774G>C polymorphism and sperm motility in normozoospermic nor oligospermic men (data not shown).
Association of CHDH +432G>T polymorphism with sperm parameters
Our analysis of the CHDH +432G>T polymorphism found noteworthy associations between the gene variant and sperm concentration in normozoospermic men. Specifically, men with the 432G/G genotype had a sperm concentration of 98.6±62.2×106 spermatozoa ml−1, whereas those with the 432G/T and 432T/T genotypes had a lower sperm concentration (58.8±33.6×106 spermatozoa ml−1, P<0.001) (Figure 2a). The analysis of each CHDH +432G>T genotype separately revealed noteworthy differences in the respective sperm concentrations (CHDH 432G/G: 98.6±62.2×106 spermatozoa ml−1, CHDH 432G/T: 64.1±37.9×106 spermatozoa ml−1, CHDH 432T/T: 53.8±30.5×106 spermatozoa ml−1, P<0.001).
Figure 2.
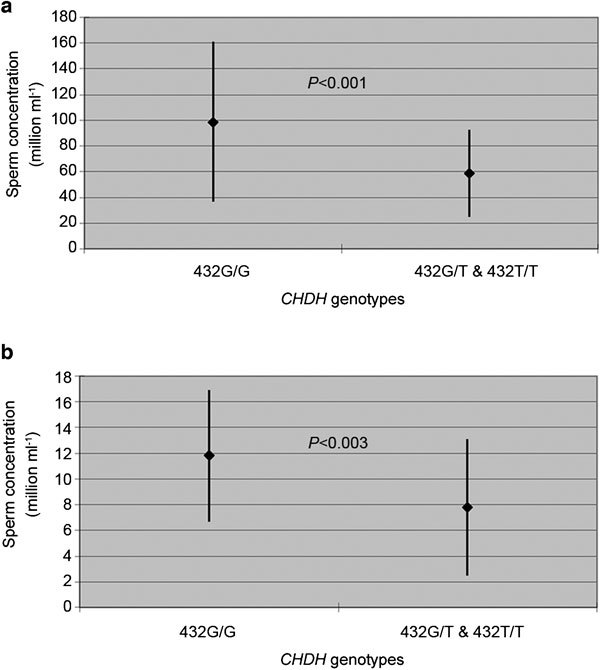
Association of the CHDH +432G>T genotypes with sperm concentration in (a) normozoospermic men (P<0.001) and (b) oligospermic men (P<0.003). Data are shown as mean±s.d. CHDH, choline dehydrogenase.
A similar association was observed in oligospermic men. Specifically, men with the 432G/G genotype had a sperm concentration of 11.8±5.1×106 spermatozoa ml−1, whereas those with the 432G/T and 432T/T genotypes had a lower sperm concentration (7.8±5.3×106 spermatozoa ml−1, P<0.003) (Figure 2b).
No associations were found between the CHDH +432G>T polymorphism and sperm motility in oligospermic nor normozoospermic men (data not shown).
Association of PEMT −774G>C/CHDH +432G>T combined genotypes with sperm parameters
The combined genotype analysis of the PEMT and CHDH gene variants failed to identify any synergistic effect on sperm concentration (data not shown), suggesting a potential independent influence of each polymorphism on sperm concentration.
Discussion
Choline constitutes an important source of methyl groups in the human diet.26 Choline and its metabolites play an essential role in the formation of the methyl donor S-adenosylmethionine, which is implicated in more than 80 biological methylation reactions, including the methylation of proteins, DNA and RNA. Approximately 56% of sperm plasma membrane phospholipids consist of choline and ethanolamine phosphoglycerides.3 Additionally, glycerophosphorylcholine and choline, synthesized by the epithelial cells of the epididymis, are necessary for spermatozoa maturation.27 These facts support a possibly important role of choline metabolism in spermatogenesis. Alterations in the enzymes participating in choline metabolism, such as PEMT and choline dehydrogenase, due to gene sequence variations and single nucleotide polymorphisms can probably affect spermatogenesis.
The aim of the current study was to examine the genotype distribution of the PEMT −774G>C and CHDH +432G>T polymorphisms in oligospermic and normozoospermic men and their association with sperm parameters. Our data show that the polymorphisms described above may influence human spermatogenesis, and consequently, sperm quality. Specifically, the PEMT 774G/G genotype was associated with higher sperm concentration compared to the PEMT 774G/C and 774C/C genotypes in both oligospermic and normozoospermic men. However, the genotypic and allelic distribution of the CHDH +432G>T polymorphism presented notable differences between oligospermic and normozoospermic men, whereas the CHDH 432G/G genotype was associated with higher sperm concentration compared to the CHDH 432G/T and 432T/T genotypes in oligospermic and normozoospermic men.
Considering that choline is synthesized de novo or obtained from the diet, we took into account the data showing that healthy men fed a choline-deficient diet became choline-depleted and developed hepatic steatosis and damage,28 and that animals developed growth retardation, bone abnormalities and renal dysfunction.29, 30, 31 We therefore only recruited men with normal choline intakes so we could investigate the direct effects of the PEMT −774G>C and CHDH +432G>T polymorphisms on sperm quality. Men who ate a choline-deficient diet were excluded from the study because their phenotype could have been a consequence of their dietary choline absence and not the result of the PEMT/CHDH genotypes. Indeed, the regression analysis showed that the effect of the PEMT −774G>C and CHDH +432G>T polymorphisms on sperm concentration in our study population was independent of choline intake. However, we did not measure choline and betaine levels in peripheral blood or sperm plasma in the current study because, according to a previous study, these measurements may reflect short-term dietary intake and do not accurately represent the effects of long-term exposure.32 Nonetheless, future studies that measure choline and betaine levels may reveal important associations between these nutrients and the PEMT −774G>C and CHDH +432G>T genotypes.
A noteworthy association of the PEMT−774G/G genotype with higher sperm concentration was observed in normozoospermic and oligospermic men, whereas homozygosity or heterozygosity for the PEMT −774C allele was associated with lower sperm concentrations. The PEMT −774C/C genotype-normozoospermic men presented with the lowest sperm concentrations, illustrating the negative effect of the PEMT −774C allele on sperm concentration. Previous studies have implicated the PEMT −774C allele in pathogenic situations. Specifically, the minor PEMT −774C allele has been associated with organ dysfunction in premenopausal and postmenopausal women when choline is removed from their diet,19 and an increased dietary requirement for choline has been recommended for women carrying a PEMT −774C allele. The PEMT −774C allele has also been associated with an increased risk of breast cancer.20 Finally, it has been proposed that PEMT −744C/C genotype carriers have a greater dependency on the CDP/choline pathway for phosphatidylcholine biosynthesis compared to those with a PEMT −744G/G genotype,7 likely due to their defective PEMT pathway.
Taking the above findings into consideration, we speculate that the PEMT −774C allele affects choline metabolism. However, functional studies investigating the influence of the PEMT −774C allele are lacking, and consequently, we cannot predict the effect of this allele on gene activity. PEMT catalyses the methylation of phosphatidylethanolamine to PC, the principal phospholipid of eukaryotic cellular membranes, which is responsible for the maintenance of membrane integrity. It has been shown that PEMT knockout mice fed a choline-deficient diet present with noteworthy decreases in plasma phosphatidylcholine levels,7 abnormal choline metabolite levels13 and membrane integrity loss12 due to impaired PC synthesis. Furthermore, there is much evidence to implicate PEMT in spermatogenesis, such as the presence of PEMT transcripts in the testis, which constitutes proof of phosphatidylcholine biosynthesis in this tissue;9 the crucial structural role of PC in membranes and lipoproteins of spermatozoa;3 and the important correlation between sperm maturity and the glycerylphosphorylcholine ratio,33 which supports the observation that spermatozoa do not achieve full maturation and fertilizing capacity until passage through the epididymis.27 Consequently, alterations in PEMT activity cause impairments in spermatogenesis. The decreased sperm concentration of the PEMT −774G/C and −774C/C men observed in the current study could be associated with lower phosphatidylcholine biosynthesis due to alterations in PEMT activity. However, a larger series of studies is needed to verify the above findings. In addition, studies investigating the reproductive tract of male PEMT knockouts would reveal the actual role of the PEMT enzyme on spermatogenesis.
The CHDH +432G>T polymorphism analysis revealed a noteworthy association of the CHDH +432G allele with higher sperm concentration in normozoospermic and oligospermic men. The CHDH 432G/G genotype and consequently, the CHDH +432G allele presented with higher frequency in oligospermic compared to normozoospermic men. Although oligospermic men have a higher frequency of the CHDH 432G/G genotype, their sperm concentration is extremely low. This finding could be explained by the fact that spermatogenesis is a very complicated process; CHDH possibly participates in but does not completely regulate spermatogenesis. Moreover, in the case of oligospermia, many genetic and environmental factors participate in the development of the final phenotype. The negative effect of the CHDH +432T allele on sperm concentration was confirmed when we focused on normozoospermic men who had no oligospermic factors. Among normozoospermic men, the CHDH 432T/T genotype-carriers had the lowest sperm concentration. Conversely, no association was found between CHDH +432G>T genotypes and sperm motility, although Johnson et al.18 have demonstrated that CHDH deletion causes diminished sperm motility, probably due to alterations in mitochondrial morphology, function and ATP content. CHDH converts choline to betaine aldehyde, which is then oxidized to betaine, a methyl donor for homocysteine. A previous study has shown that carriers of the minor CHDH 432T allele present with an increased susceptibility to developing organ dysfunction on a low choline diet.19 The CHDH +432G>T single nucleotide polymorphism produces an amino-acid substitution that could alter the enzyme activity, affecting the methyl moiety availability for the methylation reaction, and consequently, choline metabolism. However, given that there is no present evidence about the functional effects of the CHDH +432G>T polymorphism, we do not know how the CHDH 432 alleles influence CHDH gene or protein activity.
The extremely high betaine concentrations in the testis compared to the liver18 (the organ thought to be the principal choline metabolism site), along with the generation of five ATP molecules during the oxidation of one choline molecule to betaine,34 suggest that betaine probably plays a crucial role in testicular function. In addition, the increased testicular CHDH activity, the almost undetectable testicular choline and betaine concentrations in the case of the CHDH gene deletion and the asthenospermic phenotype of the male CHDH knockout mice 18 support the significance of CHDH in the male reproductive system. In the current study, a noteworthy association of the CHDH 432T allele with low sperm concentration was observed. Taking the above findings into consideration, we hypothesize that the CHDH 432T allele may have negative effects on CHDH activity, leading to reduced testicular betaine concentrations, lower testicular ATP content and consequently, to poorer sperm concentrations. However, further studies that investigate the association of the CHDH 432T allele with testicular CHDH activity and betaine levels are needed to clarify the correlation of this allele with impaired spermatogenesis.
To our knowledge, this study is the first to show that the association of the PEMT −774G>C and CHDH +432G>T polymorphisms with sperm concentration is probably due to alterations in testicular PEMT and choline dehydrogenase transcripts or activity. Although the number of cases enrolled in this study may limit the power of our conclusion, our findings are nevertheless indicative of the association of PEMT and CHDH enzymes with sperm concentration. The validation of our preliminary results in larger patient groups, the simultaneous measurement of testicular PEMT/CHDH levels, transcripts and activity and the assessment of sperm nuclear maturation may provide evidence for the contribution of these genes to the efficiency of spermatogenesis.
Author contributions
LL was the main investigator, wrote the article and participated in the conception of the study. NX performed the acquisition, analysis and interpretation of data. EH performed the semen analysis and participated in the execution of the study. AK made important contributions to the study design and sample collection. GM performed the patient recruitment, follow-up and drafting of the article. AT made important contributions to the study design and to the revision of the article. NS made important contributions to patient recruitment and the revision of the article. TS participated in patient recruitment and the drafting of the article. KZ participated in sample collection and the drafting of the article. IG was responsible for the conception and design of the study, the semen analysis and the coordination, drafting and revision of the article. All authors read and approved the final manuscript.
Acknowledgments
The authors would like to thank Mr. Prodromos Sakaloglou for his contribution in the laboratory work-up and the sample collection.
The authors declare no competing financial interests.
References
- Zeisel SH, Blusztajn JK. Choline and human nutrition. Annu Rev Nutr. 1994;14:269–96. doi: 10.1146/annurev.nu.14.070194.001413. [DOI] [PubMed] [Google Scholar]
- Hinkovska VT, Dimitrov GP, Koumanov KS. Phospholipid composition and phospholipid asymmetry of ram spermatozoa plasma membranes. Int J Biochem. 1986;18:1115–21. doi: 10.1016/0020-711x(86)90085-6. [DOI] [PubMed] [Google Scholar]
- Hall JC, Hadley J, Doman T. Correlation between changes in rat sperm membrane lipids, protein, and the membrane physical state during epididymal maturation. J Androl. 1991;12:76–87. [PubMed] [Google Scholar]
- Vance DE, Ridgway ND. The methylation of phosphatidylethanolamine. Prog Lipid Res. 1988;27:61–79. doi: 10.1016/0163-7827(88)90005-7. [DOI] [PubMed] [Google Scholar]
- Cui Z, Vance JE, Chen MH, Voelker DR, Vance DE. Cloning and expression of a novel phosphatidylethanolamine N-methyltransferase. A specific biochemical and cytological marker for a unique membrane fraction in rat liver. J Biol Chem. 1993;268:16655–63. [PubMed] [Google Scholar]
- Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem. 1990;266:7248–56. [PubMed] [Google Scholar]
- Walkey CJ, Donohue LR, Bronson R, Agellon LB, Vance DE. Disruption of the murine gene encoding phosphatidylethanolamine N-methyltransferase. Proc Natl Acad Sci USA. 1997;94:12880–5. doi: 10.1073/pnas.94.24.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway ND. Phosphatidylcholine Metabolism. Boca RatonFL; CRC Press; 1989. pp. 103–20. [Google Scholar]
- Shields DJ, Agellon LB, Vance DE. Structure, expression profile and alternative processing of the human phosphatidylethanolamine N-methyltransferase (PEMT) gene. Biochim Biophys Acta. 2001;1532:105–14. doi: 10.1016/s1388-1981(01)00122-6. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Su B, Jacobs RL, Kennedy B, Francis GA, et al. Lack of phosphatidylethanolamine N-methyltransferase alters plasma VLDL phospholipids and attenuates atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2009;29:1349–55. doi: 10.1161/ATVBAHA.109.188672. [DOI] [PubMed] [Google Scholar]
- Watkins SM, Zhu X, Zeisel SH. Phosphatidylethanolamine-N-methyltransferase activity and dietary choline regulate liver-plasma lipid flux and essential fatty acid metabolism in mice. J Nutr. 2003;133:3386–91. doi: 10.1093/jn/133.11.3386. [DOI] [PubMed] [Google Scholar]
- Li Z, Agellon LB, Allen TM, Umeda M, Jewell L, et al. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab. 2006;3:321–31. doi: 10.1016/j.cmet.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Zhu X, Song J, Mar MH, Edwards LJ, Zeisel SH. Phosphatidylethanolamine N-methyltransferase (PEMT) knockout mice have hepatic steatosis and abnormal hepatic choline metabolite concentrations despite ingesting a recommended dietary intake of choline. Biochem J. 2003;370 Pt 3:987–93. doi: 10.1042/BJ20021523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Zeisel SH. Gene expression profiling in phosphatidylethanolamine N-methyltransferase knockout mice. Brain Res Mol Brain Res. 2005;134:239–55. doi: 10.1016/j.molbrainres.2004.10.040. [DOI] [PubMed] [Google Scholar]
- Hamamah S, Seguin F, Bujan L, Barthelemy C, Mieusset R, et al. Quantification by magnetic resonance spectroscopy of metabolites in seminal plasma able to differentiate different forms of azoospermia. Hum Reprod. 1998;13:132–5. doi: 10.1093/humrep/13.1.132. [DOI] [PubMed] [Google Scholar]
- Huang S, Lin Q. Functional expression and processing of rat choline dehydrogenase precursor. Biochem Biophys Res Commun. 2003;309:344–50. doi: 10.1016/j.bbrc.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Garrow TA. Purification, kinetic properties, and cDNA cloning of mammalian betaine-homocysteine methyltransferase. J Biol Chem. 1996;271:22831–8. doi: 10.1074/jbc.271.37.22831. [DOI] [PubMed] [Google Scholar]
- Johnson AR, Craciunescu CN, Guo Z, Teng YW, Thresher RJ, et al. Deletion of murine choline dehydrogenase results in diminished sperm motility. FASEB J. 2010;24:2752–61. doi: 10.1096/fj.09-153718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa KA, Kozyreva OG, Song J, Galanko JA, Fischer LM, et al. Common genetic polymorphisms affect the human requirement for the nutrient choline. FASEB J. 2006;20:1336–44. doi: 10.1096/fj.06-5734com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Gammon MD, Zeisel SH, Lee YL, Wetmur JG, et al. Choline metabolism and risk of breast cancer in a population-based study. FASEB J. 2008;22:2045–52. doi: 10.1096/fj.07-101279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Cambridge; Cambridge University Press;; 1999. WHO Laboratory Manual for the Examination of Human Semen and Sperm–Cervical Mucus Interaction. 4th ed. [Google Scholar]
- Gaudet MM, Britton JA, Kabat GC, Steck-Scott S, Eng SM, et al. Fruits, vegetables, and micronutrients in relation to breast cancer modified by menopause and hormone receptor status. Cancer Epidemiol Biomarkers Prev. 2004;13:1485–94. [PubMed] [Google Scholar]
- Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr. 2003;133:1302–7. doi: 10.1093/jn/133.5.1302. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine . WashingtonDC; National Academy Press; 1998. Dietary Reference Intakes for Folate, Thiamin, Riboflavin, Niacin, Vitamin B12, Panthothenic Acid, Biotin, and Choline. Vol. 1. [PubMed] [Google Scholar]
- Lazaros L, Xita N, Kaponis A, Hatzi E, Plachouras N, et al. The association of aromatase (CYP19) gene variants with sperm concentration and motility. Asian J Androl. 2011;13:292–7. doi: 10.1038/aja.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu MD, Zeisel SH. Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J Nutr. 2002;132 8 Suppl: 2333S–5S. doi: 10.1093/jn/132.8.2333S. [DOI] [PubMed] [Google Scholar]
- Arrata WS, Burt T, Corder S. The role of phosphate esters in male fertility. Fertil Steril. 1978;30:329–33. [PubMed] [Google Scholar]
- Zeisel SH, da Costa KA, Franklin PD, Alexander EA, Lamont JT, et al. Choline, an essential nutrient for humans. FASEB J. 1991;5:2093–8. [PubMed] [Google Scholar]
- Newberne PM, Rogers AE. Labile methyl groups and the promotion of cancer. Annu Rev Nutr. 1986;6:407–432. doi: 10.1146/annurev.nu.06.070186.002203. [DOI] [PubMed] [Google Scholar]
- Handler P, Bernheim F. Choline deficiency in the hamster. Proc Soc Exp Med. 1949;72:569. doi: 10.3181/00379727-72-17502. [DOI] [PubMed] [Google Scholar]
- Fairbanks BW, Krider JL. Significance of B vitamins in swine nutrition. N Am Vet. 1945;26:18–23. [Google Scholar]
- Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006;26:229–50. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Banerjee A, Pandey HC, Singh G, Kumari GL. Application of seminal germ cell morphology and semen biochemistry in the diagnosis and management of azoospermic subjects. Asian J Androl. 2001;3:55–62. [PubMed] [Google Scholar]
- Kagawa T, Wilken DR, Lardy HA. Control of choline oxidation in liver mitochondria by adenine nucleotides. J Biol Chem. 1965;240:1836–42. [PubMed] [Google Scholar]


